Abstract
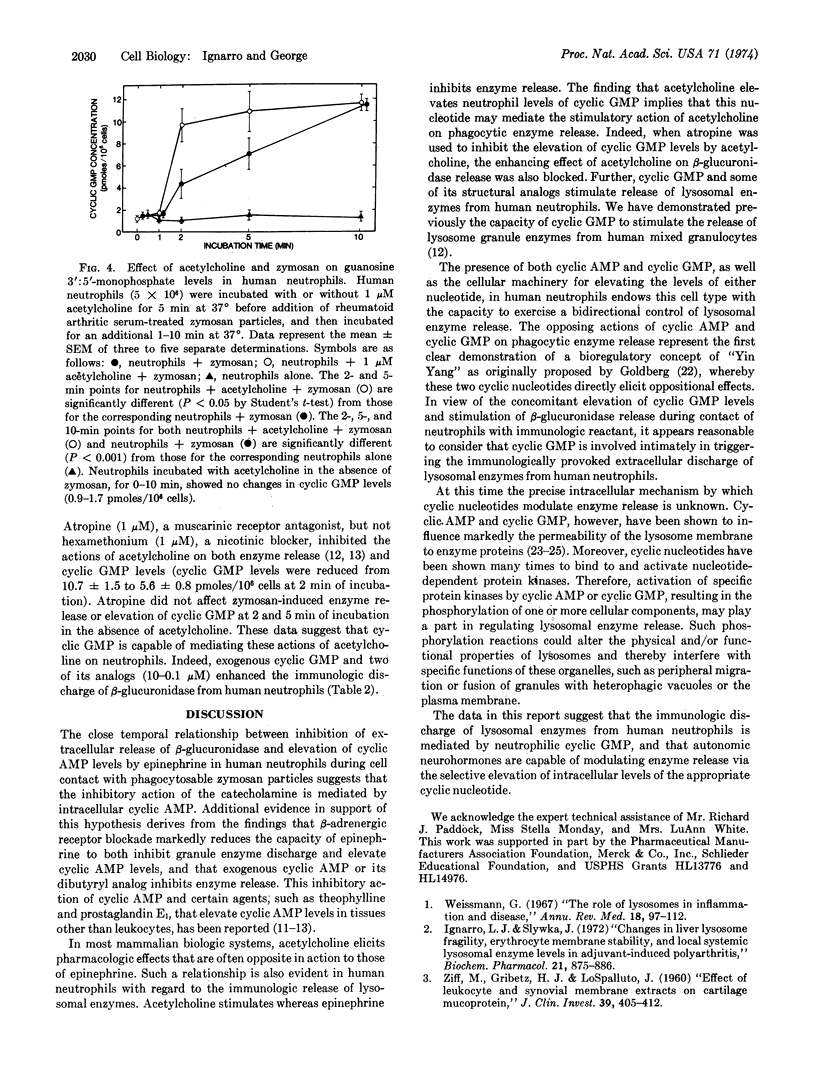
The influence of autonomic neurohormones on the immunologic release of β-glucuronidase (EC 3.2.1.31) from, and the cyclic nucleotide levels in, human neutrophils was determined. Interaction of neutrophils with rheumatoid arthritic, serum-treated zymosan particles in a neutral balanced salt solution at 37° resulted in the extracellular discharge of β-glucuronidase without any loss of cell viability, as indicated by the failure of incubated cells to take up eosin Y or to release cytoplasmic lactate dehydrogenase (EC 1.1.1.27). Epinephrine reduced the release of β-glucuronidase from neutrophils in the presence of zymosan during 2-30 min of incubation and elicited a concomitant elevation of adenosine 3′:5′-monophosphate levels. Propranolol, a β-adrenergic receptor antagonist, but not phentolamine, an α-adrenergic receptor antagonist, blocked both actions of epinephrine. Acetylcholine stimulated the release of β-glucuronidase, but not lactate dehydrogenase, and provoked a concomitant elevation of guanosine 3′:5′-monophosphate levels. Atropine, a muscarinic receptor antagonist, but not hexamethonium, a ganglionic blocker, inhibited both actions of acetylcholine. Interaction of neutrophils and zymosan particles resulted in an elevation of guanosine 3′:5′-monophosphate levels within 2 min. These data suggest that intracellular guanosine 3′:5′-monophosphate may be involved in mediating the immunologic release of lysosomal enzymes from human neutrophils whereas adenosine 3′:5′-monophosphate may inhibit enzyme release. Moreover, autonomic neurohormones appear to be capable of modulating lysosomal enzyme release by virtue of their capacity to elevate neutrophil cyclic nucleotide levels.
Keywords: guanosine 3′:5′-monophosphate, adenosine 3′:5′-monophosphate, lysosome membrane, acetylcholine, epinephrine
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cochrane C. G., Aikin B. S. Polymorphonuclear leukocytes in immunologic reactions. The destruction of vascular basement membrane in vivo and in vitro. J Exp Med. 1966 Oct 1;124(4):733–752. doi: 10.1084/jem.124.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIANETTO R., DE DUVE C. Tissue fractionation studies. 4. Comparative study of the binding of acid phosphatase, beta-glucuronidase and cathepsin by rat-liver particles. Biochem J. 1955 Mar;59(3):433–438. doi: 10.1042/bj0590433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George W. J., Polson J. B., O'Toole A. G., Goldberg N. D. Elevation of guanosine 3',5'-cyclic phosphate in rat heart after perfusion with acetylcholine. Proc Natl Acad Sci U S A. 1970 Jun;66(2):398–403. doi: 10.1073/pnas.66.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George W. J., Wilkerson R. D., Kadowitz P. J. Influence of acetylcholine on contractile force and cyclic nucleotide levels in the isolated perfused rat heart. J Pharmacol Exp Ther. 1973 Jan;184(1):228–235. [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman J. G., Robison G. A., Sutherland E. W. Cyclic nucleotides. Annu Rev Physiol. 1971;33:311–336. doi: 10.1146/annurev.ph.33.030171.001523. [DOI] [PubMed] [Google Scholar]
- Hawkins D., Cochrane C. G. Glomerular basement membrane damage in immunological glomerulonephritis. Immunology. 1968 May;14(5):665–681. [PMC free article] [PubMed] [Google Scholar]
- Hawkins D. Neutrophilic leukocytes in immunologic reactions: evidence for the selective release of lysosomal constituents. J Immunol. 1972 Feb;108(2):310–317. [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971 Dec;107(6):1535–1546. [PubMed] [Google Scholar]
- Ignarro L. J., Colombo C. Enzyme release from polymorphonuclear leukocyte lysosomes: regulation by autonomic drugs and cyclic nucleotides. Science. 1973 Jun 15;180(4091):1181–1183. doi: 10.1126/science.180.4091.1181. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Krassikoff N., Slywka J. Release of enzymes from a rat liver lysosome fraction: inhibition by catecholamines and cyclic3', 5'-adenosine monophosphate, stimulation by cholinergic agents and cyclic 3', 5'-guanosine monophosphate. J Pharmacol Exp Ther. 1973 Jul;186(1):86–99. [PubMed] [Google Scholar]
- Ignarro L. J. Neutral protease release from human leukocytes regulated by neurohormones and cyclic nucleotides. Nat New Biol. 1973 Oct 3;245(144):151–154. doi: 10.1038/newbio245151a0. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Oronsky A. L., Perper R. J. Breakdown of noncollagenous chondromucoprotein matrix by leukocyte lysosome granule lysates from guinea pig, rabbit, and human. Clin Immunol Immunopathol. 1973 Nov;2(1):36–51. doi: 10.1016/0090-1229(73)90034-2. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Preservation of structural integrity of liver lysosomes and membrane-stabilizing action of anti-inflammatory drugs, catecholamines and cyclic adenosine monophosphate in isotonic salt media. Biochem Pharmacol. 1973 Jun 1;22(11):1269–1282. doi: 10.1016/0006-2952(73)90301-8. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Slywka J. Changes in liver lysosome fragility, erythrocyte membrane stability, and local and systemic lysosomal enzyme levels in adjuvant-induced polyarthritis. Biochem Pharmacol. 1972 Mar 15;21(6):875–886. doi: 10.1016/0006-2952(72)90131-1. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Stimulation of phagocytic release of neutral protease from human neutrophils by cholinergic amines and cyclic 3',5'-guanosine monophosphate. J Immunol. 1974 Jan;112(1):210–214. [PubMed] [Google Scholar]
- Oronsky A., Ignarro L., Perper R. Release of cartilage mucopolysaccharide-degrading neutral protease from human leukocytes. J Exp Med. 1973 Aug 1;138(2):461–472. doi: 10.1084/jem.138.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABINOWITZ Y. SEPARATION OF LYMPHOCYTES, POLYMORPHONUCLEAR LEUKOCYTES AND MONOCYTES ON GLASS COLUMNS, INCLUDING TISSUE CULTURE OBSERVATIONS. Blood. 1964 Jun;23:811–828. [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Weissmann G. The role of lysosomes in inflammation and disease. Annu Rev Med. 1967;18:97–112. doi: 10.1146/annurev.me.18.020167.000525. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Zurier R. B., Hoffstein S. Leukocytic proteases and the immunologic release of lysosomal enzymes. Am J Pathol. 1972 Sep;68(3):539–564. [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Zurier R. B., Spieler P. J., Goldstein I. M. Mechanisms of lysosomal enzyme release from leukocytes exposed to immune complexes and other particles. J Exp Med. 1971 Sep 1;134(3 Pt 2):149s–165s. [PubMed] [Google Scholar]
- ZIFF M., GRIBETZ H. J., LOSPALLUTO J. Effect of leukocyte and synovial membrane extracts on cartilage mucoprotein. J Clin Invest. 1960 Feb;39:405–412. doi: 10.1172/JCI104051. [DOI] [PMC free article] [PubMed] [Google Scholar]


