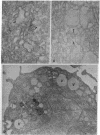
Abstract
An improved procedure was used to conjugate ferritin to antibodies specific for the NH2-terminal extensions on the precursor form of collagen known as procollagen. The ferritin-antibody conjugates were then used to determine the localization of procollagen in fibroblasts isolated from chick-embryo tendons. Procollagen was found in the cisternae of the endoplasmic reticulum, indicating that the protein passes into this compartment early in its biosynthesis. Specific labeling of large Golgi vacuoles was also observed, suggesting that this compartment is also involved in the secretory process. When cells were incubated with colchicine so that the secretion of procollagen was delayed, there was an increase of large, smooth-surfaced vacuoles in the cells and these vacuoles were labeled with the ferritin-antibody conjugate.
Keywords: ferritin-antibody conjugates, Golgi vacuoles, protein secretion
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper G. W., Prockop D. J. Intracellular accumulation of protocollagen and extrusion of collagen by embryonic cartilage cells. J Cell Biol. 1968 Sep;38(3):523–537. doi: 10.1083/jcb.38.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombre A. J., Coulombre J. L. Corneal development. IV. Interruption of collagen excretion into the primary stroma of the cornea with L-azetidine-2-carboxylic acid. Dev Biol. 1972 May;28(1):183–190. doi: 10.1016/0012-1606(72)90136-4. [DOI] [PubMed] [Google Scholar]
- Dehm P., Prockop D. J. Time lag in the secretion of collagen by matrix-free tendon cells and inhibition of the secretory process by colchicine and vinblastine. Biochim Biophys Acta. 1972 Apr 21;264(2):375–382. doi: 10.1016/0304-4165(72)90302-9. [DOI] [PubMed] [Google Scholar]
- Diegelmann R. F., Peterkofsky B. Inhibition of collagen secretion from bone and cultured fibroblasts by microtubular disruptive drugs. Proc Natl Acad Sci U S A. 1972 Apr;69(4):892–896. doi: 10.1073/pnas.69.4.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H. P., Bornstein P. Microtubules in transcellular movement of procollagen. Nat New Biol. 1972 Aug 30;238(87):257–260. doi: 10.1038/newbio238257a0. [DOI] [PubMed] [Google Scholar]
- HUNTER M. J., COMMERFORD S. L. Pressure homogenization of mammalian tissues. Biochim Biophys Acta. 1961 Mar 4;47:580–586. doi: 10.1016/0006-3002(61)90553-4. [DOI] [PubMed] [Google Scholar]
- Hay E. D., Dodson J. W. Secretion of collagen by corneal epithelium. I. Morphology of the collagenous products produced by isolated epithelia grown on frozen-killed lens. J Cell Biol. 1973 Apr;57(1):190–213. doi: 10.1083/jcb.57.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. I. Role of the peripheral elements of the Golgi complex. J Cell Biol. 1967 Aug;34(2):577–596. doi: 10.1083/jcb.34.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Synthesis, intracellular transport, and discharge of secretory proteins in stimulated pancreatic exocrine cells. J Cell Biol. 1971 Jul;50(1):135–158. doi: 10.1083/jcb.50.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraehenbuhl J. P., Jamieson J. D. Solid-phase conjugation of ferritin to Fab-fragments of immunoglobulin G for use in antigen localization on thin sections. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1771–1775. doi: 10.1073/pnas.69.7.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B. R., Berg R. A., Kishida Y., Prockop D. J. Collagen synthesis: localization of prolyl hydroxylase in tendon cells detected with ferritin-labeled antibodies. Science. 1973 Nov 23;182(4114):825–827. doi: 10.1126/science.182.4114.825. [DOI] [PubMed] [Google Scholar]
- REVEL J. P., HAY E. D. AN AUTORADIOGRAPHIC AND ELECTRON MICROSCOPIC STUDY OF COLLAGEN SYNTHESIS IN DIFFERENTIATING CARTILAGE. Z Zellforsch Mikrosk Anat. 1963 Oct 8;61:110–144. doi: 10.1007/BF00341524. [DOI] [PubMed] [Google Scholar]
- Reith E. J. Collagen formation in developing molar teeth of rats. J Ultrastruct Res. 1967 Dec;21(5):383–414. doi: 10.1016/s0022-5320(67)80148-5. [DOI] [PubMed] [Google Scholar]
- Ross R., Benditt E. P. Wound healing and collagen formation. V. Quantitative electron microscope radioautographic observations of proline-H3 utilization by fibroblasts. J Cell Biol. 1965 Oct;27(1):83–106. doi: 10.1083/jcb.27.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter M. M. H3-proline incorporation into cartilage: electron microscope autoradiographic observations. J Morphol. 1968 Apr;124(4):387–421. doi: 10.1002/jmor.1051240402. [DOI] [PubMed] [Google Scholar]
- Schofield J. D., Prockop D. J. Procollagen-a precursor form of collagen. Clin Orthop Relat Res. 1973 Nov-Dec;(97):175–195. doi: 10.1097/00003086-197311000-00026. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Collagen formation--observations on its intracellular packaging and transport. Z Zellforsch Mikrosk Anat. 1972;129(4):455–470. doi: 10.1007/BF00316743. [DOI] [PubMed] [Google Scholar]