Abstract
Mesenchymal stromal cells (MSCs) are multipotential adult cells present in all tissues. Paracrine effects and differentiating ability make MSCs an ideal cell source for tissue regeneration. However, little is known about how interactions between implanted MSCs and native cells influence cellular growth, proliferation, and behaviour. By using an in vitro three-dimensional (3D) co-culture assay of normal or scarred human vocal fold fibroblasts (VFFs) and bone marrow-derived MSCs (BM-MSCs) in a uniquely suited hyaluronan hydrogel (HyStem–VF), we investigated cell morphology, survival rate, proliferation and protein and gene expression of VFFs and BM-MSCs. BM-MSCs inhibited cell proliferation of both normal and scarred VFFs without changes in VFF morphology or viability. BM-MSCs demonstrated decreased proliferation and survival rate after 7 days of co-culture with VFFs. Interactions between BM-MSCs and VFFs led to a significant increase in protein secretion of collagen I and hepatocyte growth factor (HGF) and a decrease of vascular endothelial growth factor (VEGF), monocyte chemotactic protein-1 (MCP-1) and interleukin-6 (IL-6). In particular, BM-MSCs significantly upregulated matrix metalloproteinase 1 (MMP1) and HGF gene expression for scarred VFFs compared to normal VFFs, indicating the potential for increases in extracellular matrix remodelling and tissue regeneration. Application of BM-MSCs-hydrogels may play a significant role in tissue regeneration, providing a therapeutic approach for vocal fold scarring.
Keywords: BM-MSCs, VFFs, three-dimensional co-culture, cell regulation, hydrogel
1. Introduction
Wound healing is a complex, dynamic process of restoring cellular and tissue structure. It consists of inflammatory, proliferative and remodelling phases with well-organized interactions among various types of cells and cytokines, including platelets, macrophages, mesenchymal stromal cells (MSCs), resident cells (fibroblasts and epithelial cells), released platelet-derived growth factor (PDGF), interleukin-6 (IL-6), monocyte chemotactic protein-1 (MCP-1), transforming growth factor (TGF), vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) (Hollinger et al., 2008). This intricate process is susceptible to interruption or failure, leading to the formation of non-healing chronic wounds and scar. Specific to vocal folds, scarring causes abnormal tissue structure and function resulting in vocal hoarseness and fatigue, significantly decreasing one’s quality of life (Ma and Yiu, 2001). To date, a number of regenerative medicine strategies have been investigated for the prophylaxis and treatment of vocal fold scarring. These include the application of synthetic extracellular matrix (ECM) hyaluronan hydrogel (Duflo et al., 2006b; Thibeault et al., 2010), cell implantation (Halum et al., 2007) and utilization of biomaterials and cells concurrently (Ohno et al., 2011). Further in vitro investigation is necessary to provide support for future in vivo regenerative medicine based therapies for vocal fold tissue fibrosis.
Because wound healing and tissue regeneration involves interaction and regulation between cells, it is essential to understand how communication between different cell types can affect regenerative outcomes. Vocal fold fibroblasts (VFFs), the main cellular component of vocal fold lamina propria, plays a vital role in the maintenance, development and repair of the ECM of vocal fold lamina propria (Gray et al., 2000). MSCs are multipotential adult stromal cells present in all tissues, which can be activated upon entering wounds or other damaged tissue, producing a broad range of bioactive molecules that have roles in immunomodulation, anti-apoptosis, angiogenesis, support of the growth and differentiation of local stem and progenitor cells, anti-scarring and chemoattraction (Caplan, 2010). Since 1995, bone marrow-derived MSCs (BM-MSCs) have been used in Phase I/II clinical trials in other parts of the body to treat scarring and tissue deficits (Langston, 2005; Mazo et al., 2012). Moreover, attention has been directed at the potential of BM-MSCs therapy for the prevention and treatment of vocal fold scar (Hong et al., 2011; Svensson et al., 2011). To date there is a paucity of data defining interactions between BM-MSCs and native tissue cells, specifically in a biomaterial environment. Investigations regarding in vitro stromal cell communication and therapeutics for vocal fold scar require complex multicellular structures – multiple cell types and a three-dimensional (3D) ECM. For this investigation, we developed an in vitro 3D co-culture assay using VFFs, BM-MSCs and hyaluronan hydrogel HyStem–VF. HyStem–VF has been shown previously to be biocompatible with human VFFs (Chen and Thibeault, 2010a) to regulate human VFFs function, enhance ECM remodelling (Chen and Thibeault, 2010b) and improve tissue regeneration and vocal fold scarring (Duflo et al., 2006b). The purpose of this investigation was to elucidate in vitro cooperative aspects of VFFs and BM-MSCs in HyStem–VF and to characterize cellular behaviour parameters, including cell morphology, proliferation, viability and profiling of various bioactive proteins and genes. Our hypothesis was that in 3D, BM-MSCs and VFFs regulate each other’s proliferation rates without a significant effect on cell morphology and viability. We further hypothesize that through paracrine effects BM-MSCs regulate VFFs ECM production to promote tissue regeneration, providing in vitro support for our long-term goal – employing BM-MSCs in combination with hydrogels as an injectable therapeutic for vocal fold scarring.
2. Materials and methods
2.1. Human vocal fold fibroblasts and BM-MSCs
BM-MSCs were derived from bone marrow of healthy donors, based on protocols approved by the University of Wisconsin Health Science Institutional Review Board (IRB) after obtaining informed consent from the donors (Hanson et al., 2010). VFFs were isolated from normal and scarred vocal folds of human donors (Chen and Thibeault, 2008; Jette et al., 2013), based on protocols approved by the University of Wisconsin Health Science IRB. BM-MSCs and VFFs were expanded using Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS). Passages 4–8 of these primary cells were used for all experiments.
2.2. Hyaluronan–gelatin hydrogel
HyStem–VF is an injectable chemically modified hyaluronan-gelatin hydrogel (Biotime Inc., Alameda, CA, USA), which was obtained by mixing 1 ml 1.4% w/v thiol-modified semi-synthetic glycosaminolycan analogous (Glycosil) with 75 μl 1.0% w/v thio-modified gelatin (Gelin-S) and crosslinking this mixture with 8.2% w/v Extralink (PEGDA), as previous described (Shu et al., 2003). The final concentration of HyStem–VF was 1.2% Glycosil, 0.06% Gelin-S and 0.8% PEGDA. All components were dissolved in sterile water in a cell culture hood to ensure sterility. At room temperature, HyStem–VF casts in about 5 min.
2.3. 3D co-culture of VFFs and BM-MSCs
In co-culture, primary normal or scarred VFFs were seeded at 1 × 105 cells/well in a six-well plate [tissue culture polystyrene (TCP)] in DMEM–10% FBS medium. After incubation at 37 °C for 4 h, a transwell insert with 500 μl mixture of BM-MSCs and HyStem–VF (2 × 106 cells/ml) was plated into the well with VFFs (Figure 1). All cells and hydrogel were covered by medium and co-cultures were maintained for 7 days. 3D co-culture allows cells to maintain contact distance without physical contact. Cell concentration was calculated to maintain constant cell–cell distance; a cell concentration of 1 × 106/ml in 3D is equivalent to a plating density of 1 × 104 cells/cm2 (Semino et al., 2003). As controls, monocultures of VFFs on TCP or BM-MSCs in 3D HyStem–VF were established using the same methods as noted above. All experiments were performed in triplicate.
Figure 1.
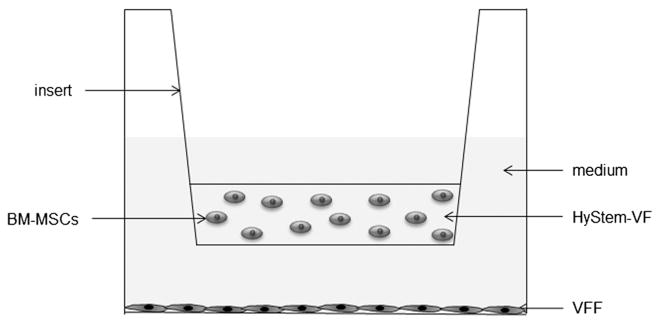
Schematic of 3D co-culture
2.4. Immunostaining and confocal microscopy
After 1 week of co-culture, VFFs and BM-MSCs were separately fixed in 4% paraformaldehyde for 30 min. After washing, the cells were permeabilized three times with 1× PBS with 0.1% Triton X-100 for 5 min. Following blocking (in 5% normal goat serum), the cells were incubated with 1:200 mouse anti-human-prolyl 4-hydroxylase antibody for 90 min (hPH; Millipore, Billerica, MA, USA) and detected with 1:100 Alexa488-conjugated goat anti-mouse secondary antibody (Molecular Probes, Eugene, OR, USA) for 60 min at room temperature. Nuclei were labelled with DAPI (mounting medium with DAPI, Vector Laboratories, Burlingame, CA, USA). Cell morphological features were examined and the images were captured using an inverted confocal microscope (Nikon A1R, Melville, NY, USA).
2.5. Cell viability assay
Cell viability of BM-MSCs and VFFs was separately semi-quantified using a Live/Dead Viability/Cytotoxicity Assay (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. This assay is based on the simultaneous determination of live and dead cells with two-colour fluorescence probes (calcein acetoxymethyl ester–calcein AM and ethidium homodimer 1-EthD-1) that measures recognized parameters of cell viability–intracellular esterase activity and plasma membrane integrity. This assay has been used to quantify apoptotic cell death and cell-mediated cytotoxicity (Lichtenfels et al., 1994). After 7 days of co-culture, VFFs and BM-MSCs incubated separately with staining solution (2 μM calcein AM and 4 μM EthD-1 in PBS) at room temperature for 30 min. Following incubation, cells were imaged using a Nikon E600 fluorescence microscopy-equipped (Nikon Instruments, Melville, NY, USA) Olympus DP71 CCD (Olympus America, San Jose, CA, USA) at ×10 magnification, using green and red filters. The percentage of live and dead cells was determined using MetaMorph software for each condition in quadruplicate. A minimum of 100 cells were counted for each image.
2.6. Cell proliferation assay
VFFs were seeded at 1 × 104 cells/well in 24-well plates and grown for 4 h prior to adding the insert with BM-MSCs and HyStem–VF (1 × 105 cells/well in 200 μl HyStem–VF). After 1, 4 and 7 days of co-culture, cell numbers of VFFs and BM-MSCs were separately monitored in quadruplicate, using CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI, USA). Briefly, 200 μl medium was gently removed from each well and 200 μl CellTiter-Glo reagent was added into each well with VFFs or BM-MSCs. After 10 min of incubation at room temperature, the luminescent output was read on a Flex Station III plate-reader (Molecular Devices, Sunnyvale, CA, USA). The luminescent signal reflects the ATP level and is proportional to the number of viable cells (Chen and Thibeault, 2010a; Crouch et al., 1993).
2.7. Gene expression analysis
Total RNA was separately extracted from VFFs and BM-MSCs after 24 h of co-culture, using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and reverse-transcribed using a QuantiTect Reverse Transcription Kit (Qiagen). mRNA from the cDNA sample was applied with specific primer pairs (Table 1) for matrix metalloproteinase 1 (MMP1), tissue inhibitor of metalloproteinase 3 (TIMP3), collagen I α-2 (Col1), collagen III α-1 (Col3), IL-6, VEGF, HGF and the housekeeping gene β-actin (internal control). Reactions were performed using SYBR-Green PCR Master Mix (Roche, Basel, Switzerland) in the Light Cycler System (Roche) with a standard curve method, as described previously (Chen and Thibeault, 2010a). Results are calculated by each target gene mRNA (ng/μl) normalized to the housekeeping gene β-actin mRNA (ng/μl).
Table 1.
Primer sequences and products of RT–PCR
| Gene | GeneBank no. | Forward primer | Reverse primer | Size (bp) |
|---|---|---|---|---|
| Collagen I-α2 | NM_000089 | 5′-AACAAATAAGCCATCACGCCTGCC-3′ | 5′-TGAAACAGACTGGGCCAATGTCCA-3′ | 101 |
| Collagen III-α1 | NM_000090 | 5′-CCATTGCTGGGATTGGAGGTGAAA-3′ | 5′-TTCAGGTCTCTGCAGTTTCTAGCGG-3′ | 187 |
| MMP1 | NM_002421 | 5′-TGCAACTCTGACGTTGATCCCAGA-3′ | 5′-ACTGCACATGTGTTCTTGAGCTGC-3′ | 122 |
| TIMP3 | NM_000362 | 5′-TGATGCAGCACACACAATTCCC-3′ | 5′-AAGCTCTGTTATTCTGGCCTGGGT-3′ | 102 |
| VEGF | NM_003376 | 5′-ACACATTGTTGGAAGAAGCAGCCC-3′ | 5′-AGGAAGGTCAACCACTCACACACA-3′ | 179 |
| HGF | NM_000601 | 5′-GGCCCACTTGTTTGTGAGCAACAT-3′ | 5′-TGGTGGGGTGCTTCAGACACACTTA-3′ | 84 |
| β-Actin | NM_001101 | 5′-ACGTTGCTATCCAGGCTGTGCTAT-3′ | 5′-CTCGGTGAGGATCTTCATGAGGTAGT-3′ | 188 |
2.8. Secreted cytokines and proteins
After 48 h of co-culture, conditioned media were collected for all conditions. Secreted cytokines and proteins, which included MCP-1, IL-6, VEGF, HGF, TGFβ1, HGF, collagen I and collagen III, were analysed by enzyme-linked immunosorbent assay (ELISA; Invitrogen; except for collagen I, MD Bioproducts, St. Paul, MN, USA; and collagen III, My Biosource, San Diego, CA, USA), according to manufacturers’ instructions.
2.9. Statistical analyses
Values of cellular ATP, cell survival rate, protein and gene expression were expressed as mean ± standard deviation (SD). Analysis of variance (ANOVA) with Fisher’s protected least significant difference tests was performed to examine: (a) effect of co-culture on cell proliferation at different time points: (b) effect of co-culture on cell survival rate; (c) effect of co-culture on protein expression of cytokines and collagens; and (d) effect of co-culture on gene expression of cytokines, collagens and growth factors. Prior to all analyses, data were rank-transformed. p ≤ 0.05 was considered significant. All analyses were performed using SAS statistical software (SAS Institute, Cary, NC, USA).
3. Results
3.1. Morphological features of co-cultured VFFs and BM-MSCs
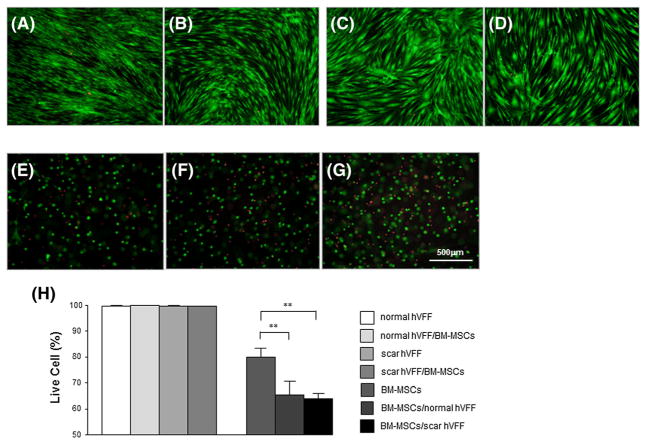
Representative photographs for each type of cell under different culture conditions are presented in Figure 2. After 1 week of culture with and without BM-MSCs, normal and scarred VFFs maintained their typical spindle shape (Figure 2A–D). BM-MSCs in 3D HyStem–VF demonstrated rounded morphological features (Figure 2E); after co-culture with VFFs (normal and scarred) BM-MSCs sustained this rounded morphology (Figure 2F, G). After a 2 week culture period, VFFs and BM-MSCs (including controls and co-cultured cells) maintained similar morphological features as described for 1 week (data not shown).
Figure 2.
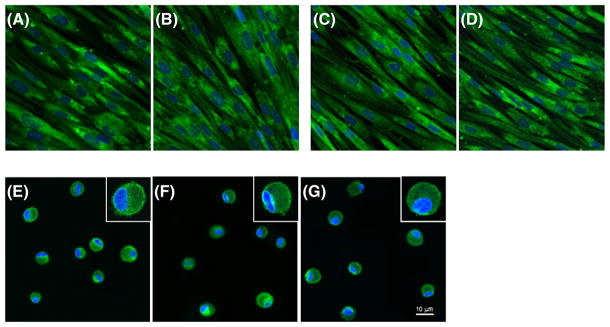
Cell morphology after 1 week of culture: (A) monoculture normal VFFs; (B) normal VFFs after co-culture with BM-MSCs; (C) monoculture scarred VFFs; (D) scarred VFFs after co-culture with BM-MSCs; (E) monoculture BM-MSCs in 3D HyStem–VF; (F) BM-MSCs in 3D HyStem–VF co-culture with normal VFFs; (G) BM-MSCs in 3D HyStem–VF co-culture with scarred VFFs. Cells were stained with p4h (green) and nuclei were counterstained with DAPI (blue). Scale bar = 10 μm
3.2. Effect of co-culture on cell proliferation
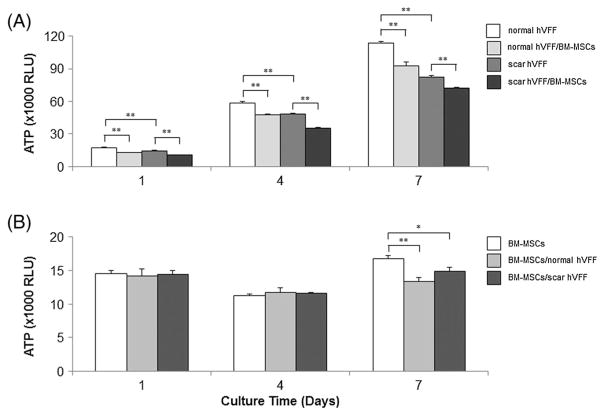
In order to investigate the effect of co-culture on cell proliferation, total ATP values, which are proportional to the number of viable cells, were investigated separately for VFFs and hydrogel-encapsulated BM-MSCs (Figure 3). After 1 week of culture, scarred VFFs growth was slower than normal VFFs (p <0.001), proliferation rates for both normal and scarred VFFs were significantly suppressed by BM-MSCs compared to their monoculture controls (p <0.001; Figure 3A). In contrast, proliferation of BM-MSCs on days 1 and 4 was not significantly affected by either VFFs (Figure 3B). On day 7, proliferation of BM-MSCs was significantly inhibited by both normal and scarred VFFs compared to control (p <0.001 and p <0.05, respectively).
Figure 3.
Effect of co-culture on cell proliferation. VFFs were seeded at 10 000 cells/well and grown for 4 h prior to co-culture with BM-MSCs (100 000 cells/well in 3D HA). On days 1, 4 and 7, viable cell numbers of VFFs and BM-MSCs were separately evaluated by ATP amount (RLU) in quadruplicate. Data shown represent mean ± SD of a single representative experiment. (A) ATP levels of VFFs (normal and scarred VFFs) in monoculture and BM-MSCs co-culture conditions; (B) ATP levels of BM-MSCs in monoculture and co-culture conditions. *p < 0.05; **p < 0.01
3.3. Effect of co-culture on cell survival rate
After 1 week of co-culture, cellular live/dead indicator dyes (calcein AM and EthD-1) were directly added to each kind of cell from different conditions and after 30 min images were captured and analysed (Figure 4). For day 7, normal and scarred VFFs with or without co-cultured BM-MSCs demonstrated few dead (red) cells among many live (green) cells (Figure 4A–D, H). There was no significant differences in VFFs survival rate between monoculture and co-culture (p >0.05). For BM-MSCs monoculture there was a significant increase in the number of dead cells compared to monocultured VFFs (Figure 4E; p <0.0001). Co-culture with both normal and scarred VFFs (Figure 4F, G) significantly reduced BM-MSCs survival percentage from 80.5% to 64.0–65.4%, respectively (Figure 4H; p <0.01).
Figure 4.
Effect of 3D co-culture on cell viability. After 1 week of monoculture and co-culture, live/dead microscopy images of VFFs and BM-MSCs in HyStem–VF were taken: (A) monocultured normal VFFs; (B) co-cultured normal VFFs; (C) monocultured scarred VFFs; (D) co-cultured scarred VFFs; (E) monocultured BM-MSCs in HyStem–VF; (F) normal VFFs co-cultured BM-MSCs in HyStem–VF; (G) scarred VFFs co-cultured BM-MSCs in HyStem–VF. Green, viable cells; red, dead cells. Scale bar = 500 μm. (H) Live cells (%) were analysed by Metamorph software. *p <0.05; **p <0.01
3.4. Effect of co-culture on the gene expression of VFFs and BM-MSCs
In order to examine the effect of co-culture on gene expression of VFFs and BM-MSCs, mRNA transcript expression was measured independently for VFFs and BM-MSCs, using real-time PCR. Investigated genes included wound healing-related VEGF and HGF ECM regulation-related collagen type I α-2 (Col1), collagen type III α-1 (Col3), MMP1 and TIMP3, and an inflammatory cytokine, IL-6. After 24 h, monocultured scarred VFFs demonstrated significantly higher expression of Col1, Col3, MMP1 and TIMP3 compared to monocultured normal VFFs (p <0.05, p <0.01, p <0.01 and p <0.05, respectively), and co-culture with BM-MSCs caused further increases in expression of Col1, MMP1 and TIMP3 compared to scarred VFFs monoculture (p <0.05, p <0.01 and p <0.05, respectively; Figure 5A and Table 2). In particular, MMP1 expression from scarred VFFs increased 4.19 times in the presence of BM-MSCs. We also observed that significantly lower levels of VEGF and HGF genes were expressed in monocultured scarred VFFs compared to monocultured normal VFFs (both p <0.05), and BM-MSCs significantly upregulated their expression levels for both VFFs compared to their monoculture (p <0.05 and p <0.01, respectively). IL-6 gene expression from monocultured scarred VFFs was significantly higher than normal VFFs (p <0.01) and BM-MSCs significantly decreased IL-6 gene expression in scarred VFFs (p <0.05).
Figure 5.
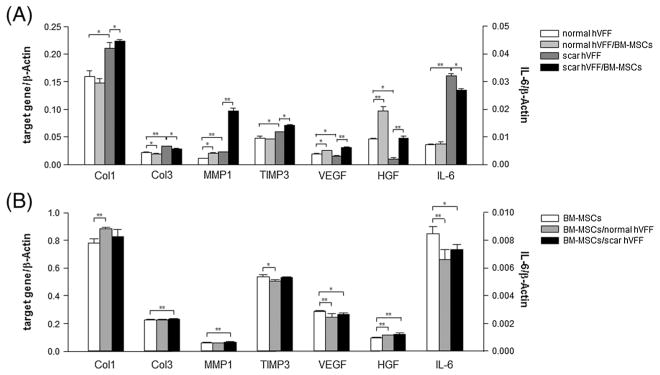
Effect of co-culture on gene expression. After 24 h of monoculture and co-culture, gene expression was separately assayed for VFFs and BM-MSCs. Data are expressed as mRNA expression for collagen I (Col1), III (Col3), MMP-1, TIMP3, VEGF, HGF and IL-6 genes (ng/μl) relative to β-Actin (ng/μl). Values are expressed as mean ± SD of triplicate assays. *p <0.05; **p <0.01. (A) mRNA levels of monoculture normal and scarred VFFs, and BM-MSC co-cultured normal and scarred VFFs; (B) mRNA levels of monocultured BM-MSCs, and VFFs (normal and scarred) co-cultured BM-MSCs
Table 2.
Summary of relative changes in gene expression levels for VFFs and BM-MSCs during monoculture and after co-culture
| Collagen I | Collagen III | MMP1 | TIMP3 | VEGF | HGF | IL-6 | |
|---|---|---|---|---|---|---|---|
| Monocultured normal VFFs control | |||||||
| Monocultured scarred VFFs | ▲* | ▲** | ▲** | ▲* | ▼* | ▼* | ▲** |
| Monocultured normal VFFs control | |||||||
| Co-cultured normal VFFs with BM-MSCs | — | ▼* | ▲* | — | ▲* | ▲** | — |
| Monocultured scarred VFFs control | |||||||
| Co-cultured scarred VFFs with BM-MSCs | ▲* | ▼* | ▲** | ▲* | ▲** | ▲** | ▼* |
| Monocultured BM-MSCs control | |||||||
| Co-cultured BM-MSCs with normal VFFs | ▲** | — | — | ▼* | ▼** | ▲** | ▼** |
| Co-cultured BM-MSCs with scarred VFFs | — | ▲** | ▲** | — | ▼* | ▲** | ▼* |
▲, Upregulation
▼, downregulation
—, no significant change
p <0.05
p <0.01.
In addition to the effect of BM-MSCs on VFFs, VFFs influenced BM-MSCs gene expression (Figure 5B and Table 2). After 24 h, Col1 gene expression from BM-MSCs was significantly upregulated by the presence of normal VFFs (p <0.01) and Col3 and MMP1 genes were also significantly increased by co-culture with scarred VFFs compared with monoculture BM-MSCs (both p <0.01). HGF transcript level was significantly upregulated when co-cultured with both VFFs (both p <0.01). Finally, VEGF and IL-6 gene expressions of BM-MSCs were downregulated by both types of VFFs (p <0.01 and <0.05) and TIMP3 expression was significantly decreased by normal VFFs (p <0.05).
3.5. Collagens, cytokines and growth factors released from co-cultured VFFs and BM-MSCs
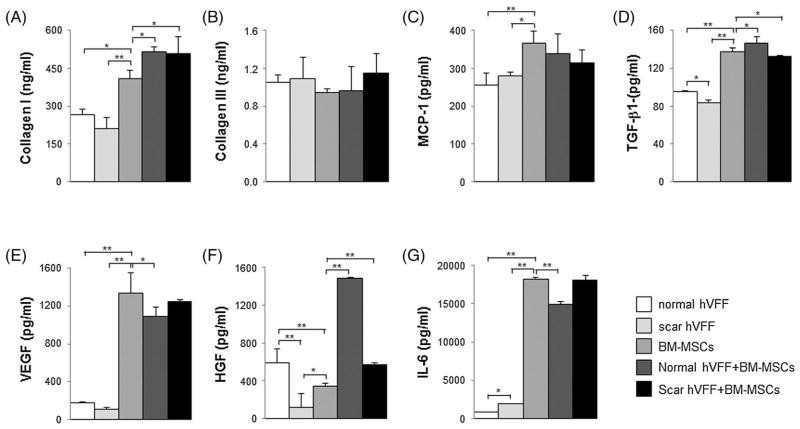
To examine protein levels of collagens, inflammatory cytokines and growth factors secreted from VFFs and BM-MSCs, ELISA assays were performed on conditioned media from 48 h monocultures and co-cultures (Figure 6 and Table 3). Monocultured scarred VFFs produced significantly lower TGFβ1 and HGF and higher IL-6 than normal VFFs (p <0.05, p <0.01 and p <0.05, respectively). Both types of VFFs have similar levels of collagens (I and III), MCP-1 and VEGF. However, monocultured BM-MSCs secreted more collagen I, MCP-1, TGFβ1, VEGF and IL-6 and less HGF than monocultured VFFs (p <0.05 and p<0.01, respectively). Interestingly, monocultured BM-MSCs produced significantly greater protein levels for all markers assayed except collagen III. After 48 h for VFFs (scarred and normal) BM-MSCs co-culture, protein levels of collagen I and HGF significantly increased compared to monocultured BM-MSCs (p <0.05 and p <0.01, respectively). Co-culture of VFFs and BM-MSCs did not result in significant changes in collagen III and MCP-1 levels (p >0.05). BM-MSCs incubated with normal VFFs caused significant increases in TGFβ1 (p <0.05) but co-culture with scarred VFFs resulted in significantly less TGFβ1 release (p <0.05). Co-culture of normal VFFs and BM-MSCs significantly decreased the production of VEGF and IL-6 compared to monocultured BM-MSCs (p <0.05 and p <0.01, respectively), but co-culture of scarred VFFs and BM-MSCs did not cause significant changes in VEGF and IL-6 production (Table 3).
Figure 6.
Effect of co-culture on secreted proteins, growth factors and cytokines. After 48 h of monoculture and co-culture, conditioned media were collected and tested by ELISA for: (A) collagens I; (B) collagen III; (C) MCP-1; (D) TGFβ1; (E) VEGF; (F) HGF; and (G) IL-6. *p <0.05; **p <0.01
Table 3.
Summary of relative changes in collagen, growth factors and inflammatory cytokine production for VFFs and BM-MSCs in the presence conditional media during monoculture and co-culture
| Collagen I | Collagen III | MCP-1 | TGFβ1 | VEGF | HGF | IL-6 | |
|---|---|---|---|---|---|---|---|
| Monocultured normal VFFs control | |||||||
| Monocultured scarred VFFs | — | — | — | ▼* | — | ▲** | ▲* |
| Monocultured BM-MSCs | ▲* | — | ▲** | ▲** | ▲** | ▼** | ▲** |
| Monocultured scarred VFFs control | |||||||
| Monocultured BM-MSCs | ▲** | — | ▲* | ▲** | ▲** | ▲* | ▲** |
| Monocultured BM-MSCs control | |||||||
| Co-cultured normal VFFs + BM-MSCs | ▲* | — | — | ▲* | ▼* | ▲** | ▼** |
| Co-cultured scarred VFFs + BM-MSCs | ▲* | — | — | ▼* | — | ▲** | — |
▲, upregulation
▼, downregulation
—, no significant change
p <0.05
p <0.01.
4. Discussion
Vocal fold scar remains one of the most perplexing, frustrating and resistant conditions to treat among all conditions affecting voice (Hirano, 1995; Benninger et al., 1996; Hansen and Thibeault, 2006). Treatment outcomes for patients with vocal fold scarring remain grim despite remediative efforts that have been undertaken to date (see reviews, Benninger et al., 1996; Bless and Welham, 2010; Hansen and Thibeault, 2006). HyStem–VF, a HA-based hydrogel, has been extensively studied and shown to have the ideal biomechanical properties for the vocal fold (Chen and Thibeault, 2010a; Qian et al., 2012; Serban et al., 2008) and improves vocal fold wound healing in several animal models (Duflo et al., 2006a; Thibeault et al., 2010). Seeding this injectable hydrogel with cells such as BM-MSCs that may be able regenerate the lost/fibrotic ECM of the vocal fold directly or through paracrine effects is a treatment strategy that requires in vitro validation prior to moving to in vivo trials. The goal of this investigation was to provide insight as to how BM-MSCs embedded in 3D hydrogel would interact with native VFFs – normal and scarred VFFs, cells that would interact with BM-MSCs once injected in HyStem–VF. We were particularly interested in determining differential regulation on cell proliferation and collagen synthesis as well as cytokine production as indicators of wound healing and tissue regeneration.
Increasing the percentage of live and proliferating cells post-implantation is critical to the success of the cell transplantation and tissue regeneration. As cell proliferation was assessed as a means to normalize cytokine production, we measured significant decreases in proliferation for all cells when co-cultured vs monocultured, with overall proliferation increasing over a 7 day period. Concomitantly, there was significant increase in the number of dead BM-MSCs when co-cultured vs monocultured. The literature supports the attenuation of proliferation in cardiac fibroblasts treated with BM-MSCs conditioned media (Ohnishi et al., 2007a) and of ferret vocal fold scar fibroblasts co-cultured with adipose-derived stromal cells (Kumai et al., 2010). Conversely, decreased viability of MSCs has been measured when BM-MSCs were co-cultured with gingival fibroblasts (Proksch et al., 2012). Given that all cell types demonstrated decreased proliferation, there appears to be cross-talk in 3D co-culture between BM-MSCs and VFFs. Normal and scar fibroblasts decreased the viability of BM-MSCs through unknown mechanisms that may be associated to the fact that BM-MSCs and tissue-resident fibroblasts display similar morphology, surface markers, gene profile and differentiation ability (Haniffa et al., 2009; Hanson et al., 2010) and have some functional overlap (Francois et al., 2006). Further, fibroblasts from various tissue sites have been shown to inhibit mitogen- and allo-antigen-stimulated T cell proliferation (Donnelly et al., 1993; Korn, 1981; Sarkhosh et al., 2003; Shimabukuro et al., 1992) and IFNγ production (Le and Vilcek, 1987) in exactly the same vein as more recent reports using MSCs (Krampera et al., 2003). Indeed VFFs, characterized as a type of MSCs, may be attenuating proliferation in a manner similar to BM-MSCs. Further investigation is warranted.
BM-MSCs may exert paracrine antifibrotic effects, at least in part, through inhibition of collagen synthesis. Ohnishi et al. (2007a) reported that BM-MSCs conditioned medium significantly attenuated inhibited collagen I and III expression in cardiac fibroblasts. Adipose-derived stromal cells significantly decreased collagen production in scar fibroblasts (Kumai et al., 2010). Previous studies have shown that in scarred fibroblasts, mRNAs for type I procollagen (Kopp et al., 2005), type I collagen (Song et al., 2011) and type XI collagen (Jette et al., 2013) are intrinsically elevated. Therefore, there has been considerable interest in agents that can inhibit or modulate collagen synthesis in fibrotic diseases. Here, we found that in scarred VFFs gene expression levels of collagen I and III were higher than with normal VFFs. This data matches pathophysiological features of vocal fold scar with excess collagen synthesis and deposition (Hirano et al., 2009). However, we did not observe significantly higher collagen protein levels from scarred VFFs medium. Our use of 48 h conditioned media for collagen protein assays may have been too short a time period to measure protein changes. Moreover, we also demonstrated that scarred VFFs produced and secreted higher IL-6 transcript levels than normal VFFs. Excessive secretion of IL-6 is thought to contribute to the pathogenesis of many diseases, such as asthma (Doganci et al., 2005), idiopathic pulmonary fibrosis (Saito et al., 2008) and rheumatoid arthritis (Nishimoto, 2006). IL-6 may have direct role in mediating tissue damage and in patients an elevated level of IL-6 correlates with disease activity (Linker-Israeli et al., 1991). Therefore, inhibitors of IL-6 production or IL-6 receptor-mediated signal transduction may be a potential treatment of fibrosis. Our findings that co-cultured BM-MSCs significantly downregulated IL-6 expression in scarred VFFs suggests that use of BM-MSCs may be an alternative fibrosis treatment approach via modulation of IL-6.
When VFFs were co-cultured with BM-MSCs, HGF protein levels were significantly increased, particularly for BM-MSCs VFFs conditions. HGF is thought to have strong antifibrotic activity through its stimulation of hyaluronic acid (HA) and suppression of collagen I production (Hirano et al., 2003, 2004; Matsumoto and Nakamura, 1997). Using real-time PCR, we were able to show more conclusively that a high level of HGF protein mainly results from the influence of BM-MSCs on VFFs. As previous studies have shown, adipose-derived stromal cells could potentially ameliorate vocal fold scar by acting as long-term, intrinsic sources of HGF (Kumai et al., 2010). Further, BM-MSCs increased HGF may play a role in regeneration, as increases in HGF have caused tissue regeneration in the liver, kidney and lung (Matsumoto and Nakamura, 1997; Ueki et al., 1999; Xue et al., 2003). Interestingly, we measured higher production of HGF and VEGF by normal VFFs than that produced by scarred VFFs. The lower HGF and VEGF in scarred VFFs may be part of the molecular mechanism of scar formation (Kumai et al., 2010) and is consistent with lower HGF levels measured in vocal fold scarring, and injections of HGF has been shown to improve tissue structure and function in various animal vocal fold scar models.
Besides HGF, other paracrine factors released by BM-MSCs may alter the ECM, resulting in favourable remodelling. A number of molecules expressed in BM-MSCs involved in biogenesis of ECM, such as collagens, MMPs and TIMPs, suggest that transplanted MSCs can inhibit fibrosis (Ohnishi et al., 2007b). Xu et al. (2005) evaluated the effects of grafting BM-MSCs on ECM in infarcted rat hearts, and showed that BM-MSCs transplantation significantly attenuated the increased cardiac expression of collagens I and III, TIMP-1 and TGFβ observed in infarcted control hearts. Here, we found that co-cultured BM-MSCs upregulated MMP1, TIMP3 and VEGF gene expression for both types of VFFs, without evidence of increasing IL-6 expression for VFFs/BM-MSCs co-culture. In particular, co-cultured BM-MSCs significantly up-regulated MMP1 expression in scarred VFFs, leading to an enhanced MMP1:TIMP3 ratio. This suggests that co-culture-induced MMP1, TIMP3 and VEGF gene expression may potentially enhance ECM regulation, remodelling and angiogenesis without increasing inflammation, whereby promoting normal wound-healing processes (Chen and Thibeault, 2010b; Wolfram et al., 2009).
A limitation of our study warrants discussion. Transwell inserts were used to physically separate our cell types in co-culture. Where our gene expression results are from individual cell types, protein expression results are from both cell types in co-culture, whereby it is not possible to determine the specific cell contribution to total ELISA levels, as the ECM markers and cytokines may be produced by both cell types. We utilized monoculture controls as a mechanism to understand collective protein production.
5. Conclusion
We developed an in vitro fibroblast and hydrogel-embedded BM-MSCs co-culture assay suitable for investigating the complex interactions among different cells and implanted biomaterials. This system has contributed to a better understanding of the molecular biology mechanisms related to the cell–cell interactions within a hyaluronan biomaterial environment. Our findings provide quantitative evidence to support the hypothesis that fibroblasts and hydrogel-embedded BM-MSCs are capable of modulating cellular behaviour via cytokines and growth factor production, thereby providing an in vitro regenerative milieu for vocal fold scarring.
Acknowledgments
The authors thank Dr Peiman Hematti, University of Wisconsin Madison, for providing human BM-MSCs and Dr Glen Leverson, University of Wisconsin Madison, for statistical support. This work was funded by the NIDCD at the National Institutes of Health (Grant No. R01DC4336).
Footnotes
Conflict of interest
The authors have declared that there is no conflict of interest.
References
- Benninger MS, Alessi D, Archer S, et al. ; Vocal fold scarring: current concepts and management. Otolaryngol Head Neck Surg. 1996;115:474–482. doi: 10.1177/019459989611500521. [DOI] [PubMed] [Google Scholar]
- Bless DM, Welham NV. ; Characterization of vocal fold scar formation, prophylaxis, and treatment using animal models. Curr Opin Otolaryngol Head Neck Surg. 2010;18:481–486. doi: 10.1097/MOO.0b013e3283407d87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. ; What’s in a name? Tissue Eng Part A. 2010;16:2415–2417. doi: 10.1089/ten.TEA.2010.0216. [DOI] [PubMed] [Google Scholar]
- Chen X, Thibeault SL. ; Characteristics of age-related changes in cultured human vocal fold fibroblasts. Laryngoscope. 2008;118:1700–1704. doi: 10.1097/MLG.0b013e31817aec6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Thibeault SL. ; Biocompatibility of a synthetic extracellular matrix on immortalized vocal fold fibroblasts in 3D culture. Acta Biomater. 2010a;6:2940–2948. doi: 10.1016/j.actbio.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Thibeault SL. ; Role of tumor necrosis factor-α in wound repair in human vocal fold fibroblasts. Laryngoscope. 2010b;120:1819–1825. doi: 10.1002/lary.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch SP, Kozlowski R, Slater KJ, et al. ; The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J Immunol Methods. 1993;160:81–88. doi: 10.1016/0022-1759(93)90011-u. [DOI] [PubMed] [Google Scholar]
- Doganci A, Sauer K, Karwot R, et al. ; Pathological role of IL-6 in the experimental allergic bronchial asthma in mice. Clin Rev Allergy Immunol. 2005;28:257–270. doi: 10.1385/CRIAI:28:3:257. [DOI] [PubMed] [Google Scholar]
- Donnelly JJ, Xi MS, Rockey JH. A soluble product of human corneal fibroblasts inhibits lymphocyte activation. Enhancement by interferon-γ. Exp Eye Res. 1993;56:157–165. doi: 10.1006/exer.1993.1023. [DOI] [PubMed] [Google Scholar]
- Duflo S, Thibeault SL, Li W, et al. ; Effect of a synthetic extracellular matrix on vocal fold lamina propria gene expression in early wound healing. Tissue Eng. 2006a;12:3201–3207. doi: 10.1089/ten.2006.12.3201. [DOI] [PubMed] [Google Scholar]
- Duflo S, Thibeault SL, Li W, et al. ; Vocal fold tissue repair in vivo using a synthetic extracellular matrix. Tissue Eng. 2006b;12:2171–2180. doi: 10.1089/ten.2006.12.2171. [DOI] [PubMed] [Google Scholar]
- Francois S, Bensidhoum M, Mouiseddine M, et al. ; Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells. 2006;24:1020–1029. doi: 10.1634/stemcells.2005-0260. [DOI] [PubMed] [Google Scholar]
- Gray SD, Titze IR, Alipour F, et al. ; Biomechanical and histologic observations of vocal fold fibrous proteins. Ann Otol Rhinol Laryngol. 2000;109:77–85. doi: 10.1177/000348940010900115. [DOI] [PubMed] [Google Scholar]
- Halum SL, Naidu M, Delo DM, et al. ; Injection of autologous muscle stem cells (myoblasts) for the treatment of vocal fold paralysis: a pilot study. Laryngoscope. 2007;117:917–922. doi: 10.1097/MLG.0b013e31803e8c8d. [DOI] [PubMed] [Google Scholar]
- Haniffa MA, Collin MP, Buckley CD, et al. ; Mesenchymal stem cells: the fibroblasts’ new clothes? Haematologica. 2009;94:258–263. doi: 10.3324/haematol.13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JK, Thibeault SL. ; Current understanding and review of the literature: vocal fold scarring. J Voice. 2006;20:110–120. doi: 10.1016/j.jvoice.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Hanson SE, Kim J, Johnson BH, et al. ; Characterization of mesenchymal stem cells from human vocal fold fibroblasts. Laryngoscope. 2010;120:546–551. doi: 10.1002/lary.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M. Phonosurgery: past, present, future. Chevalier Jackson Lecture. 1995:25–30. [Google Scholar]
- Hirano S, Bless D, Heisey D, et al. ; Roles of hepatocyte growth factor and transforming growth factor-β1 in production of extracellular matrix by canine vocal fold fibroblasts. Laryngoscope. 2003;113:144–148. doi: 10.1097/00005537-200301000-00027. [DOI] [PubMed] [Google Scholar]
- Hirano S, Bless DM, Rousseau B, et al. ; Prevention of vocal fold scarring by topical injection of hepatocyte growth factor in a rabbit model. Laryngoscope. 2004;114:548–556. doi: 10.1097/00005537-200403000-00030. [DOI] [PubMed] [Google Scholar]
- Hirano S, Minamiguchi S, Yamashita M, et al. ; Histologic characterization of human scarred vocal folds. J Voice. 2009;23:399–407. doi: 10.1016/j.jvoice.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Hollinger JO, Hart CE, Hirsch SN, et al. ; Recombinant human platelet-derived growth factor: biology and clinical applications. J Bone Joint Surg Am. 2008;90(suppl 1):48–54. doi: 10.2106/JBJS.G.01231. [DOI] [PubMed] [Google Scholar]
- Hong SJ, Lee SH, Jin SM, et al. ; Vocal fold wound healing after injection of human adipose-derived stem cells in a rabbit model. Acta Otolaryngol. 2011;131:1198–1204. doi: 10.3109/00016489.2011.599816. [DOI] [PubMed] [Google Scholar]
- Jette ME, Hayer SD, Thibeault SL. ; Characterization of human vocal fold fibroblasts derived from chronic scar. Laryngoscope. 2013;123:738–745. doi: 10.1002/lary.23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp J, Preis E, Said H, et al. ; Abrogation of transforming growth factor-β signaling by SMAD7 inhibits collagen gel contraction of human dermal fibroblasts. J Biol Chem. 2005;280:21570–21576. doi: 10.1074/jbc.M502071200. [DOI] [PubMed] [Google Scholar]
- Korn JH. ; Modulation of lymphocyte mitogen responses by cocultured fibroblasts. Cell Immunol. 1981;63:374–384. doi: 10.1016/0008-8749(81)90016-2. [DOI] [PubMed] [Google Scholar]
- Krampera M, Glennie S, Dyson J, et al. ; Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- Kumai Y, Kobler JB, Park H, et al. ; Modulation of vocal fold scar fibroblasts by adipose-derived stem/stromal cells. Laryngoscope. 2010;120:330–337. doi: 10.1002/lary.20753. [DOI] [PubMed] [Google Scholar]
- Langston JW. ; The promise of stem cells in Parkinson disease. J Clin Invest. 2005;115:23–25. doi: 10.1172/JCI24012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le JM, Vilcek J. ; Accessory function of human fibroblasts in mitogen-stimulated interferon-γ production by T lymphocytes. Inhibition by interleukin 1 and tumor necrosis factor. J Immunol. 1987;139:3330–3337. [PubMed] [Google Scholar]
- Lichtenfels R, Biddison WE, Schulz H, et al. ; CARE-LASS (calcein-release assay), an improved fluorescence-based test system to measure cytotoxic T lymphocyte activity. J Immunol Methods. 1994;172:227–239. doi: 10.1016/0022-1759(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Linker-Israeli M, Deans RJ, Wallace DJ, et al. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol. 1991;147:117–123. [PubMed] [Google Scholar]
- Ma EP, Yiu EM. ; Voice activity and participation profile: assessing the impact of voice disorders on daily activities. J Speech Lang Hear Res. 2001;44:511–524. doi: 10.1044/1092-4388(2001/040). [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Nakamura T. ; Hepatocyte growth factor (HGF) as a tissue organizer for organogenesis and regeneration. Biochem Biophys Res Commun. 1997;239:639–644. doi: 10.1006/bbrc.1997.7517. [DOI] [PubMed] [Google Scholar]
- Mazo M, Arana M, Pelacho B, et al. Mesenchymal stem cells and cardiovascular disease: a bench to bedside roadmap. Stem Cells Int. 2012;2012:11. doi: 10.1155/2012/175979. Article ID 175979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N. ; Interleukin-6 in rheumatoid arthritis. Curr Opin Rheumatol. 2006;18:277–281. doi: 10.1097/01.bor.0000218949.19860.d1. [DOI] [PubMed] [Google Scholar]
- Ohnishi S, Sumiyoshi H, Kitamura S, et al. ; Mesenchymal stem cells attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. FEBS Lett. 2007a;581:3961–3966. doi: 10.1016/j.febslet.2007.07.028. [DOI] [PubMed] [Google Scholar]
- Ohnishi S, Yasuda T, Kitamura S, et al. ; Effect of hypoxia on gene expression of bone marrow-derived mesenchymal stem cells and mononuclear cells. Stem Cells. 2007b;25:1166–1177. doi: 10.1634/stemcells.2006-0347. [DOI] [PubMed] [Google Scholar]
- Ohno S, Hirano S, Kanemaru S, et al. ; Implantation of an atelocollagen sponge with autologous bone marrow-derived mesenchymal stromal cells for treatment of vocal fold scarring in a canine model. Ann Otol Rhinol Laryngol. 2011;120:401–408. doi: 10.1177/000348941112000610. [DOI] [PubMed] [Google Scholar]
- Proksch S, Steinberg T, Stampf S, et al. ; Crosstalk on cell behavior in interactive cocultures of hMSCs with various oral cell types. Tissue Eng Part A. 2012;18:2601–2610. doi: 10.1089/ten.TEA.2012.0041. [DOI] [PubMed] [Google Scholar]
- Qian L, Shim W, Gu Y, et al. ; Hemodynamic contribution of stem cell scaffolding in acute injured myocardium. Tissue Eng Part A. 2012;18:1652–1663. doi: 10.1089/ten.TEA.2011.0591. [DOI] [PubMed] [Google Scholar]
- Saito F, Tasaka S, Inoue K, et al. ; Role of interleukin-6 in bleomycin-induced lung inflammatory changes in mice. Am J Respir Cell Mol Biol. 2008;38:566–571. doi: 10.1165/rcmb.2007-0299OC. [DOI] [PubMed] [Google Scholar]
- Sarkhosh K, Tredget EE, Li Y, et al. ; Proliferation of peripheral blood mononuclear cells is suppressed by the indoleamine 2,3-dioxygenase expression of interferon-γ-treated skin cells in a co-culture system. Wound Repair Regen. 2003;11:337–345. doi: 10.1046/j.1524-475x.2003.11505.x. [DOI] [PubMed] [Google Scholar]
- Semino CE, Merok JR, Crane GG, et al. ; Functional differentiation of hepatocyte-like spheroid structures from putative liver progenitor cells in three-dimensional peptide scaffolds. Differentiation. 2003;71:262–270. doi: 10.1046/j.1432-0436.2003.7104503.x. [DOI] [PubMed] [Google Scholar]
- Serban MA, Liu Y, Prestwich GD. ; Effects of extracellular matrix analogues on primary human fibroblast behavior. Acta Biomater. 2008;4:67–75. doi: 10.1016/j.actbio.2007.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro Y, Murakami S, Okada H. ; Interferon-γ-dependent immunosuppressive effects of human gingival fibroblasts. Immunology. 1992;76:344–347. [PMC free article] [PubMed] [Google Scholar]
- Shu XZ, Liu Y, Palumbo F, et al. ; Disulfide-crosslinked hyaluronan–gelatin hydrogel films: a covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials. 2003;24:3825–3834. doi: 10.1016/s0142-9612(03)00267-9. [DOI] [PubMed] [Google Scholar]
- Song R, Bian HN, Lai W, et al. ; Normal skin and hypertrophic scar fibroblasts differentially regulate collagen and fibro-nectin expression as well as mitochondrial membrane potential in response to basic fibroblast growth factor. Braz J Med Biol Res. 2011;44:402–410. doi: 10.1590/S0100-879X2011007500041. [DOI] [PubMed] [Google Scholar]
- Svensson B, Nagubothu SR, Cedervall J, et al. ; Injection of human mesenchymal stem cells improves healing of vocal folds after scar excision – a xenograft analysis. Laryngoscope. 2011;121:2185–2190. doi: 10.1002/lary.22143. [DOI] [PubMed] [Google Scholar]
- Thibeault SL, Klemuk SA, Chen X, et al. ; In vivo engineering of the vocal fold ECM with injectable HA hydrogels – late effects on tissue repair and biomechanics in a rabbit model. J Voice. 2010;25:249–253. doi: 10.1016/j.jvoice.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki T, Kaneda Y, Tsutsui H, et al. ; Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5:226–230. doi: 10.1038/5593. [DOI] [PubMed] [Google Scholar]
- Wolfram D, Tzankov A, Pulzl P, et al. ; Hypertrophic scars and keloids – a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg. 2009;35:171–181. doi: 10.1111/j.1524-4725.2008.34406.x. [DOI] [PubMed] [Google Scholar]
- Xu X, Xu Z, Xu Y, et al. ; Effects of mesenchymal stem cell transplantation on extra-cellular matrix after myocardial infarction in rats. Coron Artery Dis. 2005;16:245–255. doi: 10.1097/00019501-200506000-00006. [DOI] [PubMed] [Google Scholar]
- Xue F, Takahara T, Yata Y, et al. ; Hepatocyte growth factor gene therapy accelerates regeneration in cirrhotic mouse livers after hepatectomy. Gut. 2003;52:694–700. doi: 10.1136/gut.52.5.694. [DOI] [PMC free article] [PubMed] [Google Scholar]