Abstract
MyoD1 is a key regulator that orchestrates skeletal muscle differentiation through the regulation of gene expression. Although many studies have focused on its role in transcriptional control at gene promoters, less is known regarding the role of MyoD1 in the assembly of active enhancers. Here, we discuss novel data that point to the ability of MyoD1 to mediate the assembly of active enhancers that augment the transcription of genes essential for muscle development and lineage specification. Based on genome-wide studies of epigenetic marks that typify active enhancers, we recently identified the compendium of distal regulatory elements that dictate transcriptional programs during myogenesis. Superimposition of MyoD1 binding sites upon the locations of muscle enhancers revealed its unequivocal binding to a core region of nearly a third of condition-specific muscle enhancers. Further studies exploring deposition of enhancer-related epigenetic marks in myoblasts lacking MyoD1 demonstrate the dependence of muscle enhancer assembly on the presence of MyoD1. We propose a model wherein MyoD1 mediates recruitment of Set7, H3K4me1, H3K27ac, p300, and RNAP II to MyoD1-bound enhancers to establish condition-specific activation of muscle genes. Moreover, muscle enhancers are modulated through coordinated binding of transcription factors, including c-Jun, Jdp2, Meis, and Runx1, which are recruited to muscle enhancers in a MyoD1-dependent manner. Thus, MyoD1 and enhancer-associated transcription factors function coordinately to assemble and regulate enhancers, thereby augmenting expression of muscle-related genes.
Keywords: muscle enhancers, transcriptional enhancers, ChIP-seq, skeletal muscle, differentiation, transcription, MyoD1, myogenesis
Lineage specification demands precise and tightly coordinated patterns of gene expression that dictate cell fate choices. In metazoans, cell type specificity of gene expression patterns is largely regulated by distal regulatory elements, called enhancers. Similar to promoters, enhancers harbor arrays of sequences that are recognized by multiple transcription factors (TFs), but unlike promoters, enhancers can activate transcription independent of their location, distance, or orientation with respect to the transcription start site (TSS) of each gene (reviewed in refs. 1–4). Seminal studies have revealed that chromatin states at promoters are highly correlated across different cell types, whereas histone modifications at enhancers are frequently cell type-specific and tightly associated with gene expression patterns.5,6 This fundamental observation suggests that enhancers can be regarded as a major determinant of cell type-specific gene expression in mammalian genomes. Indeed, evidence is emerging that due to their potent effect on gene transcription, enhancers are capable of influencing a variety of fundamental cellular phenomena, including stem cell multipotency7,8 and nuclear and chromosomal architecture.9,10
Along these lines, aberrant enhancer function was recently demonstrated to have important implications for human disease and development. It has been estimated that the total coverage of human enhancers amounts to nearly 10% of the human genome, representing a 5-fold greater coverage compared with protein-coding sequences.2 Although most well established disease-related mutations are within protein-coding sequences, it is logical to assume that mutations within enhancers could similarly result in human disease. These mutations could impair recruitment of TFs to enhancers, potentially disrupting tissue- and lineage-specific gene expression and, consequently, development. Indeed, sequencing analyses and epigenetic comparisons have implicated mutations in non-coding distal enhancers as a pathological basis of multiple diseases and cancer.11-15 In addition, single-nucleotide polymorphisms (SNPs) can confer genetic variation within enhancers as a result of altered TF binding to enhancer sites. For example, recent studies have demonstrated that a single nucleotide alteration in the enhancer of the sodium channel gene, SCN10A, functionally disrupts TBX3/TBX5 binding and leads to reduced cardiac activity of this enhancer in vivo.16 In another report, allelic variation in a common non-coding SNP located in the distal enhancer of the pigment gene, OCA2, was shown to disrupt its regulatory function and promoter interactions, resulting in allelic gene expression differences that influence pigmentation.17
Given the essential function of enhancers, it is important to comprehensively deduce the locations of these elements in each cell type. It is now clear that deposition of monomethylated H3K4 (H3K4me1), coupled with robust acetylation of histone H3K27 (H3K27ac), constitutes a predominant chromatin “signature” for transcriptional enhancers associated with actively transcribed genes.18,19 Recruitment of enzymes that facilitate this unique set of histone modifications, such as histone acetyltransferases, p300 and CREB binding protein (CBP),20 which acetylate H3K27, and methyltransferases Set7,13 MLL3 and MLL4,14 which monomethylate H3K4, are also associated with active enhancers. Transcriptional enhancers were further shown to recruit RNA polymerase II (RNAP II), and such recruitment is associated with transcription of small RNAs and large non-coding RNAs.20,21 These observations, catalyzed by the advent of massively parallel sequencing, have accelerated enhancer identification and analysis and allowed enhancer mapping in numerous cell types, including forebrain,22 cortical neurons,20 bone marrow macrophages,23 heart,24,25 and adipocytes.26 Tissue-specific TF binding represents an additional attribute of active enhancers, and it is logical to postulate that such TFs facilitate recruitment of histone-modifying enzymes.8 Motif searches on genomic regions identified by ChIP-seq analysis as potential enhancers reveal the recruitment of cell type-specific TFs that were previously implicated as essential regulators of lineage-specific differentiation. This list of factors includes Foxa1 and Foxa2 in liver cells,8,27 PU.1 in pro-B lymphocytes,23 Rfx1 in neural progenitors,8 and STAT1, 4 and 6 proteins in T cells,28 among others. Thus, the recent discovery of the role of these TFs positions them as critical master regulators of enhancer activity.
Based on the combination of chromatin marks observed in a given state, distal enhancers can be separated into several classes: “active” enhancers marked by H3K4me1 and H3K27ac, “inactive” (or “poised”) enhancers associated with H3K4me1 but not H3K27ac, and “latent” enhancers, which, in terminally differentiated cells, are not bound by TFs and lack the histone marks characteristic of enhancers but are capable of rapidly acquiring both features in response to specific stimuli.8,29-31 Thus, prior to their differentiation into pro-B lymphocytes, liver, or neuronal cells, ES cells contain poised enhancers that are converted to their active state upon differentiation.8 Complementary evidence showed that in addition to H3K4me1 (and the absence of H3K27ac), poised enhancers in ES or undifferentiated cells are associated with the repressive modification H3K27me3.30,32 Interestingly, epigenetic studies in pluripotent ES cells have shown that during the earliest stages of development, poised enhancers are bound by pioneer factors (also known as “place-holders”) that act to maintain developmental enhancers in a poised/inactive state.2,3,33 Upon differentiation, and depending upon the lineage that is adopted, the presence of such factors at enhancers decays, concomitant with the appearance of a master regulator and chromatin marks signifying an active enhancer. Thus, upon differentiation of ES cells into endoderm, FoxD3 is replaced by the activating factor, FoxA127; in addition, upon differentiation to neurons, Sox2 is replaced by Sox3 and Sox11.34 Although the biology of placeholders needs further exploration, one critical aspect is their timely replacement by enhancer activating factors and the acquisition of active enhancer signature. It is possible that defects in this transition could be deleterious to differentiation, as they are likely to lock cells in a proliferative state, which could promote cancer.
MyoD1 as a Central Regulator of Active Muscle Enhancers
MyoD1 is a key master regulator of skeletal muscle differentiation,35 and its role in myogenic differentiation36 and trans-differentiation of fibroblasts37 and other cell types38 has been well established. Indeed, enforced expression of MyoD1 alone is sufficient to trans-differentiate fibroblasts and other cell types to muscle.36-38 Upon induction of differentiation, MyoD1 interacts with E-proteins to form DNA-binding heterodimers that synergistically activate promoter-specific transcription of myogenic genes39 in a temporally defined manner.40 The MyoD1:E-protein heterodimer complex exhibits preference for an E-box element with the VCASCTGT consensus sequence (where V stands for A, C or G, and S stands for C or G), which was found to be enriched within promoter and enhancer regions associated with muscle-related genes.41-44 In addition, in vivo45 and in vitro46,47 reporter assays established that MyoD1 activates transcription of genes containing multiple E-box motifs. Although the mechanisms by which MyoD1 contributes to muscle differentiation have primarily focused on promoter regulation, classical enhancer-reporter assays revealed a dependence on MyoD1 for enhancer activity as well. Enhancer regions critical for the myogenic program and enriched with E-box elements that recruit MyoD1 have been identified distal to the myogenin,48,49 MyoD1,50 myosin chain 1,51-53 Ckm,54,55 Myf5 and Mrf4,56,57 γ-sarcoglycan,58 Id159 and Adamts560 genes.
To identify active enhancers in skeletal muscle genome-wide and to elucidate the molecular mechanisms that regulate their activity during differentiation, we recently performed ChIP-seq analysis (high-throughput sequencing of DNA enriched by chromatin immunoprecipitation) on four of the well-established enhancer marks (H3K4Me1, H3K27ac, p300 and RNAP II) before and after myogenic differentiation.61 Our analysis revealed that the total number of muscle enhancers increased throughout differentiation from approximately 4,000 in myoblasts to as many as 1.5-times that number in myotubes. Of these, ~3,000 were exclusively active before differentiation, and ~5,000 were only active after differentiation (whereas the remaining enhancers were constitutively marked in both conditions). Interestingly, this finding is in line with the previously known increase in active enhancer assembly in the transition from blastula to gastrula stages31 and from embryonic stem cells (ESC) to neuronal progenitor cells (NPC),8 and thus, it may reflect a general feature of enhancers related to differentiation. Our compendium of distal enhancers recovered many previously identified enhancer regions, and overall there was a strong correlation between the activity of condition-specific enhancers and the transcription level of their adjacent genes, indicating that these enhancers augment transcription of thousands of protein-coding genes. Consistent with other tissue-specific elements whose evolution occurs at higher rates,62,63 we have shown that muscle-specific enhancers are strongly- but not ultra-conserved.61
Furthermore, by linking condition-specific enhancers to their nearest associated condition-specific genes, we showed that the median enhancer-promoter distance for myotubes was significantly shorter (by more than 13 kb) than the corresponding distance in myoblasts, suggesting that reduction in the distances between enhancers and their active, linked promoters may also be a characteristic of muscle differentiation. Future studies will determine whether this reduction could be related to differentiation-dependent, architectural changes in chromatin. In addition, approximately 10% of our condition-specific enhancers were associated with non-coding transcripts,64 and ~60% of these enhancers displayed significant levels of RNAP II recruitment. These findings are in line with previous reports that suggest a cis-regulatory role for transcripts emanating from enhancers.20,21
In-silico analysis indicated that nearly half of myoblast-specific and approximately 80% of myotube-specific enhancers exhibited predicted MyoD1 binding sites.61 However, overlap of our enhancer data sets with experimentally determined MyoD1-binding events44 revealed that approximately 30% of condition-specific enhancers were bound with MyoD1, suggesting that additional epigenetic cues, such as binding of sequence-specific factors and/or chromatin architecture, dictated by histone modifications, play an important role in limiting MyoD1 recruitment to muscle enhancers. The strong trans-activation potential of MyoD1 at muscle enhancers is likely augmented through its ability to interact with multiple transcriptional regulatory factors. Motif enrichment analysis of ChIP-seq data sets indicated that sequence-specific TFs can be recruited in a spatially constrained manner around MyoD1-binding sites. Importantly, several of these identified TFs, such as Jdp2,65 Meis,66 c-Jun,67 and Runx1,68 are well known regulators of myogenesis, and in the absence of MyoD1, the recruitment of these factors, as well as RNAP II, was dramatically reduced at MyoD1-bound enhancers (Fig. 1). These findings suggest that MyoD1 plays an essential role in enhancer assembly by recruiting TFs that have established roles in muscle differentiation. Indeed, genome-wide analysis of c-Jun binding sites in myoblasts indicated that MyoD1 and c-Jun co-localize within a narrow window on 54% of muscle enhancers.61 Further, suppression of c-Jun expression in myoblasts led to strong reductions in the levels of H3K4me1 and H3K27ac at selected myoblast enhancers bound by c-Jun but not at other enhancers that were not bound by this TF.61 These studies, which strongly suggest that MyoD1 and c-Jun coordinately regulate enhancer assembly, are consistent with biochemical studies showing that these proteins interact in vitro.67 It will be interesting in the future to determine whether mutations and SNPs that map to our compendium of enhancers—particularly those that map to MyoD1, c-Jun, and other factor binding sites—could underlie human skeletal muscle disease.
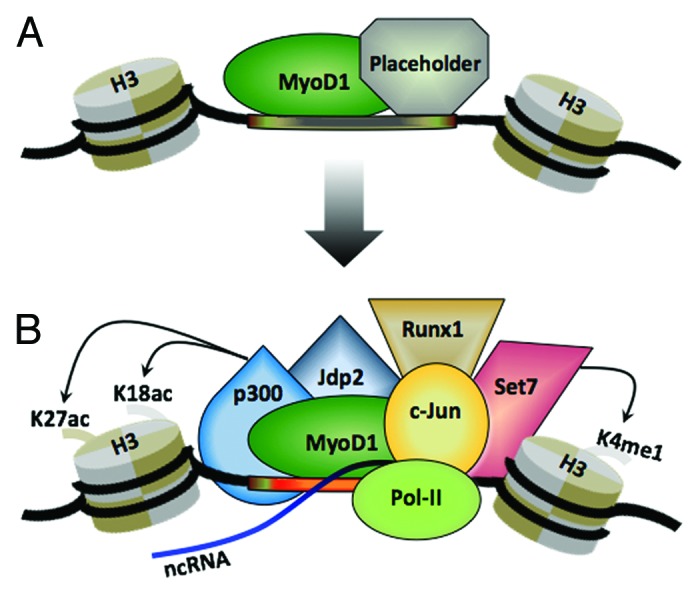
Figure 1. Model for the coordinate assembly of active MyoD1 enhancers in muscle. (A) MyoD1 co-binds to enhancers in conjunction with a putative pioneer factor (“placeholder”) that maintains them in a poised/inactive state. (B) Eviction (or inactivation) of enhancer-bound placeholder allows the recruitment of other transcription factors that positively regulate enhancer activity, leading to acquisition of a transcriptionally active state, characterized by deposition of H3K4me1 and H3K27ac and often in non-coding transcription. See text for further details.
Several studies have indicated that once tethered to its binding sites at promoters, MyoD1 recruits the acetyltransferases, p300 and PCAF, leading to acetylation of histones H3 and H444,69-73 and MyoD,47,74 respectively. Our analysis indicated that the overwhelming majority of condition-specific enhancers bound by MyoD1 were co-occupied by p300. Statistically, the fraction of MyoD1-bound enhancers that recruit p300 was significantly higher than the fraction of MyoD1 enhancers that lack p300, suggesting that MyoD1 might have a strong impact on p300 recruitment to active muscle enhancers. Indeed, MyoD1-null myoblasts exhibited sharply diminished levels of p300 and H3K27ac at each of the MyoD1-bound enhancers that we tested.61 qChIP experiments revealed that Set7 was recruited to muscle enhancers in a MyoD1-dependent manner, since recruitment of Set7 and deposition of H3K4me1 were significantly diminished at MyoD1-bound enhancers in MyoD1−/− myoblasts, as compared with their wild-type counterparts.61 These results are consistent with another study that demonstrated direct MyoD1-Set7 interactions on the MCK enhancer and delineated the importance of Set7 for promoting myoblast differentiation via regulation of H3K4me1 deposition.75 Furthermore, we detected MyoD1-independent recruitment of Set7 to enhancers, suggesting that this methyltransferase may be recruited by TFs other than MyoD1. Despite these observations, one important question—the functional role of mono-methylation of H3K4 in enhancer regulation—will need to be addressed to complete our understanding of MyoD1 function.
Another intriguing question pertains to the role of placeholders as regulators of the myogenic program. It is interesting to speculate that myogenic muscle precursor cells (myoblasts) found within the developing limb bud and regenerating satellite cells might show widespread binding of such putative factors to their enhancers (Fig. 1). In each of these cases, MyoD1 is expressed at high levels, yet these populations remain undifferentiated until appropriate environmental conditions are satisfied. Attempts to identify a specific modification that can convert MyoD1 from an inactive to an active form have not succeeded.76 Of note, our studies have shown that among MyoD1-bound enhancers that become exclusively active in myotubes, the majority of them (~72%), are already bound by MyoD1 in myoblasts, prior to their assembly into active enhancers.61 These results suggest that in many cases, binding of MyoD1 by itself is not sufficient to promote the transition to an active enhancer, and it is plausible that an additional trigger is required to facilitate the removal or acquisition of a specific factor that co-occupies the enhancer with MyoD1. It is possible that co-repressors (or other placeholders) can suppress the action of MyoD1 bound to muscle enhancers before it becomes active.33,77,78 Future investigations into how placeholders restrain the transcriptional activity of MyoD1 (and possibly other myogenic regulatory factors, MRFs) will greatly advance our understanding of mechanisms through which the myogenic program is induced.
Signaling to Enhancers: Conversion to an Active or Inactive State
Recent studies have delineated the signaling pathways that govern specification of mesodermal precursor cells into myoblasts and subsequently into differentiated myotubes (reviewed in ref. 79). Such specification of these precursor cells, located in the dorsal region of the somites (dermomyotome), into myoblasts is mediated by a variety of molecular signals emanating from surrounding tissues. A combination of stimulatory signals, including Wnts, Sonic hedgehog (Shh), and Noggin, and suppressive signals, such as BMP4, ultimately dictate expression patterns of the primary MRFs, MyoD1 and Myf5, and therefore propel commitment to the myogenic fate. In the lateral part of the dermomyotome, myoblasts that preferentially express MyoD1 migrate to form the myotome, which eventually forms the skeletal musculature. Increased expression of the secondary MRFs—myogenin and MRF4—stimulates the next step in myogenesis, as skeletal myoblasts fuse and eventually form bundles of multinucleated myofibers.79
Several studies suggest that extracellular signals could ultimately be converted into epigenetic modifications that directly affect transcription. For example, one of the major pathways associated with muscle differentiation is the p38 MAP kinase signaling cascade. At the onset of differentiation, p38 is activated and phosphorylates E47 at Ser140, leading to its dimerization with MyoD1 and to subsequent binding to E-box motifs of different muscle promoters.80 In addition, phosphorylation of the SWI/SNF component, BAF60, by p38α/β stimulates the recruitment of this multi-subunit chromatin-remodeling complex to regulatory regions of muscle-specific genes.71 Further, forced inhibition of p38α/β blocks the engagement between MyoD1 and BRG1 and BRM, two ATPase subunits of SWI/SNF.71 Another aspect of p38 activity is its ability to directly phosphorylate the transactivation domains of MEF2A and MEF2C, which, through interactions with MyoD1, results in stimulation of their transcriptional activity.81-84 By contrast, p38γ is known to phosphorylate MyoD1, resulting in its enhanced promoter occupancy but reduced transcriptional activity.85 Another signaling pathway that is known to mediate intracellular events in response to external growth factors, such as IGF1, is PI3K/AKT signaling. This pathway, known to function in parallel with the p38 cascade during early myogenic differentiation, is critically involved in activation of muscle differentiation,86 muscle cell survival,87 and regeneration.88,89 Consistent with this notion, suppression of each of these pathways was shown to alter the patterns of specific chromatin modifiers on the Mck enhancer.90
Given the fact that MyoD1 plays a prominent role at enhancers, and since several of these signaling pathways impinge on MyoD1 activity, it is reasonable to postulate that these cascades may indeed regulate MyoD1 activity at enhancers, converting them to an activated or inactivated state. Thus, although the mechanisms through which these signaling pathways participate in regulation of enhancer activity remain largely unknown, future studies will determine the extent to which these signaling pathways play a role at enhancers, before and during muscle differentiation.
Conclusion
In summary, we propose that while MyoD1 binds to a large number of promoters that augment expression of genes essential for specifying the muscle lineage, a key role for this factor appears to be its ability to bind to enhancers. Our model establishes a role for MyoD1 in mediating the co-recruitment of several chromatin modifying enzymes, among them Set7 and p300, which subsequently deposit the histone marks that typify active enhancers (Fig. 1). Importantly, several TFs, including c-Jun, are recruited to active enhancers bound by MyoD1, suggesting that interactions between MyoD1 and other TFs are necessary for assembly of active enhancers. Interestingly, unlike MyoD1, whose expression is restricted to skeletal muscle, c-Jun is expressed in multiple tissue types. Thus, it is interesting to speculate that in other tissues, alternative bHLH-type transcriptional activators could collaborate with c-Jun to regulate enhancer activity through a similar mechanism.
Acknowledgments
We thank members of the Dynlacht laboratory, in particular J. Cheng and C. Bowman, for helpful discussions. Work in the Dynlacht laboratory was supported by NIH grant 2R01 GM067132.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/epigenetics/article/25441
References
- 1.Ong CT, Corces VG. Enhancer function: new insights into the regulation of tissue-specific gene expression. Nat Rev Genet. 2011;12:283–93. doi: 10.1038/nrg2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bulger M, Groudine M. Functional and mechanistic diversity of distal transcription enhancers. Cell. 2011;144:327–39. doi: 10.1016/j.cell.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spitz F, Furlong EE. Transcription factors: from enhancer binding to developmental control. Nat Rev Genet. 2012;13:613–26. doi: 10.1038/nrg3207. [DOI] [PubMed] [Google Scholar]
- 4.Calo E, Wysocka J. Modification of enhancer chromatin: what, how, and why? Mol Cell. 2013;49:825–37. doi: 10.1016/j.molcel.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A, Harp LF, et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459:108–12. doi: 10.1038/nature07829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visel A, Blow MJ, Li Z, Zhang T, Akiyama JA, Holt A, et al. ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature. 2009;457:854–8. doi: 10.1038/nature07730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui K, Zang C, Roh TY, Schones DE, Childs RW, Peng W, et al. Chromatin signatures in multipotent human hematopoietic stem cells indicate the fate of bivalent genes during differentiation. Cell Stem Cell. 2009;4:80–93. doi: 10.1016/j.stem.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci U S A. 2010;107:21931–6. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackwood EM, Kadonaga JT. Going the distance: a current view of enhancer action. Science. 1998;281:60–3. doi: 10.1126/science.281.5373.60. [DOI] [PubMed] [Google Scholar]
- 10.Chepelev I, Wei G, Wangsa D, Tang Q, Zhao K. Characterization of genome-wide enhancer-promoter interactions reveals co-expression of interacting genes and modes of higher order chromatin organization. Cell Res. 2012;22:490–503. doi: 10.1038/cr.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akhtar-Zaidi B, Cowper-Sal-lari R, Corradin O, Saiakhova A, Bartels CF, Balasubramanian D, et al. Epigenomic enhancer profiling defines a signature of colon cancer. Science. 2012;336:736–9. doi: 10.1126/science.1217277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emison ES, McCallion AS, Kashuk CS, Bush RT, Grice E, Lin S, et al. A common sex-dependent mutation in a RET enhancer underlies Hirschsprung disease risk. Nature. 2005;434:857–63. doi: 10.1038/nature03467. [DOI] [PubMed] [Google Scholar]
- 13.Kleinjan DA, van Heyningen V. Long-range control of gene expression: emerging mechanisms and disruption in disease. Am J Hum Genet. 2005;76:8–32. doi: 10.1086/426833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visel A, Rubin EM, Pennacchio LA. Genomic views of distant-acting enhancers. Nature. 2009;461:199–205. doi: 10.1038/nature08451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noonan JP, McCallion AS. Genomics of long-range regulatory elements. Annu Rev Genomics Hum Genet. 2010;11:1–23. doi: 10.1146/annurev-genom-082509-141651. [DOI] [PubMed] [Google Scholar]
- 16.van den Boogaard M, Wong LY, Tessadori F, Bakker ML, Dreizehnter LK, Wakker V, et al. Genetic variation in T-box binding element functionally affects SCN5A/SCN10A enhancer. J Clin Invest. 2012;122:2519–30. doi: 10.1172/JCI62613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visser M, Kayser M, Palstra RJ. HERC2 rs12913832 modulates human pigmentation by attenuating chromatin-loop formation between a long-range enhancer and the OCA2 promoter. Genome Res. 2012;22:446–55. doi: 10.1101/gr.128652.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39:311–8. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 19.Zentner GE, Tesar PJ, Scacheri PC. Epigenetic signatures distinguish multiple classes of enhancers with distinct cellular functions. Genome Res. 2011;21:1273–83. doi: 10.1101/gr.122382.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim TK, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–7. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Santa F, Barozzi I, Mietton F, Ghisletti S, Polletti S, Tusi BK, et al. A large fraction of extragenic RNA pol II transcription sites overlap enhancers. PLoS Biol. 2010;8:e1000384. doi: 10.1371/journal.pbio.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Visel A, Taher L, Girgis H, May D, Golonzhka O, Hoch RV, et al. A high-resolution enhancer atlas of the developing telencephalon. Cell. 2013;152:895–908. doi: 10.1016/j.cell.2012.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghisletti S, Barozzi I, Mietton F, Polletti S, De Santa F, Venturini E, et al. Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity. 2010;32:317–28. doi: 10.1016/j.immuni.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 24.May D, Blow MJ, Kaplan T, McCulley DJ, Jensen BC, Akiyama JA, et al. Large-scale discovery of enhancers from human heart tissue. Nat Genet. 2012;44:89–93. doi: 10.1038/ng.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narlikar L, Sakabe NJ, Blanski AA, Arimura FE, Westlund JM, Nobrega MA, et al. Genome-wide discovery of human heart enhancers. Genome Res. 2010;20:381–92. doi: 10.1101/gr.098657.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mikkelsen TS, Xu Z, Zhang X, Wang L, Gimble JM, Lander ES, et al. Comparative epigenomic analysis of murine and human adipogenesis. Cell. 2010;143:156–69. doi: 10.1016/j.cell.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J, Watts JA, Pope SD, Gadue P, Kamps M, Plath K, et al. Transcriptional competence and the active marking of tissue-specific enhancers by defined transcription factors in embryonic and induced pluripotent stem cells. Genes Dev. 2009;23:2824–38. doi: 10.1101/gad.1861209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vahedi G, Takahashi H, Nakayamada S, Sun HW, Sartorelli V, Kanno Y, et al. STATs shape the active enhancer landscape of T cell populations. Cell. 2012;151:981–93. doi: 10.1016/j.cell.2012.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostuni R, Piccolo V, Barozzi I, Polletti S, Termanini A, Bonifacio S, et al. Latent enhancers activated by stimulation in differentiated cells. Cell. 2013;152:157–71. doi: 10.1016/j.cell.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 30.Rada-Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA, Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470:279–83. doi: 10.1038/nature09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bogdanovic O, Fernandez-Miñán A, Tena JJ, de la Calle-Mustienes E, Hidalgo C, van Kruysbergen I, et al. Dynamics of enhancer chromatin signatures mark the transition from pluripotency to cell specification during embryogenesis. Genome Res. 2012;22:2043–53. doi: 10.1101/gr.134833.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asp P, Blum R, Vethantham V, Parisi F, Micsinai M, Cheng J, et al. Genome-wide remodeling of the epigenetic landscape during myogenic differentiation. Proc Natl Acad Sci U S A. 2011;108:E149–58. doi: 10.1073/pnas.1102223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011;25:2227–41. doi: 10.1101/gad.176826.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergsland M, Ramsköld D, Zaouter C, Klum S, Sandberg R, Muhr J. Sequentially acting Sox transcription factors in neural lineage development. Genes Dev. 2011;25:2453–64. doi: 10.1101/gad.176008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tapscott SJ. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development. 2005;132:2685–95. doi: 10.1242/dev.01874. [DOI] [PubMed] [Google Scholar]
- 36.Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold HH, Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–9. doi: 10.1016/0092-8674(93)90621-V. [DOI] [PubMed] [Google Scholar]
- 37.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-X. [DOI] [PubMed] [Google Scholar]
- 38.Weintraub H, Tapscott SJ, Davis RL, Thayer MJ, Adam MA, Lassar AB, et al. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc Natl Acad Sci U S A. 1989;86:5434–8. doi: 10.1073/pnas.86.14.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lassar AB, Davis RL, Wright WE, Kadesch T, Murre C, Voronova A, et al. Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell. 1991;66:305–15. doi: 10.1016/0092-8674(91)90620-E. [DOI] [PubMed] [Google Scholar]
- 40.Bergstrom DA, Penn BH, Strand A, Perry RL, Rudnicki MA, Tapscott SJ. Promoter-specific regulation of MyoD binding and signal transduction cooperate to pattern gene expression. Mol Cell. 2002;9:587–600. doi: 10.1016/S1097-2765(02)00481-1. [DOI] [PubMed] [Google Scholar]
- 41.Murre C, McCaw PS, Vaessin H, Caudy M, Jan LY, Jan YN, et al. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989;58:537–44. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- 42.Blackwell TK, Weintraub H. Differences and similarities in DNA-binding preferences of MyoD and E2A protein complexes revealed by binding site selection. Science. 1990;250:1104–10. doi: 10.1126/science.2174572. [DOI] [PubMed] [Google Scholar]
- 43.Blais A, Tsikitis M, Acosta-Alvear D, Sharan R, Kluger Y, Dynlacht BD. An initial blueprint for myogenic differentiation. Genes Dev. 2005;19:553–69. doi: 10.1101/gad.1281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao Y, Yao Z, Sarkar D, Lawrence M, Sanchez GJ, Parker MH, et al. Genome-wide MyoD binding in skeletal muscle cells: a potential for broad cellular reprogramming. Dev Cell. 2010;18:662–74. doi: 10.1016/j.devcel.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis RL, Weintraub H. Acquisition of myogenic specificity by replacement of three amino acid residues from MyoD into E12. Science. 1992;256:1027–30. doi: 10.1126/science.1317057. [DOI] [PubMed] [Google Scholar]
- 46.Bengal E, Flores O, Rangarajan PN, Chen A, Weintraub H, Verma IM. Positive control mutations in the MyoD basic region fail to show cooperative DNA binding and transcriptional activation in vitro. Proc Natl Acad Sci U S A. 1994;91:6221–5. doi: 10.1073/pnas.91.13.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dilworth FJ, Seaver KJ, Fishburn AL, Htet SL, Tapscott SJ. In vitro transcription system delineates the distinct roles of the coactivators pCAF and p300 during MyoD/E47-dependent transactivation. Proc Natl Acad Sci U S A. 2004;101:11593–8. doi: 10.1073/pnas.0404192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yee SP, Rigby PW. The regulation of myogenin gene expression during the embryonic development of the mouse. Genes Dev. 1993;7(7A):1277–89. doi: 10.1101/gad.7.7a.1277. [DOI] [PubMed] [Google Scholar]
- 49.Cheng TC, Wallace MC, Merlie JP, Olson EN. Separable regulatory elements governing myogenin transcription in mouse embryogenesis. Science. 1993;261:215–8. doi: 10.1126/science.8392225. [DOI] [PubMed] [Google Scholar]
- 50.Goldhamer DJ, Brunk BP, Faerman A, King A, Shani M, Emerson CP., Jr. Embryonic activation of the myoD gene is regulated by a highly conserved distal control element. Development. 1995;121:637–49. doi: 10.1242/dev.121.3.637. [DOI] [PubMed] [Google Scholar]
- 51.Donoghue M, Ernst H, Wentworth B, Nadal-Ginard B, Rosenthal N. A muscle-specific enhancer is located at the 3′ end of the myosin light-chain 1/3 gene locus. Genes Dev. 1988;2(12B):1779–90. doi: 10.1101/gad.2.12b.1779. [DOI] [PubMed] [Google Scholar]
- 52.Rosenthal N, Berglund EB, Wentworth BM, Donoghue M, Winter B, Bober E, et al. A highly conserved enhancer downstream of the human MLC1/3 locus is a target for multiple myogenic determination factors. Nucleic Acids Res. 1990;18:6239–46. doi: 10.1093/nar/18.21.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wentworth BM, Donoghue M, Engert JC, Berglund EB, Rosenthal N. Paired MyoD-binding sites regulate myosin light chain gene expression. Proc Natl Acad Sci U S A. 1991;88:1242–6. doi: 10.1073/pnas.88.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson JE, Wold BJ, Hauschka SD. Muscle creatine kinase sequence elements regulating skeletal and cardiac muscle expression in transgenic mice. Mol Cell Biol. 1989;9:3393–9. doi: 10.1128/mcb.9.8.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horlick RA, Benfield PA. The upstream muscle-specific enhancer of the rat muscle creatine kinase gene is composed of multiple elements. Mol Cell Biol. 1989;9:2396–413. doi: 10.1128/mcb.9.6.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carvajal JJ, Keith A, Rigby PW. Global transcriptional regulation of the locus encoding the skeletal muscle determination genes Mrf4 and Myf5. Genes Dev. 2008;22:265–76. doi: 10.1101/gad.442408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang TH, Primig M, Hadchouel J, Tajbakhsh S, Rocancourt D, Fernandez A, et al. An enhancer directs differential expression of the linked Mrf4 and Myf5 myogenic regulatory genes in the mouse. Dev Biol. 2004;269:595–608. doi: 10.1016/j.ydbio.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 58.Wakabayashi-Takai E, Noguchi S, Ozawa E. Identification of myogenesis-dependent transcriptional enhancers in promoter region of mouse gamma-sarcoglycan gene. Eur J Biochem. 2001;268:948–57. doi: 10.1046/j.1432-1327.2001.01954.x. [DOI] [PubMed] [Google Scholar]
- 59.Tournay O, Benezra R. Transcription of the dominant-negative helix-loop-helix protein Id1 is regulated by a protein complex containing the immediate-early response gene Egr-1. Mol Cell Biol. 1996;16:2418–30. doi: 10.1128/mcb.16.5.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barthel KK, Liu X. A transcriptional enhancer from the coding region of ADAMTS5. PLoS One. 2008;3:e2184. doi: 10.1371/journal.pone.0002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blum R, Vethantham V, Bowman C, Rudnicki M, Dynlacht BD. Genome-wide identification of enhancers in skeletal muscle: the role of MyoD1. Genes Dev. 2012;26:2763–79. doi: 10.1101/gad.200113.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ponting CP. The functional repertoires of metazoan genomes. Nat Rev Genet. 2008;9:689–98. doi: 10.1038/nrg2413. [DOI] [PubMed] [Google Scholar]
- 63.Prabhakar S, Poulin F, Shoukry M, Afzal V, Rubin EM, Couronne O, et al. Close sequence comparisons are sufficient to identify human cis-regulatory elements. Genome Res. 2006;16:855–63. doi: 10.1101/gr.4717506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–5. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ostrovsky O, Bengal E, Aronheim A. Induction of terminal differentiation by the c-Jun dimerization protein JDP2 in C2 myoblasts and rhabdomyosarcoma cells. J Biol Chem. 2002;277:40043–54. doi: 10.1074/jbc.M205494200. [DOI] [PubMed] [Google Scholar]
- 66.Knoepfler PS, Bergstrom DA, Uetsuki T, Dac-Korytko I, Sun YH, Wright WE, et al. A conserved motif N-terminal to the DNA-binding domains of myogenic bHLH transcription factors mediates cooperative DNA binding with pbx-Meis1/Prep1. Nucleic Acids Res. 1999;27:3752–61. doi: 10.1093/nar/27.18.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bengal E, Ransone L, Scharfmann R, Dwarki VJ, Tapscott SJ, Weintraub H, et al. Functional antagonism between c-Jun and MyoD proteins: a direct physical association. Cell. 1992;68:507–19. doi: 10.1016/0092-8674(92)90187-H. [DOI] [PubMed] [Google Scholar]
- 68.Wang X, Blagden C, Fan J, Nowak SJ, Taniuchi I, Littman DR, et al. Runx1 prevents wasting, myofibrillar disorganization, and autophagy of skeletal muscle. Genes Dev. 2005;19:1715–22. doi: 10.1101/gad.1318305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Puri PL, Sartorelli V, Yang XJ, Hamamori Y, Ogryzko VV, Howard BH, et al. Differential roles of p300 and PCAF acetyltransferases in muscle differentiation. Mol Cell. 1997;1:35–45. doi: 10.1016/S1097-2765(00)80005-2. [DOI] [PubMed] [Google Scholar]
- 70.Rampalli S, Li L, Mak E, Ge K, Brand M, Tapscott SJ, et al. p38 MAPK signaling regulates recruitment of Ash2L-containing methyltransferase complexes to specific genes during differentiation. Nat Struct Mol Biol. 2007;14:1150–6. doi: 10.1038/nsmb1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simone C, Forcales SV, Hill DA, Imbalzano AN, Latella L, Puri PL. p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat Genet. 2004;36:738–43. doi: 10.1038/ng1378. [DOI] [PubMed] [Google Scholar]
- 72.Cao Y, Kumar RM, Penn BH, Berkes CA, Kooperberg C, Boyer LA, et al. Global and gene-specific analyses show distinct roles for Myod and Myog at a common set of promoters. EMBO J. 2006;25:502–11. doi: 10.1038/sj.emboj.7600958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuan W, Condorelli G, Caruso M, Felsani A, Giordano A. Human p300 protein is a coactivator for the transcription factor MyoD. J Biol Chem. 1996;271:9009–13. doi: 10.1074/jbc.271.15.9009. [DOI] [PubMed] [Google Scholar]
- 74.Sartorelli V, Puri PL, Hamamori Y, Ogryzko V, Chung G, Nakatani Y, et al. Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle program. Mol Cell. 1999;4:725–34. doi: 10.1016/S1097-2765(00)80383-4. [DOI] [PubMed] [Google Scholar]
- 75.Tao Y, Neppl RL, Huang ZP, Chen J, Tang RH, Cao R, et al. The histone methyltransferase Set7/9 promotes myoblast differentiation and myofibril assembly. J Cell Biol. 2011;194:551–65. doi: 10.1083/jcb.201010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ludolph DC, Konieczny SF. Transcription factor families: muscling in on the myogenic program. FASEB J. 1995;9:1595–604. doi: 10.1096/fasebj.9.15.8529839. [DOI] [PubMed] [Google Scholar]
- 77.Wang JC, Waltner-Law M, Yamada K, Osawa H, Stifani S, Granner DK. Transducin-like enhancer of split proteins, the human homologs of Drosophila groucho, interact with hepatic nuclear factor 3beta. J Biol Chem. 2000;275:18418–23. doi: 10.1074/jbc.M910211199. [DOI] [PubMed] [Google Scholar]
- 78.Sekiya T, Zaret KS. Repression by Groucho/TLE/Grg proteins: genomic site recruitment generates compacted chromatin in vitro and impairs activator binding in vivo. Mol Cell. 2007;28:291–303. doi: 10.1016/j.molcel.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chargé SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–38. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 80.Lluís F, Ballestar E, Suelves M, Esteller M, Muñoz-Cánoves P. E47 phosphorylation by p38 MAPK promotes MyoD/E47 association and muscle-specific gene transcription. EMBO J. 2005;24:974–84. doi: 10.1038/sj.emboj.7600528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ornatsky OI, Cox DM, Tangirala P, Andreucci JJ, Quinn ZA, Wrana JL, et al. Post-translational control of the MEF2A transcriptional regulatory protein. Nucleic Acids Res. 1999;27:2646–54. doi: 10.1093/nar/27.13.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao M, New L, Kravchenko VV, Kato Y, Gram H, di Padova F, et al. Regulation of the MEF2 family of transcription factors by p38. Mol Cell Biol. 1999;19:21–30. doi: 10.1128/mcb.19.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Puri PL, Wu Z, Zhang P, Wood LD, Bhakta KS, Han J, et al. Induction of terminal differentiation by constitutive activation of p38 MAP kinase in human rhabdomyosarcoma cells. Genes Dev. 2000;14:574–84. [PMC free article] [PubMed] [Google Scholar]
- 84.Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol. 1998;14:167–96. doi: 10.1146/annurev.cellbio.14.1.167. [DOI] [PubMed] [Google Scholar]
- 85.Gillespie MA, Le Grand F, Scimè A, Kuang S, von Maltzahn J, Seale V, et al. p38-gamma-dependent gene silencing restricts entry into the myogenic differentiation program. J Cell Biol. 2009;187:991–1005. doi: 10.1083/jcb.200907037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu Z, Woodring PJ, Bhakta KS, Tamura K, Wen F, Feramisco JR, et al. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol Cell Biol. 2000;20:3951–64. doi: 10.1128/MCB.20.11.3951-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lawlor MA, Rotwein P. Insulin-like growth factor-mediated muscle cell survival: central roles for Akt and cyclin-dependent kinase inhibitor p21. Mol Cell Biol. 2000;20:8983–95. doi: 10.1128/MCB.20.23.8983-8995.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Musarò A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, et al. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- 89.Barton ER, Morris L, Musaro A, Rosenthal N, Sweeney HL. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol. 2002;157:137–48. doi: 10.1083/jcb.200108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Serra C, Palacios D, Mozzetta C, Forcales SV, Morantte I, Ripani M, et al. Functional interdependence at the chromatin level between the MKK6/p38 and IGF1/PI3K/AKT pathways during muscle differentiation. Mol Cell. 2007;28:200–13. doi: 10.1016/j.molcel.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


