Abstract
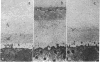
A block to polyspermy in amphibians is established at fertilization by the conversion of the vitelline envelope to the fertilization envelope. In Xenopus laevis a major ultrastructural change in the envelope at fertilization is the appearance of an electron-dense layer, termed the F layer, between the envelope and the inner-most jelly coat layer, J1. The F layer is derived, at least in part, from materials released from the cortical granules. Further definition of the origin and chemical nature of the F layer was sought by using isolated cortical granule (CG) exudate and jelly coat layer J1. In double diffusion experiments, the isolated components interacted in an agglutination reaction producing a band of precipitation. The agglutination involved α-galactoside residues and metal ions (Ca++). Employing chemically modified jelly, we demonstrated that sulfhydryl-disulfide interchanges were not involved in the agglutination and, with 35S-labeled jelly, that the agglutinating J1 component possessed sulfate esters. Both the CG exudate and the J1 components contained carbohydrate, as evidenced by their lectin reactivity. A number of ionic polymers, both natural and synthetic, were tested as chemical analogs of CG exudate and J1; none gave an agglutination band. Dissolved jelly coat material from eggs of two different species of frogs agglutinated with CG exudate, while jelly from sea urchin eggs and hyaluronic acid from mammalian eggs did not. Thus, the agglutination reaction was chemically and phylogenetically specific. An electron-dense layer, similar to the F layer, formed on the outer of the vitelline envelope when jellied unfertilized eggs were immersed in CG exudate; such eggs were not fertilizable. We suggest that in Xenopus laevis, and perhaps other organisms as well, an agglutination type of reaction between cortical granule components and egg integuments is a participant in the structural and molecular events establishing a block to polyspermy.
Keywords: sulfated glycoproteins, lectins
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burger M. M., Goldberg A. R. Identification of a tumor-specific determinant on neoplastic cell surfaces. Proc Natl Acad Sci U S A. 1967 Feb;57(2):359–366. doi: 10.1073/pnas.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Font J., Leseney A. M., Bourrillon R. Isolement et propriétés d'une phytohémagglutinine anti A 1 extraite des graines de Dolichos biflorus. Biochim Biophys Acta. 1971 Sep 28;243(3):434–446. [PubMed] [Google Scholar]
- Graham C. F. The regulation of DNA synthesis and mitosis in multinucleate frog eggs. J Cell Sci. 1966 Sep;1(3):363–374. doi: 10.1242/jcs.1.3.363. [DOI] [PubMed] [Google Scholar]
- Grey R. D., Wolf D. P., Hedrick J. L. Formation and structure of the fertilization envelope in Xenopus laevis. Dev Biol. 1974 Jan;36(1):44–61. doi: 10.1016/0012-1606(74)90189-4. [DOI] [PubMed] [Google Scholar]
- Gusseck D. J., Hedrick J. L. A molecular approach to fertilization. I. Disulfide bonds in Xenopus laevis jelly coat and a molecular hypothesis for fertilization. Dev Biol. 1971 Jul;25(3):337–347. doi: 10.1016/0012-1606(71)90035-2. [DOI] [PubMed] [Google Scholar]
- Gwatkin R. B., Williams D. T., Hartmann J. F., Kniazuk M. The zona reaction of hamster and mouse eggs: production in vitro by a trypsin-like protease from cortical granules. J Reprod Fertil. 1973 Feb;32(2):259–265. doi: 10.1530/jrf.0.0320259. [DOI] [PubMed] [Google Scholar]
- Lis H., Sharon N. The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem. 1973;42(0):541–574. doi: 10.1146/annurev.bi.42.070173.002545. [DOI] [PubMed] [Google Scholar]
- Lorenzi M., Hedrick J. L. On the macromolecular composition of the jelly coat from Strongylocentrotus purpuratus eggs. Exp Cell Res. 1973 Jun;79(2):417–422. doi: 10.1016/0014-4827(73)90461-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto I., Osawa T. Purification and characterization of an anti-H(O) phytohemagglutinin of Ulex europeus. Biochim Biophys Acta. 1969 Nov 11;194(1):180–189. doi: 10.1016/0005-2795(69)90193-7. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Shaver J. R., Barch S. H., Attinasi L. C. The effects of immune globulins on the fertilizing capacity of frog spermatozoa. Biol Reprod. 1973 Feb;8(1):112–118. doi: 10.1093/biolreprod/8.1.112. [DOI] [PubMed] [Google Scholar]
- Vacquier V. D., Tegner M. J., Epel D. Protease activity establishes the block against polyspermy in sea urchin eggs. Nature. 1972 Dec 8;240(5380):352–353. doi: 10.1038/240352a0. [DOI] [PubMed] [Google Scholar]
- Wolf D. P., Hedrick J. L. A molecular approach to fertilization. 3. Development of a bioassay for sperm capacitation. Dev Biol. 1971 Jul;25(3):360–376. doi: 10.1016/0012-1606(71)90037-6. [DOI] [PubMed] [Google Scholar]
- Wolf D. P., Hedrick J. L. A molecular approach to fertilization. II. Viability and artificial fertilization of Xenopus laevis gemetes. Dev Biol. 1971 Jul;25(3):348–359. doi: 10.1016/0012-1606(71)90036-4. [DOI] [PubMed] [Google Scholar]