Abstract
Study Objective
To estimate the cost-effectiveness of genotype-guided selection of antiplatelet therapy compared with selecting clopidogrel or prasugrel irrespective of genotype.
Design
Decision model based on event occurrence in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction (TRITON-TIMI) 38.
Patients
Simulated cohort of patients with acute coronary syndrome scheduled to undergo percutaneous coronary intervention (PCI), consisting of three arms: those receiving genotype-guided antiplatelet therapy with clopidogrel or prasugrel, those receiving clopidogrel regardless of genotype, and those receiving prasugrel regardless of genotype.
Measurements and Main Results
All three arms of the model incorporated the probability that patients would experience a cardiovascular event (death from cardiovascular causes, nonfatal myocardial infarction, or non-fatal stroke), a bleeding event (major or minor bleeding), or no event while receiving antiplatelet therapy during the 15 months after the scheduled PCI. The cytochrome P450 (CYP) 2C19 genotype determined antiplatelet drug selection in the genotyping group. Cost-effectiveness was expressed as the incremental cost-effectiveness ratio (ICER) for each event avoided in the genotype-guided therapy arm versus the other two arms. Genotype-guided antiplatelet therapy was dominant, or more effective and less costly, when compared with the selection of clopidogrel (ICER –$6760 [95% confidence interval (CI) –$6720 to –$6790]) or prasugrel (ICER –$11,710 [95% CI –$11,480 to –$11,950]) for all patients with-out regard to genotype. Genotype-guided therapy that included generic clopidogrel was dominant to prasugrel for all patients (ICER –$27,160 [95% CI –$27,890 to –$26,420]). Cost savings were not evident when genotype-guided therapy that included generic clopidogrel was compared with generic clopidogrel for all patients (ICER $2300 [95% CI $2290 to $2320]).
Conclusion
Genotype-guided antiplatelet therapy selection may be more cost-effective and may provide more clinical value due to fewer adverse outcomes.
Keywords: clopidogrel, prasugrel, cytochrome P450 2C19, pharmacogenomics, cost-effectiveness
Clopidogrel is a thienopyridine antiplatelet agent approved for the treatment of acute coronary syndrome, recent myocardial infarction, recent stroke, and established peripheral arterial disease.1 In 2010, clopidogrel was the third highest selling drug in the U.S. pharmaceutical market.1, 2
Clopidogrel is a prodrug that requires biotrans-formation to its active metabolite through two cytochrome P450 (CYP)–dependent steps. Multiple studies demonstrate that clopidogrel-treated individuals with one or more reduced-function variants in CYP2C19 are at increased risk of death from adverse cardiovascular outcomes compared with those without any reduced-function alleles.3–6 Although many reduced-function alleles of CYP2C19 exist, the *2 variant is the most common variant allele among individuals of European or African ancestry.7–9 The United States Food and Drug Administration (FDA) updated clopidogrel's label with a boxed warning to alert prescribers that the drug may be less effective in patients who are CYP2C19 poor metabolizers (PMs).1 In addition, guidelines have been published that outline the clinical use of CYP2C19 genotyping in patients who require antiplatelet therapy.10
A newer-generation thienopyridine, prasugrel, was compared with clopidogrel in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel –Thrombolysis in Myocardial Infarction (TRI-TON-TIMI) 38.11, 12 This trial was a phase III, randomized, double-blind, parallel-group, multinational trial that studied patients with an acute coronary syndrome and scheduled percutaneous coronary intervention (PCI).11 The study found that prasugrel was associated with a reduction in the primary end point (death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke) but with an increased risk of fatal and major bleeding events.12 Prasugrel is also a prodrug, but its activation occurs in a single, efficient CYP-dependent step that is not influenced by functional variants of CYP enzymes.4, 13 Therefore, although prasugrel may be more effective on average than clopidogrel, it is associated with increased bleeding risk. Secondary analyses of the TRITON-TIMI 38 data have suggested that for patients with fully functional CYP2C19 alleles (i.e., extensive metabolizers [EMs]), there was no difference in the primary outcome occurrence between those treated with prasugrel or clopidogrel (relative risk [RR] 0.98, 95% confidence interval [CI] 0.80– 1.20).14 Clopidogrel's patent is expected to expire later in 2012, thereby allowing for the marketing of a generic form; however, prasugrel will remain under patent for much longer and will presumably be more expensive than generic clopidogrel. The use of pharmacogenomic screening could allow for individualized antiplatelet therapy in which patients with fully functional CYP2C19 alleles could be treated with clopidogrel, whereas the more expensive agent (prasugrel) could be reserved for those with reduced-function CYP2C19 alleles. Whether this approach would be cost-effective has not been studied.
Recently, a cost-effectiveness analysis based on the overall TRITON-TIMI 38 study without regard to genotype was published.15 The study found prasugrel to be cost-effective compared with clopidogrel for patients with a planned PCI. The study found that average costs were $221/patient lower with prasugrel than with clopidogrel. In addition, prasugrel was associated with an anticipated life expectancy gain of 0.102 years. In examining generic clopidogrel, the study's investigators determined the incremental cost-effectiveness ratio to be $9727/life-year gained. However, the United Kingdom's National Institute for Health and Clinical Excellence found insufficient evidence to support prasugrel's clinical superiority to clopidogrel or vice versa. The evidence review group that assessed clopidogrel and prasugrel recommended a 20% reduction in the acquisition cost of prasu-grel after 1 year to accomplish clinical equipoise between the two drugs.16 Neither study included CYP2C19 genotype status of the patients.
Thus, the aim of our study was to evaluate the cost-effectiveness of genotype-guided antiplatelet therapy compared with either clopidogrel or prasugrel for all patients irrespective of genotype.
Methods
Study Design
A decision tree was developed to model selection of antiplatelet drug based on a patient's CYP2C19 genotype status compared with selection of antiplatelet drug based on standard of care (i.e., not guided by pharmacogenomic test results). The entire patient population was composed of a simulated cohort of 10,000 patients/treatment branch who had a scheduled PCI and who started antiplatelet therapy. This simulation identified the *1, *2, *3, *4, *5, *6, *7, *8, and *17 CYP2C19 alleles through pharmacogenomic screening.17, 18 In the genotyping arm ultrarapid metabolizers (UMs) or extensive metabolizers (EMs) (those with two fully functional alleles) were assumed to receive clopidogrel and those genotyped as intermediate metabolizers (IMs) or poor metabolizers (PMs) (those with at least one copy of a CYP2C19 reduced-function allele) received prasugrel. The genotyping arm was compared with a scenario in which all patients received clopidogrel and a scenario in which all patients received prasugrel, irrespective of genotype. A composite of cardiovascular events (cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke) was used to obtain the number of cardiovascular events avoided.3, 4, 12 Bleeding events were categorized as major or minor bleeding as defined by TRI-TON-TIMI 38.11 The measure of effectiveness used in this model was events avoided. The model's perspective was that of a private payer (e.g., commercial health insurance providers, pharmacy benefit managers), or an entity other than the federal government that pays for the use of health care resources by subscribed or enrolled individuals. To account for the devaluation of future costs, the current cost of drugs was discounted at a 5% rate. All costs are presented in 2011 U.S. dollars. Costs and event probabilities were obtained from publically available, peer-reviewed sources. The model was constructed using TreeAge Pro 2009 software (TreeAge Software Inc., Williamstown, MA).
Model Structure, Clinical Inputs, and Assumptions
The main decision node in the model was the selection of antiplatelet therapy represented by the following three options: drug selection based on CYP2C19 genotype, treatment with clopidogrel, or treatment with prasugrel (Figure 1). In the model, a cohort of 10,000 simulated patients was assigned to each treatment arm of the decision tree. For each treatment option, base case probabilities were obtained from the TRITON-TIMI 38 study, whereas event probabilities for the genotyping arm (i.e., distinguishing UM/EM and IM/PM) were obtained from the TRITON-TIMI 38 genetic substudies.3, 4 Simulated individuals determined to be UM/EM were prescribed clopidogrel, given that these individuals have fully functional CYP2C19 activity that can metabolize the drug to the active form; IM/PM were prescribed prasugrel, as they lack the fully functional CYP2C19 activity required to activate clopidogrel. Subsequently, simulated patients progressed through the decision tree based on the probability of experiencing a cardiovascular event or bleeding event (Figure 1).
Figure 1.
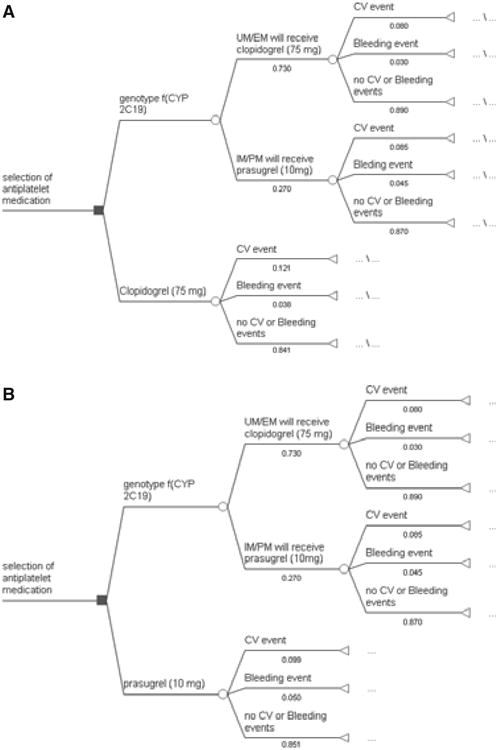
Decision tree for cost-effectiveness of genotyping to determine antiplatelet therapy selection. Squares represent decision nodes, circles represent chance nodes, and triangles represent terminal nodes. Panel 1a depicts genotyping to select medication compared to clopidogrel for all patients. Panel 1b depicts genotyping to select medication compared to prasugrel for all patients. UM/EM = ultrarapid or extensive metabolizers; IM/PM = intermediate or poor metabolizers; CV = cardiovascular.
Although multiple cardiovascular and bleeding events may have occurred in an individual patient, the outcomes reported from TRITON-TIMI 38 were time to first event for the primary outcome (cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) and safety outcome (TIMI major or minor bleeding unrelated to coronary artery bypass graft surgery).12 Our model is consistent with the TRITON-TIMI 38 trial and is based on the first trial event. The incremental cost-effectiveness ratio (ICER), defined as the cost/event avoided, was calculated as the ratio of the difference in total costs and the difference in events reached for each treatment option, or arm of the decision tree at the final event level for all events (i.e., cardiovascular event, bleeding event, or no event). The model incorporated event data for up to 15 months, as reported in the trial and substudies.3, 4, 11, 12
Cost Estimates
All event costs, including direct medical costs of hospitalization due to PCI, costs of drug therapy, and costs associated with events incorporated into the model were derived from publically available sources and are shown in Table 1.19–22 Costs for genotyping were obtained by averaging reported costs from a direct-to-consumer Web site for CYP2C19 testing (cost includes testing for CYP2C19 *1, *2, *3, *4, *5, *6, *7, *8, and *17) and costs derived from Current Procedural Terminology codes associated with molecular testing for the state of Mary-land.17, 18 To reflect the higher inflation rate associated with health services compared with goods and services of other industries, all costs incorporated into the model were transformed into 2011 costs in accordance with the medical component of the Consumer Price Index.23 Direct costs for clopidogrel and prasugrel were based on the average wholesale price of the drugs or the price paid by third-party payers. To account for future effects of costs, we assumed a 5% discount rate for all costs included in our analysis (Table 1).
Table 1. Model Parameters Used in Determining Cost-Effectiveness of Antiplatelet Therapy Selection.
| Parameter | Base-Case Value (range) |
|---|---|
| UM or EM3, 6, 25–27, 29, 30 | 0.730 (0.680–0.738)a |
| IM or PM4, 6, 26, 27 | 0.270 (0.261–0.319)a, b |
| Cardiovascular event for UM or EM with clopidogrel3, 25, 26 | 0.080 (0.059–0.139) |
| Noncardiovascular event (bleeding) for UM or EM with clopidogrel3 | 0.030 |
| Cardiovascular event for IM or PM with prasugrel4 | 0.085 |
| Noncardiovascular event (bleeding) for IM or PM with prasugrel4 | 0.045 |
| Cardiovascular event with clopidogrel12, 28 | 0.121 (0.092–0.150)b |
| Cardiovascular event with prasugrel12, 28 | 0.099 (0.057–0.141)b |
| Noncardiovascular event (bleeding) with clopidogrel12, 28 | 0.038 (0.022–0.054)c |
| Noncardiovascular event (bleeding) with prasugrel12, 28 | 0.050 (0.022–0.078)c |
| Genotyping CYP2C1917, 18 | $310d |
| Uncomplicated percutaneous coronary intervention19 | $18,000d |
| Branded clopidogrel 75 mg/day for 15 mo20 | $2660d |
| Prasugrel 10 mg/day for 15 mo20 | $2800d |
| Bleeding event21 | $19,360 |
| Cardiovascular death22 | $23,760d |
| Nonfatal myocardialinfarction22 | $40,970d |
| Nonfatal stroke22 | $27,314d |
Costs are reported in 2011 U.S. dollars.
Cardiovascular events were defined as a composite of cardiovascular deaths, nonfatal myocardial infarction, and nonfatal stroke. UM or EM = ultrarapid or extensive metabolizer; IM or PM = intermediate or poor metabolizer; CYP2C19 = cytochrome P450 2C19.
Values reflect IMs or PMs for predominantly Caucasian populations.
Value of lower range imputed from upper range.
Value of upper range imputed from lower range.
All costs reflect adjusted August 2011 costs discounted at 5% to account for future value.
Effect Estimates
The unit of effectiveness for all analyses was expressed as number of events avoided. Individuals were processed through the decision tree to determine the number of events avoided. The number needed to genotype (NNG) was calculated to determine the number of patients who would need to undergo genotyping in order to avoid the occurrence of one event. To determine the NNG, all estimated events (cardiovascular events and bleeding events) were added according to treatment option (drug selection determined by genotyping, clopidogrel for all patients, and prasugrel for all patients) by each of the four scenarios (scenario A– genotyping compared with clopidogrel for all patients; scenario B–genotyping compared with prasugrel for all patients; scenario C–genotyping [including generic clopidogrel] compared with generic clopidogrel for all patients; and scenario D–genotyping [including generic clopidogrel] compared with prasugrel for all patients). The difference between the total events was taken for each scenario and divided by the number of simulated individuals processed through each treatment option. To get the final NNG, the inverse of the difference between total events was obtained.
Scenario Analysis
In each scenario analysis, the reference group was the population prescribed an antiplatelet drug without genotyping and comparison groups were clopidogrel for all patients or prasugrel for all patients. Clopidogrel loses patent protection later in 2012; therefore, scenario analyses were extended to determine how generic clopidogrel, with an estimated price of $1/pill, would affect the results. The previously mentioned scenarios were repeated with the price of generic clopidogrel replacing the price of branded clopidogrel. The bootstrap technique was used to generate 95% CIs around ICER estimates and assumed a uniform distribution for input model parameters.
Sensitivity Analysis
Probabilistic sensitivity analyses were conducted to introduce a range of parameter estimates reported in the literature. Node probabilities were assigned a triangular distribution defined by ranges reported in the literature for terminal node events. Probabilistic sensitivity analysis was conducted to propagate uncertainty throughout the model. Model inputs, ranges, and distributions used during these analyses are presented in Table 1.
Sensitivity analyses were conducted on the scenarios derived from the decision model. Parameters that were varied included prevalence of UM/EM and IM/PM status, cardiovascular and bleeding event rates occurring in those treated with clopidogrel or prasugrel, and event rates with and without previous genotyping to select antiplatelet therapy. Sensitivity analysis incorporated varying estimates of event rates from several observational studies and two meta-analyses that examined CYP2C19 loss-of-function alleles.6, 25– 30
Cost-effectiveness acceptability curves (CEACs) were created through Monte Carlo simulations for each scenario examined and present the probability that a therapy (genotype-selected antiplatelet drug, clopidogrel for all patients, or prasugrel for all patients) is cost-effective compared with an alternative over a range of willingness-to-pay values, or the amount of money an individual is willing to trade for a good or service. These CEAC plots illustrate changes in the proportion of replicates that are cost-effective (vertical axis) as the willingness-to-pay threshold (horizontal axis) varies from $1/event avoided to approximately $90,000/event avoided assuming treatment with CYP2C19 genotype–guided therapy, clopidogrel, or prasugrel.
Result
Cost-Effectiveness of Genotyping
Genotyping to select antiplatelet therapy was the dominant strategy; that is, it was less costly and more effective compared with clopidogrel for all patients (ICER: –$6760 [95% CI –$6720 to –$6790]) and prasugrel for all patients (–$11,710 [95% CI –$11,480 to –$11,950]) (Table 2). The generic clopidogrel scenario reflected a change in clopidogrel's price from approximately $6.22/pill to an estimated $1/pill. In the generic clopidogrel scenario, genotyping was less costly compared with prasugrel for all patients (ICER: –$27,160 [95% CI –$27,890 to –$26,420]) but was not less costly compared with clopidogrel for all patients (ICER: $2300 [95% CI $2290 to $2320]) (Table 2).
Table 2. Scenario Analysis of Genotyping CYP2C19 to Determine Selection of Antiplatelet Therapy.
| Scenario | ICERa (95% confidence interval) |
|---|---|
| Branded clopidogrel and prasugrel | |
| Scenario A: genotyping to select drug therapy vs clopidogrel for all patients | −6760 (-6720 to −6790) |
| Scenario B: genotyping to select drug therapy vs prasugrel for all patients | −11,710 (−11,480 to −11,950) |
| Generic clopidogrelb | |
| Scenario C: genotyping to select drug therapy vs clopidogrel for all patients | 2300 (2290–2320) |
| Scenario D: genotyping to select drug therapy vs prasugrel for all patients | −27,160 (−27,890 to −26,420) |
Costs are reported in 2011 U.S. dollars.
ICER = incremental cost-effectiveness ratio.
Defined as cost/cardiovascular event avoided.
To account for costs associated with generic clopidogrel (clopido-grel will lose its patent protection in 2012), we estimated generic clopidogrel would be priced at $1/pill. The scenarios of comparing antiplatelet drug therapy selection based on genotype with clopidogrel or prasugrel for all patients was repeated with the estimated cost of generic clopidogrel in place of the branded clopidogrel.
Number Needed to Genotype
The model predicted a lower number of cardiovascular events associated with genotype-directed antiplatelet therapy. Genotyping produced approximately 450 fewer cardiovascular events compared with treating all patients with clopidogrel. The NNG was 23 when comparing genotype-guided therapy to clopidogrel for all. In other words, one excess cardiovascular event was avoided for every 23 patients genotyped when compared to a clopidogrel-for-all strategy. Genotyping resulted in approximately 350 fewer cardiovascular events compared with prasugrel. The NNG was 30 when comparing genotype-guided therapy to prasugrel for all. One excess cardiovascular event was avoided for every 30 patients genotyped when compared to a prasu-grel-for-all strategy.
Sensitivity Analysis
Probabilistic sensitivity analysis was used to propagate uncertainty throughout the model. Uncertainty could arise from any node depicted in our decision analytic model (Figure 1) and was modeled by varying decision node estimates based on ranges reported in the literature (Table 2). Results from probabilistic sensitivity analyses are presented in CEACs (Figures 2 and 3). Figure 2 depicts the scenario in which genotype-guided antiplatelet drug selection is compared with branded clopidogrel and prasugrel. The CEAC shows that if a private payer were willing to pay $9670/event avoided, the payer could be 95% certain to avoid one event when choosing between genotype-guided therapy and branded clopidogrel. However, when choosing between genotype-guided therapy and prasugrel, the payer could be 95% certain to avoid an event if willingness to pay is established at $225,500/event avoided. The CEAC of the generic clopidogrel scenario is presented in Figure 3. A willingness-to-pay threshold of $3700 to avoid an event will result in 95% certainty that an event will be avoided when choosing between genotype-guided therapy (that incorporates generic clopidogrel) and generic clopido-grel. Using the same level of certainty when choosing between genotype-guided therapy (that incorporates generic clopidogrel) and prasugrel, a private payer must be willing to pay $55,000 to avoid one event.
Figure 2.
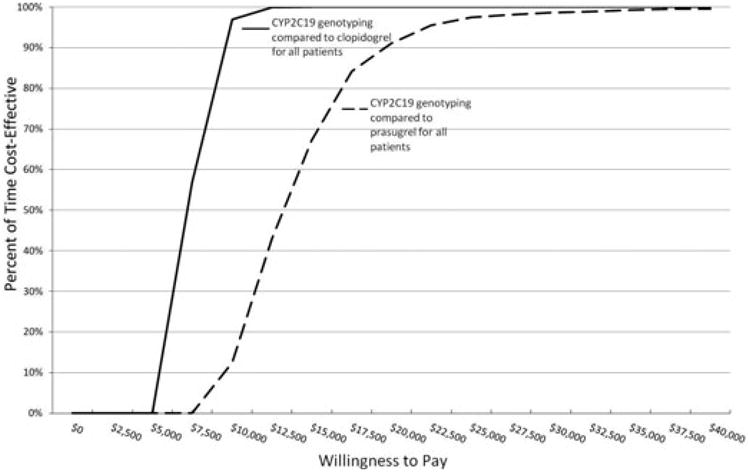
Cost-effectiveness acceptability curves derived from plots of the percentage of time a therapy is cost-effective (vertical axis) over a range of values that a private payer would be willing to pay (horizontal axis) for cytochrome P450 (CYP) 2C19 genotype-guided antiplatelet therapy compared with clopidogrel for all patients or prasugrel for all patients. This plot indicates that a willingness-to-pay threshold of $9670 to avoid an event will result in 95% certainty that one of these events will be avoided when using CYP2C19 genotyping compared with clopidogrel for all patients. However, to be 95% certain that an event will be avoided when using CYP2C19 genotyping compared with prasugrel for all patients, a private payer must be willing to pay $22,500 to avoid one event.
Figure 3.
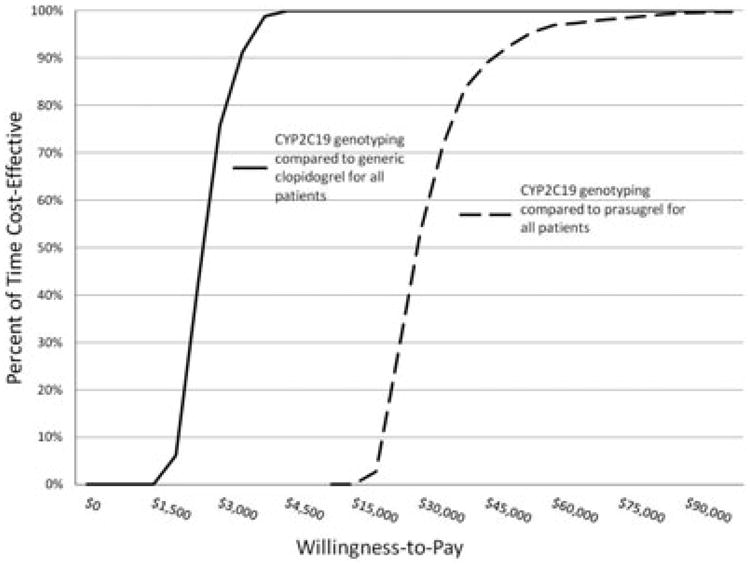
Cost-effectiveness acceptability curves derived from plots of the percentage of time a therapy is cost-effective over a range of values that a private payer would be willing to pay (horizontal axis) for cytochrome P450 (CYP) 2C19 genotype-guided antiplatelet therapy (incorporating generic clopidogrel) compared with generic clopidogrel or prasugrel. This plot indicates that a willingness-to-pay threshold of approximately $3700 to avoid an event will result in 95% certainty that one of these events will be avoided when using CYP2C19 genotyping compared with generic clopidogrel for all patients. However, to be 95% certain that an event will be avoided when using CYP2C19 genotyping compared with prasugrel for all patients, a private payer must be willing to pay approximately $55,000 to avoid one event.
Discussion
We compared a model in which patients were assigned antiplatelet drug therapy based on their genotype with patients who received either clopidogrel or prasugrel irrespective of genotype. The model predicted that genotype-guided anti-platelet therapy was dominant over scenarios in which clopidogrel therapy was prescribed for all patients or prasugrel therapy was prescribed for all patients. The incorporation of generic clopidogrel into the model found that genotype-guided antiplatelet therapy was dominant over prescribing prasugrel for all patients, but not over prescribing generic clopidogrel for all patients. For our base-case analysis, genotype-guided therapy compared with branded clopidogrel for all patients was at least 95% dominant, but when genotype-guided therapy (including generic clopidogrel) was compared with generic clopidogrel for all patients, genotyping did not result in cost savings. Ignoring events not included in these analyses, a private payer may choose to use generic clopidogrel, given its association with a relatively low willingness to pay threshold, $3700. In all of the analyses conducted, the model reflects fewer events when comparing genotype-guided therapy with non– genotype-guided therapy, with the additional costs of genotyping for CYP2C19 status offset in all but one scenario (clopidogrel with generic pricing for all patients). The generic clopidogrel scenario is driven by the reduced price of generic clopidogrel, not the value of improved clinical outcomes associated with genotyping to determine CYP2C19 metabolic status, as illustrated by Figure 3.
Today's clinical setting for personalized antiplatelet therapy is rapidly evolving. The clopidogrel patent expires later in 2012 and newer-generation antiplatelet agents not as susceptible to polymorphic drug metabolizing enzymes have gained FDA approval. During the time since we performed this analysis with prasugrel, ticagrelor has also received FDA approval and will require additional cost-effectiveness analyses. High-dose clopidogrel is also being investigated as a potential treatment alternative for those with reduced-function CYP2C19. In addition, several clinical trials and comparative effectiveness studies that are evaluating genotype-guided antiplatelet therapy (with or without platelet function testing) versus standard of care are under way. As these studies are completed, further cost-effectiveness analyses can be undertaken to better refine the scenarios in which genotype-guided antiplatelet therapy may be cost-effective.
This study did not aim to investigate whether clopidogrel or prasugrel was best suited for patients hospitalized with PCI, but examined whether pharmacogenomic testing in clinical practice (i.e., CYP2C19 genotype testing) might be a cost-effective way to individualize antiplatelet therapy. Based on these analyses, this study suggests that pharmacogenomic testing provides greater value despite its associated costs. Of course, in addition to cost-effectiveness, many other factors influence whether a particular test is incorporated into clinical care. For example, when considering CYP2C19 genotyping to individualize antiplatelet therapy, other important factors include clinical infrastructure (e.g., electronic medical records), turnaround time, and coverage and reimbursement issues, all of which will influence whether genotyping will become standard of care. Although no guidelines exist for adoption of pharmacogenomic tests into clinical practice, the Centers for Medicare and Medicaid Services have issued criteria for coverage with evidence development that is aimed at promoting the use of pharmacogenomic testing in clinical practice while also collecting data about these services.32, 33 Currently, Medicare will reimburse the cost of pharmacogenomic testing for warfarin therapy for a beneficiary if he or she is enrolled in a prospective, randomized, outcome study that examines the test's utility.33 Because Medicare insures so many individuals and holds its coverage decisions to a rigorous standard, other insurance companies often incorporate the decisions made by Medicare into their policies. The health insurer Aetna considers genotyping for certain allelic variants associated with heterogeneity in treatment response medically necessary for its beneficiaries who take specific drugs.34 However, reimbursement is only a portion of the key to clinical uptake. Health insurance companies who strive to ensure use of the most effective and cost-saving medical practices must also commit to educating their clinicians and their beneficiaries about the use of these practices.32
This study has some limitations that may affect its validity and generalizability. First, we chose to include both IMs and PMs for prasu-grel in the genotype-guided treatment arm. Although the boxed warning in the clopidogrel label includes only PMs, the majority of published data indicates that both IMs and PMs who are taking clopidogrel are at increased risk of high residual platelet reactivity and adverse outcomes. Clinical treatment guidelines have been published recently that suggest that both IMs and PMs should receive alternate therapy if no contraindications exist, with a moderate strength of recommendation.10 Providing alternate treatment for only PMs would include about 2–5% of individuals with European or African ancestry, whereas including IMs and PMs increases the percentage to approximately 25–30%.10
Second, all of the probabilities used in our base-case models came from one randomized controlled trial (TRITON-TIMI 38) and substudies of that trial, but the sensitivity analysis included studies with a wide range of patients and included two meta-analyses. The clinical course encountered by the “typical” patient enrolled in TRITON-TIMI 38 may not be reflective of patients outside of the clinical trial setting. For example, approximately 92% of TRITON-TIMI 38 participants were Caucasian, but the prevalence of CYP2C19 IM or PM status varies by ethnicity, with a higher frequency in those of Asian descent.12, 35, 36 Therefore we modeled our data to vary the prevalence of IM or PM status in order to consider how a multiethnic population could change the cost-effectiveness results. Furthermore, the ages of patients enrolled in the studies used for analysis ranged from approximately 40–70 years and thus do not provide insights into the treatment of individuals genotyped as IMs or PMs who are not recommended for treatment with prasugrel because they are older than 75 years.37 Clinical algorithms must be developed for subgroups that are unable to follow the pathway outlined in this decision analysis.
In addition, it is possible that the substudy analyses of genotype in TRITON-TIMI 38 could have introduced bias into the model given its retrospective nature and the fact that not all study participants had genotype data included in the study analysis. As data from prospective genotype-guided studies and real-world clinical settings become available, it will be important to compare our results with cost-effectiveness data generated from these other settings. Furthermore, our model used a treatment duration of 15 months based on the median duration in TRITON-TIMI 38. Treatment guidelines for acute coronary syndrome recommend antiplatelet therapy for at least 1 year in patients who receive stents and for at least 1 month, but preferably 1 year, for those who receive medical therapy without stents.38 Therefore, different durations of therapy in clinical practice could alter the cost-effectiveness estimates.
Another factor that could influence our analysis is the fact that the cost of genetic tests will likely influence their use. The costs reflected in our decision tree model were averaged from two different sources—a direct-to-consumer genetic testing firm and Current Procedural Terminology codes used to bill for clinical services reflective of genetic tests in the state of Maryland. As demand increases and more rapid turnaround time becomes available for pharmacogenomic testing, it is likely that costs will decrease and the use of pharmacogenomic testing will become more clinically feasible. In fact, one could argue that pharmacogenetic cost-effectiveness analyses should incorporate $0 for the cost of the genetic testing because these tests do not need to be repeated, could apply to multiple classes of drugs (e.g. antiplatelets, proton pump inhibitors, antiepileptics, and antidepressants), potentially reduce adverse drug events, and the costs could be averaged over a person's lifetime and become asymptotic to zero.
Conclusion
This cost-effectiveness analysis based on the TRITON-TIMI 38 trial suggests that genotyping patients before selecting antiplatelet therapy could offer more value in the clinical setting than assigning drug therapy without regard to pharmacogenomic test results. Based on this model, the value of genotyping reflects both fewer adverse events and lower costs. Additional studies, especially those using “real world” data, are needed to determine how pharmacogenomic testing will affect the proportion of individuals who are prescribed clopidogrel, prasugrel, and ticagrelor, and its cost-effectiveness.
Acknowledgments
Dr. Mullins receives grant funding from GlaxoSmithK-line, Novartis, Pfizer, and sanofi-aventis and consulting income from Amgen, Bayer, Bristol-Myers Squibb, Cubist, Eisai, Genentech, Novartis, Pfizer, and sanofi-aventis. Dr. Onukwugha receives grant funding from Bayer, Novartis and sanofi-aventis. Dr. Beitelshees is supported by a National Institutes of Health grant (K23HL091120).
Contributor Information
Ms. Emily S. Reese, School of Pharmacy, University of Maryland, Baltimore, Maryland
Dr. C. Daniel Mullins, School of Pharmacy, University of Maryland, Baltimore, Maryland
Dr. Amber L. Beitelshees, School of Medicine, University of Maryland, Baltimore, Maryland
Dr. Eberechukwu Onukwugha, School of Pharmacy, University of Maryland, Baltimore, Maryland
References
- 1.Sanofi. [Accessed March 16, 2011];PLAVIX® (clopidogrel bisulfate) tablets Prescribing Information. Available from http://products.sanofi.us/PLAVIX/PLAVIX.html.
- 2.IMS Institute for Healthcare Informatics. [Accessed February 9, 2012];The Use of Medicines in the United States: Review of 2010. Available at http://www.imshealth.com/deployedfiles/imshealth/Global/Content/IMS%20Institute/Static%20File/IHII_UseOfMed_report.pdf.
- 3.Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–62. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 4.Mega JL, Close SL, Wiviott SD, et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel: Relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009;119:2553–60. doi: 10.1161/CIRCULATIONAHA.109.851949. [DOI] [PubMed] [Google Scholar]
- 5.Brandt JT, Close SL, Iturria SJ, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasu-grel. J Thromb Haemost. 2007;5:2429–36. doi: 10.1111/j.1538-7836.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 6.Giusti B, Gori AM, Marcucci R, et al. Relation of cytochrome P450 2C19 loss-of-function polymorphism to occurrence of drug-eluting coronary stent thrombosis. Am J Cardiol. 2009;103:806–11. doi: 10.1016/j.amjcard.2008.11.048. [DOI] [PubMed] [Google Scholar]
- 7.Kim KA, Park PW, Hong SJ, Park JY. The effect of CYP2C19 polymorphism on the pharmacokinetics and pharmacodynamics of clopidogrel: A possible mechanism for clopidogrel resistance. Clin Pharmacol Ther. 2008;84:236–42. doi: 10.1038/clpt.2008.20. [DOI] [PubMed] [Google Scholar]
- 8.Giusti B, Gori AM, Marcucci R, Abbate R. Research highlights: CYP2C19 genetic variants and clopidogrel responsiveness in acute coronary syndrome: are we ready for individualized therapy? Future Med. 2009;10:1131–3. [Google Scholar]
- 9.Shuldiner A, O’Connell J, Bliden K, et al. Association of cyto-chrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–57. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott SA, Sangkuhl K, Gardner EE, et al. Clinical pharmaco-genetics implementation consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clin Pharmacol Ther. 2011;90:328–32. doi: 10.1038/clpt.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiviott SD, Antman EM, Gibson CM, et al. Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel thrombolysis in myocardial infarction 38 (TRITON-TIMI 38) Am Heart J. 2006;152:627–35. doi: 10.1016/j.ahj.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 13.Rehmel JL, Eckstein JA, Farid NA, et al. Interactions of two major metabolites of prasugrel, a thienopyridine antiplatelet agent, with the cytochromes P450. Drug Metab Dispos. 2006;34:600–7. doi: 10.1124/dmd.105.007989. [DOI] [PubMed] [Google Scholar]
- 14.Sorich MJ, Vitry A, Ward MB, Horowitz JD, McKinnon RA. Prasugrel vs clopidogrel for cytochrome P450 2C19-geno-typed subgroups: integration of the TRITON-TIMI 38 trial data. J Thromb Haemost. 2010;8:1678–84. doi: 10.1111/j.1538-7836.2010.03923.x. [DOI] [PubMed] [Google Scholar]
- 15.Mahoney EM, Wang K, Arnold SV, et al. Cost-effectiveness of prasugrel versus clopidogrel in patients with acute coronary syndromes and planned percutaneous coronary intervention: results from the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction TRITON-TIMI 38. Circulation. 2010;121:71–9. doi: 10.1161/CIRCULATIONAHA.109.900704. [DOI] [PubMed] [Google Scholar]
- 16.Greenhalgh J, Bagust A, Boland A, et al. Prasugrel for the treatment of acute cornary artery syndromes with percutaneous cornary intervention. Health Technol Assess. 2010;14:31–8. doi: 10.3310/hta14Suppl1/05. [DOI] [PubMed] [Google Scholar]
- 17.Genelex. [Accessed March 16, 2011];CYP2C19 Genotyping: Pharmacogenetic Testing. Available from http://www.healthanddna.com/healthcare-professional/p450-2c19-genotyping.html.
- 18.Centers for Medicare and Medicaid Services. [Accessed March 16, 2011];Fee Schedule Clinical Laboratory Fee Schedule. Available from http://www.cms.gov/ClinicalLabFeesched/02_clinlab.asp.
- 19.Jacobson KM, Hall Long K, McMurtry EK, Naessens JM, Rihal CS. The economic burden of complications during percutaneous coronary intervention. Qual Saf Health Care. 2007;16:154–9. doi: 10.1136/qshc.2006.019331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staff PDR. Red book: pharmacy's fundamental reference. Montvale, NJ: Thomson Reuters; 2010. [Google Scholar]
- 21.Ewen EF, Zhao L, Kolm P, et al. Determining the in-hospital cost of bleeding in patients undergoing percutaneous coronary intervention. J Interv Cardiol. 2009;22:266–73. doi: 10.1111/j.1540-8183.2009.00431.x. [DOI] [PubMed] [Google Scholar]
- 22.Schleinitz MD, Heidenreich PA. A cost-effectiveness analysis of combination antiplatelet therapy for high-risk acute coronary syndromes: clopidogrel plus aspirin versus aspirin alone. Ann Intern Med. 2005;142:251–9. doi: 10.7326/0003-4819-142-4-200502150-00007. [DOI] [PubMed] [Google Scholar]
- 23.United States Bureau of Labor and Statistics. [Accessed March 16, 2011];How BLS measures price change for Medical Care Services in the Consumer Price Index. Available from http://www.bls.gov/cpi/cpifact4.htm.
- 24.Holmes DR, Jr, Dehmer GJ, Kaul S, Leifer D, O’Gara PT, Stein CM. ACCF/AHA clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the american college of cardiology foundation task force on clinical expert consensus documents and the american heart association endorsed by the society for cardiovascular angiography and interventions and the society of thoracic surgeons. J Am Coll Cardiol. 2010;56:321–41. doi: 10.1016/j.jacc.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009;373:309–17. doi: 10.1016/S0140-6736(08)61845-0. [DOI] [PubMed] [Google Scholar]
- 26.Simon T, Verstuyft C, Mary-Krause M, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–75. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 27.Trenk D, Hochholzer W, Fromm MF, et al. Cytochrome P450 2C19 681G>A polymorphism and high on-clopidogrel platelet reactivity associated with adverse 1-year clinical outcome of elective percutaneous coronary intervention with drug-eluting or bare-metal stents. J Am Coll Cardiol. 2008;51:1925–34. doi: 10.1016/j.jacc.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 28.Pride YB, Wiviott SD, Buros JL, et al. Effect of prasugrel versus clopidogrel on outcomes among patients with acute coronary syndrome undergoing percutaneous coronary intervention without stent implantation: a trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel (TRITON)-thrombolysis in myocardial infarction (TIMI) 38 substudy. Am Heart J. 2009;158:e21–6. doi: 10.1016/j.ahj.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Hulot JS, Collet JP, Silvain J, et al. Cardiovascular risk in clopidogrel-treated patients according to cytochrome P450 2C19*2 loss-of-function allele or proton pump inhibitor coad-ministration: a systematic meta-analysis. J Am Coll Cardiol. 2010;56:134–43. doi: 10.1016/j.jacc.2009.12.071. [DOI] [PubMed] [Google Scholar]
- 30.Mega JL, Simon T, Collet JP, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–30. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fenwick E, Marshall DA, Levy AR, Nichol G. Using and interpreting cost-effectiveness acceptability curves: an example using data from a trial of management strategies for atrial fibrillation. BMC Health Serv Res. 2006;6:52. doi: 10.1186/1472-6963-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ginsburg GS, Voora D. The long and winding road to warfarin pharmacogenetic testing. J Am Coll Cardiol. 2010;55:2813–5. doi: 10.1016/j.jacc.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Medicare and Medicaid Services. [Accessed October 6, 2011];Decision memo for pharmacogenomic testing for warfarin response (CAG-00400N) Available from https://www.cms.gov/medicare-cover-age-database/details/nca-decision-memo.aspx?NCAId=224&ver=15&NcaName=Pharmacogenomic+Testing+for+Warfarin+Response&NCDId=333&ncdver=1&IsPopup=y&bc=AAAAAAAAIAAA&. [PubMed]
- 34.Aetna. [Accessed September 22, 2011];Pharmacogenetic and Pharmacodynamic Testing. Available from http://www.aetna.com/cpb/medical/data/700_799/0715.html.
- 35.Xie HG, Kim RB, Wood AJ, Stein CM. Molecular basis of ethnic differences in drug disposition and response. Annu Rev Pharmacol Toxicol. 2001;41:815–50. doi: 10.1146/annurev.pharmtox.41.1.815. [DOI] [PubMed] [Google Scholar]
- 36.Zhou SF, Liu JP, Chowbay B. Polymorphism of human cyto-chrome P450 enzymes and its clinical impact. Drug Metab Rev. 2009;41:89–295. doi: 10.1080/03602530902843483. [DOI] [PubMed] [Google Scholar]
- 37.Daiichi Sankyo and Lily. Daiichi Sankyo and Lilly Important safety information – Effient.com (prasugrel) tablets. [Accessed March 16, 2011]; Available from http://www.effient.com/Pages/important-safety-information.aspx.
- 38.Wright RS, Anderson JL, Adams CD, et al. 2011 ACCF/AHA focused update of the guidelines for the management of patients with unstable Angina/Non-ST-elevation myocardial infarction (updating the 2007 guideline): a report of the American college of cardiology foundation/American heart association task force on practice guidelines developed in collaboration with the American college of emergency physicians, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J Am Coll Cardiol. 2011;57:1959. doi: 10.1016/j.jacc.2011.02.009. [DOI] [PubMed] [Google Scholar]


