Abstract
Background
Interstitial lung disease (ILD) is associated with high morbidity and mortality in rheumatoid arthritis (RA). Citrullinated proteins are observed in RA lung tissues; however, the association of specific anticitrullinated peptide antibodies (ACPA) with ILD in RA is unknown.
Methods
RA patients underwent multidetector CT (MDCT) of the chest, from which ILD features and a semiquantitative ILD Score (ILDS; range 0–32) were assessed. Anti-CCP (CCP2) and levels of a panel of antibodies against 17 citrullinated and four noncitrullinated peptides were assessed from concurrent serum samples using a custom Bio-Plex bead array. High level ACPA was defined as ≥the group 75th percentile.
Results
Among the 177 RA patients studied, median levels of CCP2 and all specific ACPAs were 46–273% higher among RA patients with versus those without ILD (all p values <0.05), and higher levels correlated with higher ILDS. In contrast, levels of non-citrullinated protein antibodies were not higher in those with ILD. RA patients had a median of 2 high level ACPA reactivities (range 0–16), with each high level ACPA associated, on average, with a 0.10 unit higher ILDS (p=0.001). This association remained significant after adjusting for characteristics associated with ILD (age, gender, current and former smoking, Disease Activity Score for 28 joints, current prednisone and leflunomide use). More high level ACPA were observed in those with versus without pulmonary function restriction or impaired diffusion.
Conclusions
Our findings of a broader ACPA repertoire in RA ILD suggest a possible role for ACPA in the pathogenesis of ILD.
INTRODUCTION
Survival is reduced in rheumatoid arthritis (RA) compared with the general population,1 with pulmonary manifestations representing a leading contributor to RA mortality.2 Pulmonary manifestations of RA represent a heterogeneous group of disorders affecting the airways, parenchyma, pleura and pulmonary vasculature. Among these, interstitial lung disease (ILD) is associated with a particularly high burden of morbidity and mortality. For example, in a population based surveillance cohort,3 median survival was only 2.6 years after RA-ILD diagnosis, and RA-ILD represented 13% of the overall mortality of RA. Clinically significant RA-ILD is observed in 8–10% of RA patients over the course of their disease3,4; however, a much larger proportion exhibit subclinical findings on CT, observed in 20–50% of RA patients.5,6
The natural history and pathogenic determinants of RA-ILD are not well defined. A link between pulmonary inflammation and RA was noted as early as 1953, with Caplan's observation of nodular opacities in the lungs of coalminers with RA.7 In some, lung findings were noted prior to the onset of synovitis. Smoking is a risk factor for both RA related and idiopathic pulmonary fibrosis,8,9 an entity with similarities to some forms of RA-ILD. Importantly, it has been suggested that smoking, the HLA-DRB1 ‘shared epitope’ and anti-CCP interact to increase the risk of RA.8 In support, smoking was associated with higher levels of pulmonary peptidyl arginine deiminase-2 (PAD2), an enzyme that catalyses the post-translational modification of arginine to citrulline,10 and higher levels of citrullination were observed in the cells obtained on bronchoalvelolar lavage of smokers compared with non-smokers. In another study,11 citrullinated proteins were detected in the lung tissue of patients with both RA and idiopathic ILD. Despite this circumstantial evidence, it remains unclear whether citrullination of lung proteins represents a mechanistic link between respiratory exposures and autoimmunity in RA, and/or whether citrullinated proteins in the lung become immune targets for a circulating pathogenic autoantibody generated against citrullinated proteins sharing common antigenic epitopes in other tissues, such as the synovium.
Intra- and inter-molecular epitope spreading of antibodies targeting self-proteins is well described in several autoimmune disorders, including RA. In particular, epitope spreading of autoantibodies targeting citrullinated proteins was found to precede RA diagnosis by several years, and the accumulation of greater numbers of specific anticitrullinated protein antibodies (ACPA) was associated with higher levels of multiple systemic inflammatory cytokines.12 However, neither specific ACPA nor epitope spreading, in general, has been described with ILD in RA. Therefore, we sought to explore the relationships between the number and fine specificity of ACPA with the presence and extent of RA-ILD. We hypothesised that patients with RA-ILD would exhibit a broader repertoire of ACPA compared with those without ILD features on pulmonary imaging, perhaps reflecting epitope spreading in the ACPA response.
METHODS
Study participants
Participants were enrolled in ESCAPE RA (Evaluation of Subclinical Cardiovascular disease And Predictors of Events in Rheumatoid Arthritis), a prospective cohort study investigating subclinical cardiovascular disease in RA described previously.13,14 Participants met 1987 RA classification criteria,15 had RA ≥6 months from diagnosis and were 45–84 years of age without known prior prespecified cardiovascular events. The study was approved by the institutional review board of the Johns Hopkins Hospital and ongoing analyses were approved by the Columbia University Medical Center institutional review board. Enrolment occurred between October 2004 and May 2006.
Outcomes
Pulmonary outcomes
As described previously,13 cardiac multidetector row CT (MDCT) scans were obtained using standard methods16 with 3 mm thickness on a Toshiba Aquilion 64 scanner. With cardiac MDCT, only lung parenchyma from the level of the carina to the lung bases was included. The validity of cardiac MDCT for pulmonary parenchymal disease has been evaluated, with correlation >90% compared with high resolution CT.17 Scans were assessable in 177 of the 195 enrolled participants (91%) by an expert pulmonary radiologist (SS) using a previously described standardised method18 and blinded to clinical characteristics. Characteristics of the subgroup with assessable scans did not differ from those of the full cohort (data not shown). An expert radiologist ILD Score (ILDS) was determined based on the presence and extent of ILD features (ie, ground glass opacification (GGO), reticulation, honeycombing (HC) and traction bronchi-ectasis (TB)) using a semiquantitative scale (0=none, 1=1–25%, 2=26–50%, 3=51–75%, 4=76–100%) with a maximum total score possible of 32. Intraobserver concordance for detecting no ILD was 90%, and 100% for ILDS ≥3. Pulmonary function testing (PFT), consisting of spirometry and assessment of diffusion capacity to carbon monoxide (DLCO), was performed according to the American Thoracic Society guidelines19 at the second study visit, occurring a mean±SD of 21±3 months post baseline. Any restriction and impaired diffusion were both defined as ≤79% of predicted for forced vital capacity and DLCO, respectively.
Measurement of circulating antibodies against citrullinated and non-citrullinated antigens
Serum samples obtained concurrently with CT scanning were assessed for anti-CCP (second generation commercial anticyclic citrullinated peptide assay (CCP2)) using a commercial kit. Levels of a panel of antibodies against four non-citrullinated proteins (fibrinogen A, heat shock protein 60, apolipoprotein A1 and apolipoprotein E) and 17 citrullinated full length proteins or peptides within these proteins (see table 2 for full list) were assayed using a custom Bio-Plex bead array, as previously described.12,20 Individual candidate citrullinated antigens were conjugated to spectrally distinct fluorescently dyed beads. Peptide conjugated beads were incubated with diluted patient sera, autoantibody binding detected with a phycoerythrin conjugated secondary antibody and levels of autoantibody binding quantitated on a Luminex 200 System. With optimisation and evaluation, an interaction between rheumatoid factor (RF) and measurement of autoantibodies using this method has not been observed. There are no population standard cut points defining positive or high levels for the specific ACPA measured. We modelled the 75th and 90th percentiles as cut points for defining high levels for individual ACPA. As results were similar for both cut points (data not shown), we present only the 75th percentile cut point for clarity.
Table 2.
Levels of antibodies against citrullinated and non-citrullinated proteins and peptides, according to strata of expert read Interstitial Lung Disease Score
| ILDS=0 (n=120) | ILDS 1 or 2 (n=25) | ILDS 3+ (n=32) | % Difference | p Value (ILDS 3+vs 0) | |
|---|---|---|---|---|---|
| Antibodies against citrullinated epitopes | |||||
| CCP2 | 89 (11–152) | 148 (73–174) | 152 (99–194) | +71 | 0.0005 |
| Antifibrinogen A-CIT | 76 (48–182) | 68 (54–257) | 142 (61–749) | +87 | 0.020 |
| FibrinogenA 41–60 cit3 cyclic | 552 (172–2115) | 1005 (304–4603) | 1306 (525–3784) | +137 | 0.0097 |
| FibrinogenA 211–230 cit sm cyclic | 397 (69–1370) | 530 (196–2908) | 936 (432–3130) | +136 | 0.0018 |
| FibrinogenA 556–575 cit sm cyclic | 907 (100–3452) | 1874 (509–3729) | 2444 (472–10 251) | +169 | 0.023 |
| FibrinogenA 616–635 cit3 small cyclic | 830 (110–5101) | 5148 (198–19439) | 2355 (688–19434) | +184 | 0.0093 |
| ApolipoproteinA1-CIT | 121 (53–444) | 213 (68–668) | 289 (94–880) | +139 | 0.033 |
| ApolipoproteinE CIT | 284 (211–489) | 325 (220–847) | 416 (258–1630) | +46 | 0.017 |
| Apolipo E 277–296 cit2 sm2 cyclic | 648 (116–2145) | 980 (266–3775) | 1827 (471–5140) | +182 | 0.0042 |
| Enolase 1A cyclic | 824 (98–3046) | 1317 (402–6854) | 2860 (738–7503) | +247 | 0.0059 |
| Vimentin–CIT | 544 (116–2284) | 932 (257–2734) | 1748 (276–3153) | +221 | 0.016 |
| Vimentin 58–77 cit3 cyclic | 513 (84–1715) | 1148 (232–4160) | 1380 (439–2302) | +169 | 0.049 |
| Histones2B–CIT | 1216 (225–3300) | 1845 (558–9734) | 3918 (1017–8817) | +222 | 0.0021 |
| H2B/a 62–81 cit cyclic | 332 (101–1228) | 588 (329–1943) | 1077 (242–1644) | +224 | 0.020 |
| H2A/a 1–20 cit sm-2 cyclic | 452 (97–3023) | 1411 (296–4809) | 1425 (404–3135) | +215 | 0.042 |
| Filaggrin 48–65 cit2 v1 cyclic | 1027 (128–4036) | 2029 (324–4460) | 3834 (1240–10588) | +273 | 0.0013 |
| Biglycan 247–266 cit sm-1 cyclic | 850 (118–3035) | 1313 (323–6477) | 2258 (996–7081) | +166 | 0.0053 |
| Clusterin 231–250 cit sm-1 cyclic | 1808 (139–7579) | 3880 (710–6864) | 3804 (1023–11246) | +110 | 0.035 |
| No of high level ACPA† | 1(0–5) | 3 (1–11) | 6 (1–11) | 0.0048 | |
| Antibodies against native epitopes | |||||
| Fibrinogen A | 48 (38–64) | 45 (37–58) | 38 (35–48) | –21 | 0.0018 |
| Heat shock protein 60 | 523 (193–1576) | 420 (128–1622) | 588 (186–1560) | +12 | 0.97 |
| Apolipoprotein A1 | 53 (48–63) | 51 (46–65) | 52 (43–57) | –2 | 0.11 |
| Apolipoprotein E | 334 (314–366) | 333 (310–356) | 316 (304–333) | –5 | 0.0035 |
All statistical comparisons made using the Kruskal–Wallis test
p values represent comparison of the ILDS=0 group versus the ILDS 3+ group.
High level ACPA defined≥the group 75th percentile.
ACPA, anticitrullinated protein antibody; CCP, cyclic citrullinated protein; ILDS, expert read Interstitial Lung Disease Score.
Other measures
Demographics, current and past smoking, and medical and RA disease history were assessed by patient self-report. Forty-four joints were examined for swelling and tenderness by a single trained assessor, and RA disease activity calculated using the Disease Activity Score for 28 joints with C reactive protein (DAS28-CRP).21 The Stanford Health Assessment Questionnaire22 was used to assess disability. Current and past use of glucocorticoids, biologic and non-biologic disease modifying antirheumatic drugs were queried by detailed examiner administered questionnaires. Radiographs of the hands and feet were scored using the van der Heijde modification of the Sharp method23 by a single experienced reader blinded to patient characteristics.
Other laboratory assessments
C reactive protein was measured by nephelometry (Dade Behring Inc, Deerfield, Illinois, USA). RF was assessed by ELISA, with seropositivity defined at or above a level of 40 units. The presence of shared epitope alleles in exon 2 of HLA-DRB1 was determined as previously described.14
Statistical methods
After exploring the distributions of all variables, group wise differences in normally distributed continuous variables were compared using t tests, in non-normally distributed continuous variables using the Kruskal–Wallis test and in categorical variables using the χ2 goodness of fit or Fisher's exact test, as appropriate. Associations of characteristics with the primary exposure variable (number of high level ACPA) and with the outcome of ILDS were explored using linear regression, with variables transformed to normality as required. Multivariable linear regression models were constructed, with potential confounders included that were associated with the primary exposure variable in univariate analyses at the p<0.20 significance level, to allow for residual confounding. Non-contributory covariates were excluded using Akaike's Information Criterion. The associations of ACPA number with patterns of CT ILD and PFT outcomes were explored using ordinary logistic regression, with multivariable models constructed similar to those described for linear regression, with the exception of the likelihood ratio test for nested models used for dimensionality reduction. All statistical calculations were performed using Intercooled Stata 12 (StataCorp, College Station, Texas, USA). A two tailed α of 0.05 was used throughout.
RESULTS
The characteristics of the 177 RA patients with CT assessment of ILD features are summarised in table 1. Compared with patients without ILD features (ie, ILDS=0), those with ILDS >0 were significantly more likely to have ever smoked and/or were current smokers. RA features significantly associated with ILDS >0 included RF and anti-CCP seropositivity, interleukin 6 level, rheumatoid nodules and current treatment with prednisone and biologic disease modifying antirheumatic drugs. The prevalence of future PFT evidence of restrictive lung disease or impaired diffusion was more than threefold greater in the group with ILDS >0 compared with those with no CT ILD features.
Table 1.
Characteristics of 177 rheumatoid arthritis patients according to the presence or absence of any CT interstitial lung disease features
| Total (n=177) | ILDS=0 (n=120) | ILDS>0 (n=57) | p Value | |
|---|---|---|---|---|
| Age (years) | 59±8 | 58±8 | 61±9 | 0.065 |
| Male (n (%)) | 71 (40) | 43 (36) | 28 (49) | 0.092 |
| Caucasian (n (%)) | 153 (86) | 103 (86) | 50 (88) | 0.73 |
| Any college (n (%)) | 134 (76) | 89 (74) | 45 (79) | 0.49 |
| Ever smoking (n (%)) | 105 (60) | 63 (53) | 43 (75) | 0.004 |
| Current smoking (n (%)) | 20 (11) | 7 (6) | 13(23) | 0.001 |
| Reported lung disease (n=169) (n (%)) | 27 (16) | 19 (17) | 8 (15) | 0.72 |
| BMI (kg/m2) | 28.5± 5.5 | 28.1±5.0 | 29.1±6.2 | 0.31 |
| RA duration (years) | 8 (4–17) | 8 (4–16) | 9 (5–19) | 0.17 |
| RF seropositivity (n (%)) | 114 (64) | 71 (59) | 43 (75) | 0.035 |
| CCP2 seropositivity (n (%)) | 133 (76) | 82 (69) | 51 (89) | 0.003 |
| Any shared epitope alleles (n (%)) | 122 (70) | 80 (67) | 42 (75) | 0.30 |
| DAS28-CRP | 3.7 (2.9–4.4) | 3.5 (2.8–4.3) | 3.8 (3.2–4.4) | 0.18 |
| CRP (mg/l) | 2.3 (1.1–7.6) | 2.3 (1.0–7.1) | 3.5 (1.2–8.4) | 0.67 |
| IL-6 (pg/ml) | 3.7 (1.8–7.8) | 3.1 (1.6–7.0) | 4.5 (2.3–10.0) | 0.042 |
| Total SHS | 8 (1 –36) | 6 (0–29) | 14 (3–55) | 0.070 |
| HAQ (0–3) | 0.63 (0.13–1.25) | 0.63 (0.13–1.25) | 0.75 (0.25–1.38) | 0.11 |
| Ever rheumatoid nodules reported (n (%)) | 33 (19) | 15 (14) | 19 (36) | 0.001 |
| Current prednisone (n (%)) | 67 (38) | 38 (32) | 29 (51) | 0.014 |
| Current non-biologic DMARDs (n (%)) | 150 (85) | 103 (87) | 47 (82) | 0.47 |
| Methotrexate (n (%)) | 114 (64) | 81 (68) | 33 (58) | 0.21 |
| Leflunomide (n (%)) | 19 (11) | 11 (9) | 8 (14) | 0.33 |
| Current biologic DMARDs (n (%)) | 82 (47) | 49 (41) | 33 (58) | 0.037 |
| TNF inhibitors (n (%)) | 79 (45) | 47 (40) | 32 (56) | 0.038 |
| PFT restriction or low DLCO* (n (%)) | 30 (21) | 13 (13) | 17 (40) | <0.001 |
Between group comparisons made with t tests (normally distributed continuous variables), the Kruskal–Wallis test (non-normally distributed continuous variables, the χ2 test or Fisher's exact test (categorical variables).
PFTs were obtained an average of 21±3 months post baseline in 141 patients with baseline ILDS.
BMI, body mass index; CCP2, second generation commercial anticyclic citrullinated peptide assay; CRP, C reactive protein; DAS, Disease Activity Score; DLCO, diffusing lung capacity for carbon monoxide; DMARD, disease modifying antirheumatic drug; HAQ, Health Assessment Questionnaire; IL, interleukin; ILDS, Interstitial Lung Disease Score; PFT, pulmonary function test; RA, rheumatoid arthritis; RF, rheumatoid factor; SHS, Sharp-van der Hiejde score; TNF, tumour necrosis factor.
ACPA level and number was associated with the extent of ILD features in RA
Median levels of CCP2 and all of the 17 measured ACPA antibodies with different specificities were significantly higher for the group with more extensive CT ILD compared with the group with no CT ILD features, with levels ranging from approximately 0.5 to nearly threefold higher (table 2). RA patients had a median of 2 high level ACPA reactivities (range 0–16). More than half of the patients with ILDS ≥3 had 6 or more high level ACPA. In contrast, high level ACPA were uncommon in patients with no ILD features (median high titre ACPA=1). In comparison, median levels of antibodies targeting non-citrullinated proteins were not higher among those with higher ILDS. Of note, levels of anti-native fibrinogen were negligible in both groups.
Patient characteristics were associated with an expanded repertoire of high level ACPA
The associations of patient characteristics with the number of high level ACPA are summarised in table 3. On average, the number of high level ACPA was significantly higher among current smokers compared with non-smokers, those with RF or CCP2 seropositivity compared with those seronegative, those with shared epitope alleles compared with those without, those with higher C reactive protein and interleukin 6 levels, and those with rheumatoid nodules.
Table 3.
Unadjusted associations of patient characteristics with the square root of the number of high titre anticitrullinated protein antibodies
| Characteristic | β * | p Value |
|---|---|---|
| Age, per year | –0.0015 | 0.89 |
| Male vs female | 0.304 | 0.11 |
| Caucasian vs other | –0.414 | 0.12 |
| Any college vs less education | –0.298 | 0.17 |
| Ever smoking vs never | 0.135 | 0.47 |
| Current smoking vs non-smoking | 0.784 | 0.006 |
| Reported lung disease vs none | 0.013 | 0.96 |
| BMI, per kg/m2 | 0.012 | 0.51 |
| RA duration, per year | 0.0067 | 0.44 |
| RF seropositivity vs seronegative | 1.25 | <0.001 |
| CCP2 seropositivity vs seronegative | 1.87 | <0.001 |
| Any shared epitope alleles vs none | 0.457 | 0.021 |
| 1 SE allele vs none | 0.410 | 0.055 |
| 2 SE alleles vs none | 0.545 | 0.028 |
| Any *01 alleles | 0.012 | 0.95 |
| Any *04 alleles | 0.307 | 0.095 |
| Any *0401 alleles | 0.393 | 0.040 |
| Any *0404 alleles | –0.065 | 0.83 |
| Any *10 alleles | 0.606 | 0.15 |
| Any *0401 or *10 alleles | 0.484 | 0.009 |
| DAS28-CRP, per unit | –0.014 | 0.87 |
| CRP, per long unit | 0.171 | 0.011 |
| IL-6, per log unit | 0.272 | <0.001 |
| Total SHS, per log unit | 0.135 | 0.067 |
| HAQ (0–3), per unit | –0.167 | 0.18 |
| Rheumatoid nodules vs none | 0.903 | <0.001 |
| Current prednisone | 0.345 | 0.068 |
| Cumulative prednisone, per gram | 0.0035 | 0.71 |
| Current non-biologic DMARDs | 0.035 | 0.89 |
| Methotrexate | 0.300 | 0.12 |
| Leflunomide | 0.175 | 0.57 |
| Hydroxychloroquine | –0.310 | 0.15 |
| Current biologic DMARDs | 0.117 | 0.53 |
| TNF inhibitors | 0.048 | 0.80 |
β coefficients represent the average change in the square root of the number of high level ACPA associated with a one unit higher value of the characteristic of interest from linear regression.
ACPA, anticitrullinated protein antibody; BMI, body mass index; CCP2, second generation commercial anticyclic citrullinated peptide assay; CRP, C reactive protein; DAS, Disease Activity Score; DMARD, disease modifying antirheumatic drug; HAQ, Health Assessment Questionnaire; IL, interleukin; RA, rheumatoid arthritis; RF, rheumatoid factor; SE, shared epitope; SHS, Sharp-van der Hiejde Score; TNF, tumour necrosis factor.
The number of high level ACPA was an indicator of the extent of CT ILD
On average, each additional high level ACPA was associated with a 0.10 higher unit ILDS score (p=0.001; table 4, model 1); however, the association was not linear, as ILDS scores in those with 1–6 high level ACPA were not significantly higher than those with no high level ACPA (table 4, model 2). In contrast, average ILDS was significantly higher in the group with 7 or more high level ACPA compared with the group with no high level ACPA, suggesting a possible threshold of ACPA diversification for the development of ILD. This association remained significant in expanded and simplified models adjusting for potential confounders (table 4, models 3 and 4). The association of CCP2 seropositivity with ILDS paralleled the association of number of high level ACPA with ILDS (table 4, model 5).
Table 4.
Crude and adjusted associations of number of citrullinated protein antibodies with CT expert read Interstitial Lung Disease Score
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
Model 5 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p* | p Value | β * | p Value | p* | p Value | β * | p Value | β * | p Value | |
| No of high level ACPA, per antibody | 0.10 | 0.001 | ||||||||
| High level ACPA=0 (n=50) | Ref | – | Ref | – | Ref | – | Ref | – | ||
| High level ACPA=1 or 2 (n=43) | 0.31 | 0.43 | 0.36 | 0.35 | 0.39 | 0.27 | -0.26 | 0.58 | ||
| High level ACPA=3–6(n=41) | 0.45 | 0.26 | 0.30 | 0.47 | 0.36 | 0.33 | -0.36 | 0.46 | ||
| High level ACPA≥ 7 (n=42) | 1.28 | 0.001 | 0.79 | 0.055 | 0.93 | 0.012 | 0.23 | 0.63 | ||
| Age, per year | 0.034 | 0.035 | 0.034 | 0.032 | 0.036 | 0.022 | ||||
| Male vs female | 0.49 | 0.12 | 0.54 | 0.055 | 0.45 | 0.12 | ||||
| Ever smoking | 0.78 | 0.010 | 0.70 | 0.017 | 0.75 | 0.011 | ||||
| Current smoking | 0.83 | 0.061 | 0.93 | 0.033 | 0.90 | 0.038 | ||||
| RF seropositivity | 0.25 | 0.43 | ||||||||
| Square root DAS28, per unit | 1.35 | 0.013 | 1.36 | 0.005 | 1.33 | 0.006 | ||||
| Log SHS, per unit | –0.06 | 0.61 | ||||||||
| Rheumatoid nodules | 0.34 | 0.22 | ||||||||
| HAQ, per unit | –0.14 | 0.53 | ||||||||
| Current prednisone | 0.44 | 0.12 | 0.53 | 0.053 | 0.44 | 0.11 | ||||
| Current methotrexate | –0.31 | 0.30 | ||||||||
| Current leflunomide | 1.20 | 0.007 | 1.24 | 0.004 | 1.24 | 0.004 | ||||
| Current biologics | 0.26 | 0.37 | ||||||||
| CCP2>20 units | 0.96 | 0.035 | ||||||||
β coefficients represent the average change in the square root of the expert read Interstitial Lung Disease Score associated with a 1 unit higher value of the characteristic of interest.
Statistical comparisons from linear regression.
ACPA, anticitrullinated protein antibody; CCP2, second generation commercial anticyclic citrullinated peptide assay; DAS, Disease Activity Score; HAQ, Health Assessment Questionnaire; RF, rheumatoid factor; SHS, Sharp-van der Hiejde Score.
Presence of an expanded ACPA repertoire was associated with radiographic ILD patterns and PFT abnormalities
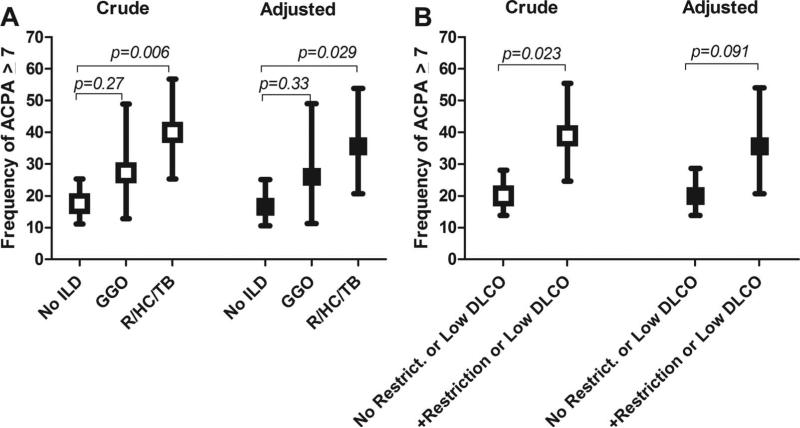
Among the 57 patients with CT ILD features, the predominant pattern was GGO in 22 (39%) and reticulation, honeycombing or traction bronchiectasis (R/HC/TB) in 35 (61%). Forty per cent of those with R/HC/TB had 7 or more high level ACPA compared with only 18% of those without ILD (p=0.006; figure 1A). The proportion with 7 or more high level ACPA was also higher in those with GGO compared those without ILD (27% vs 18%, respectively); however, this difference was not statistically significant. Adjusting for age, gender, current and past smoking, DAS28, and current prednisone and leflunomide use did not substantially alter the differences between groups.
Figure 1.
Associations of radiographic interstitial lung disease features with an expanded ACPA repertoire. Graphs represent the proportion of rheumatoid arthritis patients with 7 or more high level ACPA according to the predominant pattern of interstitial lung disease features (A) and the presence of restrictive lung disease and/or impaired diffusing capacity on pulmonary function testing (B). Depicted are means and 95% confidence intervals. Adjusted analyses from ordinary logistic regression account for age, gender, current and past smoking, Disease Activity Score for 28 joints, and current use of prednisone and leflunomide. ACPA, anticitrullinated protein antibody; DLCO, diffusing lung capacity for carbon monoxide; GGO, ground glass opacification; ILD, interstitial lung disease; ILDS, expert read ILD score; R/HC/TB, reticulation/honeycombing/traction bronchiectasis.
Among the 156 patients with subsequent PFT assessment, 36 (23%) met criteria for restriction and/or impaired diffusion. Among this group, 39% had 7 or more high level ACPA compared with only 20% without these features (p=0.023; figure 1B). Covariate adjustment reduced this difference only slightly; however, statistical significance was only marginal after adjustment (p=0.091).
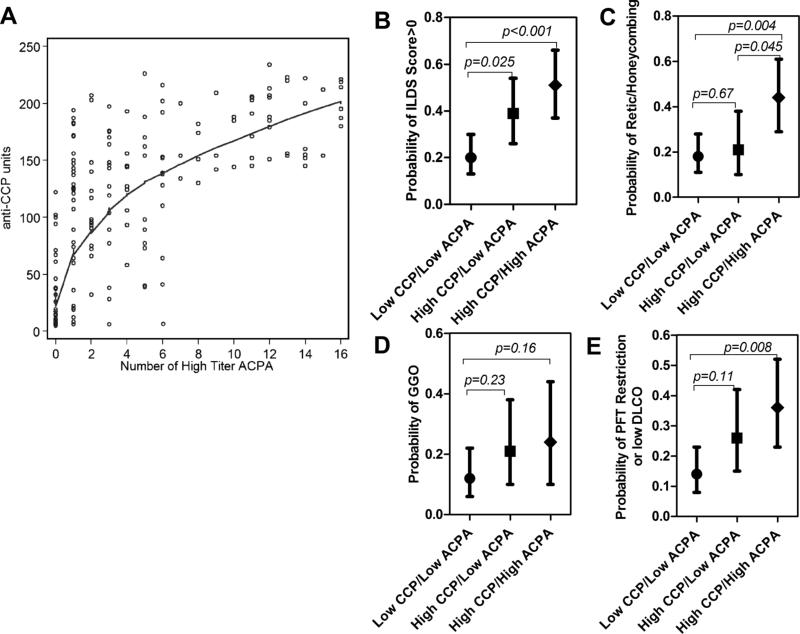
Association of an expanded ACPA repertoire with RA ILD versus anti-CCP alone
We explored whether the number of high level ACPA could discriminate ILD or PFT outcomes beyond CCP2 alone. Plotting the relationship between anti-CCP level and number of high level ACPA (figure 2A) showed an association between the two, as all patients with 7 or more high level ACPA also had a CCP2 >120 units (n=43). However, there was a similar number (n=44) with CCP2 >120 units, but with 0–6 high level ACPA, suggesting a limited capacity of higher CCP2 to discriminate within the breadth of the ACPA response within a given patient. We used these groupings to explore ILD and PFT outcomes (figures 2B–E). For each of the outcomes, the group with higher CCP2 and 7 or more high level ACPA demonstrated a numerically higher frequency of the outcome compared with the group with lower CCP2/lower ACPA number (statistically significant for all outcomes except the outcome of GGO) or the group with higher CCP2/lower ACPA number; however, the difference was only statistically significant for the outcome of R/HC/TB; figure 2C).
Figure 2.
Radiographic and pulmonary function testing evidence of interstitial lung disease according to anti-CCP level and number of high level ACPA. Anti-CCP level and number of high level ACPA were highly correlated (A; the curved line is the lowess smoothed least squares estimate of the average association). Based on this relationship, the cohort was subdivided into three mutually exclusive groups: those with CCP2 <120 units and 0–6 high level ACPA (‘Low CCP/Low ACPA’; n=89), CCP2 ≥120 units and 0–6 high level ACPA (‘High CCP/Low ACPA’; n=44) and CCP2≥120 units and 7 or more high level ACPA (‘High CCP/High ACPA’; n=43). (B–E) According to these groups, the probability of any ILD (ie, ILDS >0; B); the probability of a predominant pattern of reticulation/honeycombing/or traction bronchiectasis (C); the probability of a predominant pattern of ground glass opacification (D); and the probability of restrictive lung disease and/or impaired diffusion on pulmonary function testing (E) are shown. Depicted are means and 95% CIs for all graphs. ACPA, anticitrullinated protein antibody; CCP, cyclic citrullinated protein; CCP2, second generation commercial anticyclic citrullinated peptide assay; DLCO, diffusing lung capacity for carbon monoxide; GGO, ground glass opacification; ILDS, expert read Interstitial Lung Disease Score; PFT, pulmonary function testing.
DISCUSSION
To our knowledge, this is the first report of ACPA specificity with radiographic ILD and PFT indicators of pulmonary restriction and/or impaired diffusion commonly associated with RA-ILD. On average, we observed higher levels of all of the specific ACPA measured among patients with radiographic evidence of ILD features, often higher by several fold, compared with those without RA-ILD. In contrast, levels of antibodies against non-citrullinated proteins were not higher among those with ILD features. Within individuals, the number of high level ACPA was also a potent indicator of ILD features, even after adjusting for pertinent confounders. In particular, an expanded ACPA repertoire (ie, having 7 or more high level ACPA) was most strongly associated with ILD features of fibrosis (ie, R/HC/TB), as well as restriction and/or impaired diffusion on future PFTs. While CCP2 level alone was a potent indicator of ILD and PFT outcomes, knowledge of the number of high level ACPA was an indicator of R/HC/TB over and above the information gained from the level of CCP2. RA-ILD is heterogeneous radiographically and histologically.24 One radiographic distinction is between non-specific interstitial pneumonia (characterised by a predominant radiographic pattern of GGO) and usual interstitial pneumonia (characterised by a predominant pattern of reticulation and honeycombing). In our study, we observed a stronger association of ACPA number with radio-graphic usual interstitial pneumonia than non-specific interstitial pneumonia, perhaps suggesting a mechanistic distinction between these two entities.
Taken together, these data suggest a robust relationship between ACPA level and the presence and severity of RA-ILD; however, the proximity of these associations to causality is not clear. All of the protein antigens to which, in their citrullinated forms, the patients in our study had high titre antibodies are present in the lung.25,26 Citrullinated proteins have been identi-fied in lung tissue,11 a finding not exclusive to RA.27 In addition, PAD enzymes, necessary for the conversion of arginine to citrul-line in substrate proteins, have also been shown to be present in lung tissue.10,27 Whether these observations support the possibility of antibody mediated lung tissue damage resulting from a pathogenic interaction between autoantibodies and their cognate citrullinated peptides will require additional mechanistic studies. Circumstantially, autoantibodies are frequently observed in other forms of non-RA-ILD28 and, in the case of antiendothelial cell antibodies, have some support for a pathogenic role.29 In addition, immune complexes have been demonstrated in the circulation and bronchoalveolar lavage fluid of patients with non-RA-ILD,30 and autoimmunity against collagen type V mediates a cellular immune response leading to bronchiolitis after lung transplantation.31 Whether similar processes targeting citrullinated, rather than native, lung proteins mediate RA-ILD warrants additional study.
It is also possible that our findings of high ACPA levels and higher ACPA number among RA patients with ILD features is not related to any direct causal link between ACPA and lung inflammation, but an epiphenomenon representing two unlinked processes operating in parallel. However, if unlinked, then higher levels of all autoantibodies, not just those directed against citrullinated antigens, would be expected with ILD. This was not the case in our study, in which the antibodies against non-citrullinated proteins were not associated with ILD.
The study has notable strengths and limitations. Among the strengths, we utilised multiple measures of lung disease, both imaging and pulmonary function testing. Scans were interpreted by the same pulmonary radiologist with decades of ILD experience. Among the limitations, cardiac MDCT differs from high resolution CT typically obtained for ILD assessment, primarily in slice thickness and the lung apices were not imaged. However, while we may have missed additional ILD features exclusive to the apices (uncommon for RA-ILD), our findings are internally consistent as the same techniques were used for all patients. Owing to the characteristics of the cohort, our findings may only be generalisable to RA patients >45 years of age without prior cardiovascular events. The study was cross sectional, limiting any ability to establish temporality in the associations. Finally, the citrullinated antigens in our ACPA panel were derived from rheumatoid synovium12 and not specifically with lung antigens in mind. Thus we may have missed relevant citrullinated lung epitopes not represented in our panel of antigens.
In summary, our findings of higher levels of a variety of ACPA and an expanded ACPA repertoire among RA patients with radiographic ILD and PFT restriction and/or impaired DLCO suggest a link between autoimmunity against citrullinated proteins and a high impact outcome in RA. Future studies will help define the potential causality of this association.
Acknowledgements
We would like to thank the Johns Hopkins Bayview Medical Center General Clinical Research Center and staff, the field center of the Baltimore MESA cohort and the MESA Coordinating Center at the University of Washington, Seattle. We are indebted to the dedication and hard work of the ESCAPE RA Staff: Marilyn Towns, Michelle Jones, Patricia Jones, Marissa Hildebrandt, Shawn Franckowiak and Brandy Miles, and to the participants of the ESCAPE RA study who graciously agreed to take part in this research. Drs Uzma Haque, Clifton Bingham III, Carol Ziminski, Jill Ratain, Ira Fine, Joyce Kopicky-Burd, David McGinnis, Andrea Marx, Howard Hauptman, Achini Perera, Peter Holt, Alan Matsumoto, Megan Clowse, Gordon Lam and others generously recommended their patients for this study.
Funding This work was supported by NIH NIAMS AR050026–01 (to JMB) and RC1 AR058713 (to WHR) awards from the American College of Rheumatology Research and Education Foundation Within-Our-Reach Campaign (to SKD, JTG and WHR) and Veterans Affairs Health Care System funding (to WHR). Additional support was provided by the Johns Hopkins Bayview Medical Center General Clinical Research Center (grant No M01RR02719).
Footnotes
Contributors All authors contributed materially to the study and approved the final version. Specific contributions include: study design and conception: JTG, SKD, JS, WHR and JMB; acquisition of the data: JTG, JS, DAP, Cramb-Wagner, SS, GC and JMB; data analysis: JTG; data interpretation: JTG, SKD, JS, RW, DAP, WHR and JMB; manuscript writing: all authors.
To cite: Giles JT, Danoff SK, Sokolove J, et al. Ann Rheum Dis Published Online First: [please include Day Month Year] doi:10.1136/annrheumdis-2012-203160
Competing interests None.
Ethics approval The study was approved by the institutional review board of the Johns Hopkins Hospital and ongoing analyses were approved by the Columbia University Medical Center institutional review board.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Gonzalez A, Maradit Kremers H, Crowson CS, et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007;56:3583–7. doi: 10.1002/art.22979. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez A, Icen M, Kremers HM, et al. Mortality trends in rheumatoid arthritis: the role of rheumatoid factor. J Rheumatol. 2008;35:1009–14. [PMC free article] [PubMed] [Google Scholar]
- 3.Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62:1583–91. doi: 10.1002/art.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olson AL, Swigris JJ, Sprunger DB, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med. 2011;183:372–8. doi: 10.1164/rccm.201004-0622OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawson JK, Fewins HE, Desmond J, et al. Fibrosing alveolitis in patients with rheumatoid arthritis as assessed by high resolution computed tomography, chest radiography, and pulmonary function tests. Thorax. 2001;56:622–7. doi: 10.1136/thorax.56.8.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujii M, Adachi S, Shimizu T, et al. Interstitial lung disease in rheumatoid arthritis: assessment with high-resolution computed tomography. J Thorac Imaging. 1993;8:54–62. [PubMed] [Google Scholar]
- 7.Caplan A. Certain unusual radiological appearances in the chest of coal-miners suffering from rheumatoid arthritis. Thorax. 1953;8:29–37. doi: 10.1136/thx.8.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kallberg H, Ding B, Padyukov L, et al. Smoking is a major preventable risk factor for rheumatoid arthritis: estimations of risks after various exposures to cigarette smoke. Ann Rheum Dis. 2011;70:508–11. doi: 10.1136/ard.2009.120899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumgartner KB, Samet JM, Coultas DB, et al. Occupational and environmental risk factors for idiopathic pulmonary fibrosis: a multicenter case-control study. Collaborating Centers. Am J Epidemiol. 2000;152:307–15. doi: 10.1093/aje/152.4.307. [DOI] [PubMed] [Google Scholar]
- 10.Makrygiannakis D, Hermansson M, Ulfgren AK, et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis. 2008;67:1488–92. doi: 10.1136/ard.2007.075192. [DOI] [PubMed] [Google Scholar]
- 11.Bongartz T, Cantaert T, Atkins SR, et al. Citrullination in extra-articular manifestations of rheumatoid arthritis. Rheumatology (Oxford) 2007;46:70–5. doi: 10.1093/rheumatology/kel202. [DOI] [PubMed] [Google Scholar]
- 12.Sokolove J, Bromberg R, Deane KD, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 2012;7:e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giles JT, Szklo M, Post W, et al. Coronary arterial calcification in rheumatoid arthritis: comparison to the multi-ethnic study of atherosclerosis. Arthritis Res Ther. 2009;11:R36. doi: 10.1186/ar2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi H, Giles JT, Polak JF, et al. Increased prevalence of carotid artery atherosclerosis in rheumatoid arthritis is artery-specific. J Rheumatol. 2010;37:730–9. doi: 10.3899/jrheum.090670. [DOI] [PubMed] [Google Scholar]
- 15.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 16.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman EA, Jiang R, Baumhauer H, et al. Reproducibility and validity of lung density measures from cardiac CT Scans—The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16:689–99. doi: 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldin JG, Lynch DA, Strollo DC, et al. High-resolution CT scan findings in patients with symptomatic scleroderma-related interstitial lung disease. Chest. 2008;134:358–67. doi: 10.1378/chest.07-2444. [DOI] [PubMed] [Google Scholar]
- 19.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J. 2005;26:153–61. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 20.Monach PA, Hueber W, Kessler B, et al. A broad screen for targets of immune complexes decorating arthritic joints highlights deposition of nucleosomes in rheumatoid arthritis. Proc Natl Acad Sci USA. 2009;106:15867–72. doi: 10.1073/pnas.0908032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prevoo ML, van 't Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe F, Kleinheksel SM, Cathey MA, et al. The clinical value of the Stanford Health Assessment Questionnaire Functional Disability Index in patients with rheumatoid arthritis. J Rheumatol. 1988;15:1480–8. [PubMed] [Google Scholar]
- 23.van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 2000;27:261–3. [PubMed] [Google Scholar]
- 24.Lee HK, Kim DS, Yoo B, et al. Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest. 2005;127:2019–27. doi: 10.1378/chest.127.6.2019. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen MD, Simpson-Haidaris PJ. Cell type-specific regulation of fibrinogen expression in lung epithelial cells by dexamethasone and interleukin-1beta. Am J Respir Cell Mol Biol. 2000;22:209–17. doi: 10.1165/ajrcmb.22.2.3746. [DOI] [PubMed] [Google Scholar]
- 26.Kim TH, Lee YH, Kim KH, et al. Role of lung apolipoprotein A-I in idiopathic pulmonary fibrosis: antiinflammatory and antifibrotic effect on experimental lung injury and fibrosis. Am J Respir Crit Care Med. 2010;182:633–42. doi: 10.1164/rccm.200905-0659OC. [DOI] [PubMed] [Google Scholar]
- 27.Baka Z, Barta P, Losonczy G, et al. Specific expression of PAD4 and citrullinated proteins in lung cancer is not associated with anti-CCP antibody production. Int Immunol. 2011;23:405–14. doi: 10.1093/intimm/dxr026. [DOI] [PubMed] [Google Scholar]
- 28.Khalil N, O'Connor R. Idiopathic pulmonary fibrosis: current understanding of the pathogenesis and the status of treatment. CMAJ. 2004;171:153–60. doi: 10.1503/cmaj.1030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsui T, Inui N, Suda T, et al. Anti-endothelial cell antibodies in patients with interstitial lung diseases. Respir Med. 2008;102:128–33. doi: 10.1016/j.rmed.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 30.Dall'Aglio PP, Pesci A, Bertorelli G, et al. Study of immune complexes in bronchoalveolar lavage fluids. Respiration. 1988;54(Suppl 1):36–41. doi: 10.1159/000195495. [DOI] [PubMed] [Google Scholar]
- 31.Sumpter TL, Wilkes DS. Role of autoimmunity in organ allograft rejection: a focus on immunity to type V collagen in the pathogenesis of lung transplant rejection. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1129–39. doi: 10.1152/ajplung.00330.2003. [DOI] [PubMed] [Google Scholar]