Abstract
Airway lining fluid contains relatively high concentrations of nitrite and arterial blood levels of nitrite are higher than venous levels, suggesting the lung epithelium may represent an important source of nitrite in vivo. To investigate whether lung epithelial cells possess the ability to convert NO to nitrite by oxidation, and the effect of oxygen reactions on nitrite formation, the NO donor DETA NONOate was incubated with or without A549 cells or primary human bronchial epithelial (HBE) cells for 24 hrs under normoxic (21% O2) and hypoxic (1% O2) conditions. Nitrite production was significantly increased under all conditions in the presence of A549 or HBE cells, suggesting that both A549 and HBE cells have the capacity to oxidize NO to nitrite even under low oxygen conditions. The addition of oxy-hemoglobin (oxy-Hb) to the A549 cell media decreased the production of nitrite, consistent with NO scavenging limiting nitrite formation. Heat-denatured A549 cells produced much lower nitrite and bitrate, suggesting an enzymatic activity is required. This NO oxidation activity was found to be highest in membrane bound proteins with molecular sizes < 100 kDa. In addition, 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one] (ODQ) and cyanide inhibited formation of nitrite in A549 cells. It has been shown that ceruloplasmin (Cp) possesses an NO oxidase and nitrite synthase activity in plasma based on NO oxidation to nitrosonium cation (NO+). We observed that Cp is expressed intracellularly in lung epithelial A549 cells and secreted into medium under basal conditions and during cytokine stimulation. However, an analysis of Cp expression level and activity measured via ρ-phenylenediamine oxidase activity assay revealed very low activity compared with plasma, suggesting that there is insufficient Cp to contribute to detectable NO oxidation to nitrite in A549 cells. Additionally, Cp levels were knocked down using siRNA by more than 75% in A549 cells, with no significant change in either nitrite or cellular S-nitrosothiol (SNO) formation compared to scrambled siRNA control under basal conditions or cytokine stimulation. These data suggest that lung epithelial cells possess NO oxidase activity, which is enhanced in cell membrane associated proteins and not regulated by intracellular or secreted Cp, indicating that alternative NO oxidases determine hypoxic and normoxic nitrite formation from NO in human lung epithelial cells.
Keywords: ceruloplasmin, NO oxidase, nitrite, nitric oxide, human lung epithelial cells, hypoxia
Introduction
Once considered merely a toxic food additive and oxidation product of NO, nitrite has now been shown to exhibit important cell signaling functions in health and disease [1, 2]. Recent studies reveal that nitrite functions as an endocrine reservoir of NO present in plasma and tissues that can be reduced to NO during physiological and pathological hypoxia, to regulate important functions such as hypoxic vasodilation and cytoprotection [3, 4]. During physiological and pathological hypoxia, nitrite is converted to NO via reactions with hemoglobin, myoglobin, neuroglobin, xanthine oxidoreductase and other heme- and thiol-containing enzymes, and by acidic reduction [1, 2, 5–13]. Although nitrite is present in human plasma and organs, the mechanisms of nitrite formation in vivo are not clear. Traditionally, auto-oxidation of NO was thought to be a primary route of nitrite formation; however, these reactions are too slow to compete with other faster reactions, such as the reaction of NO with oxygenated hemoglobin (oxy-Hb) [14]. Recent data from our laboratory suggests that NO can react with the plasma multi-copper oxidase ceruloplasmin (Cp) to form nitrosonium cation (NO+), which subsequently forms nitrite [15]. The high concentrations and high oxidase activity of Cp in plasma has been shown to effectively oxidize NO to nitrite and compete with the reaction of red cell oxy-Hb with NO to form nitrite [15].
Cp is a blue-colored plasma protein that binds up to 95% of circulating copper [16]. The proposed physiological functions of Cp include copper transport, oxidation of organic amines, ferroxidase activity, regulation of cellular iron levels, glutathione peroxidase and ascorbate oxidase activities, as well as an antioxidant activity [17]. Structurally, Cp is an a-glycoprotein composed of a single chain polypeptide of 1046 amino acids and has a molecular weight of approximately 132 kDa [18]. There are two forms of Cp, one is a secretory form that is an acute-phase soluble protein mainly synthesized in the liver and found predominantly in the plasma. The other is expressed in the central nervous system of humans and other mammals as a membrane-bound form [19]. In addition to the liver, Cp is also expressed in various other tissues including lung, brain, heart, spleen, testis, retina, thymus and placenta, as well as monocytic and epithelial cells [20, 21].
In the current study, we explored mechanisms by which lung epithelium can oxidize NO to nitrite. This question is of interest based on observations that airway lining fluid contains high concentrations of nitrite and because plasma nitrite levels are higher in the arterial blood than the venous blood, suggesting increased nitrite formation in blood as it passes through the lung [15].
Materials and Methods
Chemicals and reagents
We purchased all chemicals from Sigma-Aldrich unless otherwise stated. Lipolysaccharide (LPS, from Escherichia coli 0111:B4) was obtained from List Biological Laboratories, Inc. Recombinant human IFN-γ, TNF-α, IL-1β, and monoclonal anti-humnan iNOS antibody were obtained from R & D Systems. Purified mouse anti-ceruloplasmin, mouse anti-COX-2 and anti-AOX1 (aldehyde oxidase) antibodies were obtained from BD Transduction Laboratories. Rabbit anti-β-actin antibody was purchased from Cell Signaling. We purchased antibodies of sulfite oxidase (SO), xanthine oxidase (XO) from Santa Cruz Biotechnology, Inc. The secondary antibodies for chemiluminescence detections were obtained from LI-COR Biosciences. Purified human ceruloplasmin was acquired from Athens Research & Technology. NO donor DETA NONOate was purchased from Cayman Chemical. Human oxy-Hb was purified from human blood in this laboratory.
Cell culture and treatments
Human lung adenocarcinoma epithelial cell line A549 was purchased from the ATCC (American Type Culture Collection) and grown in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% (v/v) FBS (fetal bovine serum, Invitrogen), 2 mM glutamine and 1x penicillin-streptomycin (Invitrogen) in an atmosphere of 95% air/5% CO2 at 37°C. The cells were treated with or without LPS (1 ug/ml) and cytokine mixture (TNF-α, IFN-γ and IL-1β, 20 ng/ml each) for up to 24 hrs before harvesting the conditioned media and cells for nitrite measurement and Western blotting. Primary human bronchial epithelial (HBE) cells were cultured from excess pathological tissue following lung transplantation and organ donation under a protocol approved by the University of Pittsburgh Investigational Review Board. HBE cells were cultured on human placental collagen-coated Costar Transwell filters in bronchial epithelial growth medium as previously described by Myerburg et al [22]. The cells were used for experimentation following 4–6 wk of culture at an air-liquid interface.
For the heat-denatured A549 cells and heme protein inhibition experiments, A549 cells (1×106/well) were cultured in 6-well plates with regular DMEM growth medium (10% FBS and glucose) for 24 hrs. The cells were then washed twice with PBS and cultured with serum deprived DMEM medium. For heat-denatured A549 cells group, the cells were trypsinized, resuspended in complete DMEM growth medium. After washing the cells for two times with PBS, A549 cells were heat-denatured at 95°C for 10 minutes. The cells were then added back to the culture plate wells with serum deprived DMEM medium. Each well was added with DETA NONOate (50 µM). ODQ (30 uM) and sodium cyanide (NaCN, 50 µM) were also added to respective wells with A549 cells. The culture plate was incubated in CO2 incubator (21% O2, 5% CO2) for 24 hrs.
Western blotting analysis
Cells were washed twice with ice-cold PBS and lysed in NP-40 buffer, supplemented with a protease inhibitor cocktail (Roche). The cell lysates were resolved using 4–12% Bis-Tris SDS-PAGE gels (Invitrogen) and transferred to nitrocellulose membranes (Bio-Rad). The membrane was then probed with primary antibodies specific to the target proteins. After incubation with the secondary antibodies from LI-COR Biosciences, the fluorescent signals were detected by an Odyssey scanner.
Nitrite, Nitrate and S-nitrosothiol (SNO) measurement
Nitrite, nitrate and S-nitrosothiols were measured using reductive chemiluminescence in a Sievers 280i Nitric Oxide Analyzer (NOA, from GE Analytical Instruments) by chemiluminescence [23–26]. The protocol published in Free Radical Research by Yang et al [27] was followed for SNO measurement.
Cp siRNA transfection
Dharmacon Accell siRNA against human Cp and scrambled siRNA control were purchased from Thermo Scientific. The Accell delivery media (Thermo Scientific) was used for the transfection according to the manufacturer’s instructions.
Oxygen exposure assay
A549 2×106 cells/ml/well were plated in 6-well culture plates for up to 24 hrs under normoxic (21% O2) and hypoxic (1% O2) conditions. The cells were treated with or without LPS/cytokine mixture for 24 hrs. The cells and conditioned media were harvested for Western blotting and nitrite measurement, respectively.
Measurement of amine oxidase activity
We measured ceruloplasmin activity spectrophotometrically by monitoring the oxidation of p -phenylenediamine (PPD) at 530 nm in the presence of A549 cell lysates, purified human Cp, and concentrated medium as described by Sunderman et al [28].
Statistical analysis
All data are presented as mean ± SD. Significance was estimated using Student’s t-test or ANOVA followed by the Tukey’s multiple comparison procedure with ρ < 0.05 being considered significant.
Results
NO oxidase activity of A549 and HBE cells generates nitrite and nitrate
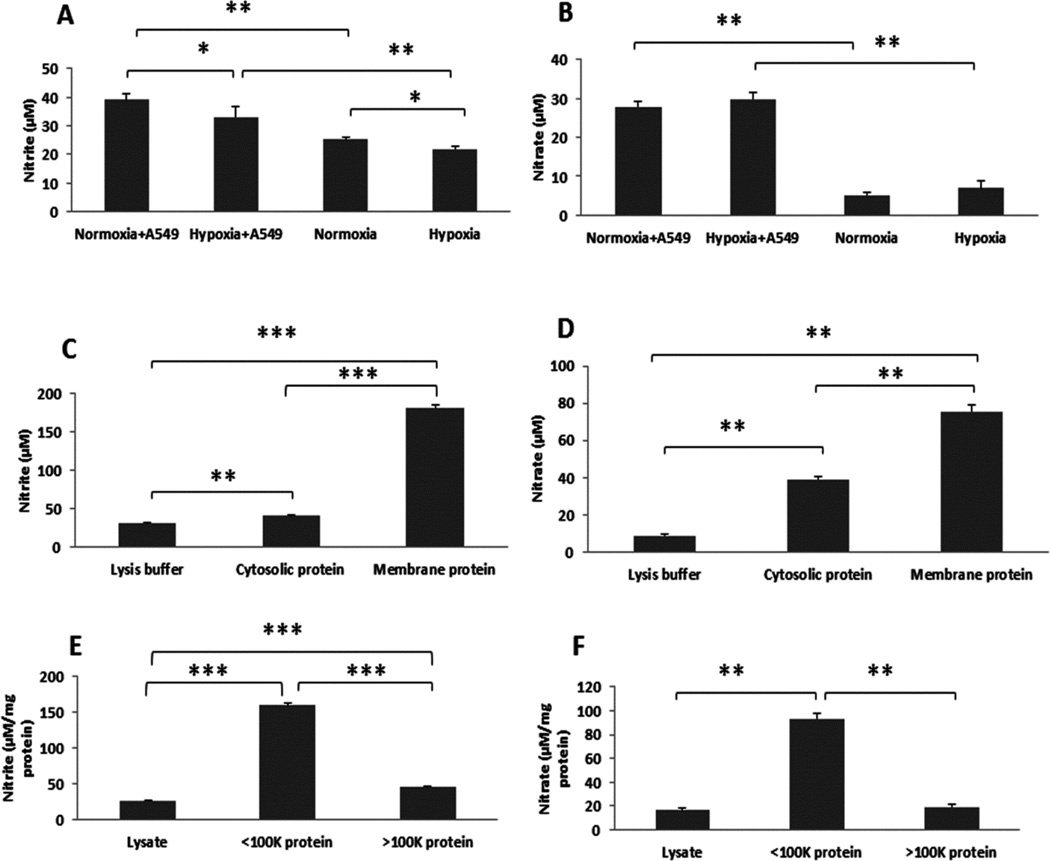
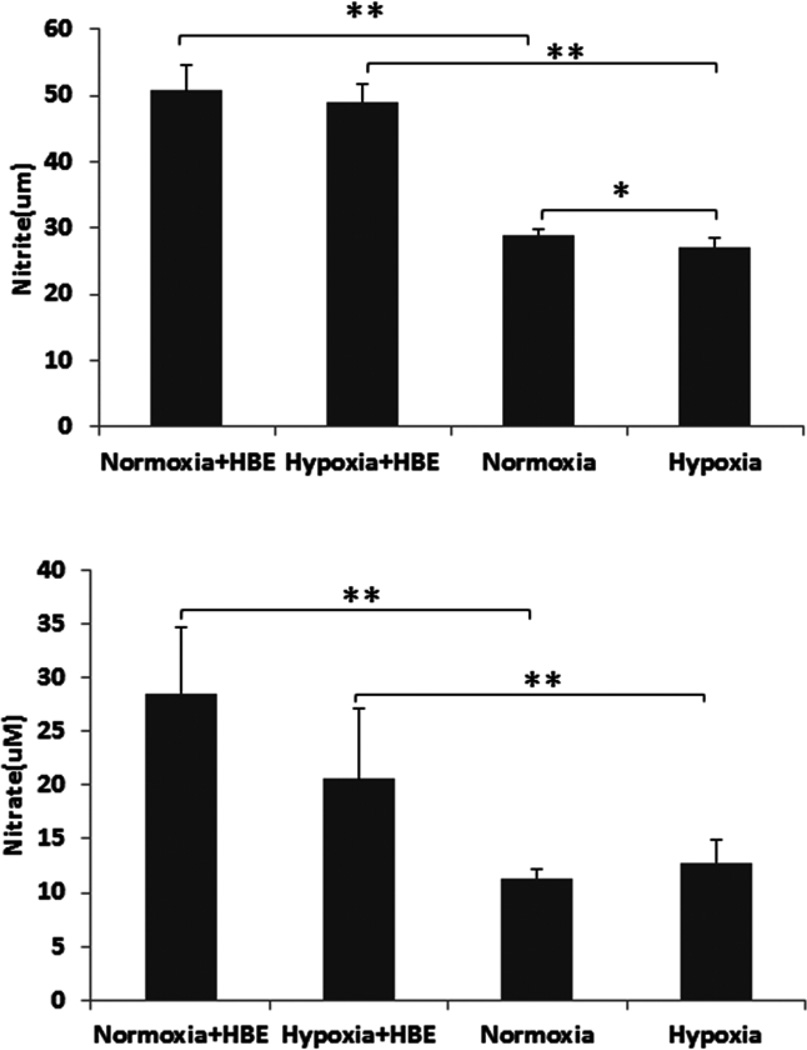
To investigate whether A549 and HBE cells possess the ability to convert NO to nitrite/nitrate, and the effect of oxygen reactions on nitrite/nitrate formation, we incubated the NO donor DETA NONOate (50 µM, with a half-life of about 20 hrs at 37°C) with or without A549 or HBE cells for 24 hrs under normoxic (21% O2) and hypoxic (1% O2) conditions. As shown in Fig. 1 and 2, nitrite (Fig. 1A, Fig. 2 top panel) and nitrate (Fig. 1B, Fig. 2 bottom panel) production was significantly increased in the presence of A549 or HBE cells compared to NO donor exposure in the absence of cells under different oxygen tensions, suggesting that both lung epithelial cell line and primary cells have the capacity to oxidize NO to nitrite/nitrate even under low oxygen conditions. Using a protocol to isolate A549 cell membrane proteins and cytosolic proteins we next evaluated which fraction exhibited the NO oxidase activity. After correction for protein concentration in the fractions the results suggested that the membrane proteins had the highest NO oxidase activity, converting NO to nitrite (Fig. 1C) and nitrate (Fig. 1D). Protein size fractionation suggested the highest oxidase activity was in the fraction < 100 kDa in size (Fig. 1E and 1F).
Fig. 1. A549 cells had ability to make nitrite and nitrate from NO.
NO donor was incubated with and the other without A549 cells. A549 cells (1×106/well) were cultured in 6-well plates with regular DMEM growth medium (10% FBS and glucose) for 24 hrs. Prior to treat the cells with DETA NONOate (50 µM), the cells were washed twice with PBS and the culture medium was replaced with serum deprived DMEM medium. Cells were then placed in a regular CO2 incubator (21% O2, 5% CO2) or hypoxic chamber (1% O2, 5% CO2) for 24 hrs after adding DETA NONOate. Nitrite (A) and nitrate (B) levels in conditioned media were then measured with Sievers 280i NOA (see Materials and Methods). The cell protein was extracted from the cytoplasmic and membrane fraction and similarly exposed to the NO donor for 24 hours and the nitrite and nitrate measured. The membrane fraction had the highest NO oxidase, converting NO to nitrite (C) and nitrate (D). Protein size fractionation suggested the highest oxidase activity was in the fraction < 100 kDa in size (E, F) *ρ < 0.05 and **ρ < 0.01 compared to respective controls.
Fig. 2. Primary human bronchial epithelial (HBE) cells converted NO to Nitrite and Nitrate.
NO donor was incubated with and the other without HBE cells. The cells were cultured on human placental collagen-coated Costar Transwell filters with complete DMEM/F-12 growth medium for 24 hrs. Prior to treat the cells with DETA NONOate (50 µM), the cells were washed twice with PBS and the culture medium was replaced with serum deprived DMEM/F-12 medium. Cells were then placed in a regular CO2 incubator (21% O2, 5% CO2) or hypoxic chamber (1% O2, 5% CO2) for 24 hrs after adding DETA NONOate (50 µM). Nitrite (top panel) and nitrate (bottom panel) levels in conditioned media were then measured (see Materials and Methods).
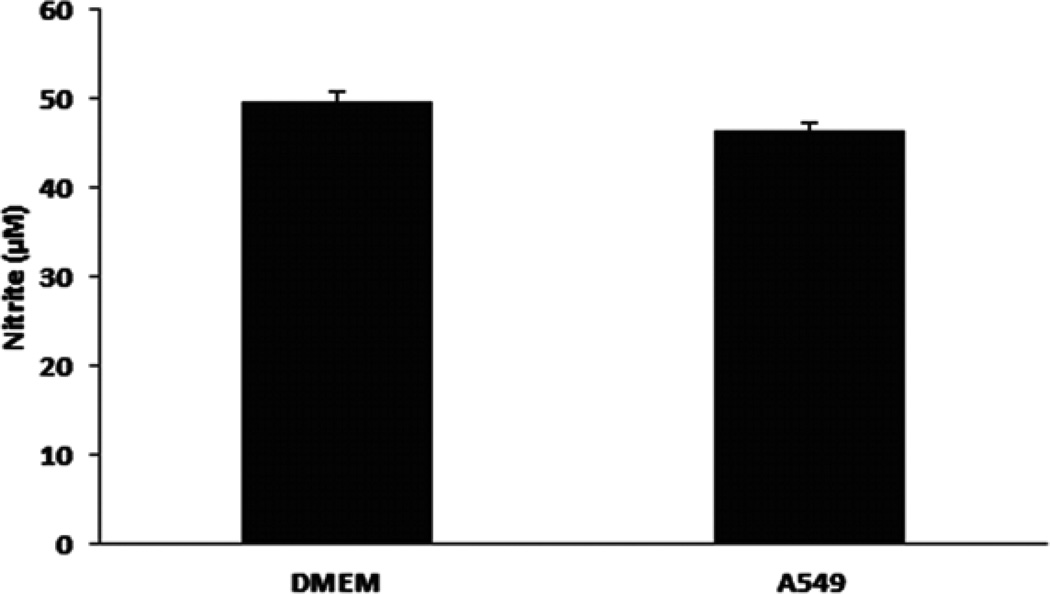
A549 cells exhibit minimal nitrite consumption
In order to investigate a role for differential nitrite reduction or oxidation (i.e. nitrite consumption), A549 cells (1×106/well) were cultured in 6-well plates with regular DMEM growth medium (10% FBS and glucose) for 24 hrs. The cells were then washed twice with PBS and cultured with serum deprived DMEM medium. Cell free well with DMEM medium only was also set up as a control. Sodium nitrite at 50 µM was added to the wells with or without A549 cells. The culture plate was incubated in CO2 incubator (21% O2, 5% CO2) for 24 hrs. As shown in Fig. 3, there was minimal nitrite consumption by A549 cells.
Fig. 3. Nitrite consumption in A549 cells.
A549 cells (1×106/well) were cultured in 6-well plates with regular DMEM growth medium (10% FBS and glucose) for 24 hrs. The cells were then washed twice with PBS and cultured with serum deprived DMEM medium. Cell free well with DMEM medium only was also set up as a control. Sodium nitrite at 50 µM was added to the wells with or without A549 cells. The culture plate was incubated in CO2 incubator (21% O2, 5% CO2) for 24 hrs. Nitrite levels in conditioned media were then measured (see Materials and Methods).
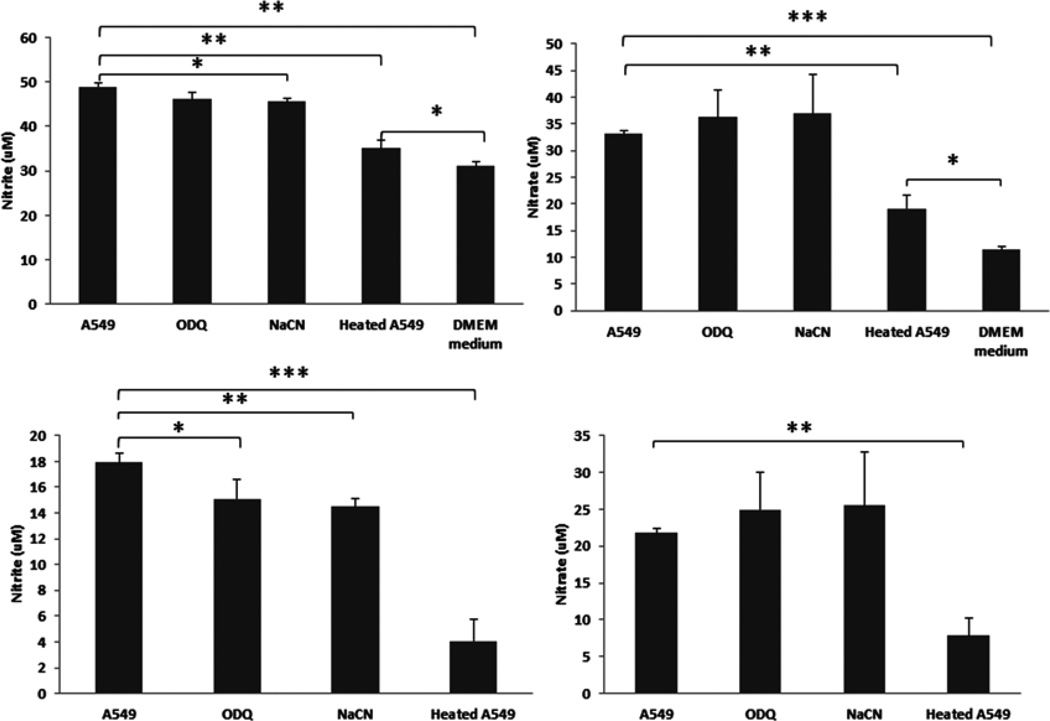
Effects of ODQ, cyanide and heat-denatured A549 cells on nitrite and nitrate production in A549 cells
To demonstrate that if an enzymatic activity is involved in the conversion of NO to nitrite, we heat-denatured A549 cells and measured nitrite and nitrate formation. We found that heat-denatured A549 cells almost abolished both nitrite and nitrate formation, suggesting enzymatic activity is required for cellular NO oxidation (Fig. 4). Furthermore, we treated A549 cells with heme inhibitors (ODQ and cyanide) and observed significantly inhibition of nitrite formation, indicating heme containing proteins may partially contribute to NO oxidation (Fig. 4)
Fig. 4. Effects of ODQ, cyanide and heat-denatured A549 cells on nitrite and nitrate production in A549 cells.
A549 cells (1×106/well) were cultured in 6-well plates with regular DMEM growth medium (10% FBS and glucose) for 24 hrs. The cells were then washed twice with PBS and cultured with serum deprived DMEM medium. For heat-denatured A549 cells group, the cells were trypsinized, resuspended in complete DMEM growth medium. After washing the cells for two times with PBS, A549 cells were heat-denatured at 95°C for 10 minutes. The cells were then added back to the culture plate wells with serum deprived DMEM medium. Each well was added with DETA NONOate (50 µM). ODQ (30 uM) and sodium cyanide (NaCN, 50 µM) were also added to respective wells with A549 cells. The culture plate was incubated in CO2 incubator (21% O2, 5% CO2) for 24 hrs. Nitrite and Nitrate levels in conditioned media were then measured (see Materials and Methods). The top panel shows nitrite and nitrate levels before DMEM medium normalization. The normalized levels were shown on the bottom panel. *ρ < 0.05, **ρ < 0.01 and ***ρ < 0.001 compared to respective controls.
Purified human Cp converts NO to nitrite/nitrate in A549 cells
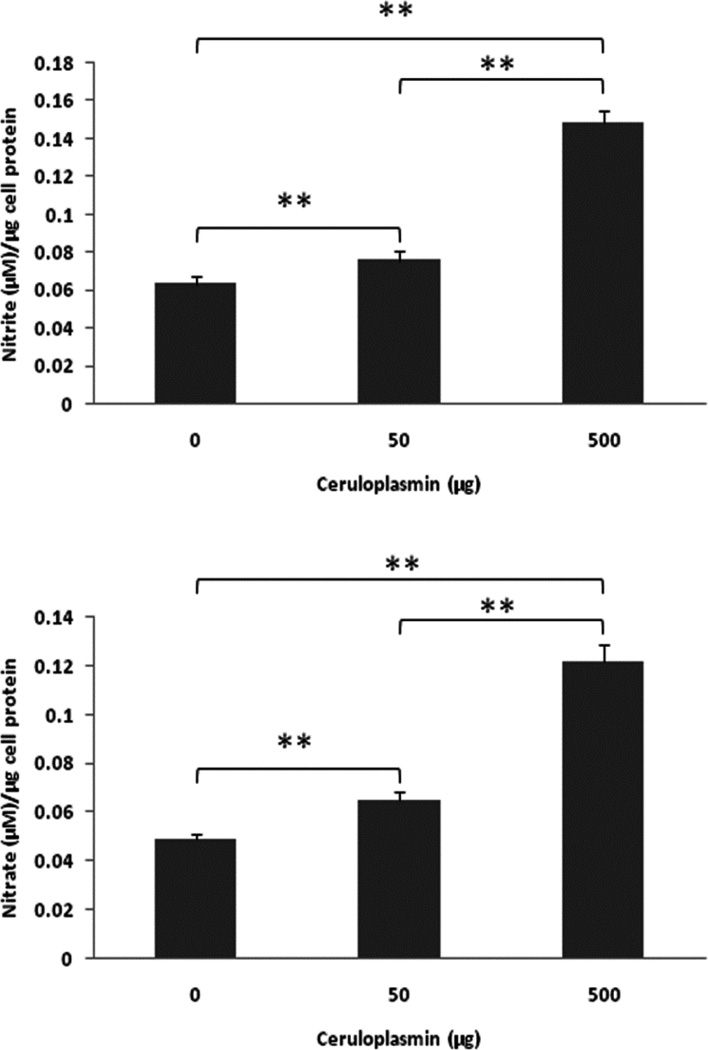
To study the possibility of NO oxidation to nitrite/nitrate by purified human Cp in A549 cells, The cells (1×106/well) were cultured in 6-well plates with regular DMEM growth medium (10% FBS and glucose) for 24 hrs. A549 cells were then washed twice with PBS and cultured with serum deprived DMEM medium. After adding purified human Cp (0 – 500 µg/ml) and DETA NONOate (50 µM), A549 cells were placed in a regular CO2 incubator (21% O2, 5% CO2) for 24 hrs. As shown in Fig. 5, purified human Cp increased nitrite (Fig. 5A) and nitrate (Fig. 5B) production in a dose-dependent manner, which strongly suggests that Cp has NO oxidase activity in vitro and that this effect could be measured in this system.
Fig. 5. Ceruloplasmin converts NO to nitrite and nitrate.
A549 cells (1×106/well) were cultured in 6-well plates with regular DMEM growth medium (10% FBS and glucose) for 24 hrs. The cells were then washed twice with PBS and cultured with serum deprived DMEM medium. After adding purified human ceruloplasmin (0 – 500 µg/ml) and DETA NONOate (50 µM), A549 cells were placed in a regular CO2 incubator (21% O2, 5% CO2) for 24 hrs. Nitrite and nitrate levels in conditioned media were then measured with Severs 280i NOA (see Materials and Methods). **ρ < 0.01 compared to respective controls.
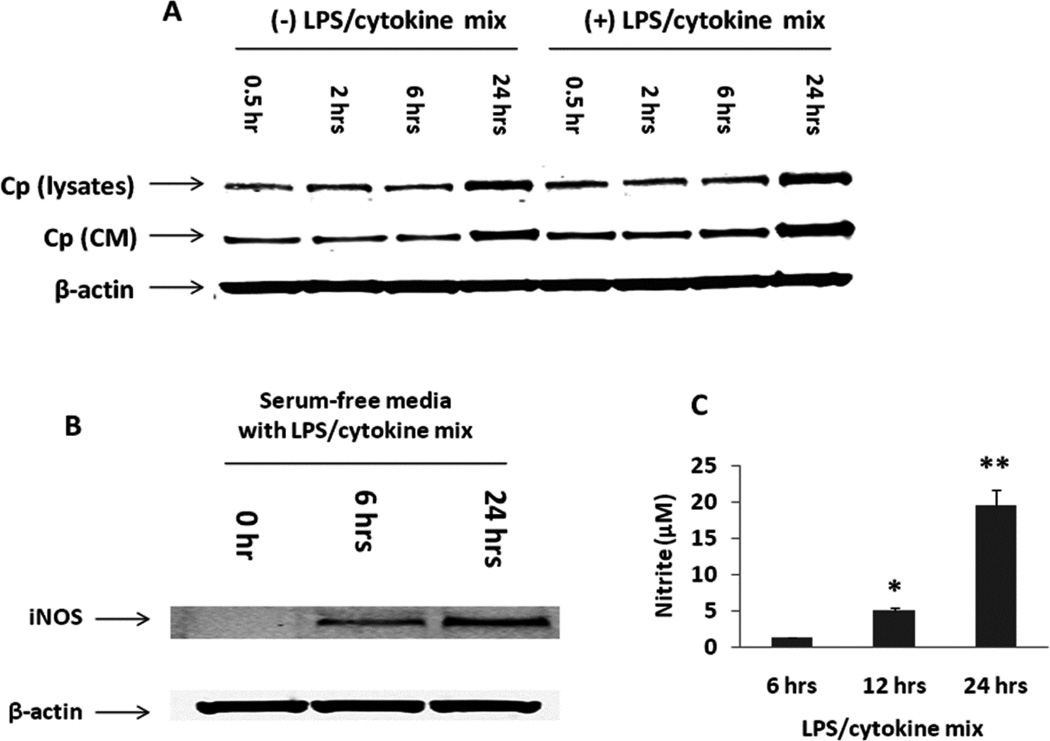
Expressions of Cp and iNOS and nitrite production in A549 cells
Cp has been shown to possess NO oxidase and nitrite synthase activity in plasma [15] and NO oxidase and S-nitrosothiol synthase activity in cells [29–31], we hypothesized that Cp may function as an NO oxidase in A549 cells. We first measured Cp and iNOS expression in A549 cells, in the presence or absence of LPS/cytokine mixtures over a 24 hour time course. The expression of Cp both in the cell lysates and the conditioned media were measured by Western blotting. There was a time-dependent increase in Cp expression both in the cell lysates and the conditioned media with or without LPS/cytokine stimulation (Fig. 6A). In contrast, iNOS was expressed in A549 cells only after LPS/cytokine stimulation (Fig. 6B). We further observed a time-dependent increase in nitrite production in conditioned media from A549 cells after LPS/cytokine stimulation (Fig. 6C), related to increases in iNOS dependent NO generation. These studies indicate that A549 cells serve as an appropriate model for studying the role of Cp as an NO oxidase in cell systems under basal and high iNOS expression conditions.
Fig. 6. Expression of Cp and iNOS and nitrite production in A549 cells under normoxia.
A549 cells were cultured in the presence or absence of LPS/cytokine mixture for up to 24 hrs with serum deprived DMEM medium. Cp expression in both the cell lysates and concentrated conditioned media was determined by Western blotting (A, CM – conditioned media). We also measured iNOS expression in cell lysates (B) and nitrite production in conditioned media (C) by Western blotting and NOA, respectively. *ρ < 0.05 and **ρ < 0.01 compared to 6 hrs control.
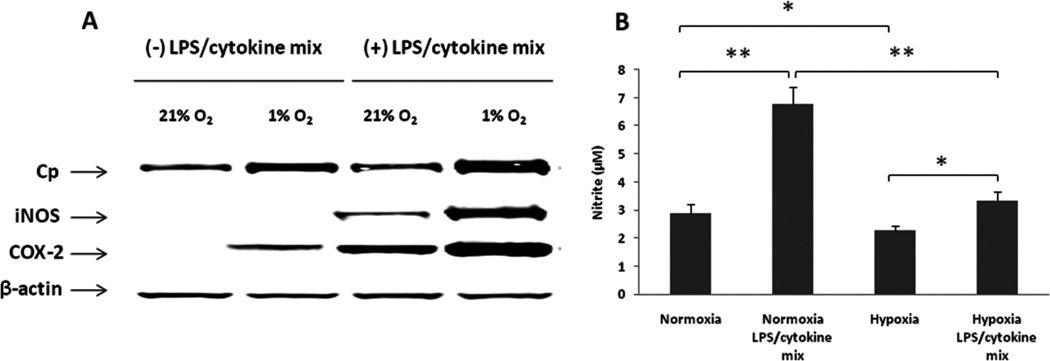
Enhanced expressions of Cp, iNOS and COX-2 and decreased nitrite concentration under hypoxic conditions in A549 cells
Because oxygen is a determinant of NO production rates based on requirement for de novo NO synthesis (oxygen and L-arginine representing obligate substrates for NO synthesis by NO synthase), we characterized the role of hypoxia in regulating the expression of Cp, iNOS and COX-2, as well as nitrite production in A549 cells. The cells were treated with or without LPS/cytokines for 24 hrs under normoxic (21% O2) or hypoxic (1% O2) conditions. As illustrated in Fig. 7A, we found that the expression of Cp and COX-2 were increased by hypoxia both with and without LPS/cytokine stimulation. We also observed that hypoxia enhanced the expression of iNOS after stimulation. There was no expression of iNOS without stimulation, both under normoxic and hypoxic conditions. In addition, nitrite production was significantly attenuated (Fig. 7B) under hypoxic conditions, despite increases in iNOS protein levels. These data indicate that oxygen can modulate both the expression of proteins such as Cp, iNOS and COX-2, and the formation of nitrite. Based on the limited effects of hypoxia on the formation of nitrite during NO donor exposure (Fig. 1), the changes in nitrite formation appear to relate to oxygen availability for de novo NO formation from iNOS (reported Km for oxygen of 6.3 ± 0.9 µM, [32, 33]), rather than reduced NO reactions with oxygen (auto-oxidation reaction).
Fig. 7. Hypoxia enhanced expressions of Cp, iNOS and COX-2 and decreased nitrite production in A549 cells.
A549 cells were treated with or without LPS/cytokine mixture for 24 hrs under normoxic (21% O2) or hypoxic (1% O2) conditions with serum deprived DMEM medium. The expressions of Cp, iNOS and COX-2 were detected by Western blotting (A). The nitrite levels in conditioned media were measured by NOA (B). *ρ < 0.05 and **ρ < 0.01 compared to respective controls.
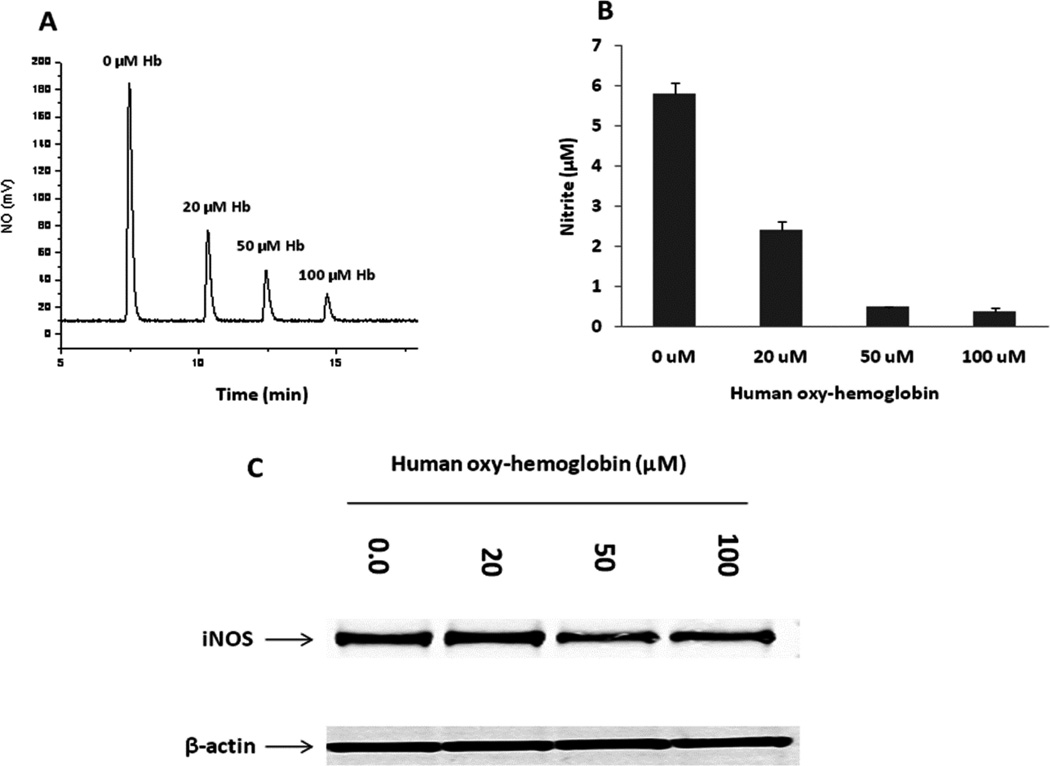
Human oxy-Hb scavenges NO production in A549 cells to reduce nitrite
To confirm that nitrite formed directly from NO we exposed cells to increasing concentrations of oxy-Hb. Human oxy-Hb was evaluated to test its effect on scavenging NO and expression of iNOS. Nitrite concentrations in conditioned media were measured with NOA. The recorded NO (mV) trace by NOA from NO produced in the headspace of the reaction chamber was shown in Fig. 8A. Corresponding nitrite concentration in the conditioned media for each sample was calculated accordingly and shown in Fig. 5B. As shown in Fig. 8, NO was scavenged by oxy-Hb that led decreased nitrite formation in a dose-dependent manner (Fig. 8A and B). However, lower concentration of oxy-Hb showed little effect on iNOS protein expression in A549 cells (Fig. 8C).
Fig. 8. NO was scavenged by human oxy-Hb in a dose-dependent manner with little effect on iNOS expression.
A549 cells (5×105/ml/well) were plated in 12-well plates and cultured with complete DMEM growth medium for 24 hrs. The medium was then replaced with serum deprived DMEM medium before treated with different concentrations of human oxy-Hb for 24 hrs in the presence of LPS/cytokine mixture. Nitrite levels in conditioned media were measured with Sievers 280i NOA (A, B). iNOS expression was determined by Western blotting (C).
Effect of Cp siRNA knockdown on nitrite and SNO formation in A549 cells
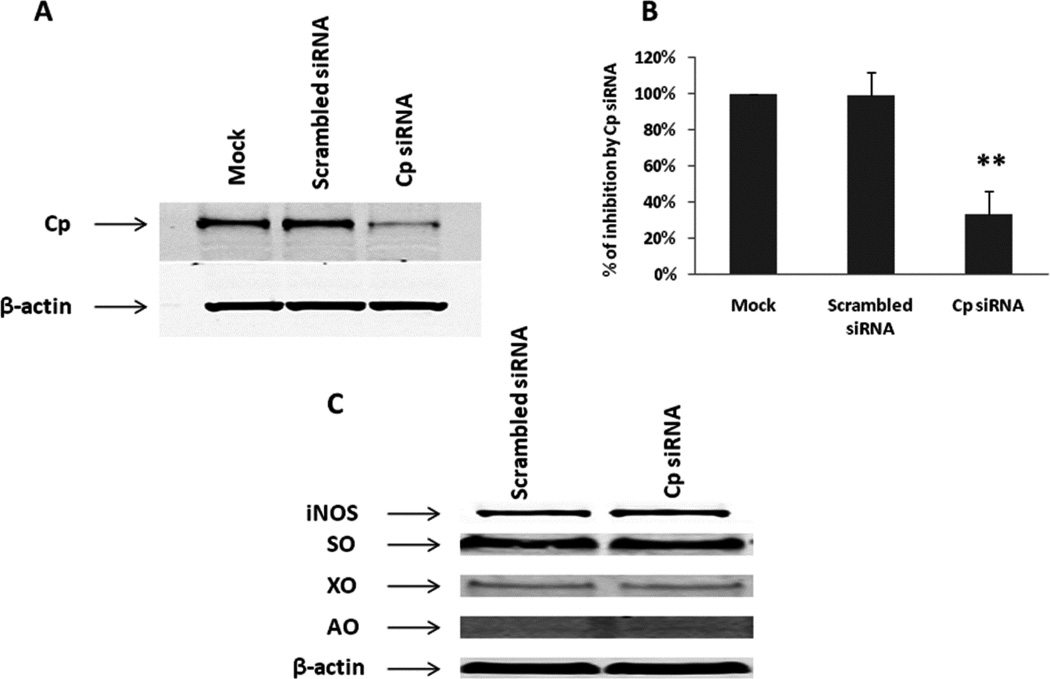
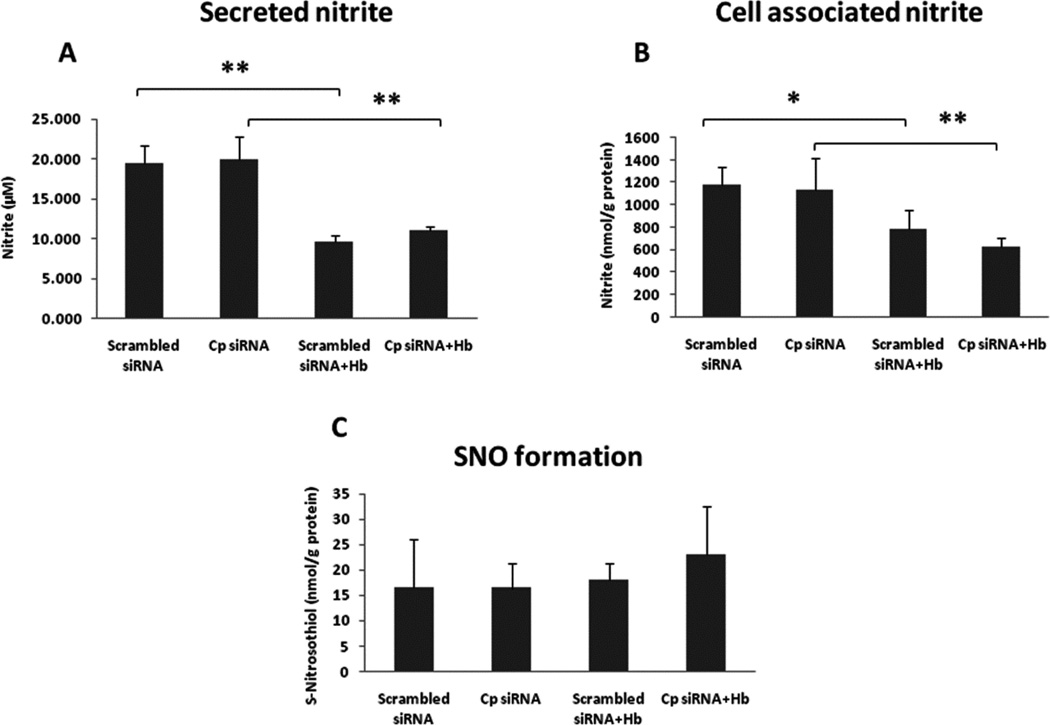
In order to identify whether Cp is a NO oxidase that converts NO to nitrite, we established a Cp knockdown cell model with A549 cells that can reduce the expression of Cp to approximately 25% compared to the scrambled siRNA control (Fig. 9A and B), without affecting expressions of other enzymes such as iNOS, SO, XO and AO (Fig. 9C). Since our previous in vivo study revealed that Cp can compete with the NO-hemoglobin reaction to oxidize NO to nitrite [15], we also performed a competition experiment with Cp and oxy-Hb in A549 cells. Previous studies demonstrated that NO oxidase activity of Cp increased nitrite formation in plasma [15] and SNO formation in cells [29–31]. In order to test our hypothesis that Cp is a NO oxidase in A549 cells, we measured both nitrite and SNO concentrations in conditioned media and cell lysates following treatment of Cp siRNA, with or without human oxy-Hb (20 µM). After 24 hrs of LPS/cytokine stimulation, together with or without oxy-Hb, production of nitrite and SNO was determined by ozone-based chemiluminescent agalysis. Oxy-Hb significantly inhibited nitrite production, but Cp siRNA showed no effect on nitrite formation both in conditioned media and cell lysates (Fig. 10A and B). We observed no significant difference in SNO formation by Cp siRNA and oxy-Hb in cell lysates (Fig. 10C).
Fig. 9. Cp siRNA significantly knocked down Cp protein expression (A, B) with no detectable effects on the expression of iNOS, SO, XO and AO (C).
A549 cells 1×105/ml/well were plated in 12-well plates. The cells were transfected with Dharmacon Accell siRNA against human Cp (1 µM) and scrambled siRNA control by using Accell delivery media (Thermo Scientific) for five days before the cells were harvested for Western blotting analysis.
Fig. 10. Effect of Cp siRNA and human oxy-Hb on nitrite and SNO formation in A549 cells.
A549 cells were transfected with Cp siRNA (1 µM) or scrambled siRNA control by using Accell delivery media (Thermo Scientific) in 12-well plates with cells of 1×105/ml/well. After transfection, the complete culture medium was replaced with serum deprived DMEM medium and human oxy-Hb (20 µM) was added to the cells together with LPS/cytokine mixture for 24 hrs. Nitrite levels in conditioned media (A) and cells (B), as well as SNO production in cells (C) were measured with NOA. *ρ < 0.05 and **ρ < 0.01 compared to controls.
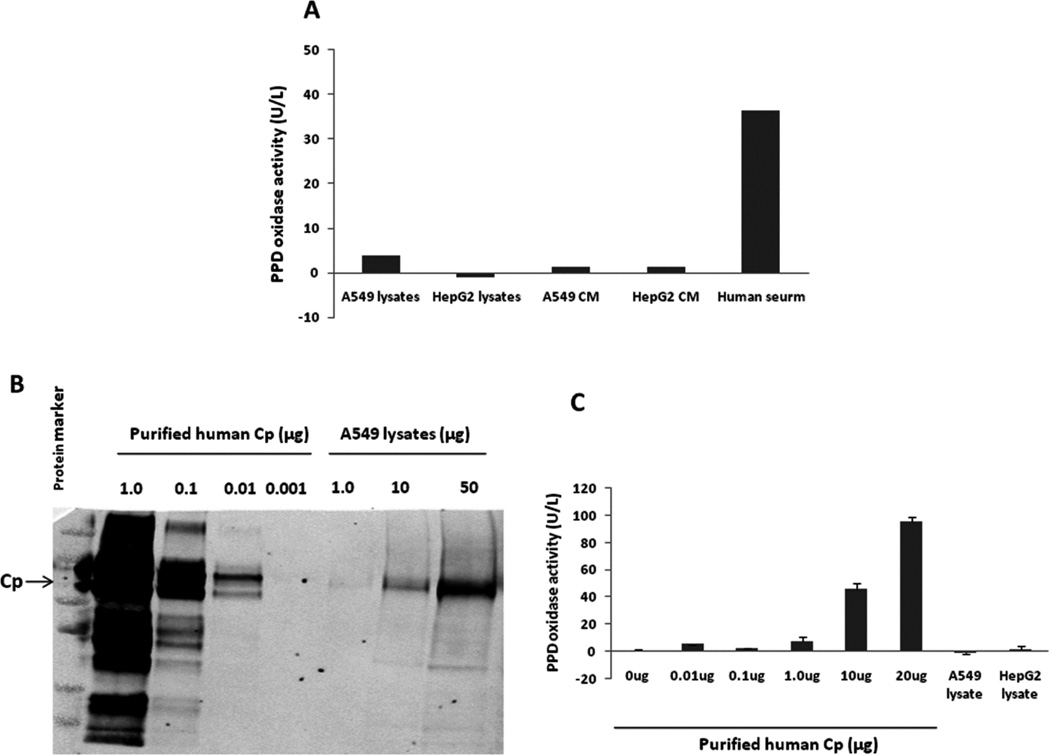
A549 cells exhibit little p-phenylenediamine (PPD) oxidase activity due to limited Cp expression
PPD oxidation is used to quantify the activity of Cp by measuring the purple oxidation product of PPD directly via spectrophotometry [34]. We therefore measured PPD oxidase activity by using both cell lysates and conditioned media from A549 cells. No PPD oxidase activity was detected from A549 cells, while human serum, a positive control, showed robust activity (Fig. 11A), which is consistent with our previous reports [15]. We further determined the protein amount of Cp presented in A549 cells by comparing it to purified human Cp. As illustrated in Fig. 11B, we found that the content of Cp in A549 cells was very low, which resulted in limited PPD activity (Fig. 11C). Therefore, it is reasonable to conclude that Cp may not exhibit sufficient NO oxidase activity in A549 cells due to a very low amount of Cp present in these cells. These studies suggest alternative NO oxidases determine hypoxia and normoxic nitrite formation from NO in human lung epithelial cells.
Fig. 11. A549 cells exhibited no detectable PPD oxidase activity due to limited Cp expression.
Each 75 cm2 cell culture flask containing either A549 or HepG2 cells was incubated in a hypoxic chamber (1% O2) for 24 hrs. Cell lysates and concentrated conditioned media from each flask were used for PPD oxidase activity assay with human serum as a positive control. PPD oxidase activity was barely detectable from either cell lysates or conditioned media (CM), while high activity was observed in human serum (A). Cp protein expression from A549 cell lysates was then detected by Western blotting using purified human Cp protein as a positive control (B). The Cp content in A549 cells was estimated about less than 0.05% of total protein by comparing with the purified human Cp protein. PPD oxidase activity was observed by purified human Cp in a dose-dependent manner, with no activity found in A549 and HepG2 cells (500 µg total protein per lysate sample) (C), which indicates that a Cp amount of at least more than 1 |g is required for detectable PPD oxidase activity.
Discussion
It is now appreciated that nitrite (NO2−) is a bioactive endocrine form of NO that circulates in plasma and is concentrated in red cells and organ tissues, that can mediate hypoxic physiological responses and modulate the cellular responses to ischemia [5, 35–36]. Nitrite is reduced to NO by several mechanisms in vivo, including acidic disproportionation and enzymatic reduction by heme containing globins (hemoglobin, myoglobin, neuroglobin) and by molybdenum containing oxidases (xanthine oxidase, aldehyde oxidase) [6–8, 35, 38–43]. These mechanisms contribute to hypoxic vasodilation, cytoprotection after ischemia-reperfusion injury, and modulation of mitochondrial respiration [2, 35, 37, 44–47]. Given the role of nitrite as an important hypoxic signaling molecule, a better understanding of the mechanisms responsible for nitrite homeostasis (both formation and metabolism) is essential.
Although nitrite concentrations in human plasma and erythrocytes are known to be maintained within a narrow range (121 ± 9 nM and 288 ± 47 nM, respectively), are conserved across many mammalian species, and are present in airway lining fluid, the mechanisms of nitrite formation in cells has not been determined. The classical pathway for nitrite formation is auto-oxidation of NO which has been considered the primary route of nitrite formation, based on the fact that this reaction occurs in oxygenated aqueous buffers. However, this route of formation is kinetically implausible at physiological oxygen concentrations, especially in the presence of NO scavenger molecules like hemoglobin. Hemoglobin present in blood reacts with NO at nearly diffusionlimited rates (3.4 × 107 M−1sec−1) to form inert nitrate (NO3−) [48, 49]. For comparison, the calculated half-time for the third order NO auto-oxidation reaction (K = 2 × 106 M−2 s−1) at physiological NO and oxygen concentrations (400 nM and 150 µM, respectively) is greater than 30 min, suggesting that this pathway is not favorable in vivo where molecules like hemoglobin would react with NO much faster. Note also that this reaction pathway becomes even less important under hypoxic conditions studied here. These considerations suggest that mechanism for metal-based catalysis of nitrite synthesis and a specific NO oxidase activity must exist to account for formation of nitrite in cell systems and organs. Consistent with such a pathway, we previously reported that the multi-copper oxidase, ceruloplasmin, can serve this purpose, competitively oxidizing NO to NO+ and nitrite in plasma [15]. Using ceruloplasmin knock-out mice we found that this enzyme regulated nitrite levels and the biological response to ischemia and reperfusion injury. In this study we investigated the NO oxidase activity of lung epithelium and explored a putative role for NO oxidases and specifically for the multi-copper oxidase ceruloplasmin in nitrite homeostasis.
In the current study we found that upon exposure of NO to buffer and cell culture systems, the nitrite levels were increased in the presence of cells, independent of oxygen tension. The finding that nitrite formation is not affected by oxygen upon addition of NO solutions suggests that NO auto-oxidation is unlikely to significantly account for observed formation of nitrite and that cells help catalyze nitrite formation from NO. The loss of ability to oxidize NO to nitrite and nitrate by heat-denatured A549 cells suggests enzymatic activity accounts for cellular NO oxidation in lung epithelium. We then evaluated endogenous NO formation from iNOS using cytokine stimulated conditions. We found that hemoglobin inhibited nitrite formation, confirming that nitrite derived from NO and the formation could be blocked with a NO scavenger. The levels of nitrite produced from iNOS were reduced at low oxygen, consistent with published Km values of iNOS for oxygen, with reduced NO formation as oxygen levels drop. In order to test a role for cerulosplasmin in the NO oxidation by cells, we knocked it down with specific siRNA. Despite a clear knock-down effect, the nitrite levels were unchanged under basal condition and in competition assays with oxyhemoglobin. Consistent with a lack of effect on nitrite levels, the measured ceruloplasmin oxidase activity in cells was very low, much lower than that observed in plasma where this activity has been shown to regulate nitrite levels [15], suggesting there is not enough Cp in lung epithelial cells to functionally regulate nitrite formation. We further found that membrane protein in A549 cells had the highest NO oxidase activity. In addition, nitrite formation was inhibited by heme inhibitors (ODQ and cyanide), indicating heme containing proteins may partially contribute to NO oxidation in lung epithelial cells.
In conclusion, these studies suggest that while NO production by lung epithelial cells by iNOS is regulated by oxygen tension, required as substrate for de novo NO synthesis, the actual metabolic fate of NO is largely independent of oxygen concentrations. The NO is oxidized by cellular proteins which are concentrated in the membrane fraction and less than 100 kDa in size. The enzyme which oxidizes NO to nitrite in lung epithelial cells is not Cp, based on the low concentrations and oxidase activity in these cells, compared with plasma. Future studies are required to identify the specific oxidase that is responsible for NO conversion to nitrite and nitrate in lung epithelial cells.
Highlights.
Human lung epithelial cells possess oxygen-independent NO oxidase activity that converts NO to nitrite.
Enzymatic activity accounts for cellular NO oxidation and nitrite formation.
Heme containing proteins contribute to NO oxidation and nitrite formation.
Ceruloplasmin is a plasma NO oxidase that forms nitrite, but not responsible for lung epithelial NO oxidation
NO oxidation and nitrite formation activity is highest in cell membrane bound proteins.
Acknowledgments
Dr. Gladwin receives research support from NIH Grants R01HL098032, R01HL096973, RC1DK085852, P01HL103455, and the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania. We thank Dr. Yinna Wang for her excellent technical assistance.
The abbreviations used are
- Cp
Ceruloplasmin
- NO
Nitric oxide
- iNOS
Inducible nitric oxide synthase
- LPS
Lipolysaccharide
- TNF-α
Tumor necrosis factor-α
- IFN-γ
Interferon-γ
- IL-1β
Interleukin-1β
- siRNA
Small-interfering ribonucleic acid
- Hb
Hemoglobin
- PPD
ρ-Phenylenediamine
- SO
Sulfite oxidase
- XO
Xanthine oxidase
- AO
Aldehyde oxidase
- COX-2
cyclooxygenase 2
- DETA NONOate
Diethylenetriamine NONOate
- NOA
Nitric oxide analyzer
- SNO
S-nitrosothiol
- NO+
nitrosonium cation
- ODQ
1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, Cannon RO, III, Kelm M, Wink DA, Espey MG, Oldfield EH, Pluta RM, Freeman BA, Lancaster JR, Jr, Feelish M, Lundberg JO. The emerging biology of the nitrite anion. Nat. Chem. Biol. 2005;1:308–314. doi: 10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
- 2.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 3.Dezfulian C, Raat N, Shiva S, Gladwin MT. Role of the anion nitrite in ischemia-reperfusion cytoprotection and therapeutics. Cardiovasc. Res. 2007;75:327–338. doi: 10.1016/j.cardiores.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC, Freeman BA, Frenneaux M, Friedman J, Kelm M, Kevil CG, Kim-Shapiro DB, KOzlov AV, Lancaster JR, Jr, Lefer DJ, McColl K, McCurry K, Patel RP, Petersson J, Rassaf T, Reutov VP, Richter-Addo GB, Schechter A, Shiva S, Tsuchiya K, van Faassen EE, Webb AJ, Zuckerbraun BS, Zweier JL, Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nat. Chem. Biol. 2009;5:865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang KT, Keszler A, Patel N, Patel RP, Gladwin MT, Kim-Shapiro DB, Hogg N. The reaction between nitrite and deoxyhemoglobin Reassessment of reaction kinetics and stoichiometry. J. Biol. Chem. 2005;280:31126–31131. doi: 10.1074/jbc.M501496200. [DOI] [PubMed] [Google Scholar]
- 6.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, Xu X, Murphy E, Darley-Usmar VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ. Res. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J. Biol. Chem. 2001;276:24482–24489. doi: 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- 8.Gladwin MT, Kim-Shapiro DB. The functional nitrite reductase activity of the hemeglobins. Blood. 2008;112:2636–2647. doi: 10.1182/blood-2008-01-115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samouilov A, Kuppusamy P, Zweier JL. Evaluation of the magnitude and rate of nitric oxide production from nitrite in biological systems. Arch. Biochem. Biophys. 1998;357:1–7. doi: 10.1006/abbi.1998.0785. [DOI] [PubMed] [Google Scholar]
- 10.Tozeck M, Hendgen-Cotta UB, Luedike P, Berenbrink M, Klare JP, Steinhoff HJ, Semmler D, Shiva S, Williams D, Kipar A, Gladwin MT, Schrader J, Kelm M, Cossins AR, Rassaf T. Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation. 2012;126:325–334. doi: 10.1161/CIRCULATIONAHA.111.087155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiso M, Tejero J, Kenney C, Frizzell S, Gladwin MT. Nitrite reductase activity of nonsymbiotic hemoglobins from Arabidopsis thaliana. Biochemistry. 2012 doi: 10.1021/bi300570v. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayaraman T, Tejero J, Chen BB, Blood AB, Frizzell S, Shapiro C, Tiso M, Hood BL, Wang X, Zhao X, Conrads TP, Mallampalli RK, Gladwin MT. 14-3-3 binding and phosphorylation of neuroglobin during hypoxia modulate six-to-five heme pocket coordination and rate of nitrite reduction to nitric oxide. J. Biol. Chem. 2011;286:42679–42689. doi: 10.1074/jbc.M111.271973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiso M, Tejero J, Basu S, Azarov I, Wang X, Simplaceanu V, Frizzell S, Jayaraman T, Geary L, Shapiro C, Ho C, Shiva S, Kim-Shapiro DB, Gladwin MT. Human neuroglobin functions as a redox-regulated nitrite reductase. J. Biol. Chem. 2011;286:18277–18289. doi: 10.1074/jbc.M110.159541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Jr, Phillips GN, Olson JS. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–6983. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 15.Shiva S, Wang X, Ringwood LA, Xu X, Yuditskaya S, Annavajjhala V, Miyajima H, Hogg N, Harris ZL, Gladwin MT. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat. Chem. Biol. 2006;2:486–493. doi: 10.1038/nchembio813. [DOI] [PubMed] [Google Scholar]
- 16.Fox PL, Mukhopadhyay C, Ehrenwald E. Structure, oxidant activity and cardiovascular mechanisms of human ceruloplasmin. Life Sciences. 1995;56:1749–1758. doi: 10.1016/0024-3205(95)00146-w. [DOI] [PubMed] [Google Scholar]
- 17.Healy J, Tipton K. Ceruloplasmin and what it might do. J. Neural. Transm. 2007;114:777–781. doi: 10.1007/s00702-007-0687-7. [DOI] [PubMed] [Google Scholar]
- 18.Koschinsky ML, Funk WD, van Oost BA, MacGillivray RT. Complete cDNA sequence of human preceruloplasmin. Proc. Natl. Acad. Sci. U S A. 1986;83:5086–5090. doi: 10.1073/pnas.83.14.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel BN, David S. A novel glycosylphosphatidylinositol-anchored form of ceruloplasmin is expressed by mammalian astrocytes. J. Biol. Chem. 1997;272:20185–20190. doi: 10.1074/jbc.272.32.20185. [DOI] [PubMed] [Google Scholar]
- 20.Mazumder B, Mukhopadhyay CK, Prok A, Cathcart MK, Fox PL. Induction of ceruloplasmin synthesis by IFN-γ in human monocytic cells. J. Immunol. 1997;159:1938–1944. [PubMed] [Google Scholar]
- 21.Wiggins JE, Goyal M, Wharram BL, Wiggins RC. Antioxidant ceruloplasmin is expressed by glomerular parietal epithelial cells and secreted into urine in association with glomerular aging and high-calorie diet. J. Am. Soc. Nephrol. 2006;17:1382–1387. doi: 10.1681/ASN.2005111239. [DOI] [PubMed] [Google Scholar]
- 22.Myerburg MM, Latoche JD, McKenna EE, Stabile LP, Siegfried JS, Feghali-Bostwick CA, Pilewski JM. Hepatocyte growth factor and other fibroblast secretions modulate the phenotype of human bronchial epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;292:L1352–L1360. doi: 10.1152/ajplung.00328.2006. [DOI] [PubMed] [Google Scholar]
- 23.Basu S, Wang X, Gladwin MT, Kim-Shapiro DB. Chemiluminescent detection of S-nitrosated proteins: comparision of tri-iodide, copper/CO/cysteine and modified copper/cysteine methods. Methods. 2008;440:137–156. doi: 10.1016/S0076-6879(07)00808-7. [DOI] [PubMed] [Google Scholar]
- 24.MacArthur PH, Shiva S, Gladwin MT. Measurement of circulating nitrite and S-nitrosothiols by reductive chemiluminescence. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2007;851:93–105. doi: 10.1016/j.jchromb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Bryan NS, MacArthur PH, Rodriguez J, Gladwin MT, Feelisch M. Measurement of nitric oxide levels in the red cell: validation of tri-iodide-based chemiluminescence with acid-sulfanilamide pretreatment. J. Biol. Chem. 2006;281:26994–27002. doi: 10.1074/jbc.M603953200. [DOI] [PubMed] [Google Scholar]
- 26.Piknova B, Gladwin MT, Schechter AN, Hogg N. Electron paramagnetic resonance analysis of nitrosylhemoglobin in humans during NO inhalation. J. Biol. Chem. 2006;280:40583–40588. doi: 10.1074/jbc.M506292200. [DOI] [PubMed] [Google Scholar]
- 27.Yang BK, Vivas EX, Reiter CD, Gladwin MT. Methodologies for the sensitive and specific measurement of S-nitrosothiols, iron-nitrosyls, and nitrite in biological samples. Free Radic. Res. 2003;37:1–10. doi: 10.1080/1071576021000033112. [DOI] [PubMed] [Google Scholar]
- 28.Sunderman FW, Jr, Nomoto S. Measurement of human serum ceruloplasmin by its ρ-phenylenediamine oxidase activit. Clin. Chem. 1970;16:903–910. [PubMed] [Google Scholar]
- 29.Inoue K, Akaike T, Miyamoto Y, Okamoto T, Sawa T, Otagiri M, Suzuki S, Yoshimura T, Maeda H. Nitrosothiol formation catalyzed by ceruloplasmin. J. Biol. Chem. 1999;274:27069–27075. doi: 10.1074/jbc.274.38.27069. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Hogg N. Formation and stability of S-nitrosothiols in RAW 264.7 cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287:L467–L474. doi: 10.1152/ajplung.00350.2003. [DOI] [PubMed] [Google Scholar]
- 31.Bosworth CA, Jr, Toledo JC, Zmijewski JW, Li Q, Jr, Lancaster JR. Dinitrosyliron complexes and the mechanism(s) of cellular protein nitrosothiol formation from nitric oxide. Proc. Natl. Acad. Sci. U S A. 2009;106:4671–4676. doi: 10.1073/pnas.0710416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rengasamy A, Johns RA. Determination of Km for oxygen of nitric oxide synthase isoforms. J. Pharmacol. Exp. Ther. 1996;276:30–33. [PubMed] [Google Scholar]
- 33.Otto CM, Baumgardner JE. Effect of culture Po2 on macrophage (RAW 264.7) nitric oxide production. Am. J. Physiol. Cell Physiol. 2001;280:C280–C287. doi: 10.1152/ajpcell.2001.280.2.C280. [DOI] [PubMed] [Google Scholar]
- 34.Holmberg CG, Laurell CB. Investigation in serum copper. III. Ceruloplasmin as an enzyme. Acta. Chem. Scand. 1951;5:476. doi: 10.3891/acta.chem.scand.01-0944. [DOI] [PubMed] [Google Scholar]
- 35.Cosby K, Partovi KS, Crawford JH, Patel RP, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, 3rd, Cannon RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 36.Gladwin MT, Raat NJ, Shiva S, Dezfulian C, Hogg N, Kim-Shapiro DB, Patel RP. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H2026–H2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 37.Gladwin MT, Shelhamer JH, Schechter AN, Pease-Fye ME, MWaclawiw MA, Panza JA, Ognibene FP, 3rd, Cannon RO. Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc. Natl. Acad. Sci. U S A. 2000;97:11482–11487. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J. Clin. Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Cui H, Kundu TK, Alzawahra W, Zweier JL. Nitric oxide production from nitrite occurs primarily in tissues not in the blood: Critical role of xanthine oxidase and aldehyde oxidase. J. Biol. Chem. 2008;283:17855–17863. doi: 10.1074/jbc.M801785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998;427:225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- 41.Modin A, Bjorne H, Herulf M, Alving K, Weitzberg E, Lundberg JO. Nitrite-derived nitric oxide: a possible mediator of 'acidic-metabolic' vasodilation. Acta. Physiol. Scand. 2001;171:9–16. doi: 10.1046/j.1365-201X.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- 42.Nagababu E, Ramasamy S, Abernethy DR, Rifkind JM. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J. Biol. Chem. 2003;278:46349–46356. doi: 10.1074/jbc.M307572200. [DOI] [PubMed] [Google Scholar]
- 43.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat. Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 44.Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Jr, Lang JD, Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dalsgaard T, Simonsen U, Fago A. Nitrite-dependent vasodilation is facilitated by hypoxia and is independent of known NO-generating nitrite reductase activities. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H3072–H3078. doi: 10.1152/ajpheart.01298.2006. [DOI] [PubMed] [Google Scholar]
- 46.Maher AR, Milsom AB, Gunaruwan P, Abozquia K, Ahmed, Weaver RA, Thomas P, Ashrafian H, Born GV, James PE, Frenneaux MP. Hypoxic Modulation of Exogenous Nitrite-Induced Vasodilation in Humans. Circulation. 2008;117:670–677. doi: 10.1161/CIRCULATIONAHA.107.719591. [DOI] [PubMed] [Google Scholar]
- 47.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet S, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J. Clin. Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooper CE. Nitric oxide and iron proteins. Biochimica. Et. Biophysica. Acta-Bioenergetics. 1999;1411:290–309. doi: 10.1016/s0005-2728(99)00021-3. [DOI] [PubMed] [Google Scholar]
- 49.Kim-Shapiro DB, Schechter AN, Gladwin MT. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler. Thromb. Vasc. Biol. 2006;26:697–705. doi: 10.1161/01.ATV.0000204350.44226.9a. [DOI] [PubMed] [Google Scholar]