Abstract
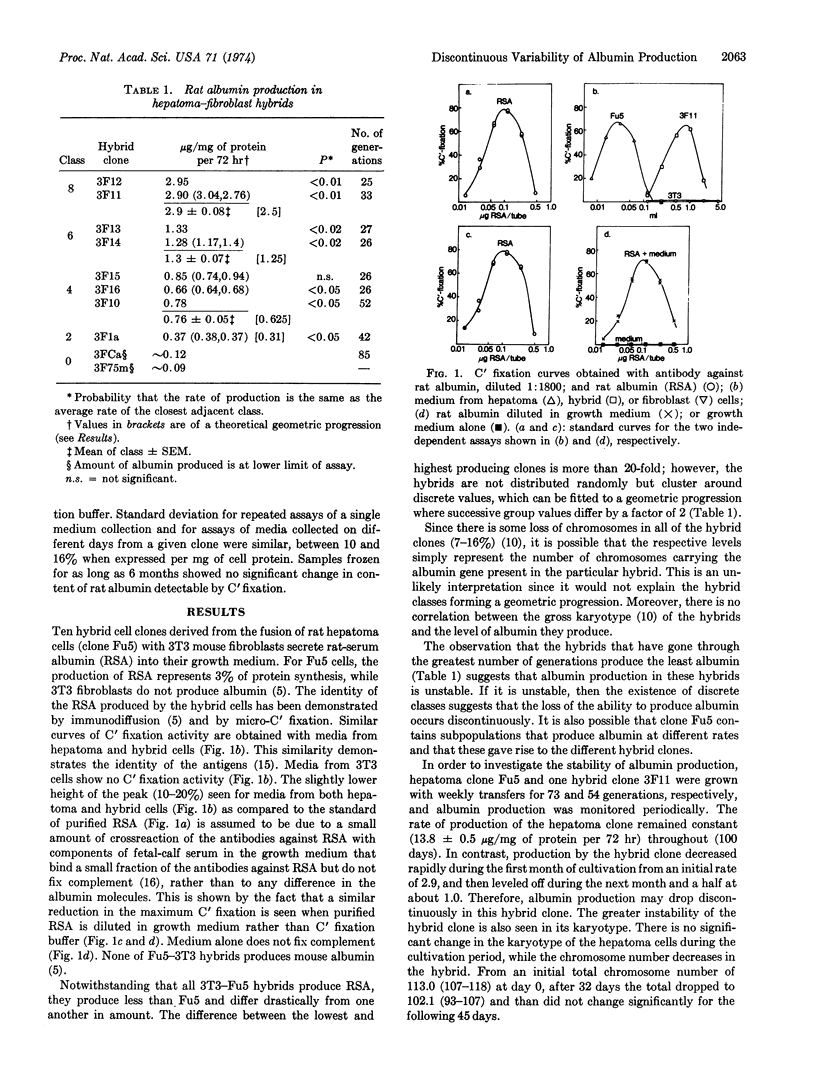
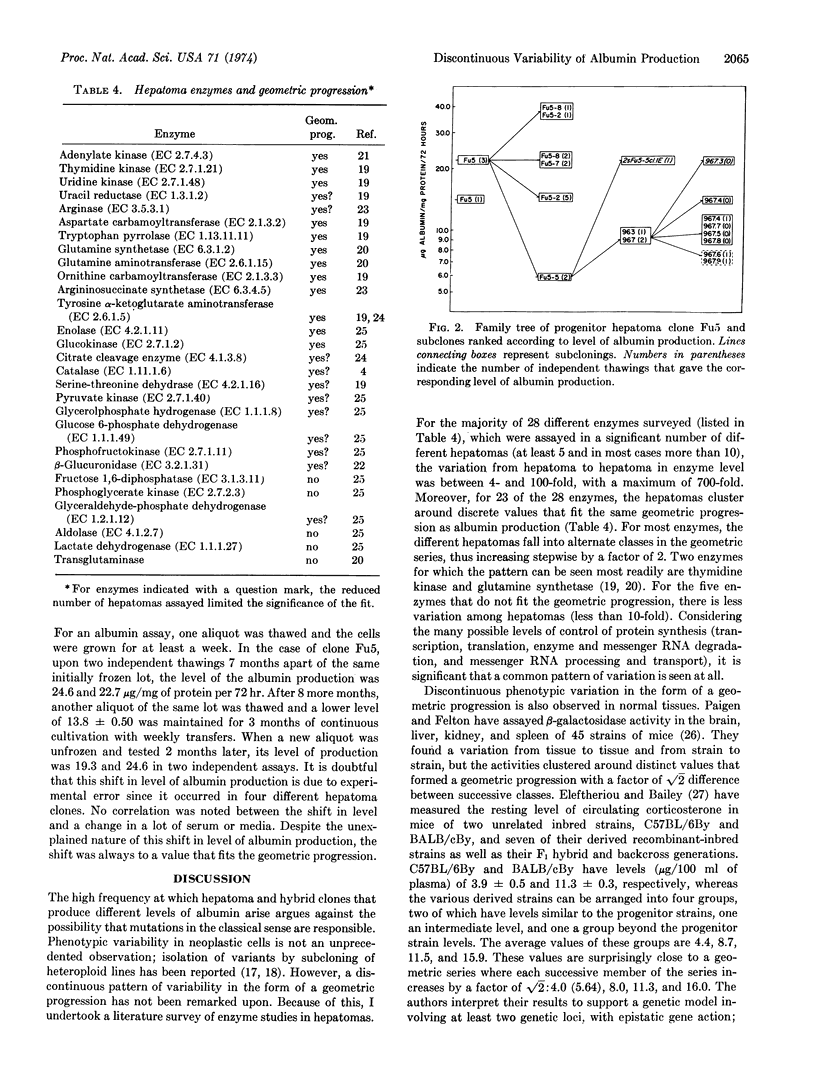
A clonal rat hepatoma cell line (Fu5) produces rat serum albumin at a constant rate over at least 3 months of continuous cultivation. Ten hybrid cell clones derived from the fusion of Fu5 cells and mouse fibroblasts, as well as 14 hepatoma subclones of Fu5 cells, all produce albumin but at different rates, ranging from about 0.09 to 36.7 μg/mg of protein per 72 hr. Despite this variability in albumin production, the distribution of clones is not random but discontinuous, with both hepatoma and hybrid clones clustering around discrete values that can be fitted to the geometric progression: a, a(√2)1, a(√2)2..... a(√2)n. The values of the majority of clones fall into alternate members of this geometric progression, which differ by a factor of 2. Hepatoma subclones with indistinguishable karyotypes differ in level of albumin production by as much as 4-fold. In contrast to hepatoma clones, albumin production in hybrid clones decreases with increasing cell generations. A survey of 28 enzymes of different hepatomas reveals a large variability in enzyme levels which, for most enzymes, can be arranged into classes that form a geometric progression. The apparent widespread nature of this discontinuous phenotypic variability suggests that it may reflect a basic mechanism of control of gene expression in animal cells.
Keywords: reiterated genes, control of gene expression and differentiation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv D., Thompson E. B. Variation in tyrosine aminotrasferase induction in HTC cell clones. Science. 1972 Sep 29;177(4055):1201–1203. doi: 10.1126/science.177.4055.1201. [DOI] [PubMed] [Google Scholar]
- Bakay B., Croce C. M., Koprowski H., Nyhan W. L. Restoration of hypoxanthine phosphoribosyl transferase activity in mouse 1R cells after fusion with chick-embryo fibroblasts. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1998–2002. doi: 10.1073/pnas.70.7.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnstiel M. L., Chipchase M., Speirs J. The ribosomal RNA cistrons. Prog Nucleic Acid Res Mol Biol. 1971;11:351–389. doi: 10.1016/s0079-6603(08)60332-3. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Pemberton R., Baglioni C. Reiteration frequency of haemoglobin genes in the duck. Nat New Biol. 1972 Feb 23;235(60):231–234. doi: 10.1038/newbio235231a0. [DOI] [PubMed] [Google Scholar]
- Bresnick E., Mayfield E. D., Jr, Liebelt A. G., Liebelt R. A. Enzyme patterns in a group of transplantable mouse hepatomas of different growth rates. Cancer Res. 1971 Jun;31(6):743–751. [PubMed] [Google Scholar]
- Buongiorno-Nardelli M., Amaldi F., Lava-Sanchez P. A. Amplification as a rectification mechanism for the redundant rRNA genes. Nat New Biol. 1972 Aug 2;238(83):134–137. doi: 10.1038/newbio238134a0. [DOI] [PubMed] [Google Scholar]
- Coffino P., Scharff M. D. Rate of somatic mutation in immunoglobulin production by mouse myeloma cells. Proc Natl Acad Sci U S A. 1971 Jan;68(1):219–223. doi: 10.1073/pnas.68.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colten H. R., Parkman R. Biosynthesis of C4 (fourth component of complement) by hybrids of C4-deficient guinea pig cells and HeLa cells. Science. 1972 Jun 2;176(4038):1029–1031. doi: 10.1126/science.176.4038.1029. [DOI] [PubMed] [Google Scholar]
- Criss W. E., Litwack G., Morris H. P., Weinhouse S. Adenosine triphosphate: adenosine monophosphate phosphotransferase isozymes in rat liver and hepatomas. Cancer Res. 1970 Feb;30(2):370–375. [PubMed] [Google Scholar]
- Crouse H. V., Keyl H. G. Extra replications in the "DNA-puffs" of Sciara coprophila. Chromosoma. 1968 Nov;25(3):357–364. doi: 10.1007/BF01183126. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson E. H., Hough B. R., Amenson C. S., Britten R. J. General interspersion of repetitive with non-repetitive sequence elements in the DNA of Xenopus. J Mol Biol. 1973 Jun 15;77(1):1–23. doi: 10.1016/0022-2836(73)90359-8. [DOI] [PubMed] [Google Scholar]
- Eleftheriou B. E., Bailey D. W. Genetic analysis of plasma corticosterone levels in two inbred strains of mice. J Endocrinol. 1972 Nov;55(2):415–420. doi: 10.1677/joe.0.0550415. [DOI] [PubMed] [Google Scholar]
- Glynn J. P., Halpern B. L., Fefer A. An immunochemotherapeutic system for the treatment of a transplanted Moloney virus-induced lymphoma in mice. Cancer Res. 1969 Mar;29(3):515–520. [PubMed] [Google Scholar]
- Harris M. Mutation rates in cells at different ploidy levels. J Cell Physiol. 1971 Oct;78(2):177–184. doi: 10.1002/jcp.1040780204. [DOI] [PubMed] [Google Scholar]
- Kedes L. H., Birnstiel M. L. Reiteration and clustering of DNA sequences complementary to histone messenger RNA. Nat New Biol. 1971 Apr 7;230(14):165–169. doi: 10.1038/newbio230165a0. [DOI] [PubMed] [Google Scholar]
- Keyl H. G. A demonstrable local and geometric increase in the chromosomal DNA of Chironomus. Experientia. 1965 Apr 15;21(4):191–193. doi: 10.1007/BF02141878. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mezger-Freed L. Effect of ploidy and mutagens on bromodeoxyuridine resistance in haploid and diploid frog cells. Nat New Biol. 1972 Feb 23;235(60):245–246. doi: 10.1038/newbio235245a0. [DOI] [PubMed] [Google Scholar]
- Morris H. P., Dyer H. M., Wagner B. P., Miyaji H., Rechcigl M., Jr Some aspects of the development, biology and biochemistry of rat hepatomas of different growth rate. Adv Enzyme Regul. 1964;2:321–333. doi: 10.1016/s0065-2571(64)80023-6. [DOI] [PubMed] [Google Scholar]
- Peterson J. A., Weiss M. C. Expression of differentiated functions in hepatoma cell hybrids: induction of mouse albumin production in rat hepatoma-mouse fibroblast hybrids. Proc Natl Acad Sci U S A. 1972 Mar;69(3):571–575. doi: 10.1073/pnas.69.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson U. I., Tashjian A. H., Jr, Levine L. Establishment of a clonal strain of hepatoma cells which secrete albumin. J Cell Biol. 1969 Jan;40(1):236–247. doi: 10.1083/jcb.40.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritossa F. M. Unstable redundancy of genes for ribosomal RNA. Proc Natl Acad Sci U S A. 1968 Jun;60(2):509–516. doi: 10.1073/pnas.60.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHONK C. E., MORRIS H. P., BOXER G. E. PATTERNS OF GLYCOLYTIC ENZYMES IN RAT LIVER AND HEPATOMA. Cancer Res. 1965 Jun;25:671–676. [PubMed] [Google Scholar]
- Sarich V. M., Wilson A. C. Quantitative immunochemistry and the evolution of primate albumins: micro-complement fixation. Science. 1966 Dec 23;154(3756):1563–1566. doi: 10.1126/science.154.3756.1563. [DOI] [PubMed] [Google Scholar]
- Schneider J. A., Weiss M. C. Expression of differentiated functions in hepatoma cell hybrids. I. Tyrosine aminotransferase in hepatoma-fibroblast hybrids. Proc Natl Acad Sci U S A. 1971 Jan;68(1):127–131. doi: 10.1073/pnas.68.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Gage L. P., Brown D. D. The genes for silk fibroin in Bombyx mori. J Mol Biol. 1972 Oct 14;70(3):637–649. doi: 10.1016/0022-2836(72)90563-3. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Mankovitz R., Baker R. M., Till J. E., Siminovitch L., Whitmore G. F. Isolation of temperature-sensitive mutants of L-cells. Proc Natl Acad Sci U S A. 1970 Jun;66(2):377–384. doi: 10.1073/pnas.66.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]
- Wagner R. L., Roth J. S. Some properties of beta-glucuronidase activity in normal rat liver and in several transplantable rat hepatomas. Cancer Res. 1967 Nov;27(11):2053–2059. [PubMed] [Google Scholar]
- Watson B., Gormley I. P., Gardiner S. E., Evans H. J., Harris H. Reappearance of murine hypoxanthine guanine phosphoribosyl transferase activity in mouse A9 cells after attempted hybridisation with human cell lines. Exp Cell Res. 1972 Dec;75(2):401–409. doi: 10.1016/0014-4827(72)90446-6. [DOI] [PubMed] [Google Scholar]
- Wu C., Bauer J. M., Morris H. P. Responsiveness of two urea cycle enzymes in Morris hepatomas to metabolic modulations. Cancer Res. 1971 Jan;31(1):12–18. [PubMed] [Google Scholar]
- Wu C., Morris H. P. Responsiveness of glutamine-metabolizing enzymes in Morris hepatomas to metabolic modulations. Cancer Res. 1970 Nov;30(11):2675–2684. [PubMed] [Google Scholar]


