Abstract
Context
Sleep restriction alters responses to food. However, the underlying neural mechanisms for this effect are not well understood.
Objective
The purpose of this study was to determine whether there is a neural system that is preferentially activated in response to unhealthy compared with healthy foods.
Participants
Twenty-five normal-weight individuals, who normally slept 7–9 h per night, completed both phases of this randomized controlled study.
Intervention
Each participant was tested after a period of five nights of either 4 or 9 h in bed. Functional magnetic resonance imaging (fMRI) was performed in the fasted state, presenting healthy and unhealthy food stimuli and objects in a block design. Neuronal responses to unhealthy, relative to healthy food stimuli after each sleep period were assessed and compared.
Results
After a period of restricted sleep, viewing unhealthy foods led to greater activation in the superior and middle temporal gyri, middle and superior frontal gyri, left inferior parietal lobule, orbitofrontal cortex, and right insula compared with healthy foods. These same stimuli presented after a period of habitual sleep did not produce marked activity patterns specific to unhealthy foods. Further, food intake during restricted sleep increased in association with a relative decrease in brain oxygenation level-dependent (BOLD) activity observed in the right insula.
Conclusion
This inverse relationship between insula activity and food intake and enhanced activation in brain reward and food-sensitive centers in response to unhealthy foods provides a model of neuronal mechanisms relating short sleep duration to obesity.
Keywords: sleep duration, body mass index, fMRI
Introduction
The neural mechanism by which sleep restriction can affect food intake has become an active area of investigation due to mounting evidence relating short sleep to obesity,1,2 increased weight gain,3,4 higher risk of cardiometabolic disorders and altered glucose metabolism.1,5–7 Clinical studies suggest that short sleep leads to greater desire for high-fat foods and sweets8 and our laboratory has shown that restricting sleep increases food intake, particularly fat intake, in normal sleepers.9 Others have also reported that the increased caloric intake during periods of restricted sleep is in large part due to increased snacking.10 However, the specific neural mechanism explaining the role of sleep duration on eating behavior and obesity remains unknown.
Some studies have shown that reducing sleep duration can alter levels of hormones involved in energy balance regulation,5,11,12 such as leptin and ghrelin. In some studies, leptin is reduced by sleep restriction11,13 and this could result in a hormonal cascade of events leading to increased dopamine release, sucrose preference and increased food intake.14 Short sleep may also increase ghrelin levels,8,11 suggesting increased appetite6 as well as increased incentive value of rewarding foods.15 The effect of sleep restriction on these two hormones suggests that reducing sleep would lead to a state that would promote increased food intake and positive energy balance.
Our previous study suggested that food stimuli activate areas involved in reward to a greater extent during a period of restricted sleep compared with habitual sleep.16 Various studies have shown that highly palatable foods have strong effects on brain regions associated with reward. Specifically, Bragulat et al.17 found activation in the insula and medial frontal cortex in response to food odors and Pelchat et al.18 found insular activation in response to thoughts of high-fat and high-sugar foods. These two brain regions are known to be important in compulsive and drug seeking behavior.19 There is also further evidence relating the brain's reward system to food intake. Of relevance to the present study is that foods high in fats and sugars activate the brain's dopaminergic system through the mesoaccumbens-dopaminergic pathway.20 Recently, Benedict et al.21 suggested that total sleep deprivation enhances hedonic stimulus processing of foods, which is consistent with a greater drive to consume food under sleep deprivation compared with regular sleep. However, neuronal responses were assessed after consumption of a liquid meal and did not differ between high calorie and low calorie food stimuli.
The goal of this study was to determine how sleep restriction modulates the neuronal response to healthy and unhealthy food stimuli in the fasted state. We hypothesized that brain activation in areas known to be associated with pleasure seeking and appetitive behaviors, such as the insula and dorsolateral prefrontal cortex, will be activated to a greater extent after a period of short sleep in response to unhealthy as opposed to healthy food stimuli.
Materials and Methods
Participants
Participants were recruited from New York City and the surrounding area by advertisements on approved media. Eligibility requirements included age 30–45 years, body mass index 22–26kg m−2, right handedness and weight stability for at least 3 months as determined by questionnaire. Subjects were also required to sleep, on average, 7–9 h per night, as assessed by actigraphy (ActiGraph LLC, Pensacola, FL, USA) and sleep diary over a 2-week period, with no more than four nights with <7 h of sleep. Exclusion criteria included abnormal scores on the Pittsburg Quality of Sleep Questionnaire, Epworth Sleepiness Scale, Berlin Questionnaire, Sleep Disorders Inventory Questionnaire, Beck Depression Inventory or Composite Scale of Morningness/Eveningness. Further exclusion criteria included smoking, diabetes, neurological, sleep, or eating disorders, history of drug or alcohol abuse, shift work, travel across time zones within 4 weeks of the study, habitual caffeine intake >300mgday−1, regular naps, history of drowsy driving, pregnancy within 1 year of the study or contraindications for MRI scanning. The study was approved by the Institutional Review Boards of St. Luke's/Roosevelt Hospital Center and Columbia University (New York, NY). All subjects provided informed consent before the study.
Design
Details of the study design have been published9,22 and this is a secondary analysis of the data published previously examining neuronal responses to food stimuli after sleep restriction.22 Briefly, subjects underwent two inpatient periods of controlled time in bed: either restricted (4h per night; 0100–0500h) or habitual (9h per night; 2200–0700h), in random order with a 3-week washout period. A restricted sleep condition of 4 h per night was chosen because this length of time has previously been used in other short-term intervention trials8 and 9 h per night was used for habitual sleep as recommended by Van Dongen et al.23 Participant adherence to this sleep scheduled was maintained by research coordinators. This washout period length was considered sufficient for sleep to recover after the first study period and for women to be tested in the same phase of their menstrual cycle for both study periods, assuming a 28-day menstrual cycle. In the washout period, subjects were told to maintain their normal sleep schedules and this was verified using actigraphy. During the first 4 days of each phase, subjects consumed a controlled, weight maintaining diet. During the day preceding the study measurements, participants were allowed to eat ad libitum. Their food intake during that time was recorded. Results of the food intake data are reported elsewhere;9 of relevance to this analysis, participants consumed more energy and fat during the period of restricted sleep relative to habitual sleep. Sleep duration and composition were assessed for sleep episodes with polysomnographic recordings. Details of recordings and effects of restricted compared with habitual sleep have been previously reported.24 Briefly, restricted sleep reduced the amount of time spent in all sleep stages except for slow-wave sleep (SWS), which was conserved. Based on the proportion of sleep, however, there was a lower percentage of sleep in all sleep stages, except for SWS, which was proportionally higher during restricted compared with habitual sleep.
Functional magnetic resonance imaging
On the morning of day 6, after an overnight fast, subjects were taken to the Columbia University fMRI Research Center to undergo functional magnetic resonance imaging (fMRI) scanning. Each participant viewed 2 separate functional runs of 10 blocks (5 food and 5 non-food), each run lasting 5 min 48 s. Blocks consisted of four food or non-food images shown for 4 s each and were separated by a 16-s basal period. In each run, approximately half of the food blocks presented images of healthy foods (carrots, yogurt, oatmeal and grapes; Supplementary Table) and half presented images of unhealthy foods (pepperoni pizza, doughnuts, chocolate bars and candy). All images were shown to subjects with Presentation software (Neurobehavioral Systems, http://nbs.neuro-bs.com) viewed through Avotec goggles (http://www.avotecinc.com/eyeTracking.htm).
Functional images were acquired along the anterior-posterior commissure line with a T2*-weighted echo-planar imaging sequence of 27 contiguous axial slices (repetition time = 2000 ms, echo time = 35 ms, flip angle = 90°, field of view = 192 × 192mm) of 4.5mm thickness and 3 mm in-plane resolution. Structural data were acquired with a highresolution T1-weighted SPGR scan (repetition time = 19 ms, echo time = 5 ms, flip angle = 20°, field of view = 220 × 220 mm), recording 124 slices at a slice thickness of 1.5 mm and an in-plane resolution of 0.86 × 0.86 mm.
All pre-processing and data analyses were performed at the fMRI Research Center using SPM5 (Wellcome Department of Imaging Neuroscience, London, England) as previously described.16 Contrasts for healthy foods >non-foods and unhealthy foods >non-foods during the each sleep period were created for each subject. Brain regions were considered as statistically significantly activated when an extent threshold of at least 10 voxels with an uncorrected signal level of P<0.05 was observed. Following individual analyses, we performed group-level analyses comparing the two sleep periods using the same statistical parameters.
A post hoc region of interest was performed to assess the correlation between neuronal brain oxygenation level-dependent (BOLD) signaling and food intake. We performed Pearson correlation between the difference in BOLD (beta) signal change in our region of interest between restricted and habitual sleep and the difference in energy intake between restricted and habitual sleep using Statistical Analysis Software (SAS version 9.2, Cary, NC, USA). A similar analysis was done for fat intake. Beta values when viewing unhealthy relative to healthy foods were extracted for each participant using Marseille Boîte à Région d′intérêt (MarsBaR).25 Because the insula was differentially activated by unhealthy compared with healthy foods during restricted sleep in this study, and was found to be specifically responsive to food stimuli in our previous studies,16,26 we chose to perform an analysis in the insula (x, y, z: 40–47, − 20 to − 8, 10–20). The same analysis was also performed for the anterior cingulate cortex, as defined by the Automated Anatomical Labeling atlas and identical to that used by Benedict et al.21 (x, y, z: 12, − 13, 40). Pearson correlations were conducted between the difference in BOLD (beta) signal change in the insula between restricted and habitual sleep and the difference in sleep stages (minutes and percent of total sleep time for stage 1, stage 2, SWS and rapid eye movement (REM) sleep, and the number of REM sleep periods during the sleep episode) between restricted and habitual sleep using data from night 5 (that is, the night preceding fMRI scans).
Results
A total of 15 men and 15 women were enrolled in this study and complete image sets were acquired for 25 (13 men and 12 women, mean age (s.d.) 34.7±4.7 years and body mass index 23.6 ± 1.3 kg m−2). Of the three women with incomplete data, one was unable to undergo fMRI due to hair extensions that caused interference with the scanning protocol, one voluntarily withdrew after completing the first phase of the study and the other was dismissed before starting the first phase due to disclosure of an exclusion factor for the study. Two men have missing data: one was excluded after periodic limb movement disorder diagnosis during the first phase of the study and the other completed both study phases but his fMRI data were subsequently corrupted and could not be analyzed.
Unhealthy foods > healthy foods
Restricted sleep
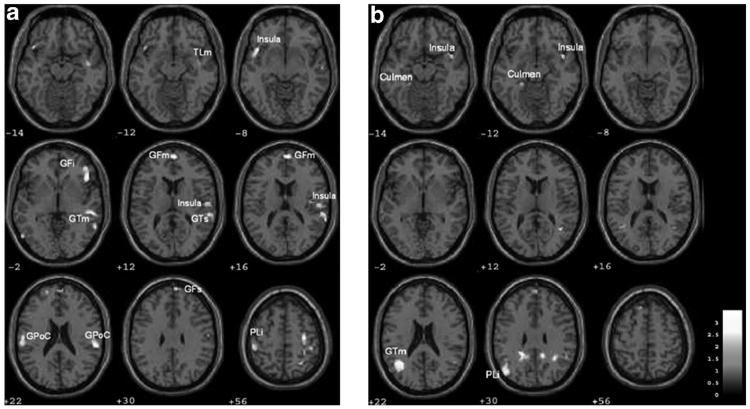
The neuronal response to unhealthy foods, in contrast to healthy foods, included the insula, middle and superior frontal gyri, right inferior frontal gyrus, left inferior parietal lobules and postcentral gyrus (Table 1; Figure 1a).
Table 1. Comparison of regional brain activation in response to unhealthy food but not healthy food after a period of restricted sleep.
| Anatomical region | Brodmann area | Clusters (voxels) | P-value (cluster) | Montreal Neurological Institute coordinates | ||
|---|---|---|---|---|---|---|
|
| ||||||
| xa | yb | zc | ||||
| Inferior frontal gyrus | 47 | 159 | 2.87 | 50 | 30 | −4 |
| 46 | 2.21 | 46 | 40 | −2 | ||
| Inferior frontal gyrus | 47 | 131 | 2.43 | −44 | 16 | −8 |
| Superior temporal gyrus | 38 | 2.30 | −44 | 20 | −16 | |
| Inferior temporal gyrus | 37 | 30 | 2.42 | 62 | −54 | −4 |
| Inferior temporal gyrus | 49 | 2.11 | −68 | 0 | ||
| Insula | 13 | 16 | 1.91 | 42 | −14 | 14 |
| Medial frontal gyrusd | 10 | 270 | 2.98 | 6 | 60 | 16 |
| Superior frontal gyrus | 10 | 2.40 | 8 | 64 | 28 | |
| Medial frontal gyrus | 10 | 2.25 | 0 | 70 | 6 | |
| Middle temporal gyrus | 21 | 11 | 0.875 | 62 | −14 | −8 |
| Middle temporal gyrus | 21 | 12 | 1.89 | −54 | −20 | −18 |
| Postcentral gyrus | 40 | 579 | 2.61 | 60 | −28 | 22 |
| Superior temporal gyrus | 22 | 2.48 | 66 | −34 | 6 | |
| Superior temporal gyrus | 22 | 2.44 | 66 | −42 | 18 | |
| Postcentral gyrus | 3 | 55 | 2.56 | −16 | 36 | |
| 3 | 1.85 | 58 | 12 | 44 | ||
| Postcentral gyrus | 2 | 109 | 2.30 | −58 | −18 | 22 |
| Postcentral gyrus | 2 | 166 | 2.22 | −48 | −30 | 56 |
| 2 | 2.14 | −46 | −26 | 44 | ||
| Postcentral gyrus | 40 | 35 | 2.06 | 44 | −32 | 56 |
| Postcentral gyrus | 2 | 20 | 1.93 | 40 | −24 | 38 |
| 2 | 1.77 | 50 | −22 | 42 | ||
| Postcentral gyrus | 5 | 19 | 1.88 | 32 | −40 | 58 |
| 3 | 1.86 | 30 | −32 | 50 | ||
| Precentral gyrus | 6 | 130 | 2.71 | 32 | −14 | 58 |
| 4 | 2.00 | 32 | −24 | 56 | ||
| Sub-gyral | 20 | 44 | 2.18 | 44 | −8 | −16 |
| Sub-gyral | 21 | 1.74 | 40 | −2 | −12 | |
| Superior frontal gyrus | 10 | 32 | 2.10 | −20 | 58 | 24 |
| Superior parietal gyrus | 7 | 32 | 2.32 | 26 | −56 | 58 |
Abbreviation: fMRI, functional magnetic resonance imaging. Data were analyzed at the fMRI Research Center using SPM 5 and a 128-s temporal high-pass filter was applied to the data to remove low-frequency artifacts. Brain regions were considered statistically significantly activated when an extent threshold of at least 10 voxels with an uncorrected voxel level at P<0.05 was observed, n = 25. Coordinates with no cluster size or P-value are separate peaks within the previously labeled cluster.
Positive x coordinate indicates right hemisphere.
Positive y coordinate indicates anterior to commissure landmark.
Positive z coordinate indicates above anterior–posterior commissure line.
Region with voxel level P = 0.001.
Figure 1.
Brain regions in normal-weight men and women (n=25) with higher activation when exposed to unhealthy>healthy food following restricted (a) and habitual (b) sleep are represented in color. Brain left is image left. Brain regions were considered significant with an extent threshold of at least 10 voxels, uncorrected voxel level at P<0.05. These results highlight different neural systems responding to unhealthy relative to healthy food under periods of restricted and habitual sleep. GFi, inferior frontal gyrus; GFm, medial frontal gyrus; GFs, superior frontal gyrus; GPoC, post central gyrus; GTm, middle temporal gyrus; GTs, superior frontal gyrus; PLi, inferior parietal lobe; TLm, medial temporal lobe. A full color version of this figure is available at the International Journal of Obesity online.
Habitual sleep
Brain activity within the inferior parietal lobe and medial temporal gyrus (Brodmann's areas (BAs) 40 and 39) was most strongly activated in response to unhealthy foods relative to healthy foods following habitual sleep (Table 2; Figure 1b).
Table 2. Comparison of regional brain activation in response to unhealthy food but not healthy after a period of habitual sleep.
| Anatomical region | Brodmann area | Clusters (voxels) | Z-score (voxel) | Montreal Neurological Institute coordinates | ||
|---|---|---|---|---|---|---|
|
| ||||||
| xa | yb | zc | ||||
| Cingulate gyrus | 31 | 163 | 2.92 | −20 | −42 | 28 |
| Precuneus | 39 | −20 | −50 | 32 | ||
| 39 | −12 | −48 | 32 | |||
| Culmen | 17 | 2.07 | −22 | −40 | −12 | |
| Inferior parietal lobule | 40 | 32 | 2.46 | 48 | −56 | 50 |
| 40 | 1.71 | 52 | −42 | 30 | ||
| Middle frontal gyrus | 8 | 39 | 2.31 | −22 | 20 | 38 |
| 8 | 1.85 | −24 | 12 | 34 | ||
| Middle temporal gyrusd | 39 | 635 | 3.11 | −34 | −58 | 22 |
| Superior temporal gyrus | 39 | 2.80 | −42 | −54 | 20 | |
| Middle temporal gyrus | 39 | 2.53 | −42 | −72 | 28 | |
| Middle temporal gyrus | 19 | 30 | 2.34 | 40 | −58 | 12 |
| Precuneus | 31 | 86 | 2.65 | 14 | −46 | 32 |
| Superior frontal gyrus | 10 | 13 | 2.09 | −12 | 66 | 6 |
| Superior frontal gyrus | 6 | 30 | 2.07 | −12 | 34 | 54 |
| 8 | 1.77 | −16 | 42 | 48 | ||
| Superior frontal gyrus | 9 | 33 | 1.96 | 2 | 58 | 30 |
| Superior temporal gyrus | 39 | 88 | 2.90 | 36 | 28 | |
| Superior temporal gyrus | 38 | 37 | 2.33 | 44 | 6 | −12 |
| Supramarginal gyrus | 40 | 52 | 2.01 | 54 | −50 | 28 |
Abbreviation: fMRI, functional magnetic resonance imaging. Data were analyzed at the fMRI Research Center using SPM 5 and a 128-s temporal high-pass filter was applied to the data to remove low-frequency artifacts. Brain regions were considered statistically significantly activated when an extent threshold of at least 10 voxels with an uncorrected voxel level at P<0.05 was observed, n =25. Coordinates with no cluster size or P-value are separate peaks within the previously labeled cluster.
Positive x coordinate indicates right hemisphere.
Positive y coordinate indicates anterior to commissure landmark.
Positive z coordinate indicates above anterior-posterior commissure line.
Region with cluster level P=0.05 and voxel level P =0.001.
Unhealthy foods > healthy foods during habitual sleep relative to restricted sleep
Direct comparisons between habitual and restricted sleep (habitual > restricted) for the unhealthy greater than healthy food conditions reveal greater activity following habitual sleep observed in right thalamus (18, 12, 0), left precuneus (−12, −56, 14 and 0, −66, 42), and middle cingulate gyrus (14, −44, 36) (P<0.01, cluster size of 100), which is consistent with relative upregulation of these neural mechanisms following habitual sleep. The reverse contrast (restricted >habitual sleep) did not yield significant differences.
Correlation between BOLD signal and food intake and sleep architecture during restricted vs. habitual sleep
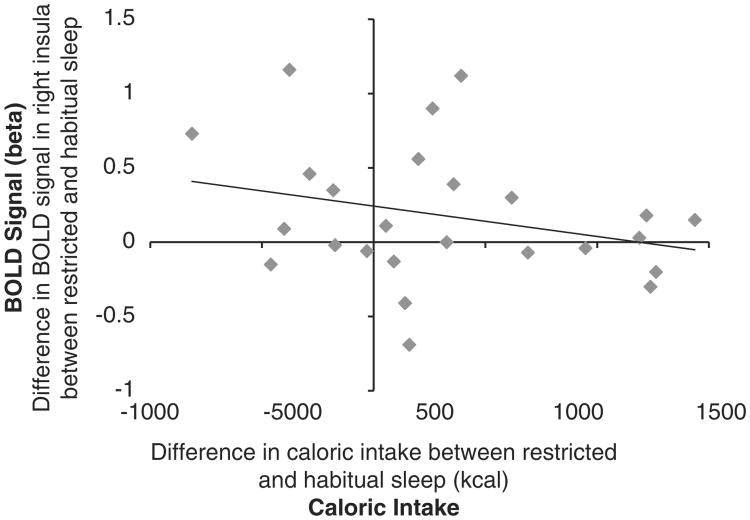
We examined the relationship between the difference in food intake during the period of restricted sleep and habitual sleep and the difference in BOLD signal in the right insula in response to unhealthy relative to healthy foods, and found an inverse association between the difference in food intake and the difference in the BOLD signal between restricted and habitual sleep (r= −0.280; Figure 2). That is, the relative BOLD signal for unhealthy foods was reduced in association with increased caloric intake, suggesting a downregulation during restricted sleep. A similar, but weaker, relationship was observed for fat intake (r= − 0.191).
Figure 2.
Right insular cortex, restricted>habitual sleep for unhealthy>healthy food. Relative change in the BOLD signal plotted against the relative change in caloric intake for each subject. For this correlation analysis, the region of interest (insula) was defined by an activation cluster derived from the group analysis (x, y, z: 40–47, −20 to −8, 10–20). Pearson's r= −0.280; n = 25.
The BOLD signal in the right insula in response to unhealthy relative to healthy foods was correlated with sleep architecture from the previous night in restricted and habitual sleep conditions. There was a slight trend for a positive relationship between insula activation and time spent in REM sleep (r=0.31, P = 0.13). This finding suggests a role of this sleep architecture parameter in the increased insula response to unhealthy food under restricted conditions, since REM sleep was reduced under restricted compared with habitual sleep duration in these participants and percent time spent in REM sleep was inversely related to fat intake.24 In this case, individuals with a greater reduction in REM sleep between restricted and habitual sleep tended to have lesser difference in insula activation after restricted relative to habitual sleep.
Because Benedict et al.21 found greater activation of the anterior cingulate cortex in response to food images (mixed healthy and unhealthy) after total sleep deprivation (complete lack of sleep) relative to habitual sleep (8h), and that the difference in BOLD signal in this region was correlated with the difference in appetite ratings (not actual food intake), we assessed the correlation with difference in food intake using the same region of interest. In our study, there was no evidence for a correlation between the difference in BOLD signal in the anterior cingulate cortex in response to unhealthy relative to healthy foods and food intake between restricted and habitual sleep (r= −0.099). This difference may relate to appetite ratings21 vs. actual intake measures in this study.
Correlations were also done between the BOLD signal in the anterior cingulate cortex in response to unhealthy relative to healthy foods and sleep architecture from the previous night in restricted and habitual sleep conditions. No significant correlations were observed between any sleep stages and the BOLD signal in the anterior cingulate cortex.
Discussion
This research extends earlier work showing that food stimuli provoke greater activity patterns in brain areas previously associated with reward under periods of restricted compared with habitual sleep.16 Here, we specifically explored the neuronal circuitry related to unhealthy and healthy food types during periods of restricted sleep. In line with our hypothesis, the insular cortex as well as areas thought to be involved in hedonic functions, such as the orbitofrontal cortex and dorsolateral prefrontal cortex, displayed the strongest activation in response to unhealthy food compared with healthy food stimuli, specifically during the period of restricted sleep.
The insula was integral to our hypothesis because of its known role in energy homeostasis, regulation of pleasure-seeking behaviors,27 integration of external stimuli, signal salience and internal bodily states of arousal.28 In the present study, the insula was activated by unhealthy foods to a greater extent than healthy foods, especially under sleep restricted states and was inversely related to food intake. Enhanced insular activation during restricted sleep is consistent with an amplification of the salience of unhealthy foods relative to healthy foods and recent reports of an inverse correlation between change in hunger after consumption of a high-fat yogurt and cerebral blood flow in the insula relative to consumption of a low-fat yogurt.29 Although preliminary, these results support a role for the insula as part of a regulatory mechanism encompassing the subjective emotional context responsive to signal salience and bodily state. Interestingly, in this study, when we assessed food intake on a day of ad libitum feeding before the fMRI scan, participants ate more often and ate more fat during the restricted sleep period than the habitual sleep period.30
It is interesting to note that, in this study, the right insula was preferentially activated by unhealthy foods during restricted sleep. There has been evidence of right-sided bias for chemosensory stimuli and in gustatory imaging studies.31,32 Also, neuroimaging studies suggest that the right orbitofrontal cortex is involved in short-term memory tasks.33
In addition to the insula, various sections of the prefrontal cortex, such as the medial frontal cortex, also displayed stronger activation in response to unhealthy as compared with healthy food stimuli. Activation of the medial frontal cortex, which is thought to be involved in higher reasoning as well as attention,34 suggests that unhealthy food stimuli may trigger neural responses related to high level cognitive process.
Unhealthy food, relative to nonfoods, elicited specific responses in the orbitofrontal cortex, a brain area known to be associated with pleasure and hedonic responses27 as well as the anticipation of reward.28,35 This association is corroborated by Schloegl et al.,36 who described the orbitofrontal cortex as a hedonic control center for food.
The comparison of regional brain activity in response to unhealthy foods during habitual relative to restricted sleep yielded interesting results. They showed an expected advantage of sleep on neuronal responses to food stimuli, such as upregulation of some arousal areas (thalamus and precuneus), and upregulation of the cingulate gyrus suggests that a cognitive control mechanism may also be more active following habitual sleep than restricted sleep. This could possibly signify improved food restraint behavior.
Benedict et al.21 reported greater activation in the anterior cingulate cortex in response to food images after a night of total sleep deprivation compared with a night of 8 h sleep with no difference in response to high calorie and low calorie foods. In this study, the anterior cingulate cortex was not found to be differentially activated by unhealthy and healthy foods under restricted and habitual sleep. However, we used actual food intake as an objective measure of hunger rather than subjective ratings. These methodological distinctions may have contributed to this difference in findings. Disparities in study design, such as the degree of sleep restriction, the length of the study, the study population and sample size, as well as the methods used to measure hunger/food intake, may also account for the differences in results.
A unique feature of this study is the categorization of foods into healthy and unhealthy as opposed to high and low calorie.21,37–40 The reason behind our classification system was to focus on the perceived healthfulness of foods rather than their calorie content. This is important as some high calorie foods, such as nuts and avocado, are healthy (recommended to be consumed as part of a healthful diet) and some low calorie foods, such as candy, are not (their consumption should be restricted to ‘once in a while’). Results of a recent study by Mehta et al41 support the use of this paradigm as ‘fattening’ foods, in their study, akin to our unhealthy foods, produced stronger alterations in neuronal response when compared with less fattening foods. Although we believe that all foods have a place as part of a healthy diet, foods established as being unhealthy such as candy and ice cream may produce feelings of guilt regardless of their calorie content.42 Therefore, it seems plausible that some of the neuronal responses observed in the present study may have been a result of an emotional context associated with the foods presented. In shifting the paradigm from high calorie to unhealthy foods, we were able to differentiate between the neuronal response to healthy and unhealthy stimuli during restricted sleep to observe a connection associated with different types of foods. When 17 individuals not associated with the study were asked to categorize the foods shown in this study as healthy and unhealthy, they correctly categorized 36.9±2.2 (s.d.) food items (92.2% success rate).
Nevertheless, there are strong similarities in results between our study and those examining brain responses to high and low calorie foods. For example, the dorsolateral prefrontal cortex, an area of the brain heavily implicated in the dopaminergic system, was activated in response to unhealthy foods in the present study and by high calorie foods in other studies.38 This suggests that the brain responds similarly to some high calorie and some unhealthy foods. In the present study, the average calorie content of the foods in the healthy group was 1.19±1.33 kcal g−1 compared with 3.59±1.10 kcal g−1 for the unhealthy group (P<0.0001), confirming the similarity of the unhealthy and high calorie groupings. On the other hand, in this study, unhealthy food stimuli activated the cingulate gyrus, inferior parietal lobe and insula while high calorie foods have not.38 These results suggest that the salience of a particular food can be based on individual perceptions of healthfulness, or lack thereof, rather than its calorie content.
There are some limitations to this study that are worth noting. First, all of our subjects were normal weight. Normal-weight individuals have been shown to display a more robust neuronal response to visual food stimuli in brain regions noted in this study such as the insula and orbitofrontal cortex compared with obese subjects.43 Additional research has also shown that the neuronal differences in response to food stimuli in reduced weight individuals extend to differences in actual consumption.44,45 Therefore, we can theorize that we would have observed more robust results with overweight or obese participants. Our participants were tested after a day of ad libitum feeding. We have shown that food intake was increased by ∼300kcal on the day before fMRI testing during restricted sleep compared with habitual sleep and that the number of eating occasions was greater.9 Responses to food stimuli may have been attenuated by allowing participants to self-select their food intake the day before the fMRI scanning. Also, our study was not a priori designed to test the effects of sleep duration on neuronal responses to specific groupings of food. We only had five food blocks per run, resulting in five unhealthy and five healthy runs. This limited our power to detect changes in neuronal responses. Similarly, it would be interesting to test whether neuronal responses to healthy and unhealthy foods would differ between men and women. Due to the small sample size that would result from such an analysis, we did not examine this question. Our results should therefore be taken as exploratory and warrant further study.
In conclusion, after a period of restricted sleep, regions involved in pleasure-seeking and food-related behaviors were activated to a greater extent by unhealthy compared with healthy foods, a pattern that was distinct from that observed after a period of habitual sleep. Further, an inverse relationship was found between the BOLD signal in the insula and food intake following restricted sleep. These results highlight a potential neuronal mechanism in which, under restricted sleep, unhealthy foods may be more salient than healthy foods, and are consistent with overeating behaviors and greater weight gain observed in short sleepers.
Supplementary Material
Acknowledgments
This study was funded by the National Institutes of Health grant #1R01HL091352-01A1 and 1 UL1 RR024156 from the National Center for Research Resources (NCRR), the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at the NCRR Website. Information on Re-engineering the Clinical Research Enterprise can be obtained from NIH Roadmap website. The Almond Board of California provided almonds and Cabot Cheese provided cheese for the study. Trial registration on http://www.clinicaltrials.gov, #NCT00935402.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Author Contributions: MPSO, JH and MS designed research; MPSO, SW and MS conducted research; MPSO and MS obtained data; SW, MS, AS and MPSO analyzed data; SW, MS, JH and MPSO interpreted data; MPSO, SW and JH wrote the paper and also shared primary responsibility for its final content. All authors read and approved the final manuscript. Special thanks to our participants as well as Andrew McReynolds, Zalak Trivedi and Amy Roberts for their work collecting data.
Supplementary Information accompanies this paper on International Journal of Obesity website (http://www.nature.com/ijo)
References
- 1.Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garaulet M, Ortega FB, Ruiz JR, Rey-Lopez JP, Beghin L, Manios Y, et al. Short sleep duration is associated with increased obesity markers in European adolescents: effect of physical activity and dietary habits. The HELENA study Int J Obes (Lond) 2011;35:1308–1317. doi: 10.1038/ijo.2011.149. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe M, Kikuchi H, Tanaka K, Takahashi M. Association of short sleep duration with weight gain and obesity at 1-year follow-up: a large-scale prospective study. Sleep. 2010;33:161–167. doi: 10.1093/sleep/33.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morselli L, Leproult R, Balbo M, Spiegel K. Role of sleep duration in the regulation of glucose metabolism and appetite. Best Pract Res. 2010;24:687–702. doi: 10.1016/j.beem.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prinz P. Sleep, appetite, and obesity--what is the link? PLoS Med. 2004;1:e61. doi: 10.1371/journal.pmed.0010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 8.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 9.St-Onge MP, Roberts A, Chen J, Kelleman M, O'Keeffe M, Jones P. Short sleep duration increases energy intakes but does not change expenditure in normal weight individuals. Am J Clin Nutr. 2011;94:410–416. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodosi B, Gardi J, Hajdu I, Szentirmai E, Obal F, Jr, Krueger JM. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1071–R1079. doi: 10.1152/ajpregu.00294.2004. [DOI] [PubMed] [Google Scholar]
- 12.Cirelli C. Cellular consequences of sleep deprivation in the brain. Sleep Med Rev. 2006;10:307–321. doi: 10.1016/j.smrv.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berthoud HR. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr Opin Neurobiol. 2011;21:888–896. doi: 10.1016/j.conb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egecioglu E, Jerlhag E, Salome N, Skibicka KP, Haage D, Bohlooly YM, et al. Ghrelin increases intake of rewarding food in rodents. Addict Biol. 2010;15:304–311. doi: 10.1111/j.1369-1600.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St-Onge MP, McReynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr. 2012;95:818–824. doi: 10.3945/ajcn.111.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bragulat V, Dzemidzic M, Bruno C, Cox CA, Talavage T, Considine RV, et al. Food-related odor probes of brain reward circuits during hunger: a pilot FMRI study. Obesity (Silver Spring) 2010;18:1566–1571. doi: 10.1038/oby.2010.57. [DOI] [PubMed] [Google Scholar]
- 18.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23:1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc London B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69:664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benedict C, Brooks SJ, O'Daly OG, Almen MS, Morell A, Aberg K, et al. Acute sleep deprivation enhances the brain's response to hedonic food stimuli: an fMRI Study. J Clin Endocrinol Metab. 2012;97:E443–E447. doi: 10.1210/jc.2011-2759. [DOI] [PubMed] [Google Scholar]
- 22.St-Onge MP, McReynolds A, Trivedi Z, Roberts A, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr. 2012;95:818–824. doi: 10.3945/ajcn.111.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 24.Shechter A, O'Keeffe M, Roberts AL, Zammit GK, Roychoudhury A, St-Onge MP. Alterations in sleep architecture in response to experimental sleep curtailment are associated with signs of positive energy balance. Am J Physiol Regul Integr Comp Physiol. 2012;303:R883–R889. doi: 10.1152/ajpregu.00222.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox; 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002; Available from CD-ROM in NeuroImage. [Google Scholar]
- 26.St-Onge MP, Sy M, Heymsfield SB, Hirsch J. Human cortical specialization for food: a functional magnetic resonance imaging investigation. J Nutr. 2005;135:1014–1018. doi: 10.1093/jn/135.5.1014. [DOI] [PubMed] [Google Scholar]
- 27.Goodman A. Neurobiology of addiction. An integrative review Biochem Pharmacol. 2008;75:266–322. doi: 10.1016/j.bcp.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 28.Critchley HD, Mathias CJ, Dolan RJ. Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron. 2002;33:653–663. doi: 10.1016/s0896-6273(02)00588-3. [DOI] [PubMed] [Google Scholar]
- 29.Frank S, Laharnar N, Kullmann S, Veit R, Canova C, Hegner YL, et al. Processing of food pictures: influence of hunger, gender and calorie content. Brain Res. 2010;1350:159–166. doi: 10.1016/j.brainres.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 30.St-Onge MP, Roberts AL, Chen J, Kelleman M, O'Keeffe M, RoyChoudhury A, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94:410–416. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zatorre RJ, Jones-Gotman M, Evans AC, Meyer E. Functional localization and lateralization of human olfactory cortex. Nature. 1992;360:339–340. doi: 10.1038/360339a0. [DOI] [PubMed] [Google Scholar]
- 32.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Royet JP, Plailly J. Lateralization of olfactory processes. Chem Senses. 2004;29:731–745. doi: 10.1093/chemse/bjh067. [DOI] [PubMed] [Google Scholar]
- 34.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 35.Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- 36.Schloegl H, Percik R, Horstmann A, Villringer A, Stumvoll M. Peptide hormones regulating appetite-focus on neuroimaging studies in humans. Diabetes Metab Res Rev. 2011;27:104–112. doi: 10.1002/dmrr.1154. [DOI] [PubMed] [Google Scholar]
- 37.Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 2009;198:149–158. doi: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 38.Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 2003;19:1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- 39.Gordon CM, Dougherty DD, Rauch SL, Emans SJ, Grace E, Lamm R, et al. Neuroanatomy of human appetitive function: a positron emission tomography investigation. Int J Eat Disord. 2000;27:163–171. doi: 10.1002/(sici)1098-108x(200003)27:2<163::aid-eat4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 40.Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes. 2009;33:653–661. doi: 10.1038/ijo.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehta S, Melhorn SJ, Smeraglio A, Tyagi V, Grabowski T, Schwartz MW, et al. Regional brain response to visual food cues is a marker of satiety that predicts food choice. Am J Clin Nutr. 2012;96:989–999. doi: 10.3945/ajcn.112.042341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steenhuis I. Guilty or not? Feelings of guilt about food among college women. Appetite. 2009;52:531–534. doi: 10.1016/j.appet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Rojas DC, Tregellas JR. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS ONE. 2009;4:e6310. doi: 10.1371/journal.pone.0006310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenbaum M, Kissileff HR, Mayer LE, Hirsch J, Leibel RL. Energy intake in weight-reduced humans. Brain Res. 2010;1350:95–102. doi: 10.1016/j.brainres.2010.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nijs IM, Muris P, Euser AS, Franken IH. Differences in attention to food and food intake between overweight/obese and normal-weight females under conditions of hunger and satiety. Appetite. 2010;54:243–254. doi: 10.1016/j.appet.2009.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.