Abstract
Conventional creatinine-based glomerular filtration rate (GFR) equations are insufficiently accurate for estimating GFR in cirrhosis. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) recently proposed an equation to estimate GFR in subjects without cirrhosis using both serum creatinine and cystatin C levels. Performance of the new CKD-EPI creatinine-cystatin C equation (2012) was superior to previous creatinine- or cystatin C-based GFR equations. To evaluate the performance of the CKD-EPI creatinine-cystatin C equation in subjects with cirrhosis, we compared it to GFR measured by non-radiolabeled iothalamate plasma clearance (mGFR) in 72 subjects with cirrhosis. We compared the “bias”, “precision” and “accuracy” of the new CKD-EPI creatinine-cystatin C equation to that of 24-hour urinary creatinine clearance (CrCl), Cockcroft-Gault (CG) and previously reported creatinine- and/or cystatin C-based GFR-estimating equations. Accuracy of CKD-EPI creatinine-cystatin C equation as quantified by root mean squared error of difference scores [differences between mGFR and estimated GFR (eGFR) or between mGFR and CrCl, or between mGFR and CG equation for each subject] (RMSE=23.56) was significantly better than that of CrCl (37.69, P=0.001), CG (RMSE=36.12, P=0.002) and GFR-estimating equations based on cystatin C only. Its accuracy as quantified by percentage of eGFRs that differed by greater than 30% with respect to mGFR was significantly better compared to CrCl (P=0.024), CG (P=0.0001), 4-variable MDRD (P=0.027) and CKD-EPI creatinine 2009 (P=0.012) equations. However, for 23.61% of the subjects, GFR estimated by CKD-EPI creatinine-cystatin C equation differed from the mGFR by more than 30%.
CONCLUSIONS
The diagnostic performance of CKD-EPI creatinine-cystatin C equation (2012) in patients with cirrhosis was superior to conventional equations in clinical practice for estimating GFR. However, its diagnostic performance was substantially worse than reported in subjects without cirrhosis.
Keywords: End-stage liver disease, liver transplantation, cystatin C, glomerular filtration rate, gender disparity
INTRODUCTION
Cirrhosis was reported to be the twelfth leading cause of death in the U.S. along with chronic liver disease1. It also constitutes one of the most common indications for liver transplantation2. One of the most common and lethal complications of cirrhosis is hepatorenal syndrome characterized by an acute or subacute deterioration in renal filtration function3. Therefore, early diagnosis and treatment of kidney dysfunction in cirrhosis is of great importance.
Measuring glomerular filtration rate (GFR) by gold standard methods [e.g. renal clearance of inulin, 125I-iothalamate or 99mTc-diethylene triamine pentaacetic acid (DTPA)] in clinical practice is laborious, time intensive and may involve radiation exposure. Several serum creatinine-based equations to estimate measured 24-hour urinary creatinine clearance (CrCl) and GFR have been developed including the Cockcroft-Gault (CG)4, 4- and 6-variable Modification of Diet in Renal Disease (MDRD) Study5 and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine (2009)6 equations.
Several lines of evidence indicate that serum creatinine is not an accurate biomarker to detect kidney dysfunction in persons with cirrhosis. This inaccuracy is due to several factors: 1) synthesis of creatine, the precursor of creatinine, requires intact liver function7. Therefore, creatine production is reduced in cirrhosis7; 2) deconditioned subjects with cirrhosis with reduced skeletal muscle mass have disproportionately low serum creatinine levels relative to reductions in GFR7-9; 3) increased tubular secretion of creatinine in cirrhosis further lowers serum creatinine levels, resulting in underestimation of kidney dysfunction by CrCl7 and 4) spuriously low levels of serum creatinine are reported in subjects with hyperbilirubinemia and hemolysis10. Due to this low sensitivity of serum creatinine elevation, it is not surprising that both the CG4 and MDRD11, 12 equations have been shown to significantly overestimate GFR and thus mask renal impairment in subjects with cirrhosis13-15.
A more accurate equation than those that are creatinine-based GFR-estimating can provide a more sensitive means to detect early renal functional decline and lead to earlier and more successful treatment of this condition. In addition, it can correctly identify patients with cirrhosis and severe renal dysfunction that warrants consideration for simultaneous liver-kidney transplantation. It may also prevent iatrogenic injury (e.g. GFR-adjusted dosing can reduce drug-induced adverse events and prevent contrast-induced nephropathy). In recent years, an alternative biomarker, cystatin C has shown promise for estimating kidney function in cirrhosis. Cystatin C, a cysteine proteinase inhibitor is an ideal GFR marker as it has a constant secretion rate by all nucleated cells and due to its low molecular weight, passes freely through the glomeruli16, 17. Almost the entire amount of cystatin C filtered by the glomeruli is reabsorbed and catabolized in proximal renal tubules16, 17. Several equations based on serum cystatin C levels have been developed by Larsson et al.18 (LARSSON equation), Hoek et al.19 (HOEK equation) and Inker et al.20 [CKD-EPI cystatin C equation (2012)] to estimate GFR. In recent equations by Stevens et al.21 (STEVENS equation) and Inker et al.20 [(CKD-EPI creatinine-cystatin C equation (2012)], both serum creatinine and cystatin C, more accurately estimated kidney function compared to models that included serum creatinine or cystatin C alone in patients with a variety of disorders. Few studies have used serum cystatin C to estimate kidney function in cirrhosis 22-28. Plus, the equations developed by Stevens et al.21 and Inker et al.20 have not been validated in subjects with cirrhosis.
To address these limitations, we tested the performance of the new CKD-EPI creatinine-cystatin C equation (2012)20 to estimate GFR that directly measured by non-radiolabeled iothalamate clearance (‘gold standard’) in subjects with cirrhosis and compared its bias, precision and accuracy with that of CrCl, the CG4, creatinine-based [4-variable MDRD5, 6-variable MDRD5 and CKD-EPI creatinine (2009)6], cystatin C-based [LARSSON18, HOEK19 and CKD-EPI cystatin C (2012)20] and creatinine-cystatin C-based (STEVENS21) GFR-estimating equations.
METHODS
Study Design and Participants
Subjects with cirrhosis were recruited from the University of Maryland Medical Center and University of Maryland Faculty Physicians, Inc. between September 2010 and March 2013. To be eligible, inclusion criteria included the following: presence of cirrhosis based on either liver biopsy or clinical, laboratory and radiological data; 18 years of age or older. Exclusion criteria included the following: inability to provide an informed consent; pregnancy or breastfeeding; iothalamate or iodine allergy; hepatocellular carcinoma that was not treated; hyperthyroidism (e.g. Graves’ disease, multinodular goiter, thyroid autonomy or active treatment with radioactive iodine); inability to collect or void urine; being on dialysis or having an estimated GFR (eGFR) < 15 ml/min/1.73m2; treatment with corticosteroids within 1 week prior to enrollment; treatment with non-steroidal anti-inflammatory drugs (NSAIDs) except aspirin lower than 325 mg daily within 1 week prior to enrollment; treatment with angiotensin-converting enzyme (ACE) inhibitors within 1 week prior to enrollment; new onset of diuretics or dose change in diuretics within 1 week prior to enrollment; acute infection, exacerbation of encephalopathy, gastrointestinal bleeding and kidney injury within 1 week prior to enrollment; acute cardiovascular or cerebrovascular event within 3 weeks prior to enrollment; cognitive impairment.
The study was approved by the University of Maryland, Baltimore, Institutional Review Board (IRB). Written informed consent was obtained from all study participants.
Study Visits and Procedures
The study was conducted at the University of Maryland Medical Center, General Clinical Research Center and consisted of a screening (Visit 1) and procedure (Visit 2) visit. During the screening visit, a history and physical examination were performed. Every participant was educated on proper techniques for 24-hour urine collection and provided with a detailed patient education pamphlet. One day prior to the visit 2, each subject completed a 24-hour urine collection for CrCl and protein. One to three weeks later, the following procedures were performed at Visit 2 after an overnight fast (except diabetic patients who fasted only for 2 hours):
1) Blood and Urine Tests
Blood samples taken prior to the GFR measurement were analyzed for cystatin C, creatinine, basic metabolic panel [sodium, potassium, chloride, bicarbonate, blood urea nitrogen (BUN), creatinine and glucose], complete blood count, liver panel, coagulation tests and C-reactive protein (CRP). Urinalysis with microscopy was performed before the GFR measurement.
2) Measurement of GFR by Non-Radiolabeled Iothalamate Plasma Clearance
GFR was measured using non-radiolabeled iothalamate plasma clearance. In order to minimize the risk of dehydration, subjects consumed 250 ml of water. At time 0, each subject had 1.5 ml of iothalamate (Conray-60, Mallinckrodt, St. Louis, MO) by intravenous infusion over 2 min and 3 ml of blood samples were drawn in heparinized tubes before and after iothalamate administration at 5, 15, 30, 45, 60, 120, 240 and 360 min
Laboratory Methods
Plasma Iothalamate Concentrations
Plasma for iothalamate concentration was harvested by centrifugation, aliquots were stored at −70°C until analysis and iothalamate concentrations were determined using reversed-phase high-performance liquid chromatography (HPLC) with ultraviolet (UV) detection previously described by Dowling et al. 29.
Measurement of Serum Cystatin C and Creatinine Concentrations
Cystatin C was measured by a nephelometric technique using a Dimension Vista® System Flex® reagent cartridge (Siemens Healthcare Diagnostics, Newark, DE) was performed to measure serum cystatin C. Standardization of serum creatinine measurement technique was traceable to the isotope-dilution mass spectrometry (IDMS) materials30.
Modeling Methods
A two-compartment model was used to model plasma iothalamate concentration data against time31. An iterative least-square method was performed to fit the model to the plasma iothalamate concentration data 31 using WinNonlin® Version 5.1 (Certara L.P. (Pharsight), St. Louis, MO).
Statistical Analysis
Statistical analyses were performed using SAS software, Version 9.2 (Cary, NC)32 and Minitab statistical software (Minitab, Inc., State College, PA)33. Categorical and quantitative variables were compared between groups using Chi-square or Fisher’s exact tests and Wilcoxon two-sample test34, independent t-test or one-way analysis of variance (ANOVA) test, respectively. Independent predictors of serum creatinine and cystatin C levels were assessed using multivariable linear regression analysis.
Performance of CrCl, CG Equation and GFR-Estimating Equations in Cirrhosis
Body-surface area was calculated using formula developed by DuBois and DuBois35. mGFR, CrCl, CG and LARSSON equations were corrected for body surface area.
The performance of CKD-EPI creatinine-cystatin C equation was compared to CrCl, the CG equation, creatinine-based (4- and 6-variable MDRD and CKD-EPI creatinine), cystatin C-based (LARSSON, HOEK, CKD-EPI cystatin C) and creatinine-cystatin C-based (STEVENS) GFR-estimating equations. The principal analyses were based on “difference scores”, i.e., the differences between mGFR and eGFR [(or the differences between mGFR and CrCl, or between mGFR and CrCl estimated by CG equation) for each subject. “Bias” of the CrCl, CG equation and GFR-estimating equations was determined by calculating the mean of difference scores, “precision” was determined by calculating the standard deviation of the difference scores36. The overall “accuracy” of CrCl, CG and GFR-estimating equations for mGFR was determined by two different methods. First, we assessed the percentage of eGFRs that differed by greater than 20% (1- P20) and 30% (1- P30) with respect to mGFR as per Inker et al.20. Second, we assessed the accuracy by the root mean squared error (RMSE), the square root of the mean of the squares of the difference scores36.
Paired t-tests36 were employed to determine whether CrCl, CG and GFR-estimating equations differed significantly from CKD-EPI creatinine-cystatin C equation with respect to bias, precision and accuracy assessed by RMSE. McNemar test37 was used to determine whether CrCl, CG and GFR-estimating equations differed significantly from CKD-EPI creatinine-cystatin C equation with respect to accuracy assessed by (1- P20) and (1- P30).
ANOVA and independent t-tests were used to determine whether the performance of the CKD-EPI creatinine-cystatin C equation differed significantly among subgroups (subjects with pre-ascites, diuretic-sensitive ascites, and diuretic-refractory ascites; those with mGFR < 60 ml/min/1.73m2 and mGFR ≥ 60 ml/min/1.73m2; and those with and without diabetes) with respect to bias, precision and accuracy assessed by RMSE. Chi-square and Fisher exact tests were used to determine whether CKD-EPI creatinine-cystatin C equation differed among subgroups with respect to accuracy assessed by (1- P20) and (1- P30).
RESULTS
Characteristics of 72 Subjects with Cirrhosis
Between September 2010 and March 2013, 85 subjects were enrolled of whom 72 completed the study. Table 1 and 2 show demographic, clinical and laboratory characteristics of 72 subjects. The majority of subjects (40%) had cirrhosis resulting from hepatitis C. Type of ascites was defined according to Arroyo et al.38; 25 (35%) had no ascites (pre-ascites), 27 (37%) had diuretic-sensitive ascites and 20 (28%) had diuretic-refractory ascites. The MELD score was 6 to 9 in 22 (31%), 10 to 19 in 42 (58%) and ≥ 20 in 8 (11%) subjects. Only 4 subjects had urine protein > 0.5 g/24 hours.
Table 1. Demographic, Clinical and Laboratory Characteristics of 72 Subjects with Cirrhosis.
| Characteristics | Number | % | |
|---|---|---|---|
| Etiology of cirrhosis | |||
| Hepatitis C | 29 | 40 | |
| Hepatitis B | 3 | 4 | |
| Alcohol | 18 | 25 | |
| Nonalcoholic fatty liver disease | 14 | 19 | |
| Primary biliary cirrhosis | 2 | 3 | |
| Primary sclerosing cholangitis | 1 | 1 | |
| Autoimmune hepatitis | 3 | 4 | |
| Sarcoidosis | 1 | 1 | |
| Sickle cell disease | 1 | 1 | |
| Sex | |||
| Female | 37 | 51 | |
| Male | 35 | 49 | |
| Race | |||
| Caucasian | 54 | 75 | |
| African-American | 17 | 24 | |
| Asian | 1 | 1 | |
| Ascites | |||
| Pre-ascites | 25 | 35 | |
| Diuretic-sensitive | 27 | 37 | |
| Diuretic-refractory | 20 | 28 | |
| MELD score | |||
| 6-9 | 22 | 31 | |
| 10-19 | 42 | 58 | |
| ≥20 | 8 | 11 | |
| C-reactive protein | |||
| ≤1.0 mg/dl | 48 | 67 | |
| >1.0 mg/dl | 24 | 33 | |
| Urine protein | |||
| ≤0.5 g/24 hours | 68 | 94 | |
| >0.5 g/24 hours | 4 | 6 | |
| Measured GFR | |||
| ≥90 ml/min/1.73m2 | 27 | 38 | |
| ≥60 and <90 ml/min/1.73m2 | 24 | 33 | |
| ≥30 and <60 ml/min/1.73m2 | 20 | 28 | |
| ≥15 and <30 ml/min/1.73m2 | 1 | 1 | |
| Diabetes | |||
| No | 50 | 69 | |
| Yes | 22 | 31 | |
| Hypothyroidism | |||
| No | 66 | 92 | |
| Yes | 6 | 8 | |
|
| |||
| Median | IQR | ||
|
| |||
| Age (yr) | 54.0 | 12.0 | |
| Weight (kg) | 80.6 | 27.2 | |
| Height (m) | 1.7 | 0.1 | |
| Body-surface area (m2) | 1.9 | 0.3 | |
| MELD score | 11.0 | 6.5 | |
| Serum creatinine (mg/dl) | 0.8 | 0.4 | |
| Serum cystatin C (mg/liter) | 1.1 | 0.5 | |
| Total bilirubin (mg/dl) | 1.4 | 1.9 | |
| Prothrombin time (sec) | 16.1 | 3.5 | |
| International normalized ratio | 1.3 | 0.3 | |
| Serum albumin (g/dl) | 3.1 | 0.8 | |
| BUN (mg/dl) | 10.5 | 9.0 | |
| Serum sodium (mmol/liter) | 137.0 | 5.0 | |
IQR=Interquartile range
Table 2. Measured GFR, CrCl, Estimated CrCl (CG Equation) and Estimated GFR in 72 Subjects with Cirrhosis.
| Measured GFR* | 83.6 | 35.0 | |
|---|---|---|---|
| CrCl* | 84.1 | 42.1 | |
| Estimated CrCl* | CG Equation | 106.3 | 39.0 |
| Estimated GFR* | 4- Variable MDRD Study equation | 95.5 | 34.6 |
| 6-Variable MDRD Study equation | 89.9 | 33.0 | |
| CKD-EPI creatinine equation (2009) | 91.9 | 25.0 | |
| LARSSON equation | 68.2 | 29.9 | |
| HOEK equation | 73.2 | 26.4 | |
| CKD-EPI cystatin C equation (2012) | 72.4 | 27.7 | |
| STEVENS equation | 86.5 | 31.0 | |
| CKD-EPI creatinine-cystatin C equation (2012) | 81.7 | 26.5 |
Expressed as ml/min/1.73m2 of body surface area
Twenty-one subjects (29%) had mGFR < 60 ml/min/1.73m2. There was a significant difference in mean mGFR among subjects with pre-ascites (106.9 ml/min/1.73m2), diuretic-sensitive ascites (75.8 ml/min/1.73m2) and diuretic-refractory ascites (64.8 ml/min/1.73m2) (P<0.0001). The mean mGFR was significantly lower in subjects with ascites compared to those with pre-ascites (71.1 vs. 106.9 ml/min/1.73m2, P=0.0001).
The median serum creatinine level was significantly lower in women than men (0.7 vs. 0.9 mg/dl, P=0.015), but there was no significant difference in median cystatin C levels (1.0 vs. 1.1 mg/L, P=0.545) and mGFR (74.5 vs. 77.9 ml/min/1.73 m2, P=0.973) between women and men. Controlling for age, female sex was an independent predictor of serum creatinine level (β=−0.205, P=0.007), but not of serum cystatin C level (β=−0.055, P=0.526). In a multiple regression analysis, only mGFR (β=−0.005), P<0.0001) was an independent predictor of serum cystatin C after controlling for age, presence of hepatitis C, ascites, diabetes, hypothyroidism. On the other hand, female sex (β=−0.175, P=0.007) in addition to mGFR (β=−0.004, P<0.0001) was an independent predictor of serum creatinine.
Median cystatin C levels did not differ between subjects with CRP levels ≤ 1.0 mg/dl and those with > 1.0 mg/dl (1.0 vs. 1.1 mg/L, P=0.286); between subjects with and without hypothyroidism (1.0 vs. 1.1 mg/L, P=0.484); between subjects with and without diabetes (1.1 vs. 1.1 mg/L, P=0.761); between subjects with and without hepatitis C cirrhosis (1.1 vs. 1.0 mg/L, P=0.370) and between African-Americans and non-African-Americans (1.1 vs. 1.0 mg/L, P=0.782).
Performance of CKD-EPI Creatinine-Cystatin C Equation (2012)
Table 3 shows the performance (bias, precision and accuracy) of CrCl, CG and GFR-estimating equations compared to that of the CKD-EPI creatinine-cystatin C equation in 72 study subjects.
Table 3. Bias, Precision and Accuracy of CrCl, CG and GFR-Estimating Equations in 72 Subjects with Cirrhosis.
| CrCl and Creatinine-Based Equations | Cystatin C-Based Equations | Combined Creatinine- Cystatin C-Based Equations |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CrCl | CG | 4-Variable MDRD |
6-Variable MDRD |
CKD-EPI Creatinine (2009) |
LARSSON | HOEK | CKD-EPI Cystatin C (2012) |
STEVENS | CKD-EPI Creatinine- Cystatin C (2012) |
|
| Bias | −1.06 | −22.78 | −11.94 | −6.39 | −8.33 | 15.30 | 10.31 | 11.17 | −2.90 | 1.87 |
| P value† | 0.447 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Precision* | 37.67 | 28.03 | 25.77 | 24.07 | 24.68 | 30.44 | 28.06 | 26.56 | 23.44 | 23.49 |
| P Value† | 0.001 | 0.119 | 0.320 | 0.753 | 0.357 | 0.016 | 0.045 | 0.004 | 0.961 | |
| Accuracy (1-P30)* | 41.67 | 54.17 | 38.89 | 34.72 | 40.28 | 37.50 | 27.78 | 30.56 | 27.78 | 23.61 |
| P Value† | 0.024 | 0.0001 | 0.027 | 0.077 | 0.012 | 0.099 | 0.629 | 0.359 | 0.375 | |
| Accuracy (1-P20)* | 55.56 | 69.44 | 51.39 | 47.22 | 55.56 | 58.33 | 43.06 | 47.22 | 48.61 | 44.44 |
| P Value† | 0.268 | 0.008 | 0.405 | 0.824 | 0.152 | 0.121 | 1.000 | 0.824 | 0.581 | |
| Accuracy (RMSE)* | 37.69 | 36.12 | 28.40 | 24.90 | 26.05 | 34.07 | 29.89 | 28.81 | 23.62 | 23.56 |
| P Value† | 0.001 | 0.002 | 0.139 | 0.563 | 0.164 | 0.0004 | 0.005 | 0.0003 | 0.970 | |
mGFR=GFR measured by non-radiolabeled iothalamate plasma clearance (gold standard)
eGFR=Estimated GFR
Difference score (DS)=mGFR-eGFR (or the differences between mGFR and CrCl, or between mGFR and CrCl estimated by CG equation) for each subject
Bias=Mean DS
Precision=Standard deviation of DS
Accuracy=Percentage of eGFRs that differed by greater than 30% (1-P30) or 20% (1-P20) of mGFRs or root mean square error (RMSE)
Lower values for precision and accuracy [(1-P30), (1-P20) and RMSE] indicate higher precision and accuracy for CrCl, CG and GFR-estimating equations
P values compare the performance of CKD-EPI creatinine-cystatin C (2012) to CrCl, CG and GFR-estimating equations. Note, the P-val ues for bias reflect difference in the location of bias, not the magnitude. Thus, for example, there is a significant difference in the bias between the STEVENS and CKD-EPI creatinine-cystatin C equations that reflects differences in their location (negative vs. positive), but not their magnitude.
Bias
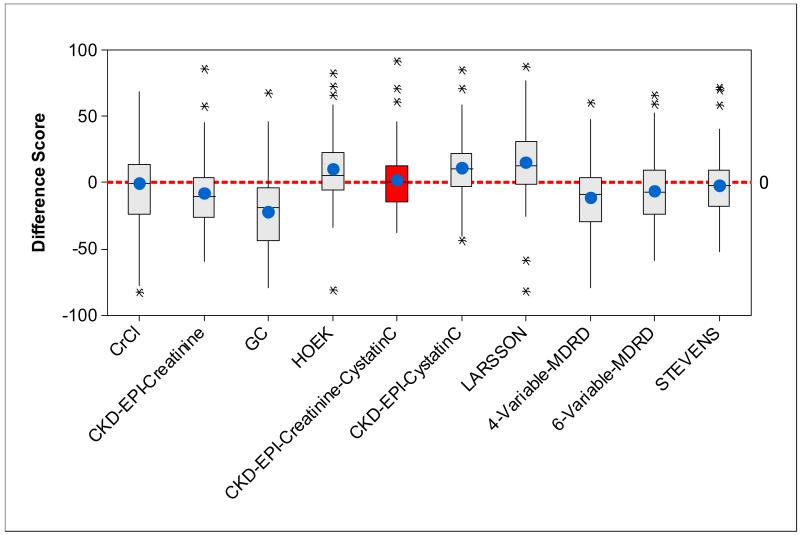
Bias was estimated by the mean of difference scores (mGFR-eGFR, mGFR-CrCl or mGFR-CrCl estimated by CG equation). The CKD-EPI creatinine-cystatin C equation had lower bias than any other serum creatinine and/or cystatin C-based GFR-estimating equations (Table 3 and Figure 1). The direct measure of CrCl had the smallest bias but it was not significantly different from the CKD-EPI creatinine-cystatin C equation. Also, while the bias of the STEVENS equation was significantly different in value from the CKD-EPI creatinine-cystatin C equation, their magnitudes were similar, but in opposite direction. In general, creatinine-based equations overestimated mGFR, cystatin C-based equations underestimated mGFR.
Figure 1.
Side-by-side boxplots of the difference scores [(DS)=mGFR-eGFR (or the differences between mGFR and CrCl, or between mGFR and CrCl estimated by CG equation) for each subject] for CrCl, CG and GFR-estimating equations; circles in each box show mean difference score (bias); black bars in each box show median difference score.
Precision
Precision of CrCl, CG and GFR-estimating equations was estimated by the standard deviation of difference scores. Lower values indicate greater precision. As shown in Table 3, the combined creatinine-cystatin C-based equations including CKD-EPI creatinine-cystatin C and STEVENS equations had the highest precision. There was no significant difference in precision between the CKD-EPI creatinine-cystatin C and CG, 4- and 6-variable MDRD, CKD-EPI creatinine and STEVENS equations. Its superior precision is reflected in the relatively smaller interquartile range (smaller box-size) for CKD-EPI creatinine-cystatin C equation in Figure 1.
Accuracy
We evaluated accuracy by three statistical methods: 1- P30 (the percentage of CrCl, CrCl estimated by the CG equation and eGFRs that differed by greater than 30% with respect to mGFR), 1- P20 (the percentage of CrCl, CrCl estimated by CG equation and eGFRs that differed by greater than 20% with respect to mGFR) and RMSE. Lower values of these parameters indicated higher accuracy (Table 3). In 23.61% of subjects with cirrhosis, the CKD-EPI creatinine-cystatin C estimate differed from mGFR by more than 30% (1- P30), and the other equations performed even more poorly. The accuracy of CKD-EPI creatinine-cystatin C equation measured by (1- P30) was significantly better than CrCl and conventional equations including CG, 4-variable MDRD and CKD-EPI creatinine equations. In 44.44% of the subjects, the CKD-EPI creatinine-cystatin C estimate differed from mGFR by more than 20% (1- P20). The accuracy of the CKD-EPI creatinine-cystatin C equation measured by (1- P20) was only significantly better than the CG equation.
When we evaluated the accuracy by RMSE, CKD-EPI creatinine-cystatin C equation had the highest accuracy (lowest RMSE) that was significantly superior to the accuracy of CrCl, CG, LARSSON, HOEK and CKD-EPI cystatin C equations (Table 3).
We examined the performance of the CKD-EPI creatinine-cystatin C equation in subgroups defined by ascites (Table 4). For most measures, the equation performed best in the group with diuretic-sensitive ascites though none of the differences reached statistical significance.
Table 4. Bias, Precision and Accuracy of CKD-EPI Creatinine-Cystatin C Equation (2012) in Subjects with Cirrhosis Stratified by Type of Ascites.
| Pre-Ascites | Diuretic-Sensitive Ascites |
Diuretic-Refractory Ascites |
P Value† | |
|---|---|---|---|---|
| Bias | 10.67 | −1.11 | −5.09 | 0.058 |
| Precision* | 28.28 | 15.36 | 22.59 | 0.154 |
| Accuracy (1-P30)* | 24.00 | 11.11 | 40.00 | 0.075 |
| Accuracy (1-P20)* | 36.00 | 44.44 | 55.00 | 0.444 |
| Accuracy (RMSE)* | 30.23 | 15.41 | 23.16 | 0.141 |
mGFR=GFR measured by non-radiolabeled iothalamate plasma clearance (gold standard)
eGFR=Estimated GFR
Difference score (DS)=mGFR-eGFR (or the differences between mGFR and CrCl, or between mGFR and CrCl estimated by CG equation) for each subject
Bias=Mean DS
Precision=Standard deviation of DS
Accuracy=Percentage of eGFRs that differed by greater than 30% (1-P30) or 20% (1-P20) of mGFRs or root mean square error (RMSE)
Lower values for precision and accuracy [(1-P30), (1-P20) and RMSE] indicate higher precision and accuracy for CrCl, CG and GFR-estimating equations
P values compare the performance of CKD-EPI creatinine-cystatin C (2012) among subjects with pre-ascites, diuretic-sensitive and -refractory ascites. Note the low P-value for bias does not reflect a difference in magnitude of bias, but rather reflects (in this case) a difference in the direction of bias.
We also examined the performance of the CKD-EPI creatinine-cystatin C equation in subgroups defined by mGFR level (Table 5). We found that precision and accuracy (measured by RMSE) were significantly superior in subjects with mGFR < 60 ml/min/1.73m2 compared to those with mGFR ≥ 60 ml/min/1.73m2.
Table 5. Bias, Precision and Accuracy of CKD-EPI Creatinine-Cystatin C Equation (2012) in Subjects with Cirrhosis Stratified by Level of mGFR.
| mGFR ≥ 60 ml/min/1.73m2 |
mGFR < 60 ml/min/1.73m2 |
P Value† | |
|---|---|---|---|
| Bias | 4.75 | −5.11 | 0.035 |
| Precision* | 26.30 | 11.96 | 0.014 |
| Accuracy (1-P30)* | 21.57 | 28.57 | 0.525 |
| Accuracy (1-P20)* | 49.02 | 33.33 | 0.223 |
| Accuracy (RMSE)* | 26.72 | 13.01 | 0.011 |
mGFR=GFR measured by non-radiolabeled iothalamate plasma clearance (gold standard)
eGFR=Estimated GFR
Difference score (DS)=mGFR-eGFR (or the differences between mGFR and CrCl, or between mGFR and CrCl estimated by CG equation) for each subject
Bias=Mean DS
Precision=Standard deviation of DS
Accuracy=Percentage of eGFRs that differed by greater than 30% (1-P30) or 20% (1-P20) of mGFRs or root mean square error (RMSE)
Lower values for precision and accuracy [(1-P30), (1-P20) and RMSE] indicate higher precision and accuracy for CrCl, CG and GFR-estimating equations
P values compare the performance of CKD-EPI creatinine-cystatin C (2012) among subjects with mGFR ≥ 60 ml/min/1.73 m2 and mGFR < 60 ml/min/1.73m2. Note the low P-value for bias does not reflect a difference in magnitude of bias, but rather reflects (in this case) a difference in the direction of bias.
There were no significant differences in bias (7.25 vs. −0.49, P=0.245), precision (26.45 vs. 21.64, P=0.411) and accuracy by (1- P30) (22.73 vs. 24.00, P=1.000), (1- P20) (45.45 vs. 44.00, P=0.910) and by RMSE (27.43 vs. 21.65, P=0.386) of the CKD-EPI creatinine-cystatin C equation between subjects with and without history of diabetes, respectively.
Performance Comparison of CKD-EPI Creatinine-Cystatin C Equation (2012) and 6-Variable MDRD Study Equation In Subjects with Cirrhosis Stratified by Type of Ascites
We compared the performance of CKD-EPI creatinine-cystatin C equation to 6-variable MDRD equation in subgroups defined by ascites (Table 6). Among subjects with diuretic-sensitive ascites, CKD-EPI creatinine-cystatin C equation had significantly lower bias (−1.11 vs. −8.60, P=0.019), superior precision (15.36 vs. 19.24, P=0.037) and accuracy (measured by RMSE) (15.41 vs. 21.08, P=0.030) compared to 6-variable MDRD equation. There was no significant difference in overall accuracy between CKD-EPI creatinine-cystatin C and 6-variable MDRD equations among subjects with pre-ascites and diuretic-refractory ascites.
Table 6. Bias, Precision and Accuracy of 6-Variable MDRD and CKD-EPI Creatinine-Cystatin C Equations in 72 Subjects with Cirrhosis Stratified by Type of Ascites.
| 6-Variable MDRD | CKD-EPI Creatinine-Cystatin C (2012) |
||
|---|---|---|---|
| Pre-Ascites | Bias | −5.14 | 10.67 |
| P value† | <0.0001 | ||
| Precision* | 30.06 | 28.28 | |
| P Value† | 0.578 | ||
| Accuracy (1-P30)* | 36.00 | 24.00 | |
| P Value† | 0.453 | ||
| Accuracy (1-P20)* | 48.00 | 36.00 | |
| P Value† | 0.453 | ||
| Accuracy (RMSE)* | 30.49 | 30.23 | |
| P Value† | 0.958 | ||
|
Diuretic-Sensitive
Ascites |
Bias | −8.60 | −1.11 |
| P value† | 0.019 | ||
| Precision* | 19.24 | 15.36 | |
| P Value† | 0.037 | ||
| Accuracy (1-P30)* | 29.63 | 11.11 | |
| P Value† | 0.125 | ||
| Accuracy (1-P20)* | 48.15 | 44.44 | |
| P Value* | 1.000 | ||
| Accuracy (RMSE)* | 21.08 | 15.41 | |
| P Value† | 0.030 | ||
|
Diuretic-
Refractory Ascites |
Bias | −4.97 | −5.09 |
| P value† | 0.968 | ||
| Precision* | 21.11 | 22.59 | |
| P Value† | 0.348 | ||
| Accuracy (I-P30)* | 40.00 | 40.00 | |
| P Value† | 1.000 | ||
| Accuracy (I-P20)* | 45.00 | 55.00 | |
| P Value† | 0.688 | ||
| Accuracy (RMSE)* | 21.69 | 23.16 | |
| P Value† | 0.449 | ||
mGFR=GFR measured by non-radiolabeled iothalamate plasma clearance (gold standard)
eGFR=Estimated GFR
Difference score (DS)=mGFR-eGFR for each subject
Bias=Mean DS
Precision=Standard deviation of DS
Accuracy=Percentage of eGFRs that differed by greater than 30% (1-P30) or 20% (1-P20) of mGFRs or root mean square error (RMSE)
Lower values for precision and accuracy [(1-P30), (1-P20) and RMSE] indicate higher precision and accuracy for 6-Variable MDRD and CKD-EPI creatinine-cystatin C equations
P values compare the performance of CKD-EPI creatinine-cystatin C (2012) to 6-variable MDRD equation. Note, the P-values for bias reflect difference in the location of bias, not the magnitude.
Performance Comparison of CKD-EPI Creatinine-Cystatin C Equation (2012) and 6-Variable MDRD Study Equation In Subjects with Cirrhosis Stratified by Level of mGFR
We also compared the performance of CKD-EPI creatinine-cystatin C equation to 6-variable MDRD equation in subgroups defined by mGFR level (Table 7). Among subjects with mGFR < 60 ml/min/1.73m2, CKD-EPI creatinine-cystatin C equation had significantly lower bias compared to 6-variable MDRD equation (−5.11 vs. −10.04, P=0.028). There was no significant difference in overall accuracy between CKD-EPI creatinine-cystatin C and 6-variable MDRD equations among subjects with mGFR < 60 ml/min/1.73m2 and ≥ 60 ml/min/1.73m2.
Table 7. Bias, Precision and Accuracy of 6-Variable MDRD and CKD-EPI Creatinine-Cystatin C Equations in 72 Subjects with Cirrhosis Stratified by Level of mGFR.
| 6-Variable MDRD | CKD-EPI Creatinine-Cystatin C (2012) |
||
|---|---|---|---|
|
mGFR ≥ 60
ml/min/1.73m2 |
Bias | −4.89 | 4.75 |
| P value† | 0.0003 | ||
| Precision* | 27.08 | 26.30 | |
| P Value† | 0.721 | ||
| Accuracy (I-P30)* | 33.33 | 21.57 | |
| P Value† | 0.109 | ||
| Accuracy (I-P20)* | 47.06 | 49.02 | |
| P Value† | 1.000 | ||
| Accuracy (RMSE)* | 27.52 | 26.72 | |
| P Value† | 0.781 | ||
|
mGFR < 60
ml/min/1.73m2 |
Bias | −10.04 | −5.11 |
| P value† | 0.028 | ||
| Precision* | 13.65 | 11.96 | |
| P Value† | 0.473 | ||
| Accuracy (I-P30)* | 38.10 | 28.57 | |
| P Value† | 0.688 | ||
| Accuracy (I-P20)* | 47.62 | 33.33 | |
| P Value† | 0.453 | ||
| Accuracy (RMSE)* | 16.95 | 13.01 | |
| P Value† | 0.213 | ||
mGFR=GFR measured by non-radiolabeled iothalamate plasma clearance (gold standard)
eGFR=Estimated GFR
Difference score (DS)=mGFR-eGFR for each subject
Bias=Mean DS
Precision=Standard deviation of DS
Accuracy=Percentage of eGFRs that differed by greater than 30% (1-P30) or 20% (1-P20) of mGFRs or root mean square error (RMSE)
Lower values for precision and accuracy [(1-P30), (1-P20) and RMSE] indicate higher precision and accuracy for 6-variable MDRD and CKD-EPI creatinine-cystatin C equations
P values compare the performance of CKD-EPI creatinine-cystatin C (2012) to 6-variable MDRD equation. Note, the P-values for bias reflect difference in the location of bias, not the magnitude.
DISCUSSION
In this study of subjects with cirrhosis, we assessed the performance of the new CKD-EPI creatinine-cystatin C equation to estimate GFR measured by “gold standard’ non-radiolabeled iothalamate clearance, and compared to CrCl, the CG equation, creatinine-based (4- and 6-variable MDRD and CKD-EPI creatinine), cystatin C-based (LARSSON, HOEK and CKD-EPI cystatin C) and creatinine-cystatin C-based (STEVENS) GFR-estimating equations. The accuracy of CKD-EPI creatinine-cystatin C equation was significantly better than that of CrCl, the CG, 4-variable MDRD, CKD-EPI creatinine, LARSSON, HOEK and CKD-EPI cystatin C equations. However, its accuracy in estimating GFR in subjects with cirrhosis [(1-P30)=23.6, (1- P20)=44.4 and RMSE=23.56] (Table 3) was markedly lower than that reported among external validation cohorts of participants without cirrhosis [(1-P30)=8.5, (1-P20)=22.8 and RMSE=0.162 to 0.200]20 [(higher values for (1-P30), (1-P20) and RMSE indicate lower accuracy)]. In subgroup analyses, we did not find significant differences in the performance of CKD-EPI creatinine-cystatin C equation among subjects with pre-ascites, diuretic-sensitive and diuretic-refractory ascites. However, the CKD-EPI creatinine-cystatin C equation performed significantly better among subjects whose mGFR was < 60 ml/min/1.73m2 compared to those whose mGFR ≥ 60 ml/min/1.73m2.
It should be emphasized that no currently available creatinine- or cystatin C-based estimating equations were derived from subjects with cirrhosis. Nonetheless, the CG and MDRD equations are the most commonly used equations to estimate CrCl and GFR in clinical practice4, 11, 12. The inferior accuracy of CrCl and creatinine-based GFR-estimating equations in subjects with cirrhosis is consistent with results of several prior studies13-15. With respect to the accuracy of cystatin C-based GFR-estimating equations in cirrhosis, Poge et al.28 reported poor agreement between GFR measured by inulin clearance and cystatin-C based equations developed by Hoek et al.19 and Larsson et al.18. Stevens et al.21 showed that an equation based on both cystatin C and creatinine, age, sex and race provided a more accurate estimate of mGFR in subjects with chronic kidney disease compared to an equation based solely on cystatin C. To our knowledge, no study has evaluated the performance of STEVENS equation in subjects with cirrhosis. In the present study, the CKD-EPI creatinine-cystatin C equation was similar in accuracy to the STEVENS equation that is also a combined creatinine-cystatin C based GFR-estimating equation.
There was no difference in accuracy of the CKD-EPI creatinine-cystatin C and 6-variable MDRD equations among subjects with cirrhosis, although the CKD-EPI creatinine-cystatin C equation performed better on all measures (Table 3). In addition, among subjects with diuretic-sensitive ascites, the accuracy of CKD-EPI creatinine-cystatin C equation was significantly superior to that of 6-variable MDRD equation (Table 6). Furthermore, among subjects with mGFR < 60 ml/min/1.73m2, the bias of CKD-EPI creatinine-cystatin C equation was significantly lower compared to that of 6-variable MDRD equation (Table 7). The 6-variable MDRD equation contains serum albumin in addition to serum creatinine, BUN, sex, age and race. Therefore, GFR estimates obtained using the 6-variable MDRD equation might have been influenced by albumin infusions among patients with cirrhosis and ascites. In addition to serum albumin, BUN can also be influenced by several factors5 and therefore the applicability of the 6-variable MDRD equation in cirrhosis is questionable.
Our results also indicate that cystatin C is a gender-neutral GFR marker in cirrhosis; while median serum creatinine level was significantly lower in women than men, there was no significant difference in median cystatin C levels nor mGFR between women and men. Even after accounting for mGFR, age, hepatitis C, diabetes and hypothyroidism; women with cirrhosis had lower serum creatinine levels compared to men with cirrhosis. Controlling for the same factors, sex was not significantly associated with cystatin C levels. These results suggest that - among cirrhotic subjects- sex differences in creatinine concentration are not explained by renal filtration but by intrinsic differences in endogenous creatinine production. We previously showed that the use of serum creatinine in calculating MELD scores resulted in gender disparity on the liver transplant waiting list; women with end-stage liver disease had higher mortality compared to men9. Based on our results, it is possible that substitution of serum cystatin C for serum creatinine in the MELD equation would eliminate this gender disparity on the liver transplant waiting list.
We found moderately lower mGFR in subjects with ascites compared to those with pre-ascites; the lowest mean mGFR having been observed among subjects with diuretic-refractory ascites. The mean mGFR was significantly lower in subjects with diuretic-sensitive and -refractory ascites compared to those with pre-ascites (71.1 vs. 106.9 ml/min/1.73m2, P=0.0001). Lower mGFR among subjects with ascites might be due to chronically reduced renal blood flow even among the clinically stable patients in this study. The lack of significant proteinuria in 46 of 47 subjects with ascites is suggestive of lower renal blood flow as the cause of lower mGFR rather than an intrinsic renal disease. Leslie et al.39 showed that subjects with refractory ascites had reduced GFR. Other studies showed that renal resistive indices were significantly elevated in subjects with cirrhosis and ascites compared to those without ascites40, 41. Increased renal resistive indices are suggestive of reduced renal blood flow in subjects with cirrhosis40, 41. Our results are in line with these studies. This may predispose these patients to hepatorenal syndrome following diverse kidney insults such excessive diuresis, sepsis, frequent paracentesis, NSAIDs and ACE inhibitors.
This study has several strengths. Carefully structured inclusion and exclusion criteria enabled us to enroll a homogenous population of subjects with cirrhosis. Subjects with exposures that might cause acute changes in renal function (recent use of ACE inhibitors42 and NSAIDs42) or influence cystatin C levels independently of eGFR (e.g. steroid use43 and thyrotoxicosis43) were excluded. GFR was measured by a well-accepted gold standard, non-radiolabeled iothalamate plasma clearance 44. IDMS traceable assay that was recommended by the National Kidney Disease Education Program was used to standardize creatinine measurement30. Plus, serum creatinine was measured with creatinase-enzymatic methodology that is less susceptible to interference by non-creatinine substances. Therefore, spuriously low serum or urine creatinine levels in subjects with cirrhosis with hyperbilirubinemia or hemolysis were avoided. Serum bilirubin levels greater than 9 mg/dl can potentially cause a negative bias exceeding 0.1 mg/dl or 10% of total with enzymatic creatinine methods45; however only 3 subjects (4%) had bilirubin levels in excess of 9 mg/dl. Furthermore, we measured serum cystatin C by advanced nephelometric methods in a Clinical Laboratory Improvement Amendments (CLIA)-certified central laboratory. This method has negligible interference with bilirubin levels up to 60 mg/dl46. Compared to using serum creatinine, this feature can potentially make cystatin C a more accurate GFR marker in subjects with cirrhosis with elevated bilirubin levels.
Study has some limitations. All participants were from a single center, and the sample size was smaller than some other validation studies of estimating equations. Nonetheless, our sample size (72 subjects) is as large as prior studies of renal function in subjects with cirrhosis, a population with unique challenges to recruitment and retention in clinical trials. The study population included Caucasian and African American subjects. Therefore, we were unable to assess the accuracy of the CKD-EPI creatinine-cystatin C equation (2012) in other races or ethnic groups. Importantly, 89% of our study population had low MELD score (MELD < 20) and a fairly well preserved renal function (71% with mGFR ≥ 60 ml/min/1.73m2 and only 1 subject with mGFR < 30 ml/min/1.73m2). Therefore, these results need to be interpreted with caution in subjects with more severe hepatic and renal dysfunction.
In conclusion, the overall diagnostic performance of the CKD-EPI creatinine-cystatin C equation in cirrhosis was superior to that of conventional, commonly-used methods including CrCl, CG and MDRD equations. However, the diagnostic performance of this equation was markedly lower than that reported in validation populations without cirrhosis. A more accurate cystatin C-based or combined creatinine-cystatin C-based GFR-estimating equation can be developed if the equation is specifically derived from subjects with cirrhosis. Until a more accurate GFR-estimating equation is developed to estimate kidney function in cirrhosis, we recommend the use of the CKD-EPI creatinine-cystatin C equation in place of conventional equations for early diagnosis and treatment of kidney dysfunction and prevention of drug-induced adverse events in subjects with cirrhosis.
ACKNOWLEDGEMENT
The authors thank Charles Howell, M.D. (Professor of Medicine, Division of Gastroenterology and Hepatology, University of Maryland School of Medicine) and Jean-Pierre Raufman, M.D. (Professor of Medicine, Department of Medicine, Division of Gastroenterology and Hepatology, University of Maryland School of Medicine) for editing our manuscript and for their valuable input; Heather L. Rebuck, MT (ASCP), CLS (NCA) and Sharon Y. Huang, MT for analysis of blood samples, Myra T. Collins, B.A. (study coordinator) and the University of Maryland General Clinical Research Center Staff.
FUNDING
“The project described was supported by Grant Number 5 K23 DK089008-03 from the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (to Ayse L. Mindikoglu, M.D., M.P.H.) and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the NIH”. This work was supported by the University of Maryland Clinical Translational Science Institute and the University of Maryland General Clinical Research Center.
REFERENCES
- 1. [Accessed on May 18, 2013];Deaths: Preliminary Data for 2011. Available at http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_06.pdf.
- 2. [Accessed on May 18, 2013];Scientific Registry of Transplant Recipients. http://www.srtr.org/annual_Reports/2011/904a_rec-dgn_li.aspx.
- 3.Alessandria C, Ozdogan O, Guevara M, et al. MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver transplantation. Hepatology. 2005;41(6):1282–9. doi: 10.1002/hep.20687. [DOI] [PubMed] [Google Scholar]
- 4.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherman DS, Fish DN, Teitelbaum I. Assessing renal function in cirrhotic patients: problems and pitfalls. Am J Kidney Dis. 2003;41(2):269–78. doi: 10.1053/ajkd.2003.50035. [DOI] [PubMed] [Google Scholar]
- 8.Cocchetto DM, Tschanz C, Bjornsson TD. Decreased rate of creatinine production in patients with hepatic disease: implications for estimation of creatinine clearance. Ther Drug Monit. 1983;5(2):161–8. doi: 10.1097/00007691-198306000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Mindikoglu AL, Regev A, Seliger SL, Magder LS. Gender disparity in liver transplant waiting-list mortality: the importance of kidney function. Liver Transpl. 2010;16(10):1147–57. doi: 10.1002/lt.22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daugherty NA, Hammond KB, Osberg IM. Bilirubin interference with the kinetic Jaffe method for serum creatinine. Clinical chemistry. 1978;24(2):392–3. [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clinical chemistry. 2007;53(4):766–72. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 13.Gonwa TA, Jennings L, Mai ML, Stark PC, Levey AS, Klintmalm GB. Estimation of glomerular filtration rates before and after orthotopic liver transplantation: evaluation of current equations. Liver Transpl. 2004;10(2):301–9. doi: 10.1002/lt.20017. [DOI] [PubMed] [Google Scholar]
- 14.Skluzacek PA, Szewc RG, Nolan CR, 3rd, Riley DJ, Lee S, Pergola PE. Prediction of GFR in liver transplant candidates. Am J Kidney Dis. 2003;42(6) doi: 10.1053/j.ajkd.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Cholongitas E, Marelli L, Kerry A, et al. Female liver transplant recipients with the same GFR as male recipients have lower MELD scores--a systematic bias. Am J Transplant. 2007;7(3):685–92. doi: 10.1111/j.1600-6143.2007.01666.x. [DOI] [PubMed] [Google Scholar]
- 16.Filler G, Bokenkamp A, Hofmann W, Le Bricon T, Martinez-Bru C, Grubb A. Cystatin C as a marker of GFR--history, indications, and future research. Clin Biochem. 2005;38(1):1–8. doi: 10.1016/j.clinbiochem.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 17.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354(23):2473–83. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 18.Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64(1):25–30. doi: 10.1080/00365510410003723. [DOI] [PubMed] [Google Scholar]
- 19.Hoek FJ, Kemperman FA, Krediet RT. A comparison between cystatin C, plasma creatinine and the Cockcroft and Gault formula for the estimation of glomerular filtration rate. Nephrol Dial Transplant. 2003;18(10):2024–31. doi: 10.1093/ndt/gfg349. [DOI] [PubMed] [Google Scholar]
- 20.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–9. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51(3) doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demirtas S, Bozbas A, Akbay A, Yavuz Y, Karaca L. Diagnostic value of serum cystatin C for evaluation of hepatorenal syndrome. Clin Chim Acta. 2001;311(2):81–9. doi: 10.1016/s0009-8981(01)00546-0. [DOI] [PubMed] [Google Scholar]
- 23.Orlando R, Mussap M, Plebani M, et al. Diagnostic value of plasma cystatin C as a glomerular filtration marker in decompensated liver cirrhosis. Clinical chemistry. 2002;48(6 Pt 1):850–8. [PubMed] [Google Scholar]
- 24.Ustundag Y, Samsar U, Acikgoz S, et al. Analysis of glomerular filtration rate, serum cystatin C levels, and renal resistive index values in cirrhosis patients. Clin Chem Lab Med. 2007;45(7):890–4. doi: 10.1515/CCLM.2007.130. [DOI] [PubMed] [Google Scholar]
- 25.Gerbes AL, Gulberg V, Bilzer M, Vogeser M. Evaluation of serum cystatin C concentration as a marker of renal function in patients with cirrhosis of the liver. Gut. 2002;50(1):106–10. doi: 10.1136/gut.50.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woitas RP, Stoffel-Wagner B, Flommersfeld S, et al. Correlation of serum concentrations of cystatin C and creatinine to inulin clearance in liver cirrhosis. Clinical chemistry. 2000;46(5):712–5. [PubMed] [Google Scholar]
- 27.Randers E, Ivarsen P, Erlandsen EJ, et al. Plasma cystatin C as a marker of renal function in patients with liver cirrhosis. Scand J Clin Lab Invest. 2002;62(2):129–34. doi: 10.1080/003655102753611753. [DOI] [PubMed] [Google Scholar]
- 28.Poge U, Gerhardt T, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP. Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant. 2006;21(3):660–4. doi: 10.1093/ndt/gfi305. [DOI] [PubMed] [Google Scholar]
- 29.Dowling TC, Frye RF, Zemaitis MA. Simultaneous determination of p-aminohippuric acid, acetyl-p-aminohippuric acid and iothalamate in human plasma and urine by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1998;716(1-2):305–13. doi: 10.1016/s0378-4347(98)00294-1. [DOI] [PubMed] [Google Scholar]
- 30.Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clinical chemistry. 2006;52(1):5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 31.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. Marcel Dekker Inc; New York, NY: 1982. [Google Scholar]
- 32.SAS software . The data analysis for this paper was generated using SAS software, Version 9.2 of the SAS System for Windows. SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc.; Cary, NC, USA: Http://www.Sas.Com/ Copyright © 2002-2008. [Google Scholar]
- 33.Minitab 15 Statistical Software . Computer software. Minitab, Inc; State College, PA: 2007. www.minitab.com. [Google Scholar]
- 34.Wilcoxon F. Individual Comparisons by Ranking Methods. Biometrics Bulletin. 1945;1:80–83. [Google Scholar]
- 35.DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Int Med. 1916;17:863–71. [Google Scholar]
- 36.Kasitanon N, Fine DM, Haas M, Magder LS, Petri M. Estimating renal function in lupus nephritis: comparison of the Modification of Diet in Renal Disease and Cockcroft Gault equations. Lupus. 2007;16(11):887–95. doi: 10.1177/0961203307084167. [DOI] [PubMed] [Google Scholar]
- 37.McNemar Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika. 1947;12(2):153–7. doi: 10.1007/BF02295996. [DOI] [PubMed] [Google Scholar]
- 38.Arroyo V, Gines P, Gerbes AL, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23(1):164–76. doi: 10.1002/hep.510230122. [DOI] [PubMed] [Google Scholar]
- 39.Leslie SH, Johnston B, Ralli EP. Renal function as a factor in fluid retention in patients with cirrhosis of the liver. J Clin Invest. 1951;30(11):1200–7. doi: 10.1172/JCI102539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Celebi H, Donder E, Celiker H. Renal blood flow detection with Doppler ultrasonography in patients with hepatic cirrhosis. Arch Intern Med. 1997;157(5):564–6. doi: 10.1001/archinte.157.5.564. [DOI] [PubMed] [Google Scholar]
- 41.Rivolta R, Maggi A, Cazzaniga M, et al. Reduction of renal cortical blood flow assessed by Doppler in cirrhotic patients with refractory ascites. Hepatology. 1998;28(5):1235–40. doi: 10.1002/hep.510280510. [DOI] [PubMed] [Google Scholar]
- 42.Koeppen BM, Stanton BA. Glomerular Filtration and Renal Blood Flow. In: Koeppen BM, Stanton BA, editors. Renal Physiology. 4th ed. Mosby; Philadelphia: 2007. pp. 31–46. [Google Scholar]
- 43.Delanaye P, Cavalier E, Depas G, Chapelle JP, Krzesinski JM. New data on the intraindividual variation of cystatin C. Nephron Clin Pract. 2008;108(4):c246–8. doi: 10.1159/000124327. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal R, Bills JE, Yigazu PM, et al. Assessment of iothalamate plasma clearance: duration of study affects quality of GFR. Clin J Am Soc Nephrol. 2009;4(1):77–85. doi: 10.2215/CJN.03720708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenberg N, Roberts WL, Bachmann LM, et al. Specificity characteristics of 7 commercial creatinine measurement procedures by enzymatic and Jaffe method principles. Clin Chem. 2012;58(2):391–401. doi: 10.1373/clinchem.2011.172288. [DOI] [PubMed] [Google Scholar]
- 46.Package Insert/Instructions for Use, CYSC, REF K7040, Issue date 2011-03, SIEMENS, Dimension Vista® System Flex® reagent cartridge. Siemens Healthcare Diagnostics Inc; Newark, DE: [Google Scholar]