Abstract
Nearly a third of obese individuals, termed metabolically benign obese, have a low burden of adiposity-related cardiometabolic abnormalities, while a substantial proportion of normal weight individuals possess risk factors. In cross-sectional analyses of 699 normal weight and 1294 overweight/obese postmenopausal women enrolled in a nested case-control stroke study ancillary to the Women’s Health Initiative Observational Study, we compared levels of adiponectin, leptin, and resistin among metabolically benign normal weight, at-risk normal weight, metabolically benign obese, and at-risk obese women using components of the ATP III definition of the metabolic syndrome (metabolically benign: ≤1 of the 4 components; at-risk phenotype: ≥2 components or diabetes). Overall, 382/699 normal weight women (54.6%) and 328/1194 overweight/obese women (27.5%) were metabolically benign. Among normal weight women, at-risk women had higher leptin and lower adiponectin levels compared to metabolically benign women; multivariate-adjusted odds ratios were significant for having leptin (OR: 2.51; 95% CI: 1.28–5.01) and resistin (1.46; 1.03–2.07) in the top tertile and adiponectin in the bottom tertile (2.64; 1.81–3.84). Compared to metabolically benign overweight/obese women, at-risk obese women had higher odds of having leptin in the top tertile (1.62; 1.24–2.12) and adiponectin in the bottom tertile (2.78; 2.04–3.77). Overall, metabolically benign overweight/obese women had an intermediate adipokine profile (between at-risk obese and metabolically benign normal weight women), while at-risk normal weight women had a less favorable profile compared to metabolically benign normal weight women. As adiponectin was the only adipokine independent of BMI, it may be most likely to have a role in the etiological pathway of these phenotypes.
Keywords: postmenopausal women, adipose tissue, obesity, adipokines
INTRODUCTION
Several studies have shown that a substantial proportion of obese individuals (15–30%),1–3 termed metabolically benign obese, present with a low burden of adiposity-related cardiometabolic abnormalities, such as dyslipidemia, hypertension, and insulin resistance, despite excess weight.4–6 Additionally, a substantial proportion of normal weight individuals possess several cardiometabolic abnormalities typically associated with obesity (“at-risk” normal weight phenotype). Adipose tissue, a highly dynamic endocrine organ, secretes numerous adipokines that are hypothesized to play a role in the variation in cardiometabolic health observed among individuals of similar body size. It is hypothesized that while healthy adipocytes produce “optimal” levels of adipokines that support normal glucose and lipid turnover and healthy endothelial function, adipose tissue that has been significantly infiltrated by inflammatory cells produces a pro-inflammatory milieu characterized by abnormally high levels of leptin, resistin, tumor necrosis factor alpha (TNF-α), and interleukin-6 (IL-6), as well as low levels of adiponectin. This milieu promotes insulin resistance, endothelial dysfunction, increased oxidative stress, and inflammation, which may lead to atherosclerosis.7, 8,9
We have previously shown that levels of inflammatory cytokines, such as IL-6, TNF-α, plasminogen activator inhibitor-1 antigen (PAI-1), and C-reactive protein (CRP), released in part by adipose tissue but also by other tissues, are elevated in at-risk normal weight women compared to their metabolically benign normal weight counterparts, and are lower in metabolically benign obese women compared to their at-risk counterparts.10 Further, a limited literature shows that even among individuals with similar BMI, the expression profile of adipokines is not uniform.11, 12 Therefore, the purpose of the current study was to examine levels of leptin, resistin, and adiponectin among women of specific body size phenotypes participating in the Hormones and Biomarkers Predicting Stroke (HaBPS) Study, an ancillary study to the Women’s Health Initiative Observational Study (WHI-OS). We hypothesized that (1) a more favorable adipokine profile, characterized by lower levels of leptin and resistin and higher levels of adiponectin, may help explain lower cardiometabolic risk among metabolically benign obese women despite their excess weight; while (2) a less favorable adipokine profile may help explain elevated cardiometabolic risk factors in at-risk lean women despite their normal weight.
METHODS
Participants and Study Design
We used data from the HaBPS, a case-control study of incident ischemic stroke nested within the WHI-OS, aimed at examining the relationships between various biomarkers and hormones measured in the WHI-OS baseline blood specimens with the subsequent development of ischemic stroke over follow-up. WHI-OS is an ongoing multicenter prospective study examining risk factors for the development of health outcomes among US postmenopausal women between 50–79 years of age at recruitment, who had no medical conditions associated with an anticipated survival of <3 years. A total of 93,676 women were recruited from October 1993 through December 1998. The extensive inflammatory biomarker profile of HaBPS study participants provided a unique opportunity to evaluate the adipokine profile among various body size phenotype using cross-sectional analyses of the baseline data, prior to the experience of a stroke event. Therefore, although the HaBPS dataset consists of matched case-control pairs, follow-up ischemic stroke case-control status is considered as a covariate, rather than an outcome. Written informed consent and appropriate institutional review board approval were obtained by each participating WHI-OS site.
In the HaBPS study, 972 women with incident ischemic stroke diagnosed between study baseline and July 1, 2003 were matched to 972 controls on age at baseline (± 2 years), race-ethnicity, date of study enrollment (± 3 months), and follow-up time (control follow-up time ≥ case follow-up time). Women with previous history of myocardial infarction (MI) or stroke were excluded from the HaBPS study. Exclusion criteria from the HaBPS dataset for the current analyses were: missing values of body mass index (BMI; n = 23), as well as BMI in the underweight range (<18.5 kg/m2; n = 22) leaving data from 699 normal weight (18.5≤ BMI <25.0 kg/m2) and 1194 overweight/obese (BMI ≥ 25 kg/m2) women for the analyses.
Measurement of Demographic, Health Behavior, and Physical Factors
At the baseline visit, women completed self-administered questionnaires that included information on demographic and behavioral factors (education, income, smoking status, and physical activity), medication use, medical history, and family history of cardiovascular disease (CVD) and diabetes. The WHI physical activity questionnaire was self-administered at enrollment, where participants reported the usual frequency, duration and intensity of recreational and household activities and exercise. Summary variables were then created by combining frequency, duration and MET-estimated intensity in the following equation: [(Frequency of activity per week × Minutes per session × MET for that activity) / (60 min/hour)] to quantify in “METhours”, the total kilocalories expended per kilogram per week. MET units are independent of body weight. The questionnaire has been shown to have moderate to high test-retest reliability.13 Additionally, each woman underwent a physical examination that included anthropometric and blood pressure measurements, and collection of fasting blood specimens (after 8 hours or longer of fasting). Height was measured with a wall-mounted stadiometer, and weight was measured on a balance beam scale, with participants wearing light clothing. BMI was calculated as weight in kilograms divided by height in square meters. Waist circumference at the natural waist or narrowest part of the torso was measured to the nearest 0.1 cm. Blood pressure was measured using the right arm in a seated position after a short rest, and averaged across two readings.
Laboratory Measurements
Baseline serum specimens (stored at −70°C at the central repository) were measured for levels of insulin, glucose, and lipids. Serum insulin and glucose levels were measured by the Medical Research Laboratories (Highland Heights, KY, USA). Insulin resistance was estimated using homeostasis model assessment (HOMA-IR).14 Diabetes was defined as self-reported diabetes treatment or a fasting glucose level ≥ 126 mg/dL. Total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) were measured on a Hitachi 911 analyzer with reagents from Roche Diagnostics (Indianapolis, IN, USA) and Genzyme Corporation (Cambridge, MA, USA). Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation for women with TG ≤ 400 mg/dl.15 High-sensitivity CRP was measured by immunoturbidity initially, and then by immunonephelometry. Plasma levels of adiponectin, leptin, and resistin were measured by a multiplex assay (Human Adipokine Panels A and B, Millipore, Billerica, MA); the inter-assay coefficientsof variation were 11.3% for adiponectin, 5.3% for leptin, and 11.4% for resistin. Laboratory methods for all biomarkers measured in the HaBPS study have been reported previously.16, 17
Metabolically Benign and At-Risk Phenotype Definitions
The primary analyses utilized components of the Adult Treatment Panel-III (ATP-III) metabolic syndrome definition excluding the waist circumference component due to its collinearity with BMI. Specifically, women were initially categorized as normal weight (BMI < 25 kg/m2) or overweight/obese (≥ 25 kg/m2), and then further categorized by metabolic status. Both normal weight and overweight/obese women were classified as metabolically benign if they had less than 2 of the following 4 ATP-III components : (1) elevated blood pressure (systolic/diastolic BP≥130/85mmHg or antihypertensive medication), (2) elevated TG (≥150mg/dL or lipid-lowering medication), (3) low HDL-C (<50mg/dL), and (4) elevated glucose (≥100mg/dL or diabetes medication).18 Normal weight and overweight/obese women with two or more of the four components were classified as at-risk. In addition, women were classified as “at-risk” if they reported use of anti-diabetic medication or had a fasting glucose of ≥ 126 mg/dL, regardless of the number of other metabolic components. Sensitivity analyses examined the consistency of findings using three alternative definitions of the metabolically benign phenotype: (1) modified ATP-III criteria ( ≥ 1 of the 4 ATP-III criteria described above), and (2) the insulin resistance-based (IR) definition among non-diabetic women only (women were classified as metabolically benign if they had HOMA-IR values less than the HOMA-IR values corresponding to the 25th percentile of the distribution among non-diabetic obese participants (HOMA-IR < 0.853) 19, 20 and (3) using the ATP III definition of metabolic syndrome including waist circumference (with presence of ≥ 2 of the 5 components defining the metabolically benign phenotype).
Statistical Methods
Demographics, health history, median laboratory values, and median adipokine levels were compared among metabolically benign normal weight, at-risk normal weight, metabolically benign overweight/obese, and at-risk overweight/obese women, using the Kruskal-Wallis non-parametric test for continuous data, and the chi-square test for categorical data. Post-hoc between group comparisons were completed using Bonferroni correction for both continuous and categorical data. Race-ethnicity was coded as “white” (Caucasian) and “others” (including black, Hispanic, Asian, American Indian, and unspecified), since non-white race-ethnic groups were not present in sufficient numbers to be analyzed separately.
Odds ratios (ORs) and 95% Confidence Intervals (CI) of being in the top tertile of leptin and resistin, and bottom tertile of adiponectin associated with body size phenotypes were calculated using multivariate logistic regression modeling, adjusted for age, race-ethnicity, stroke case status, smoking, physical activity, and hormone treatment use (Model 1). To distinguish the effects of obesity, models were further adjusted for BMI. Due to non-overlap of BMI between the normal weight and overweight/obese groups, models were run separately for the normal weight and overweight/obese groups, using the benign phenotype as reference category. Tertile cutoffs were calculated based on the entire sample of HaBPS participants (N=1,993). A separate model was built for each adipokine.
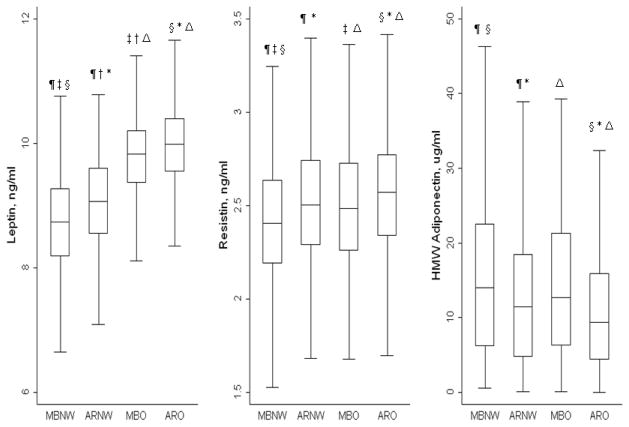
In secondary analyses, ORs were re-calculated using the modified ATP-III definition, the IR-based definition of the metabolically benign phenotype, and the ATP-III definition to evaluate the consistency of the results across several definitions. Additional sensitivity analyses were restricted to controls only (women who did not develop stroke), and (2) non-diabetic women. All statistical analyses were performed using the SAS version 9.1 software (SAS Institute Inc., Cary, NC, USA), with the exception of box plots (Figure 1), which were obtained using STATA version 11 (StataCorp LP., College Station, TX, USA). Two-tailed values of P <0.05 were considered statistically significant.
Figure 1.
Box plots representing median levels of leptin, resistin and adiponectin levels by body size phenotypes. Horizontal lines represent medians, while bottom and top of the boxes represent the 25th and 75th percentiles respectively.
Similar symbols show statistically significant differences between groups (p <0.01)using Wilcoxon Rank Sum test.
MBNW: Metabolically Benign Normal Weight (N=382); ARNW: At-risk Normal Weight (N=317); MBO: Metabolically Benign Overweight/Obese (N=328); ARO: At-risk Overweight/Obese (N=866).
The Metabolically Benign Phenotype is defined as ≤ 1 of the following ATP-III components: 1) systolic/diastolic blood pressure ≥ 130/85 mmHg or antihypertensive medication; 2) fasting triglyceride ≥ 150 mg/dL or lipid lowering medication; 3) fasting HDL ≤ 50 mg/dL; 4) fasting glucose ≥ 100 mg/dL
RESULTS
Baseline Characteristics
Baseline characteristics of normal weight and overweight/obese study participants are presented in Table 1. Overall, 382 out of 699 normal weight women (54.6%), and 328 out of 1194 obese women (27.5 %) were classified as metabolically benign. In both normal weight and overweight/obese groups, compared to metabolically benign women, at-risk women were slightly older, less likely to be white, reported lower levels of income, education, and physical activity, had higher median values of BMI, waist circumference, and CRP at baseline, and were more likely to have suffered a stroke across follow-up.
Table 1.
Baseline Characteristics among Normal Weight (NW) and Overweight/Obese Women
| Metabolically Benign Normal Weight (n = 382) | At-Risk Normal Weight (n = 317) | Metabolically Benign Overweight/ Obese (n = 328) | At-Risk Overweight/ Obese (n = 866) | P | |
|---|---|---|---|---|---|
|
|
|||||
| Age, yrs | 70.0 (8.0) | 71.0 (8.0) §# | 68.0 (8.0) † | 69.0 (9.0) | < 0.001 |
| Race-Ethnicity, n (%) | < 0.001 | ||||
| White | 355 (93) | 272 (86) § | 276 (84) † | 721 (83) | |
| Others | 27 (7) | 45 (14) § | 52 (16) † | 145 (17) | |
| Smoking, n (%) | 0.185 | ||||
| Never | 201 (53) | 151 (48) | 178 (55) | 470 (55) | |
| Former | 149 (40) | 137 (43) | 130 (40) | 344 (40) | |
| Current | 26 (7) | 27 (9) | 15 (5) | 45 (5) | |
| Education, % ≥ High School Income, n (%) | 369 (97) | 293 (93) § | 315 (96) ‡ | 788 (92) | < 0.001 |
| < $35,000 | 137 (39) | 147 (49) § | 153 (51) † | 452 (57) | |
| $35,000–$49,000 | 66 (19) | 69 (23) § | 55 (18) † | 149 (19) | |
| ≥ $50,000 | 146 (42) | 81 (27) § | 95 (31) † | 199 (25) | |
| Hormone Use, n (%) | 0.002 | ||||
| Never | 143 (37) | 122 (38) # | 140 (43) | 423 (49) | |
| Past | 75 (20) | 57 (18) # | 54 (16) | 145 (17) | |
| Current | 164 (43) | 138 (44) # | 134 (41) | 298 (34) | |
| Systolic Blood Pressure, mmHg | 121.0 (22.0) | 136.0 (25.0) § | 124.0 (23.0) ‡ | 139.0 (22.0) | < 0.001 |
| Diastolic Blood Pressure, mmHg | 71.0 (13.0) | 74.0 (12.0) §# | 74.0 (12.0) †‡ | 77.0 (14.0) | < 0.001 |
| Total Cholesterol, mg/dL | 221.0 (44.0) | 235.0 (51.0) § | 230.0 (47.0) † | 234.0 (51.0) | < 0.001 |
| HDL Cholesterol, mg/dL | 68.0 (21.0) | 55.0 (20.0) §# | 62.0 (18.0) †‡ | 49.0 (17.0) | < 0.001 |
| LDL Cholesterol, mg/dL | 126.6 (40.8) | 138.0 (46.2) § | 141.7 (45.3) † | 143.6 (51.2) | < 0.001 |
| Triglycerides, mg/dL | 110.0 (49.0) | 179.0 (86.0) § | 116.0 (46.0) ‡ | 184.0 (100.0) | < 0.001 |
| Glucose, mg/dL | 91.0 (8.0) | 100.0 (16.0) §# | 93.0 (9.0) †‡ | 103.0 (23.0) | < 0.001 |
| C-Reactive Protein, mg/mL | 1.5 (2.6) | 2.7 (3.9) §# | 2.7 (4.2) †‡ | 4.2 (5.6) | < 0.001 |
| Elevated Blood Pressure, % | 151 (40) | 252 (80) § | 136 (42) ‡ | 733 (85) | < 0.001 |
| Low HDL Cholesterol, n (%) | 28 (7) | 151 (48) §# | 19 (6) ‡ | 515 (60) | < 0.001 |
| Elevated Triglycerides, n (%) | 53 (14) | 227 (72) § | 48 (14) ‡ | 640 (74) | < 0.001 |
| Elevated Glucose, n (%) | 29 (8) | 172 (54) §# | 33 (10) ‡ | 546 (63) | < 0.001 |
| Insulin, uU/mL | 3.7 (2.3) | 5.6 (3.6) §# | 5.8 (4.2) †‡ | 9.6 (7.9) | < 0.001 |
| HOMA-IR | 0.8 (0.5) | 1.3 (1.0) §# | 1.2 (0.9) †‡ | 2.3 (2.3) | < 0.001 |
| Diabetes, n (%) | 0 | 44 (14) §# | 0‡ | 191 (22) | < 0.001 |
| Aspirin Use, n (%) | 125 (33) | 120 (38) | 119 (36) | 360 (42) | 0.023 |
| BMI, kg/m2 | 22.4 (2.6) | 23.2 (2.3) §# | 27.7 (4.3) †‡ | 29.5 (5.9) | < 0.001 |
| Waist Circumference, cm | 73.5 (8.9) | 77.5 (9.0) §# | 87.3 (14.4) †‡ | 92.0 (14.0) | < 0.001 |
| Physical Activity, METs per week | 15.3 (19.4) | 11.4 (16.4) §# | 8.8 (16.3) † | 7.5 (15.0) | < 0.001 |
| Developed Stroke during WHI follow up, n (%) | 151 (40) | 166 (52) § | 132 (40) ‡ | 495 (57) | < 0.001 |
All continuous data presented as median (IQR); all categorical data presented as number (%)
Kruskal-Wallis test used to examine differences between groups for continuous data. Bonferroni test used for post-hoc analysis
Chi-square test used to examine differences between groups for categorical data. Bonferroni test used for post-hoc analysis
P value <0.05 for metabolically benign obese vs. metabolically benign normal weight
P value <0.05 for metabolically benign obese vs. at-risk obese
P value <0.05 for at-risk normal weight vs. metabolically benign normal weight
P value <0.05 for at-risk normal weight vs. at-risk obese
The Metabolically Benign Phenotype was defined as ≤1 of: elevated blood pressure (≥130/85 mmHg or medication), elevated triglycerides (≥150mg/dL), elevated fasting glucose (≥100 mg/dL or medication), low HDL-C (<50mg/dL for women or medication).
The Metabolically At-Risk Phenotype was defined as ≥ 2 of: elevated blood pressure (≥130/85 mmHg or medication), elevated triglycerides (≥150mg/dL), elevated fasting glucose (≥100 mg/dL or medication), and low HDL-C (<50mg/dL for women or medication), OR If diabetic (self-report of diabetes treatment or fasting blood glucose ≥ 126 mg/dl).
Adipokine Levels
Adipokine levels among metabolically benign normal weight, at-risk normal weight, metabolically benign overweight/obese, and at-risk overweight/obese women are presented in Figure 1. There was a trend for increasing levels of leptin and resistin, and decreasing levels of adiponectin, going from metabolically benign and normal weight to at-risk and obese. Specifically, at-risk normal weight women overall had significantly higher median levels of leptin (9.07 vs. 8.74 ng/mL) and resistin (2.5 vs. 2.4 ng/mL), but lower levels of adiponectin (11.44 vs. 14.03 μg/mL) when compared to their metabolically benign normal weight counterparts (Figure 1). Metabolically benign overweight/obese women appeared to have intermediate levels of leptin and adiponectin, evidenced by higher levels of leptin and resistin compared to the metabolically benign normal weight group, but lower levels compared to their at-risk obese counterparts (Figure 1). Median adiponectin levels were significantly higher in the metabolically benign overweight/obese group compared to the at-risk group, but the levels were not significantly different from both the normal weight phenotypes.
Multivariable-Adjusted Associations of Tertiles of Adipokines with Body Size Phenotypes
After multivariable adjustment, using the metabolically benign normal weight phenotype as the reference category, the odd ratios of possessing leptin and resistin in the top tertile, and adiponectin in the bottom tertile, associated with being in the at-risk normal weight, metabolically benign overweight/obese, and at-risk overweight/obese phenotypes, are presented in Table 2. At-risk normal weight women had ~2.5 times greater odds of having leptin levels in the top tertile and ~2.6 times greater odds of having adiponectin in the bottom tertile, compared to their metabolically benign normal weight counterparts (Table 2). The association was not statistically significant for resistin.
Table 2.
Adjusted Odds Ratios (95% Confidence Intervals) of Having Leptin and Resistin Levels in the Top Tertile, and Adiponectin Levels in the Bottom Tertile, Associated with Body Size Phenotypes
| Top Tertile of Leptin (21.0–138.6 ng/ml) | Top Tertile of Resistin (14.2–182.6 ng/ml) | Bottom Tertile of Adiponectin ( 0.0–21.8 μg/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| MBNW (n=372) | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) | |||
| ARNW (n=310) | 2.53 | 1.28–5.01 | 0.008 | 1.39 | 0.98–1.95 | 0.064 | 2.64 | 1.81–3.84 | <0.001 |
| MBO (n=319) | 16.84 | 9.24–30.68 | < 0.001 | 1.55 | 1.11–2.18 | 0.011 | 1.46 | 0.98–2.16 | 0.062 |
| ARO (n=844) | 27.03 | 15.22–48.00 | < 0.001 | 1.78 | 1.34–2.38 | <0.001 | 4.08 | 2.95–5.65 | < 0.001 |
Model 1 = adjusted for age, race, stroke case status, smoking, physical activity, and hormone therapy use
MBNW – Metabolically Benign Normal Weight; ARNW – At-Risk Normal Weight; MBO – Metabolically Benign Overweight/Obese; ARO – At-Risk Overweight/Obese
The Metabolically Benign Phenotype was defined as ≤1 of: elevated blood pressure (≥130/85 mmHg or medication), elevated triglycerides (≥150mg/dL), elevated fasting glucose (≥100 mg/dL or medication), low HDL-C (<50mg/dL for women or medication).
The Metabolically At-Risk Phenotype was defined as ≥ 2 of: elevated blood pressure (≥130/85 mmHg or medication), elevated triglycerides (≥150mg/dL), elevated fasting glucose (≥100 mg/dL or medication), low HDL-C (<50mg/dL for women or medication). OR If diabetic (self-report of diabetes, fasting blood glucose ≥ 126 mg/dl or on antidiabetic medication).
Compared to metabolically benign normal weight women, both metabolically benign and at-risk overweight/obese women had significantly greater odds of having leptin and resistin in the top tertile. Unlike leptin and resistin, the odds of being in the lowest tertile of adiponectin were different between the benign and at-risk categories of overweight/obese women, where metabolically benign women did not show significantly greater odds of being in the lowest tertile of adiponectin, metabolically at-risk overweight/obese women had ~ 4 times higher odds.
Comparisons were then limited to within body size groups to allow for adjustment of BMI. After further adjustment for BMI, the higher odds of having leptin in the top tertile among metabolically benign vs. at-risk women noted above were attenuated, while the higher odds of having resistin in the top tertile and adiponectin in the bottom tertile remained significant (Table 3; Model 2).
Table 3.
Adjusted Odds Ratios (95% Confidence Intervals) of Having Leptin and Resistin Levels in the Top Tertile, and Adiponectin Levels in the Bottom Tertile, Associated with Body Size Phenotypes
| Top Tertile of Leptin (21.0–138.6 ng/ml) | Top Tertile of Resistin (14.2–182.6 ng/ml) | Bottom Tertile of Adiponectin (0.0–21.8 μg/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Model 1 | |||||||||
| MBNW (n=372) | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) | |||
| ARNW (n=310) | 2.51 | 1.25–5.07 | 0.014 | 1.46 | 1.03–2.07 | 0.036 | 2.87 | 1.95–4.24 | < 0.001 |
| Model 2 | |||||||||
| MBNW | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) | |||
| ARNW | 1.96 | 0.95–4.04 | 0.067 | 1.50 | 1.05–2.15 | 0.027 | 2.53 | 1.70–3.76 | < 0.001 |
Model 1 = adjusted for age, race, stroke case status, smoking, physical activity, and hormone therapy use
Model 2 = Model 1 + BMI
MBNW – Metabolically Benign Normal Weight Phenotype; ARNW – At-Risk Normal Weight Phenotype
The Metabolically Benign Phenotype was defined as ≤ 1 of: elevated blood pressure (≥130/85 mmHg or medication), elevated triglycerides (≥150mg/dL), elevated fasting glucose (≥100 mg/dL or medication), low HDL-C (<50mg/dL for women or medication).
The Metabolically At-Risk Phenotype was defined as ≥ 2 of: elevated blood pressure (≥130/85 mmHg or medication), elevated triglycerides (≥150mg/dL), elevated fasting glucose (≥100 mg/dL or medication), low HDL-C (<50mg/dL for women or medication). OR If diabetic (fasting blood glucose ≥ 126 mg/dl, or self-report of diabetes or on antidiabetic medication).
Comparing differences in adipokine levels in the overweight/obese women using the metabolically benign overweight/obese phenotype as reference, at-risk overweight/obese women had ~1.6 times significantly greater odds of having leptin in the top tertile and ~2.8 times greater odds of having adiponectin in the bottom tertile (Table 4; Model 1). After adjusting for BMI, the odds of having leptin in the top tertile were attenuated, but the association remained significant for adiponectin (Table 4; Model 2). The association with top tertile of resistin was not statistically significant.
Table 4.
Adjusted Odds Ratios (95% Confidence Intervals) of Having Leptin and Resistin Levels in the Top Tertile, and Adiponectin Levels in the Bottom Tertile, Associated with Body Size Phenotypes
| Top Tertile of Leptin (21.0–138.6 ng/ml) | Top Tertile of Resistin (14.2–182.6 ng/ml) | Bottom Tertile of Adiponectin ( 0.0–21.8 μg/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Model 1 | |||||||||
| MBO (n= 319) | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) | |||
| ARO (n= 844) | 1.62 | 1.24–2.12 | < 0.001 | 1.12 | 0.85–1.48 | 0.429 | 2.78 | 2.04–3.77 | < 0.001 |
| Model 2 | |||||||||
| MBO | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) | |||
| ARO | 1.22 | 0.91–1.62 | 0.180 | 1.02 | 0.77–1.36 | 0.876 | 2.60 | 1.91–3.55 | < 0.001 |
Model 1 = adjusted for age, race, stroke case status, smoking, physical activity, and hormone therapy use
Model 2 = Model 1 + BMI
MBO – Metabolically Benign Overweight/Obese Phenotype; ARO – At-Risk Overweight/Obese Phenotype
The Metabolically Benign Phenotype was defined as ≤ 1 of: elevated blood pressure (≥130/85 mmHg or medication), elevated triglycerides (≥150mg/dL), elevated fasting glucose (≥100 mg/dL or medication), low HDL-C (<50mg/dL for women or medication).
The Metabolically At-Risk Phenotype was defined as ≥ 2 of: elevated blood pressure (≥130/85 mmHg or medication), elevated triglycerides (≥150mg/dL), elevated fasting glucose (≥100 mg/dL or medication), low HDL-C (<50mg/dL for women or medication). OR If diabetic (fasting blood glucose ≥ 126 mg/dl, or self-report of diabetes or on antidiabetic medication).
Sensitivity Analyses
The odds ratios were similar, but results did not always reach statistical significance when the modified ATP-III definition was used (defining metabolically benign as ≤ 1 metabolic component of the ATP III criteria); when the metabolically benign phenotype was defined solely by levels of insulin resistance (HOMA-IR<25th percentile) (excluding women with self- reported diabetes treatment or fasting glucose ≥ 126mg/dL) or when the ATP III criteria including waist circumference were used (see supplementary tables). Additional sensitivity analyses restricted to controls only (excluding women who went on from these baseline data to suffer a stroke across follow-up), and non-diabetic women also produced similar findings (data not shown).
DISCUSSION
Although a growing body of scientific literature recognizes the existence of metabolically benign overweight/obese and at-risk normal weight phenotypes, the mechanisms underlying the variation in cardiometabolic health even in individuals of similar levels of adiposity remain unclear.1–3, 6 In our study, at-risk normal weight postmenopausal women presented with a less favorable adipokine profile compared to their metabolically benign normal weight counterparts, characterized by higher levels of leptin and resistin, and lower levels of adiponectin. Metabolically benign overweight/obese women had an intermediate adipokine profile, with higher levels of leptin and resistin compared to normal weight women, but lower levels of leptin and resistin and higher levels of adiponectin compared to their at-risk overweight/obese peers. After adjusting for BMI, the differences between both the normal weight and obese groups were attenuated for leptin, but remained for adiponectin. The odds of having higher levels of resistin remained in the normal weight groups but not in the overweight/obese groups after adjustment for BMI.
Among individuals with similar BMI levels, adipokine expression is variable.12, 21 Few studies have examined inflammatory marker levels in metabolically benign obese individuals, and these have provided contradictory results, partially owing to the fact that some studies have compared metabolically benign obese individuals to at-risk obese individuals, while other studies have compared them to healthy normal weight individuals.21–25 In comparing all four groups, Aguilar et al found higher levels of adiponectin in the metabolically healthy group compared to the at-risk group at each stratum of BMI.21 Similar to our results, Labruna et al did not find difference in leptin levels between metabolically benign and at-risk obese subjects.26 The role of resistin in worsening of cardiometabolic health is more discrepant than that of adiponectin and appears to be mainly due to inflammatory induction of endothelial dysfunction.27, 28 In our study, resistin was associated with metabolic health in the normal weight group, even after adjustment for BMI, but was not associated with metabolic health in the overweight/obese groups once BMI was accounted for. This difference in resistin results after BMI adjustment between normal weight and overweight/obese groups may be a statistical product resulting from the narrower BMI range in the normal weight group.
Adipokine profile comparisons between metabolically benign obese and healthy normal weight individuals are less discrepant. A cross-sectional study of 716 men and women found that metabolically benign obese individuals (defined as having HDL ≥40mg/dL and absence of type 2 diabetes or hypertension) had “higher than expected” concentrations of adiponectin, in the same range as that observed in lean subjects.21 These results are consistent with our current results also finding similar adiponectin levels in metabolically benign overweight/obese compared to healthy normal weight women. Our recent study of inflammatory marker levels (CRP, IL-6, TNF-α, PAI-1, WBC) among participants of the HaBPS cohort suggested an intermediate pro-inflammatory burden among metabolically benign obese women compared to healthy normal weight and at-risk overweight/obese women.10 Moreover, several studies, including our own, suggest that the cardiometabolic health, subclinical atherosclerosis, and clinical CVD burden of benign obese individuals seems to be slightly worse than that of their “healthy” lean counterparts (metabolically benign normal weight individuals).29–31 Our present data add to these findings showing an intermediate expression of adipokines in metabolically benign obese women, with higher levels of pro-inflammatory leptin and resistin but similar levels of anti-inflammatory adiponectin compared to metabolically benign normal weight women, and lower levels of leptin and resistin and higher levels of adiponectin compared to at-risk obese women. These findings raise the possibility that this differential expression of adipokines may contribute to the intermediate cardiometabolic health and CVD risk of the metabolically benign obese subgroup.
The at-risk normal weight phenotype (also referred to as “metabolically obese normal weight”) is another unique subgroup that has been recently gaining scientific interest. Despite having BMI in the normal range, at-risk normal weight individuals present with obesity-related phenotypic characteristics such as dyslipidemia, insulin resistance, and hypertension. Several published studies suggest that the prevalence of this phenotype ranges between 5% and 18%, depending on the criteria used to define this subgroup.32, 33 Although the literature is limited, longitudinal studies, including our own, have reported that these at-risk normal weight individuals are at higher risk for developing type 2 diabetes and CVD when compared to their metabolically benign normal weight counterparts and metabolically benign obese individuals.19, 34 Further, our prior examination of the HaBPS cohort demonstrated a pro-inflammatory state in at-risk normal weight women, with higher levels of multiple inflammatory markers such as TNF-α, IL-6, CRP, white blood cells, and e-selectin, compared to healthy normal weight women.10 The current results of higher levels of leptin and resistin, and lower levels of the anti-inflammatory adipokine adiponectin are further evidence of a heightened inflammatory state despite normal BMI values and low waist circumferences.
In the current study, leptin levels were more strongly associated with obesity status than metabolic status, since leptin levels were significantly higher in obese women than normal weight women regardless of their metabolic state, and differences between at-risk and benign women were attenuated after adjusting for BMI in both the normal weight and overweight/obese groups, whereas adiponectin levels seemed to be much more strongly influenced by the metabolic state than by BMI. High leptin levels have been associated with both insulin secretion and inflammation35 by up-regulating the expression of pro-inflammatory and pro-angiogenic factors, 36 In contrast, adiponectin, the most abundant adipose tissue-specific adipokine in circulation, improves peripheral insulin sensitivity, improves fatty acid oxidation in liver and muscle and induces the production of anti-inflammatory cytokines.37
Early evidence suggests that adipocyte hypertrophy is associated with pro-inflammatory changes in the adipokine profile.38 And, recent data suggest that omental (visceral), but not subcutaneous adipokine secretion is associated with metabolic health,39 independent of body composition.40 Therefore, it is possible that differences in adipocyte size and adipose tissue distribution, despite similar overall body size, may partially determine differences in adipokine levels between benign and at-risk phenotypes, independent of BMI. Both at-risk normal weight and obese women had larger waist circumference than their metabolically benign counterparts. However, adjustment for waist circumference in our sensitivity analyses, though not a perfect proxy for abdominal adipose tissue, had minimal effect on the estimates, suggesting that adjustment for visceral adipose tissue may not completely eliminate these differences either. Further research in these areas is warranted.
The limitations of our study should be noted. Our findings are only generalizable to postmenopausal women primarily of Caucasian race-ethnicity, who evidence a lower prevalence of overweight and obesity than the general population. Additionally, whether the adipokine variation in women of similar body size demonstrated here is a precursor to variation in cardiometabolic health, or rather, results from the cardiometabolic risk factors, themselves, or both, cannot be addressed by the cross sectional design of our analyses. In addition, we recognize that obesity is a multifactorial disorder, and we were unable to evaluate several other components such as diet, alcohol intake, genetic factors, or adipose tissue distribution.
Despite these limitations, our study has a number of strengths. The nesting of this study within the WHI-OS allowed us to assess a much larger number of overweight/obese women who had an extensive assessment of their adipokine profile. In addition, we were able to evaluate adipokine levels not only among the overweight/obese population, but also make comparisons to both metabolically benign and at-risk normal weight women.
In summary, our study supports the concept that the adipokine profile within normal weight individuals as well as within overweight/obese individuals is not uniform, and may play a role in the cardiometabolic health of metabolically benign and at-risk phenotypes. Metabolically benign overweight/obese women presented with an intermediate adipokine profile, while at-risk normal weight women had a less favorable adipokine milieu compared to their metabolically benign counterparts. The etiology of both of these unique phenotypes appears to be multifactorial, and while adipokines are likely not the only determinants, they may be a component of the etiological pathway. More basic science and epidemiologic research is needed in order to understand the complex crosstalk between inflammatory and metabolic processes among these two phenotypes.
Supplementary Material
Acknowledgments
The research on which this publication is based was funded in part by the National Heart Lung and Blood Institute Mentored Patient-Oriented Research Award 1K23HL105790-01 (to Dr. Khan); the American Heart Association 10PRE3410007 grant (to Dr. Ogorodnikova); the Hormones and Biomarkers Predicting Stroke (HaBPS) Study was supported by Grant Number R01NS042618 (to Dr. Wassertheil-Smoller), R03NS061114 (to Dr. Wildman), and N01-WH-74310 (to Dr. Ho) from the National Institutes of Neurological Disorders and Stroke. The Women’s Health Initiative (WHI) program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. The study sponsors had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Key investigators in the HaBPS Study are: Albert Einstein College of Medicine: Sylvia Wassertheil-Smoller, Robert Kaplan, Aileen McGinn; Fred Hutchinson Cancer Center: Charles Kooperberg; NIH: John Lynch; State University of New York Downstate Medical Center: Daniel Rosenbaum, Alison E. Baird; Boston University: Philip Wolf. The complete list of WHI centers and investigators can be found online at http://www.whiscience.org/collaborators/investigators.php
This publication was made possible by the CTSA Grant UL1 RR025750 and KL2 RR025749 and TL1 RR025748 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessary represent the official view of the NCRR or NIH.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Prevalence of uncomplicated obesity in an italian obese population. Obes Res. 2005;13:1116–1122. doi: 10.1038/oby.2005.130. [DOI] [PubMed] [Google Scholar]
- 2.Karelis AD, Brochu M, Rabasa-Lhoret R. Can we identify metabolically healthy but obese individuals (mho)? Diabetes Metab. 2004;30:569–572. doi: 10.1016/s1262-3636(07)70156-8. [DOI] [PubMed] [Google Scholar]
- 3.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the us population (nhanes 1999–2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 4.Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET. Metabolic and body composition factors in subgroups of obesity: What do we know? J Clin Endocrinol Metab. 2004;89:2569–2575. doi: 10.1210/jc.2004-0165. [DOI] [PubMed] [Google Scholar]
- 5.Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47:699–713. doi: 10.2337/diabetes.47.5.699. [DOI] [PubMed] [Google Scholar]
- 6.Sims EA. Are there persons who are obese, but metabolically healthy? Metabolism. 2001;50:1499–1504. doi: 10.1053/meta.2001.27213. [DOI] [PubMed] [Google Scholar]
- 7.Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11:327–332. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 8.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: Associations with obesity, insulin resistance, and endothelial dysfunction: A potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 9.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 10.Wildman RP, Kaplan R, Manson JE, Rajkovic A, Connelly SA, Mackey RH, et al. Body size phenotypes and inflammation in the women’s health initiative observational study. Obesity (Silver Spring) 2011;19:1482–1491. doi: 10.1038/oby.2010.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koster A, Stenholm S, Alley DE, Kim LJ, Simonsick EM, Kanaya AM, et al. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring) 2010;18:2354–2361. doi: 10.1038/oby.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labruna G, Pasanisi F, Nardelli C, Caso R, Vitale DF, Contaldo F, et al. High leptin/adiponectin ratio and serum triglycerides are associated with an “at-risk” phenotype in young severely obese patients. Obesity (Silver Spring) 2011;19:1492–1496. doi: 10.1038/oby.2010.309. [DOI] [PubMed] [Google Scholar]
- 13.Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, Brancaccio G, et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005;29:251–255. doi: 10.1016/j.cyto.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 16.Kaplan RC, McGinn AP, Baird AE, Hendrix SL, Kooperberg C, Lynch J, et al. Inflammation and hemostasis biomarkers for predicting stroke in postmenopausal women: The women’s health initiative observational study. J Stroke Cerebrovasc Dis. 2008;17:344–355. doi: 10.1016/j.jstrokecerebrovasdis.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wassertheil-Smoller S, Kooperberg C, McGinn AP, Kaplan RC, Hsia J, Hendrix SL, et al. Lipoprotein-associated phospholipase a2, hormone use, and the risk of ischemic stroke in postmenopausal women. Hypertension. 2008;51:1115–1122. doi: 10.1161/HYPERTENSIONAHA.107.103721. [DOI] [PubMed] [Google Scholar]
- 18.Third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 19.Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 20.Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 21.Aguilar-Salinas CA, Garcia EG, Robles L, Riano D, Ruiz-Gomez DG, Garcia-Ulloa AC, et al. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J Clin Endocrinol Metab. 2008;93:4075–4079. doi: 10.1210/jc.2007-2724. [DOI] [PubMed] [Google Scholar]
- 22.Bluher M. The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr Opin Lipidol. 2010;21:38–43. doi: 10.1097/MOL.0b013e3283346ccc. [DOI] [PubMed] [Google Scholar]
- 23.Karelis AD, Faraj M, Bastard JP, St-Pierre DH, Brochu M, Prud’homme D, et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005;90:4145–4150. doi: 10.1210/jc.2005-0482. [DOI] [PubMed] [Google Scholar]
- 24.Koster A, Stenholm S, Alley DE, Kim LJ, Simonsick EM, Kanaya AM, et al. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring) 2010 doi: 10.1038/oby.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.You T, Ryan AS, Nicklas BJ. The metabolic syndrome in obese postmenopausal women: Relationship to body composition, visceral fat, and inflammation. J Clin Endocrinol Metab. 2004;89:5517–5522. doi: 10.1210/jc.2004-0480. [DOI] [PubMed] [Google Scholar]
- 26.Labruna G, Pasanisi F, Nardelli C, Caso R, Vitale DF, Contaldo F, et al. High leptin/adiponectin ratio and serum triglycerides are associated with an “at-risk” phenotype in young severely obese patients. Obesity (Silver Spring) 2011;19:1492–1496. doi: 10.1038/oby.2010.309. [DOI] [PubMed] [Google Scholar]
- 27.Gomez-Ambrosi J, Fruhbeck G. Evidence for the involvement of resistin in inflammation and cardiovascular disease. Curr Diabetes Rev. 2005;1:227–234. doi: 10.2174/157339905774574392. [DOI] [PubMed] [Google Scholar]
- 28.Vidal-Puig A, O’Rahilly S. Resistin: A new link between obesity and insulin resistance? Clin Endocrinol (Oxf) 2001;55:437–438. doi: 10.1046/j.1365-2265.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 29.Khan UI, Wang D, Thurston RC, Sowers M, Sutton-Tyrrell K, Matthews KA, et al. Burden of subclinical cardiovascular disease in “metabolically benign” and “at-risk” overweight and obese women: The study of women’s health across the nation (swan) Atherosclerosis. 2011 doi: 10.1016/j.atherosclerosis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marini MA, Succurro E, Frontoni S, Hribal ML, Andreozzi F, Lauro R, et al. Metabolically healthy but obese women have an intermediate cardiovascular risk profile between healthy nonobese women and obese insulin-resistant women. Diabetes Care. 2007;30:2145–2147. doi: 10.2337/dc07-0419. [DOI] [PubMed] [Google Scholar]
- 31.Ogorodnikova AD, Kim M, McGinn AP, Muntner P, Khan U, Wildman RP. Incident cardiovascular disease events in metabolically benign obese individuals. Obesity (Silver Spring) 2012;20:651–659. doi: 10.1038/oby.2011.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dvorak RV, DeNino WF, Ades PA, Poehlman ET. Phenotypic characteristics associated with insulin resistance in metabolically obese but normal-weight young women. Diabetes. 1999;48:2210–2214. doi: 10.2337/diabetes.48.11.2210. [DOI] [PubMed] [Google Scholar]
- 33.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: Prevalence and associated risk factor findings in the us population from the third national health and nutrition examination survey, 1988–1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogorodnikova AD, Kim M, McGinn A, Muntner P, Khan U, Wildman PR. Incident cardiovascular disease events in metabolically benign obese individuals. Obesity (Silver Spring) 2011 doi: 10.1038/oby.2011.243. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ceddia RB, William WN, Jr, Curi R. Comparing effects of leptin and insulin on glucose metabolism in skeletal muscle: Evidence for an effect of leptin on glucose uptake and decarboxylation. Int J Obes Relat Metab Disord. 1999;23:75–82. doi: 10.1038/sj.ijo.0800762. [DOI] [PubMed] [Google Scholar]
- 36.Aleffi S, Petrai I, Bertolani C, Parola M, Colombatto S, Novo E, et al. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology. 2005;42:1339–1348. doi: 10.1002/hep.20965. [DOI] [PubMed] [Google Scholar]
- 37.Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 39.Motoshima H, Wu X, Sinha MK, Hardy VE, Rosato EL, Barbot DJ, et al. Differential regulation of adiponectin secretion from cultured human omental and subcutaneous adipocytes: Effects of insulin and rosiglitazone. The Journal of clinical endocrinology and metabolism. 2002;87:5662–5667. doi: 10.1210/jc.2002-020635. [DOI] [PubMed] [Google Scholar]
- 40.Veilleux A, Caron-Jobin M, Noel S, Laberge PY, Tchernof A. Visceral adipocyte hypertrophy is associated with dyslipidemia independent of body composition and fat distribution in women. Diabetes. 2011;60:1504–1511. doi: 10.2337/db10-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.