Abstract
Atherosclerosis (also known as arteriosclerotic vascular disease) is a chronic inflammatory disease of the arterial wall, characterized by the formation of lipid-laden lesions. The activation of endothelial cells at atherosclerotic lesion–prone sites in the arterial tree results in the up-regulation of cell adhesion molecules and chemokines, which mediate the recruitment of circulating monocytes. Accumulation of monocytes and monocyte-derived phagocytes in the wall of large arteries leads to chronic inflammation and the development and progression of atherosclerosis. The lesion experiences the following steps: foam cell formation, fatty streak accumulation, migration and proliferation of vascular smooth muscle cells, and fibrous cap formation. Finally, the rupture of the unstable fibrous cap causes thrombosis in complications of advanced lesions that leads to unstable coronary syndromes, myocardial infarction and stroke. MicroRNAs have recently emerged as a novel class of gene regulators at the post-transcriptional level. Several functions of vascular cells, such as cell differentiation, contraction, migration, proliferation and inflammation that are involved in angiogenesis, neointimal formation and lipid metabolism underlying various vascular diseases, have been found to be regulated by microRNAs and are described in the present review as well as their potential therapeutic application.
Keywords: Atherosclerosis, endothelial cell, macrophage, microRNA, vascular smooth muscle cell
MicroRNA BIOGENESIS AND FUNCTION
MicroRNA (miRNAs) are an established class of well-conserved, short non-coding RNAs that have emerged as key post-transcriptional regulators of gene expression in animals and plants. miRNAs have been described to play major roles in most, if not all, biological processes by influencing stability and translation of mRNAs [1]. miRNA genes are transcribed by RNA polymerase II as capped and polyadenylated primary miRNA transcripts (pri-miRNA) [2, 3]. Pri-miRNA processing occurs in two steps, catalyzed by two enzymes, Drosha and Dicer in cooperation with a dsRNA binding protein, DiGeorge syndrome critical region gene 8 (DGCR8) [4]. In the first step, the Drosha–DGCR8 complex processes pri-miRNA into a ~70-nucleotide precursor hairpin (pre-miRNA), which is exported to the cytoplasm. Some pre-miRNAs are produced from very short introns (mirtrons) as a result of splicing and debranching, thereby bypassing the Drosha–DGCR8 step [5, 6]. The nuclear export of pre-miRNAs is mediated by the transport receptor exportin 5 (XPO5) [7]. In the cytoplasm Dicer maturates pre-miRNA into an imperfect RNA duplex [8]. The strand of the duplex with the weakest base paring at the 5′ terminus is loaded into the miRNA-induced silencing complex (miRISC) and therefore considered to be biologically active [9]. Both strands of duplexes are produced in equal amounts by transcription; surprisingly their accumulation into the miRISC is asymmetric [10]. Initially, the other strand was assumed to be an inactive passenger strand called the miRNA*, however systemic computational analysis has demonstrated that the sequences of miRNA* strands contain, like regular miRNA, well-conserved target recognition sites indicating a functional relevance of the miRNA* molecules [10–12]. Indeed, several publications have reported the functional activity of passenger strands [13–15]. The possible impact of these initially considered non-functional passenger strands has widened the regulatory potency of miRNA-duplexes.
After the selected strand is loaded into the miRISC, the miRNA guides the miRISC to bind to the 3′UTR of its target sequence. The seed sequence (the first 2 to 8 nucleotides) is thereby the most important sequence for target recognition and the consequential silencing of the mRNA [16, 17]. Translation of the mRNA is inhibited after association of the miRISC with its target sequence in several successive steps. Efficient mRNA targeting requires continuous base pairing of the seed region to the target mRNA. Furthermore, Ago-proteins and the glycine-tryptophan protein of 182 kDa (GW182), core components of the miRISC, are directly associated with miRNAs and needed for effective translational repression. The exact mechanisms of translational arrest by the miRNA:mRNA complex are still a matter of debate, however both initiation and elongation steps of translation are thought to be affected [18–20].
ATHEROSCLEROSIS
Atherosclerosis is one of the leading causes of morbidity in Western countries. In its extreme clinical manifestations, atherosclerosis induces narrowing of vessel lumen causing ischemic symptoms, as well as plaque rupture or thrombosis that might lead to death by myocardial infarction or stroke [21].
Atherosclerosis is a chronic inflammatory and progressive pathology characterized by the accumulation of lipid and fibrous elements in the large arteries. It is caused by the interaction of many key components, like lipoproteins, macrophages, T cells and arterial wall components such as endothelial cells (ECs) and smooth muscle cells (SMCs). During the progression of atherosclerosis, lipoproteins penetrate into the intima layer of arteries, are then oxidized in the subendothelial space and together with inflammatory cytokines cause the activation of ECs. Activated ECs with high expression levels of various leukocyte adhesion molecules recruit monocytes and T cells to bind to the endothelium and migrate into the subendothelial site. Monocytes differentiate into macrophages and up-regulate pattern recognition receptors, including scavenger receptors and toll-like receptors. Scavenger receptors mediate lipoprotein internalization, which leads to foam-cell formation. Accumulation of inflammatory cells in the intima further promotes activation of ECs and in advanced stages of plaque formation SMCs proliferate and migrate from the medial portion of the arterial wall into the subintimal space where they also take up lipoproteins and become foam cells. By secreting extracellular matrix proteins, SMCs promote the formation of a fibrous cap. Apoptosis and necrosis of macrophages and SMCs in the lesion contribute to the necrotic core formation, as well as extracellular cholesterol accumulation. Plaque neovascularization in advanced lesions contributes to plaque rupture, which in turns recruits platelets and forms thrombi that lead to myocardial infarction or stroke. Numerous genetic and environmental factors account for the etiology of the disease by dynamically affecting the interplay between the involved cells. Recent body of evidence has shown that miRNAs have a prominent role in the disease progression as we discuss in the present review.
ATHEROSCLEROSIS, ENDOTHELIAL CELLS AND microRNAs
ECs form the barrier between blood and all tissues. While small blood vessels consist of ECs only, larger vessels include also pericytes and SMCs [22]. During adulthood, the quiescent endothelium has a low turnover rate and proliferates only following angiogenic or inflammatory activation. The loss of quiescence and barrier functions are common features of atherosclerosis, tumor progression and restenosis. Especially in atherosclerosis, inflammation and angiogenesis are eminently intertwined in activating the endothelium. Chronic inflammatory activation of ECs of large blood vessels induces increased proliferation of vasa vasorum, positively supporting lesion progression. Advanced human atherosclerotic plaques are characterized by neovascularization. In the atherosclerotic intima layer neovascularization proceeds with irregular but ubiquitous distribution of blood vessels, which are immature and leaky thereby contributing to disease progression. As the intima thickens, the hypoxic areas of the plaque further promote angiogenesis, newly formed blood vessels deliver more inflammatory leukocytes to the plaque, which in turn produce cytokines, chemokines and growth factors that mediate cytokine-induced angiogenic programs in a positive feedback fashion.
The relevance of miRNAs in EC physiology was showed through the disruption of Dicer and Drosha in vitro, which drastically reduce the expression of most miRNAs [23–25]. In particular, ECs in which Dicer is silenced display affected tyrosine kinase endothelial (TEK)-2, TEK-1, vascular endothelial growth factor receptor (VEGFR)-2, endothelial nitric oxide synthase (eNOS) and Interleukin 8 (IL8) expression. In particular, cord formation in matrigel was inhibited in the absence of Dicer because of increase in eNOS activation, whose repression was released upon lowering the levels of miR-221/222 [23]. The capillary ability of ECs was also affected by knocking down simultaneously Dicer and Drosha. In this system decreased levels of let-7a and miR-27b were responsible for the antiangiogenic effects in ECs. Interestingly, the generation of an EC-specific Dicer knockout mouse model confirmed that miRNAs play a fundamental role in the vessel development in adulthood in response to proangiogenic factors [26].
Multiple pathophysiological factors contribute the endothelial activation such as physical damage, hypoxia, incipient neoplasia and altered shear stress. In response to inflammatory stimuli, mainly mediated by nuclear factor of kappa light polypeptide gene enhancer in B-cells (NF-κB) activation, the vascular endothelium expressed adhesion molecules including vascular cell adhesion molecule 1 (VCAM-1), E-selectin (SELE), and intercellular adhesion molecule 1 (ICAM-1) that mediate leukocyte recruitment from the blood into extravascular tissues. MiR-181b and miR-10a affect EC activation by negatively modulating NF-κB signaling, therefore promoting an anti-inflammatory phenotype both in vitro and in vivo. In particular, the expression of miR-181b is regulated by tumor necrosis factor (TNF)α. This miRNA controls EC activation by targeting importing 3α (KPNA4), a protein required for nuclear translocation of NF-κB. By indirect targeting, miR-181b inhibits TNFα-induced expression of adhesion molecules and antagonizes the binding of leukocytes to ECs [27].
On the other hand, miR-10a targets mitogen-activated kinase kinase kinase 7 (MAP3K7) and β-transducin repeat containing gene (βTRC), both main regulators of nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor (IkB)α degradation. Interestingly, the expression of miR-10a is decreased in athero-susceptible arterial regions and it inversely correlates with MAP3K7 and βTRC expression. In addition, the knockdown of miR-10a decreased the expression of pro-inflammatory cytokines and adhesion molecules in ECs [28].
A growing body of literature highlighted the role of endothelial miRNAs in the regulation of various physiological and pathological processes linked to angiogenesis and inflammation. For instance, previous reports show that miR-126, an EC-enriched miRNA, plays a role in vascular inflammation through the negatively regulation of VCAM-1 in ECs [29, 30].
Inflammatory stimuli increase the expression of certain miRNAs in ECs. It has been shown that TNFα stimulation up-regulates miR-17-3p and miR-31, which inhibit the expression of ICAM-1 and E-selectin respectively, providing a negative feedback control of inflammation [31]. Additionally, the TNF-induced miR-155 [31] negatively regulates the expression of the transcription factor v-ets erythroblastosis virus E26 oncogene homolog 1 (Ets-1) [32], which, in addition, has been shown to be regulated by miR-221/222. The over-expression of both miRNAs results in a decrease of Ets-1 expression induced by angiotensin II (Ang II). As a consequence, the reduced expression of Ets-1 affects the expression of downstream targets such as VCAM-1, fms-related tyrosine kinase 1 (FLT1) and monocyte chemoattractant protein 1 (MCP1) impairing lymphocyte adhesion on ECs [32]. Furthermore, miR-155 also targets angiotensin II type 1 receptor (AT1R), resulting in decreases Ang II-induced migration in ECs [32].
Other cytokines regulate the expression of miRNA. For instance, IL-3 and bFGF down-regulate the expression of miR-221/222. Interestingly, signal transducer and activator of transcription 5A (STAT5A), a transcription factor that regulates the expression of genes involved in proliferation and migration, was identified as a target of miR-222. The up-regulation of STAT5A mediated by IL-3 and basic fibroblast growth factor (bFGF) is induced by the down-regulation of miR-222. This controls proliferation and migration in ECs and therefore facilitates intraplaque neovascularization during atherosclerosis. Moreover, diminished expression of miR-222 in advanced lesions correlates with an increased proliferation rate of ECs lining vessels [33]. In addition, the cluster miR-221/222 was previously shown to regulate EC migration and proliferation through the regulation of c-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog (c-Kit) [34].
ECs can also be activated by oxidized lipoproteins. Lipid deposition in the subintimal layer does not only affect foam cell formation in SMCs and macrophages, increasing inflammation and increasing the fibrous cap, but it has been shown to induce pro-inflammatory activation and apoptosis in ECs. The apoptotic phenotype in ECs is due to the oxidized-low density lipoproteins (ox-LDL)-mediated decrease in the expression of anti-apoptotic gene B-cell CLL-lymphoma 2 (Bcl-2) [35]. Interestingly, delivery of ox-LDL to ECs increases the expression of several miRNAs, including miR-365 [36] which participate in the apoptotic program induces by down-regulating Bcl-2.
Alterations associated with aging in blood vessels include a decrease in compliance and an increase in vascular inflammatory response, both of which promote atherogenesis. Several reports show the dysregulation of miRNAs during aging in ECs. In particular, miR-146 expression is decreased in senescent ECs. This miRNA is targeting the expression levels of NADPH oxidase 4 (NOX4), therefore decreasing the reactive oxygen species (ROS) production. These data suggest that the reduction of the expression of miR-146 may enhance aging by NOX4-derived ROS production [37]. In contrast, miR-217 is progressively expressed in ECs during aging [38]. MiR-217 is targeting silent information regulator 1 (SirT1), a main regulator of longevity and metabolic disorders that is reduced in different tissues during aging. Interestingly, miR-217 is negatively correlated with SirT1 expression in human atherosclerotic plaques [38].
The endothelial progenitor cells (EPCs) can be mobilized from the bone marrow to enter the peripheral circulation. EPCs are able to regenerate injured endothelium, accelerate re-endothelization, and limit the formation of atherosclerotic lesions. Aging is associated with reduced number and function of EPCs. Increasing evidence supports the role of miRNA in regulating EPCs senescence. For instance, miR-34a is involved in senescence by targeting SirT1 in EPCs [39]. On the other hand, miR-10a* and miR-21 are progressively expressed EPCs and both miRNAs suppress high mobility group AT-hook 2 (Hmga2) expression, a chromatin-associated protein that modulates transcription by altering chromatin structure. Both, miR-10a* and miR-21 over-expression decrease proliferation and impair EPC angiogenesis in vitro and in vivo [40].
Hypoxia is defined as the decrease of oxygen supply in tissues and cells and it is a potent inducer of angiogenesis and modulator of inflammation. Inflammation aggravates hypoxia by reducing oxygen availability. This effect is produced by an increase in the metabolic demand as well as by the thickening in the intimal layer, reducing oxygen supply and contributing synergistically to exacerbate hypoxia in atherosclerotic lesions [41]. Decreased oxygen tension promotes the stabilization of hypoxia inducible factors (HIF), mainly HIF-1α and HIF-2α, which act transcriptionally to induce expression of genes that promote survival of cells, anaerobic metabolism switch and angiogenic programs [42, 43]. Hypoxia also regulates miRNAs, which in turn modulate the angiogenic properties of ECs. The classical hypoxia-miR miR-210 is induced in a HIF-1α dependent manner and is up-regulated in atherosclerotic plaques [44]. Recent reports have shown that miR-210 targets EphrinA3 (EFNA3) therefore stimulating tubulogenesis and chemotaxis in ECs [45]. Intriguingly, miR-424, which is also up-regulated in hypoxic ECs, down-regulates Cullin2 (CUL2), which is responsible for HIF-1α and HIF-2α destabilization resulting in an increase proliferation and migratory capabilities in ECs [46]. Overall miRNAs that are induced in hypoxia contribute to the activation of the angiogenic properties of ECs and promote angiogenesis in atheromas.
The atherosclerotic lesions form preferentially where arteries form curves, branches or bifurcations where the blood flow is disturbed. This notion is consistent with the observation that ECs in the aortic arch, which subjected to turbulent flow and therefore is predisposed to plaque formations, has a very distinct gene expression profile compared to ECs of the descending thoracic aorta, which is a protected arterial tract where the flow is more laminar. In areas of disturbed flow, proinflammatory and proangiogenic factors such as NF-κB, bone morphogenetic protein (BMP)-4 as well as adhesion molecules are highly expressed, while in laminar flow areas there is over-expression of atheroprotective genes such Krüppel-like factor 2, Krüppel-like factor 4 (KLF2, KLF4) and eNOS. Interestingly, shear stress can modulate the expression of some miRNAs in ECs. For instance, shear stress induces miR-663 expression, which modulates the inflammatory phenotype by targeting Interleukin 6 (IL6), IL8, and E-Selectin [47]. MiR-663 is also responsible for increasing monocyte binding to ECs during shear stress. Intriguingly, KLF4, an atheroprotective effector down-regulated during oscillatory stress, is a putative target of miR-663 [47]. In addition, the expression of miR-21 is also up-regulated in response to prolonged shear stress modulating the activity of phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/v-akt murine thymoma viral oncogene (Akt) pathway resulting in an increase of nitric oxide (NO) production and reducing apoptosis in ECs [48]. Shear flow also induces the expression of the miR-17~92 cluster. Interestingly miR-92a targets the atheroprotective factors KLF4 and KLF2 in shear stress. MiR-92a exerts its proatherogenic effect by suppressing KLF2-mediated eNOS and trombomodulin expression, as well as KLF4-induced expression of MCP1, VCAM-1, E-selectin and eNOS [49]. Additionally, since KLF2 induces the expression of the atheroprotective miR-126, over-expression of miR-92a might also control the regulation of miR-126 during shear stress [49–51].
Role of Secreted Microvesicle-associated microRNAs in Atherosclerosis
A recent body of evidence suggests that cells can communicate with neighboring and distant cells by means of secreted RNA-protein-lipid complexes or extracellular vesicles [52]. Interestingly, vesicle-mediated exchange of information is achieved by delivering proteins, mRNAs and miRNA that may affect the phenotype of receiving cells [53]. Cells release extracellular vesicles originating from different intracellular pathways that determine their sizes and function. There are three main kinds of vesicles: exosomes, microvesicles and apoptotic bodies. Exosomes are membrane-bound vesicles of 30–100nm in diameter released by fusion of endocytic multivesicular bodies to the cellular surface [54]. Microvesicles are more heterogeneous and are produced by budding of plasma membrane and range between 100 nm and 1 μm in diameter [55]. Apoptotic bodies are about 50–500 nm in size and contain also DNA and histones [55]. MiRNA enclosed in vesicles are protected from RNases and can be delivered effectively to target cells in vivo [56]. It is not clear whether vesicles released from a cell have affinity for specific target cells, but it is possible that receptor-mediated mechanisms direct the uptake of vesicles and delivery of their cargo to target cells [57].
Inflammatory stimuli in monocytes trigger miR-150 release in 20–400 nm vesicles. Monocytes from atherosclerotic patients produce microvesicles enriched in miR-150 compared to microvesicles from healthy patients. Interestingly, by delivering monocytic miR-150-enriched microvesicles in vivo, ECs show decreased migratory abilities and proliferation because of decreased expression of v-myb myeloblastosis viral oncogene homolog (c-myb) [58]. Also macrophages can secrete microvesicles containing miRNA that affect recipient cells. A recent research has shown that miR-223-rich microvesicles produced by human macrophages can be taken up by a variety of cells. Interestingly, human miR-223-microvesicles can be functionally delivered to other macrophages and induce their differentiation [59]. Furthermore, miR-143/145 is secreted in microvesicles derived from ECs in which KLF2 is up-regulated to mimic shear stress. When KLF2-induced miR-143/145 rich vesicles are delivered to SMCs they induce an atheroprotective phenotype by down-regulating key target genes like member of ETS oncogene family (ELK1), KLF4, calcium/calmodulin-dependent protein kinase II delta (CAMK2d), slingshot homolog 2 (SSH2), phosphatase and actin regulator 4 (PHACTR4) and cofilin 1 (CFL1). Similarly, delivery of these KLF2 atheroprotective vesicles in an in vivo model of atherosclerosis reduces lesion size and the effect is abrogated when vesicles are depleted of miR-143/145 [60].
Apoptotic bodies derived from ECs contain miRNAs. During atherosclerosis, vesicles containing high levels of miR-126 are released into apoptotic bodies and their delivery to ECs decreases the expression of G-protein signaling 16 (RGS16) that results in the up-regulation of the chemokine (C-X-C motif) ligand 12 (CXCL12) receptor. By regulating the chemokine CXCL12 signaling, miR-126 enriched apoptotic vesicles decrease apoptosis and recruit progenitor cells at the site of lesion, therefore contributing to plaque stabilization and reducing lesion size in vivo [61].
ATHEROSCLEROSIS, VASCULAR SMOOTH MUSCLE CELLS AND microRNAs
Vascular smooth muscle cells (VSMCs) are highly specialized cells that regulate arterial tone and blood pressure [62]. During vascular development, VSMCs are required for the investment of nascent blood vessels and appropriate vascular maturation and formation of functioning vascular networks [63, 64]. In addition, they also play a critical role in secretion of extracellular matrix components, including collagen and elastin, which determine the mechanical properties of mature blood vessels [65, 66]. In adult blood vessels, differentiated VSMCs proliferate at an extremely low rate, exhibiting low synthetic activity, and expressing a unique repertoire of contractile proteins, ion channels, and signaling molecules required to fulfill VSMC main function, contraction [67, 68]. However, it is well accepted that VSMCs within adult retain a remarkable plasticity, which allows them to undergo phenotypic modulation in response to physiological and pathological environmental cues. This VSMC phenotypic modulation, referred to as phenotypic switching, is characterized by significant changes in cellular gene expression pattern [69]. In general, VSMC phenotypic switching is characterized by a marked reduced expression of VSMC-selective differentiation marker genes and increased VSMC proliferation, migration, and synthesis of extracellular matrix components required in most of cases for vascular repair in response to injury [62, 67, 70–72]. It is well established that this phenotypic switching plays a critical role in the pathogenesis of vascular proliferative syndromes including atherosclerosis, restenosis following angioplasty, and transplant arteriopathy [62, 70–72]. The complex lineage origins and lack of definitive VSMC markers have complicated the assessment of the molecular mechanisms regulating modulation of VSMC phenotype in response to arterial injury (reviewed in [73]). Recently, advances in studies of miRNA expression and function in VSMCs illustrate important effects of this class of small non-coding RNAs on cell proliferation, hypertrophy and differentiation of VSMCs and their implication to different vascular pathological conditions.
Conditional deletion of Dicer allele using Cre recombinase under control of the SM22 promoter results in embryonic lethality at E16.5 associated with extensive hemorrhage in the skin and abdomen [74]. Several vascular phenotypes were observed in SM22-Dicer KO embryos including outward hypotrophic remodeling of the aorta, defective organization of elastic lamina of the aorta, loss of contractile function in umbilical artery, as well as reduced expression of SMC-specific genes and proteins. This indicates that Dicer-generated miRNAs were essential for normal VSMC development, differentiation, and contractile function [74]. Interestingly, inducible deletion of Dicer in VSMCs produces a dramatic reduction in blood pressure [75], decreased contractile function in the bladder in response to depolarization, associated with reduced expression of contractile marker proteins and L-type Ca2+ channels [76]. Altogether, suggesting that Dicer-miRNAs were also critical for the postnatal regulation of VSMC functions [75, 76].
In addition to the global role of VSMC miRNAs, specific miRNAs have been shown to play important roles in the regulation of VSMC development, differentiation and different vascular pathological conditions, which makes these molecules interesting as potential new therapeutic targets [77–81]. MiR-21 was the first miRNA shown to regulate growth and survival by diminishing the expression of phosphatase and tensin homolog (PTEN) and increasing expression of Bcl-2 [82]. The pro-proliferative and antiapoptotic effects of miR-21 were established in a carotid injury model in rats [82]. MiR-21 was then described to promote differentiation of VSMCs in response to transforming growth factor (TGF)-β1 and BMP-4 [83]. Specifically, these factors stimulated the processing of miR-21 in human pulmonary artery smooth muscle cells from the primary transcript to the mature miRNA via Smads proteins. Interestingly, miR-21 stimulation induced the up-regulation of smooth-muscle restricted contractile proteins through the silencing of programmed cell death protein (PDCD)-4 expression, a known tumor suppressor protein. These data indicate that miR-21 regulates contractile function [83] in addition to proliferation [82]. In the context of BMP-4 regulated expression, miR-21 have been reported to target multiple members of the dedicator of cytokinesis (DOCK) superfamily and modulates the activity of ras-related C3 botulinum toxin substrate 1 (Rac1) small GTPase to regulate VSMC phenotype [77]. Additionally, miR-21 have been show to regulate hypoxia-induced pulmonary vascular smooth muscle cell proliferation and migration by regulating PDCD4, Sprouty 2 (SPRY2), and peroxisome proliferator-activated receptor-α (PPARα), known for their antiproliferative and antimigratory effects in VSMCs [84]. Interestingly, a recent report indicates that miR-21 is induced in tissue of arteriosclerosis obliterans of the lower extremities with <10% stenosis and induced in VSMCs in response to platelet-derived growth factor (PDGF)-BB or hypoxia. In this report tropomyosin 1 (TPM1) was identified as a target gene for miR-21. TPM1 reduction leads to a reduction in cytoskeletal stability, leading to vascular smooth muscle cell proliferation and migration [85]. Finally, cyclic stretch has been shown to modulate miR-21 expression at the transcription level via FBJ murine osteosarcoma viral oncogene homolog (c-fos/AP-1) in cultured human aortic SMCs [86]. Moderate stretch seems essential for maintaining vessel wall structure and vascular homeostasis [87]; however, exacerbated stretch, as in hypertension, could promote pathological vascular remodeling by stimulating VSMC proliferation, apoptosis, migration and abnormal extracellular matrix deposition [88, 89]. Although miR-21 is considered an onco-miRNA, its expression was found increased in many solid tumors with characteristics of promoting cell proliferation, migration and anti-apoptosis [90], data indicates that miR-21 is also highly expressed in VSMC vascular cells and therefore implicated in the regulation of important phenotypes of these cells and vascular disorders [91] such as vascular neointimal lesions [92]. Of note, miR-21 expression is increased during abdominal aortic aneurysm (AAA) development (AAAs induced by either porcine pancreatic elastase or infusion of angiotensin II) [93]. Interestingly, lentiviral over-expression of miR-21 induced cell proliferation and decreased apoptosis in the aortic wall, with protective effects on aneurysm expansion. Conversely, systemic injection of a locked nucleic acid–modified antagomir targeting miR-21 diminished the pro-proliferative impact of down-regulated PTEN, leading to a marked increase in the size of AAA. The data presented elegantly by Maegdefessel et al. suggests that the up-regulation of miR-21 is a physiological response to aortic expansion and that the modulation of miR-21 expression is a promising new therapeutic option to limit AAA expansion and vascular disease progression [93].
Several studies from different groups have shown that miR-143/145 play a key role in SMC phenotypic switching in response to vascular injury and that these miRNAs affect the canonical balance between the synthetic/proliferative and the contractile/differentiated states in VSMCs [94–98]. These effects are likely to be mediated, at least in part, through the targeting of multiple transcription factors, including KLF4, KLF5, and ELK-1, that are linked to the repression of SMC differentiation [95, 96, 98]. MiR-145 is the most abundant miRNA in normal differentiated blood vessels and in freshly isolated VSMC [95]. The earliest observation of changes in miRNA expression in blood vessels show that miR-145 was down-regulated in neointimal lesions following vascular injury [95]. In this report, adenovirus-mediated over-expression of miR-145 could partially restore the down-regulation of SMC marker genes and neointima formation following balloon injury of the rat carotid artery. In cultured VSMCs, miR-145 was required for PDGF-BB–induced phenotypic switching of these cells by targeting KLF5. The mechanism of miR-145 in regulating smooth muscle phenotype was subsequently more deeply defined with the phenotypic characterization in a murine model of targeted deletion of the miR-143/145 bicistronic gene cluster [96]. These miRNAs target the degradation of KLF4 and ELK-1, transcription factors known to suppress SMC differentiation and produce an indirect up-regulation of myocardin expression. MiR-143/145 were in turn shown to be activated, in differentiated SMCs, by cardiac-specific homeo box (Nkx)-2.5, serum response factor (SRF) and myocardin -now considered to be the master gene regulating VSMC differentiation [99]- thus providing a potential positive-feedback mechanism to stabilize the SMC differentiated state [96, 98]. In contrast, these miRNAs were down-regulated in injured or atherosclerotic vessels [96, 98]. In addition to the miR-143/145 cluster, miR-146a can modulate KLF4 expression in VSMC [100]. MiR-146a and KLF4 form a feedback loop to regulate each other’s expression and VSMC proliferation. Specifically, it is proposed that miR-146a regulates KLF4 and that KLF4 competes with KLF5 within the miR-146a promoter to reduce the transcription of the miR-146a gene [100]. The initial signal that activates the miR-146a-KLF4/KLF5 pathway is not established, but it would be an obvious target for reducing vascular remodeling during restenosis after angioplasty [78].
Unlike SM22-Dicer KO [74], loss of miR-143/145 either singly or in combination is not embryonic lethal [94, 97, 98] suggesting that other miRNAs are also involved in VSMC development and that miR-143/145 are not essential for VSMC differentiation in vivo. However, several abnormalities were found in miR-143/145 KO mice including a reduction in SMC marker expression and contractile function [94, 97], reduced medial thickness [94, 97, 98], increased rough endoplasmic reticulum [97, 98], and loss of actin stress fibers and dense bodies [94, 98]. Blood pressure in miR-143/145 KO mice was also reduced due to the vascular abnormalities [94, 97, 98] and consistent with the phenotype observed after the inducible deletion of Dicer in VSMCs in adult mice [75]. In miR-143/145 KO mice, some of these defects were associated with impaired contractility of femoral artery rings in response to high K+, angiotensin II, and phenylephrine [94]. Additionally, neointima formation in response to vascular injury was profoundly inhibited due at least in part to the disarray of actin stress fibers and diminished SMC migratory activity [98]. Interestingly, VSMCs isolated from SM22-Dicer KO mice, showed a remarkable loss of actin filament structures [74], an effect that was rescued by over-expression of miR-145. Importantly, over-expression of miR-145 also rescued the reduced SMC marker expression pattern observed in VSMC with deleted Dicer [74]. Despite the lack of evidence for a developmental phenotype in miR-143/145 KO mice, there is clear evidence that the up-regulation of these miRNAs is required for embryonic stem cell (ESC) differentiation at least in part by their targeting degradation of Oct4, Sox2, and KLF4, resulting in a loss of pluripotency [101]. Additional studies are needed to clarify the role of these miRNAs in SMC development from multipotent embryonic cells. In addition to regulate contractile function in VSMCs, miR-143/145 may also participate in podosome formation [102]. In fact, down-regulation of miR-143/-145 was sufficient to up-regulate PDGF receptor (PDGF-R), protein kinase C (PKC) epsilon and fascin, an actin bundling protein of podosomes which are local sites of matrix remodeling thought to be necessary for vascular wall remodeling [102]. Recently it has been shown that long-term SMC-specific up-regulation of miR-145 favorably alters plaque morphology and cellular composition in ApoE−/− mice, shifting the balance toward plaque stability versus plaque rupture. These benefits appear to be mediated through an effect of miR-145 to promote VSMC differentiation toward the contractile phenotype via a mechanism that involves reciprocal regulation of KLF4 and myocardin [103].
The relevance of miR-143/145 in human vessel pathology was suggested by down-regulation of the miR-143/145 cluster in human aortic aneurysms by comparison with normal aortic tissue [97], suggesting a role for these miRNAs in maintaining vessel homeostasis. Interestingly, extracellular miRNAs have diagnostic and prognostic potential have been used as biomarkers to classify diseases and progression of diseases [104, 105]. Remarkably, circulating levels of inflammation-associated and vascular miRNAs, including miR-145, were significantly decreased in patients with coronary artery disease [106]. As introduced in the section above, recent studies have revealed that miRNAs also serve as messengers between cells [58, 60]. To this regard, it has been proposed that shear stress-induced KLF-2 stimulates the expression of miR-143/145 in ECs [60], that do not usually express these miRNAs in resting conditions [95]. These miRNAs are then released in microvesicles (MVs), which are transferred into VSMCs that per se express high levels of miR-143/145 [95]. When ECs derived MVs, containing miR-143/145, were injected into Apolipoprotein E (ApoE) knockout the formation of atherosclerotic lesion was reduced [60].
In addition to miR-21 and miR-143/145, miR-221 and miR-222 have also been implicated in VSMC function and phenotypic plasticity [82, 83, 95–97, 107]. MiR-221 and miR-222, like miR-21, are up-regulated in neointimal lesions [108]. Knockdown of miR-221 and miR-222 in rat carotid arteries suppress SMC proliferation and neointimal lesion formation after angioplasty. MiR-221 and miR-222 appear to be essential for PDGF-mediated cell proliferation, by repressing c-Kit, the cyclin dependent kinase inhibitors p57Kip2 and p27Kip1 [108, 109]. Interestingly, another study also documents the presence of miR-133 in VSMCs and reveals essential roles of this miRNA in the control of VSMC phenotypic switch both in vitro and in vivo. Indeed, miR-133 is robustly expressed in VSMCs to levels similar to other previously characterized vascular miRNAs, whereas the expression of its cognate bicistronic gene, miR-1, is negligible. In this regard miR-133, but not miR-1, regulates VSMC growth state by inhibiting VSMC proliferation and migration in vitro via the repression of the transcription factor Sp-1, which mediates miR-133 inhibition of SMC gene down-regulation and thus VSMC proliferation. Of interest, miR-133 is down-regulated in proliferating VSMCs of carotid arteries in response to balloon injury in rats. Its adenoviral over-expression in the vascular wall reduces VSMC hyperplasia after experimental balloon injury, whereas anti-miR-133 systemic treatment exacerbates it. Altogether, pointing to a crucial role of miR-133 in regulating pathological vascular remodeling in vivo [110].
ATHEROSCLEROSIS, EXTRACELLULAR MATRIX AND microRNAs
Plaque stability can be achieved not only by increasing the extracellular matrix (ECM) production but also by decreasing the ECM degradation. ECM synthesis is tightly regulated by various factors in the cellular environment, such as growth factors, cytokines, nitric oxide and surrounding the ECM. MiR-29 is found to be up-regulated in aged arteries and its expression inversely correlates with the expression of its downstream target genes [111–115]. These include collagen, type I, α 1 (COL1A1), COL3A1, and COL5A1, matrix metalloproteinases such as, MMP2 and MMP9, as well as other key components of the aortic wall, such as fibrillin-1 (FBN1) and elastin (ELN), many of which are expressed in the abdominal aorta and have been linked to AAA formation and progression [111–115]. The miR-29 family comprises 3 members (29a, 29b, and 29c) encoded by two distinct loci that give rise to a bicistronic precursor, which includes miR-29a/b1 and miR-29b2/c. Interestingly, in 2 murine models of experimental AAA (the porcine pancreatic elastase (PPE) infusion model in C57BL/6 mice and the AngII infusion model in Apoe−/− mice). AAA development was accompanied by decreased aortic expression of miR-29b, along with increased expression of known miR-29b targets, Col1a1, Col3a1, Col5a1, and Eln, in both models [114]. In vivo administration of locked nucleic acid (LNA) anti-miR-29b greatly increased collagen expression, leading to an early fibrotic response in the abdominal aortic wall and resulting in a significant reduction in AAA progression over time in both models [114]. Similarly, miR-29 is also up-regulated in aneurysms of fibulin-4 knockout and fibrillin transgenic (Fbn1C1039G/+) mice and in angiotensin-treated mice, suggesting an important role for this miRNA family in regulating vascular integrity [111, 115]. In addition, it has recently been shown that inhibition of miR-29a can dramatically increase ELN expression in human cells and its inhibition up-regulates ELN levels in cells from patients with ELN haploinsufficiencies, including the Williams-Beuren syndrome (WBS) [113]. Importantly, atherogenic lipoproteins, such as ox-LDL, up-regulate miR-29b expression in VSMCs through the interaction with the lectin-like oxidized lipoprotein receptor-1 (LOX-1) and the activation of the mitogen-activated protein kinases (MAPK) including JNK, p38 and ERK and the subsequent activation of the miR-29 promoter by directly binding of c-Fos to an AP-1 site [116]. Furthermore, ox-LDL causes a reduction of histone deacetylases (HDAC)-1, -2, -3 and -7 expression. Moreover, HDAC-1 over-expression significantly abolishes the effect on miR-29 expression suggesting that this HDAC is likely involved in regulating miR-29 expression [116]. Altogether these results suggest that miR-29 expression is regulated by ox-LDL at different levels by a complex mechanism that includes transcription factors and epigenetic modifications [117]. Of interest is that miR-29 expression is increased in the aorta of mice fed a high fat diet, which may have potential implications for atherosclerosis. In addition, miR-29 enhances the expression of matrix metallopeptidase 9 (MMP-9) which is involved in regulating transmedial elastin degradation and ectasia in the atherosclerotic media, suggesting that anti-miR-29 therapy may be useful for treating media destruction observed in atherosclerotic plaques as well as abdominal aortic aneurysms [118].
Medial artery calcification decreases vessel elasticity, augments blood pressure, and is associated with increased incidence of many cardiovascular diseases, such as myocardial infarction and hypertension. This is becoming an increasingly common and a serious problem with population ageing and it increases plaque rupture during angioplasty procedures [119, 120]. The main feature of this process is characterized by apatite deposition on collagen fibrils and the presence of matrix vesicles [121]. In this process VSMCs acquire an osteogenic phenotype as indicated by increased expression of core-binding factor a1 (Cbfa1, Runx2), osteopontin, osteocalcin, and alkaline phosphatase (ALP) [121, 122]. MiR-204 is encoded within the transient receptor potential cation channel, subfamily M, member 3 (TRPM3) gene and is expressed in arterial smooth muscle cells and cardiomyocytes in the cardiovascular system [123]. MiR-204 inhibits osteoblastic differentiation through the down-regulat on of Runx2 [124]. Recently, it has been shown that miR-204 down-regulation may contribute to β-glycerophosphate-induced VSMC calcification through regulating Runx2. Indeed, in vivo over-expression of miR-204 by injection of miR-204 agomirs in mice attenuated vitamin D3-induced medial artery calcification [125]. MiR-204 may represent an important new regulator of VSMC calcification and a potential therapeutic target in medial artery calcification.
ATHEROSCLEROSIS, MONOCYTE/MACROPHAGE AND microRNAs
Foam cell formation due to excessive accumulation of cholesterol by macrophages is a pathological hallmark of atherosclerosis [21]. To avoid the massive accumulation of cholesterol, macrophages express adenosine tri-phosphate binding cassette (ABC) transporters including ABCA1 and ABCG1, which facilitate the cholesterol efflux to poor-lipidated apoA1 and mature high density lipoprotein (HDL) respectively [126]. In addition to the regulation of cellular cholesterol efflux, ABCA1 plays a key role in the biogenesis of HDL and its deficiency leads to a dramatic reduction of circulating HDL [127].
Different groups have recently identified miR-33a and miR-33b, intronic miRNAs located within the sterol regulatory element binding transcription factors (Srebp2 and Srebp1) genes, as important regulators of ABCA1 expression [128–132]. Both miRNAs directly target the 3′UTR of Abca1 and Abcg1, thereby inhibiting cholesterol efflux from macrophages. Most importantly, in vivo inhibition of miR-33, using a variety of strategies ranging from lentiviral-mediated antisense oligonucleotides, 2′fluoro/methoxyethyl-modified (2′F/MOE-modified) phosphorothioate backbone antisense oligonucleotides and locked nucleic acid (LNA) oligonucleotides, results in a significant increase of hepatic ABCA1 expression and circulating HDL-Cholesterol (HDL-C) [128–132]. These results were later confirmed in miR-33-deficient mice and non-human primates treated with 2′F/MOE anti-miR-33 oligonucleotides [133]. Altogether, these observations suggest that miR-33 may be an attractive target for the treatment of cardiovascular diseases.
To asses the efficacy of anti-miR-33 therapy, Rayner and colleagues studied the impact of miR-33 inhibition during atherosclerosis regression using LDLr deficient mice fed with Western diet (WD) for three months and then treated weekly for four weeks with 2′F/MOE anti-miR-33 oligonucleotides [134]. The study showed that the inhibition of miR-33 raises plasma HDL-C levels and decreases atherosclerotic plaque size [134]. Interestingly, plaques from mice treated with anti-miR-33 oligonucleotides showed increased markers of plaque stability, including more collagen content and reduced lipid accumulation and necrotic core areas. Another interesting aspect of this study is the identification of 2′F/MOE anti-miR-33 oligonucleotides located in the artery wall. MiR-33 antisense oligonucleotides co-localize with macrophages in atherosclerotic plaques and regulates the post-transcriptionally expression of ABCA1. Moreover, macrophages isolated from atherosclerotic plaques of mice treated with anti-miR-33 oligonucleotides using laser capture microdissection (LCM) showed a decrease expression of pro-inflammatory genes including inducible NOS (iNOS) and TNFα [134]. These results suggest that anti-miR-33 therapy is able to increase the expression of ABCA1, thereby enhancing cholesterol efflux and reducing inflammation by an unknown mechanism. Previous studies have shown that HDL regulates the inflammatory state of macrophages by switching them from a pro-inflammatory state characterized by iNOS and TNFα expression to the more reparative M2 phenotype characterized by Arginase 1 (Arg1) and IL-10 expression [135]. Therefore, it is possible to speculate that the anti-inflammatory effect mediated by the anti-miR-33 therapy is mostly due to an increase in the cholesterol efflux capacity rather than a direct targeting of miR-33 on the Arg1 and IL-10 3′UTR. Nevertheless, the role of miR-33 in regulating macrophage activation is somehow controversial. At this regard, a recent study has reported that miR-33 reduces the expression of the receptor interacting protein (RIP140), a co-activator of NF-κB, thereby reducing inflammation [136]. Over-expression of miR-33 in Raw264.7 murine macrophages suppresses IL-1β and TNFα secretion after lipopolysacaride (LPS) treatment [136]. Similarly, macrophages isolated form miR-33 deficient mice show an increase expression of TNFα and IL-6. Therefore, the contribution of miR-33 in regulating macrophage activation remains unclear and requires further investigation.
In addition with the regression studies, Horie and colleagues recently studied the role of miR-33 in regulating the progression of atherosclerosis using miR-33 deficient mice. Complete absence of miR-33 increases circulating HDL-C and reduces atherosclerosis in ApoE null mice. Atherosclerotic plaques isolated from miR-33−/−/ApoE−/− mice showed reduced accumulation of macrophages as well as neutral lipids. The anti-atherosclerotic effect observed in this model is likely due to an increase in circulating HDL-C rather than enhanced macrophage cholesterol efflux capacity as demonstrated by bone marrow transplantation experiments. Even though these results argue against the atheroprotective role of the absence of miR-33 in macrophages, these studies should be repeated in LDL receptor deficient mice since the absence of ApoE compromise the cholesterol efflux capacity of monocyte/macrophages [137]. MiR-33 also regulates the expression of genes involved in fatty acid oxidation including carnitine palmitoyltransferase 1A (Cpt1A), carnitine O-octanyl transferase (Crot), and hydroxyacyl-CoA dehydro-genase-3-ketoacyl-CoA thiolase-enoyl-CoA hydratase (tri-functional protein) β-subunit (Hadhb) [131, 138]. As expected, over-expression of miR-33 in human hepatic cell lines reduces the expression of CPT1A, CROT and HADHB and inhibits fatty acid β-oxidation leading to the accumulation of neutral lipids [138]. Based on these findings, Rayner and colleagues treated non-human primates with miR-33 antisense oligonucleotides and observed a significant reduction of circulating very low-density lipoproteins [133]. This observation could be easily explained by the increased lipidation of ApoB-100 and lipoprotein output from the liver. However, the effect of miR-33 silencing on fatty acid β-oxidation in vivo remains to be studied. Finally, miR-33 also regulates insulin signaling by targeting insulin receptor substrate (IRS) 2 [138]. Taken together, these studies suggest that miR-33 regulates pathways controlling three major risk factors of the metabolic syndrome, namely levels of HDL, triglycerides, and insulin signaling, and suggest that inhibitors of miR-33 may be useful in the treatment of this global health concern.
In addition to miR-33, there are a growing number of studies regarding the role of miRNAs in the biology of the human atherosclerotic plaque. Several reports have described the involvement of circulating miRNAs in vascular diseases [106]. Nonetheless whether they should be considered a diagnostic or prognostic marker, a consequence or a cause is still a matter of debate [139]. MiRNA expression profiles comparing atherosclerotic plaques versus healthy arteries [44, 140] or plaques from symptomatic and asymptomatic subjects have also been performed [141]. Even though these studies provide useful information about the homeostasis of human atherosclerotic plaques, they usually fail to decipher the specific source (cellular and/or area of the plaque) and the molecular mechanisms underlying the role of these miRNAs. New techniques such as LCM could help to elucidate the specific commitment of a single miRNA inside the cells and/or in the particular communication established by the cellular milieu in the human plaque [142]. Following this procedure Nazari-Jahantigh et al. validated miR-155 in macrophages as a key player in atherosclerosis development. MiR-155 promoted the expression of CCL2 and the direct suppression of Bcl-6, a transcription factor that inhibits NF-κB [143]. However, data regarding miR-155 are ambiguous, since it has been also described that hematopoietic deficiency of this miRNA increases atherosclerotic plaque size and instability [144], possibly by inhibition of lipid uptake and the inflammatory responses in monocytes. Thus, more studies should be performed to address this question. Another miRNA, miR-125a-5p, inhibits lipid uptake and the inflammatory responses in ox-LDLs-stimulated monocytes/macrophages through modulation of oxysterol binding protein-like 9 (OSBPL9) expression [145]. Moreover, miR-146a drives peripheral blood mononuclear cells towards Th1 polarization and the levels of this particular miRNA are increased in patients with acute coronary syndrome (ACS) [146]. Altogether these studies highlight the importance of miRNAs in regulating lipid metabolism and inflammation in macrophages and suggest that anti-miR therapies would be a promising therapy for treating atherosclerotic vascular disease.
MicroRNA THERAPEUTIC POTENTIAL IN ATHE-ROSCLEROTIC VASCULAR DISEASE
During development many miRNAs are expressed in a tissue specific manner indicating that miRNAs may be involved in specifying and maintaining tissue identity [147]. For instance, miR-1, miR-133 and miR-206 are enriched in muscle tissue [148], miR-122 is well conserved between species in the liver [147, 149, 150] and the expression of miR-126 is enriched in the endothelium of human, mice and zebrafish [147, 151]. The conservation between species suggests that the biological pathways where miRNAs play a role also may have been conserved, making them research targets of high interest with possible potency to be applied therapeutically. The therapeutic application of miRNA can be carried out by two approaches, either by inhibition of miRNAs using a variety of chemically modified oligonucleotides complementary with the sequence of the miRNA [152] or by mimicking the functional activity of miRNAs by the use of so-called miRNA mimics [153]. To date, several different methods to silence miRNAs in vitro and in vivo have been established. First, 2′-O-methyl stabilized oligonucleotides (anti-miRs) were used in D. melanogaster and C. elegans to successful inhibit the function of let-7 [152]. Although the mechanisms behind anti-miR:miRNA biology is not well studied, it has been suggested that the duplex, formed after the binding of an anti-miR to a target miRNA, is a configuration that cannot be processed by the miRISC [153, 154]. To increase cellular uptake in larger animals, these 2′-O-methyl antimiRs were conjugated with cholesterol. To prove functionality these, so-called, antagomirs were direct against miR-16 and miR-122 and subsequently systemically delivered to mice by tail vein injection. Indeed, antagomirs were shown to be powerful tools that are able to knock down miRNA function for up to 23 days in virtually all tissues [155], including the central nervous system when applied locally [156]. The use of the antagomirs and anti-miRs has been extensively studied and widely used in vitro and in vivo studies elucidating functional roles for miRNAs in a variety of biological pathways. Also, oligonucleotides containing locked nucleic acid (LNA)–modified phosphorothioate oligonucleotide DNA directed against miRNAs have been demonstrated to be effective down regulators of miRNA function in vitro as well as in vivo [157, 158].
Another approach to knock down the functional activity of miRNA is by ‘sponging’ away endogenous miRNAs by delivery of a high number of miRNA binding sites to a cell. The over-expressed sites act as decoy binding sites for target miRNA thereby reducing its function on its native targets [159]. Decoy or sponge technology was first used to knock down miR-133 in murine cardiomyocytes showing effective knock down and upregulation of three target genes: Acta1, Myh7 and Nppa [160]. Effectiveness of using sponge methods was also demonstrated in vivo, murine bone marrow cells were transduced with a sponge for miR-223 which were then subsequently used to successfully reconstitute the hematopoietic system of these mice. Mice transplanted with the lentviral vector expressing the sponge for miR-223 demonstrated clearly comprised function of this miRNA [161]. The sponge technology may have advantages in biological experiments, however using oligonucleotide-based methods to silence miRNAs is therapeutically more promising. Nevertheless, this promise depends on the continuous development of new oligonucleotide chemistries and new delivery methods of these anti-miR oligonucleotides[162]. Next to the more generally used approach to inhibit the functions of miRNAs, the alternative possibility, mimic the repressing action of miRNAs, has been studied. Since several miRNAs, miR-15a, miR-16 [163], miR-34a [164] and the let7 family [165], are demonstrated to be anti-oncogenic and anti-angiogenic [33, 166–168] restoring miRNA function in tumors might be a relevant approach to stop tumor progression but also angiogenesis. Indeed, miRNA mimics for miR-16 [169], miR-26a [170], let7a [171], and miRNA-34a [172] have been shown to suppress the development of a variety of tumors in mice. MiRNA-replacement therapy will be of high interest to be used to stop the progression of human tumors; a single miRNA can regulate multiple targets and pathways involved in tumor development. In this strength, also lies the weakness of using miRNA mimics as clinical candidates. The widespread of possible targets raises concerns about potentially toxic off-target effects, especially when miRNA mimics are delivered to natural cells and these cells are overloaded with the exogenous miRNA, leading to possible down regulation of other (vital) cellular pathways. Furthermore, excess of miRNA levels in the cell might block the endogenous miRISC leading to even more side effects. However, so far mouse studies have not reported any adverse effects after therapeutic delivery to tumor suppressing miRNA mimics [169–172], making miRNA mimics interesting targets for further development of anti-tumor medications.
Silencing or mimicking of miRNA function has been used to unravel the functional roles of miRNA in a wide variety of pathways and diseases, however these studies were mainly carried out in vitro and subsequently in vivo relevance was confirmed in mice. To ensure safety and therapeutic applicability of adjusting miRNA levels in humans, silencing and mimicking miRNA function in animals more complex then rodents has to be explored. To date, only a few studies have taken this next step and covered these aspects. One of these studies revealed the role of miR-33a and miRNA-33b in the cholesterol homeostasis of African green monkeys [133]. Previously the key role of miR-33 in cholesterol metabolism has been shown in hepatocytes as well as macrophages [128, 138]. As described before, inhibition of miR-33 in vivo showed increase levels of plasma HDL in mice, while over-expression of miR-33 showed an inverse results on HDL levels in the circulation [128]. When anti-miR-33 was used in non-human primates a significant increase of hepatic ABCA1 expression was found together with a profound increase in plasma HDL cholesterol. A highly efficient, long-lasting knock down of miR-122, a liver-specific miRNA involved in cholesterol metabolism [173], in mice and non-human primates was obtained using LNA-antimiRs. Consecutive, intravenous injections with LNA directed against miR-122 led to steep de-repression of the direct miR-122 targets Aldoa and Bckdk in the liver and subsequently reduced levels of total plasma cholesterol in green African monkeys without acute or subchronic side effects [157]. This study was followed-up by using LNA-antimiR against miR-122 in chimpanzees infected with hepatitis C virus (HCV). Down-regulation of miR-122 in vitro causes loss of viral HCV RNA, most likely due to a direct HCV RNA and miR-122 interaction, indicating that miR-122 is vital for the replication of HCV [150]. The study in chimpanzees not only copied the results on plasma cholesterol shown in the previous study with African green monkeys, but also demonstrated a prolonged suppression of viral HCV present in the circulation. During the 26-week treatment the chimpanzees were intensively monitored for a wide variety of safety parameters, and no abnormalities were recorded that could relate to the treatment with LNA-antimiR [174]. The studies described above have led to the first clinical trials using LNA-antimiRs in humans. A clinical trial with chronic hepatitis C patients with hard-to-treat HCV genotype I is momentarily being evaluated. The patients were subjected to subcutaneous injections of different doses of miravirsen, a LNA designed to inhibit the function of miR-122. Preclinical studies with miravirsen have shown a highly effective activity against the replication of all HCV genotype. Additionally, the first results of a Phase IIa study demonstrate that injection of miravirsen was linked to a dose-dependent decline of HCV RNA levels, without adverse effects in any of used doses [175]. The previously discussed studies in non-human primates and humans highlight the therapeutic promise of using the inhibition (or over-expression) of miRNAs and support the development of miRNA antagonists or agonists as potential therapeutics for human (cardiovascular) disease.
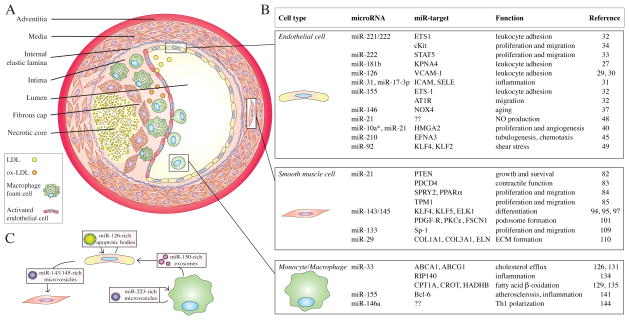
Fig. 1. MicroRNAs in atherosclerotic vascular disease.
A. Blood vessels are composed of three layers: the tunica adventitia, the tunica media and the tunica intima. During atherosclerotic progression, low-density lipoprotein (LDL) particles penetrate into the intimal layer of the arteries and they are oxidized in the subintimal space into oxidized-LDL (ox-LDL). Ox-LDL particles together with cytokines activating endothelial cells, which in turn attract monocytes and inflammatory cells into the site of lesion. Monocytes infiltrated into the subintimal space differentiate into macrophages, which internalize lipoproteins and become foam cells. In advanced stages of plaque formation, smooth muscle cells migrate and surround the lesion forming a fibrous cap around the necrotic core composed of apoptotic and necrotic macrophages and smooth muscle cells.
B. Table of the most relevant cell types involved in atherosclerosis, as well as microRNAs that modulate their phenotype in disease progression.
C. Endothelial cells, smooth muscle cells and monocytes/macrophages are engaged in cell-to-cell communication involving microRNAs transported by microvesicles, exosomes or apoptotic bodies.
Acknowledgments
This review was supported by grants from the National Institutes of Health (R01HL107953 and R01HL106063 to CF-H and R01HL105945 to YS). EA is a Howard Hughes Medical Institute International Student Research Fellow.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
References
- 1.Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441–50. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–66. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–60. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeom KH, Lee Y, Han J, Suh MR, Kim VN. Characterization of DGCR8/Pasha, the essential cofactor for Drosha in primary miRNA processing. Nucleic Acids Res. 2006;34:4622–9. doi: 10.1093/nar/gkl458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 6.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–9. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 7.Kim VN. MicroRNA precursors in motion: exportin-5 mediates their nuclear export. Trends Cell Biol. 2004;14:156–9. doi: 10.1016/j.tcb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Hammond SM. Dicing and slicing: the core machinery of the RNA interference pathway. FEBS Lett. 2005;579:5822–9. doi: 10.1016/j.febslet.2005.08.079. [DOI] [PubMed] [Google Scholar]
- 9.Hutvagner G. Small RNA asymmetry in RNAi: function in RISC assembly and gene regulation. FEBS Lett. 2005;579:5850–7. doi: 10.1016/j.febslet.2005.08.071. [DOI] [PubMed] [Google Scholar]
- 10.Okamura K, Phillips MD, Tyler DM, Duan H, Chou YT, Lai EC. The regulatory activity of microRNA* species has substantial influence on microRNA and 3′ UTR evolution. Nat Struct Mol Biol. 2008;15:354–63. doi: 10.1038/nsmb.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo L, Lu Z. The fate of miRNA* strand through evolutionary analysis: implication for degradation as merely carrier strand or potential regulatory molecule? PLoS One. 2010;5:e11387. doi: 10.1371/journal.pone.0011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai EC, Tomancak P, Williams RW, Rubin GM. Computational identification of Drosophila microRNA genes. Genome Biol. 2003;4:R42. doi: 10.1186/gb-2003-4-7-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang JS, Phillips MD, Betel D, Mu P, Ventura A, Siepel AC, et al. Widespread regulatory activity of vertebrate microRNA* species. RNA. 2011;17:312–26. doi: 10.1261/rna.2537911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang KW, Kao SY, Wu YH, Tsai MM, Tu HF, Liu CJ, et al. Passenger strand miRNA miR-31(*) regulates the phenotypes of oral cancer cells by targeting RhoA. Oral Oncol. 2012;49:27–33. doi: 10.1016/j.oraloncology.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Zhou H, Huang X, Cui H, Luo X, Tang Y, Chen S, et al. miR-155 and its star-form partner miR-155* cooperatively regulate type I interferon production by human plasmacytoid dendritic cells. Blood. 2010;116:5885–94. doi: 10.1182/blood-2010-04-280156. [DOI] [PubMed] [Google Scholar]
- 16.Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev. 2004;18:504–11. doi: 10.1101/gad.1184404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 18.Petersen CP, Bordeleau ME, Pelletier J, Sharp PA. Short RNAs repress translation after initiation in mammalian cells. Mol Cell. 2006;21:533–42. doi: 10.1016/j.molcel.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Pillai RS, Bhattacharyya SN, Artus CG, Zoller T, Cougot N, Basyuk E, et al. Inhibition of translational initiation by Let-7 MicroRNA in human cells. Science. 2005;309:1573–6. doi: 10.1126/science.1115079. [DOI] [PubMed] [Google Scholar]
- 20.Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12:99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 21.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–16. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 22.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 23.Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164–73. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 24.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 25.Shilo S, Roy S, Khanna S, Sen CK. Evidence for the involvement of miRNA in redox regulated angiogenic response of human microvascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:471–7. doi: 10.1161/ATVBAHA.107.160655. [DOI] [PubMed] [Google Scholar]
- 26.Suarez Y, Fernandez-Hernando C, Yu J, Gerber SA, Harrison KD, Pober JS, et al. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc Natl Acad Sci U S A. 2008;105:14082–7. doi: 10.1073/pnas.0804597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, et al. MicroRNA-181b regulates NF-kappaB-mediated vascular inflammation. J Clin Invest. 2012;122:1973–90. doi: 10.1172/JCI61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang Y, Shi C, Manduchi E, Civelek M, Davies PF. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci U S A. 2010;107:13450–5. doi: 10.1073/pnas.1002120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A. 2008;105:1516–21. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asgeirsdottir SA, van Solingen C, Kurniati NF, Zwiers PJ, Heeringa P, van Meurs M, et al. MicroRNA-126 contributes to renal microvascular heterogeneity of VCAM-1 protein expression in acute inflammation. Am J Physiol Renal Physiol. 2012;302:F1630–9. doi: 10.1152/ajprenal.00400.2011. [DOI] [PubMed] [Google Scholar]
- 31.Suarez Y, Wang C, Manes TD, Pober JS. Cutting edge: TNF-induced microRNAs regulate TNF-induced expression of E-selectin and intercellular adhesion molecule-1 on human endothelial cells: feedback control of inflammation. J Immunol. 2010;184:21–5. doi: 10.4049/jimmunol.0902369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu N, Zhang D, Chen S, Liu X, Lin L, Huang X, et al. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis. 2011;215:286–93. doi: 10.1016/j.atherosclerosis.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 33.Dentelli P, Rosso A, Orso F, Olgasi C, Taverna D, Brizzi MF. microRNA-222 controls neovascularization by regulating signal transducer and activator of transcription 5A expression. Arterioscler Thromb Vasc Biol. 2010;30:1562–8. doi: 10.1161/ATVBAHA.110.206201. [DOI] [PubMed] [Google Scholar]
- 34.Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, et al. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108:3068–71. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 35.Napoli C, Quehenberger O, De Nigris F, Abete P, Glass CK, Palinski W. Mildly oxidized low density lipoprotein activates multiple apoptotic signaling pathways in human coronary cells. FASEB J. 2000;14:1996–2007. doi: 10.1096/fj.99-0986com. [DOI] [PubMed] [Google Scholar]
- 36.Qin B, Xiao B, Liang D, Xia J, Li Y, Yang H. MicroRNAs expression in ox-LDL treated HUVECs: MiR-365 modulates apoptosis and Bcl-2 expression. Biochem Biophys Res Commun. 2011;410:127–33. doi: 10.1016/j.bbrc.2011.05.118. [DOI] [PubMed] [Google Scholar]
- 37.Vasa-Nicotera M, Chen H, Tucci P, Yang AL, Saintigny G, Menghini R, et al. miR-146a is modulated in human endothelial cell with aging. Atherosclerosis. 2011;217:326–330. doi: 10.1016/j.atherosclerosis.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 38.Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, et al. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120:1524–32. doi: 10.1161/CIRCULATIONAHA.109.864629. [DOI] [PubMed] [Google Scholar]
- 39.Zhao T, Li JA, Chen AF. MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. American Journal of Physiology-Endocrinology and Metabolism. 2010;299:E110–E116. doi: 10.1152/ajpendo.00192.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu S, Deng S, Ma Q, Zhang T, Jia C, Zhuo D, et al. microRNA-10A* and microRNA-21 Modulate Endothelial Progenitor Cell Senescence via Suppressing Hmga2. Circ Res. 2012;112:152–64. doi: 10.1161/CIRCRESAHA.112.280016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sluimer JC, Daemen MJ. Novel concepts in atherogenesis: angiogenesis and hypoxia in atherosclerosis. J Pathol. 2009;218:7–29. doi: 10.1002/path.2518. [DOI] [PubMed] [Google Scholar]
- 42.Semenza GL. Regulation of Oxygen Homeostasis by Hypoxia-Inducible Factor 1. Physiology. 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 43.Fraisl P, Mazzone M, Schmidt T, Carmeliet P. Developmental Cell. 2009. Regulation of Angiogenesis by Oxygen and Metabolism; pp. 167–79. [DOI] [PubMed] [Google Scholar]
- 44.Raitoharju E, Lyytikainen LP, Levula M, Oksala N, Mennander A, Tarkka M, et al. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis. 2011;219:211–7. doi: 10.1016/j.atherosclerosis.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 45.Fasanaro P, D’Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, et al. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283:15878–83. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghosh G, Subramanian IV, Adhikari N, Zhang X, Joshi HP, Basi D, et al. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-alpha isoforms and promotes angiogenesis. J Clin Invest. 2010;120:4141–54. doi: 10.1172/JCI42980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ni CW, Qiu H, Jo H. MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. Am J Physiol Heart Circ Physiol. 2011;300:H1762–9. doi: 10.1152/ajpheart.00829.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weber M, Baker MB, Moore JP, Searles CD. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochemical and Biophysical Research Communications. 2010;393:643–8. doi: 10.1016/j.bbrc.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fang Y, Davies PF. Site-specific microRNA-92a regulation of Kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler Thromb Vasc Biol. 2012;32:979–87. doi: 10.1161/ATVBAHA.111.244053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu W, Xiao H, Laguna-Fernandez A, Villarreal G, Jr, Wang KC, Geary GG, et al. Flow-Dependent Regulation of Kruppel-Like Factor 2 Is Mediated by MicroRNA-92a. Circulation. 2011;124:633–41. doi: 10.1161/CIRCULATIONAHA.110.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicoli S, Standley C, Walker P, Hurlstone A, Fogarty KE, Lawson ND. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 2010;464:1196–200. doi: 10.1038/nature08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mittelbrunn M, Sánchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012;13:328–35. doi: 10.1038/nrm3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van den Boorn JG, Daßler J, Coch C, Schlee M, Hartmann G. Exosomes as nucleic acid nanocarriers. Adv Drug Deliv Rev. 2012 doi: 10.1016/j.addr.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 54.Théry C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. Journal of Proteomics. 2010;73:1907–20. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 56.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 57.Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nature Cell Biology. 2012;14:677–85. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133–44. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 59.Ismail N, Wang Y, Dakhlallah D, Moldovan L, Agarwal K, Batte K, et al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood. 2012 doi: 10.1182/blood-2011-08-374793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–56. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 61.Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2:ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 62.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 63.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–95. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 64.Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- 65.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89:957–89. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, et al. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–80. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 67.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 68.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–58. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 69.Hoofnagle MH, Wamhoff BR, Owens GK. Lost in transdifferentiation. J Clin Invest. 2004;113:1249–51. doi: 10.1172/JCI21761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8:1249–56. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 71.Schwartz SM. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest. 1997;100:S87–9. [PubMed] [Google Scholar]
- 72.Marx SO, Totary-Jain H, Marks AR. Vascular smooth muscle cell proliferation in restenosis. Circ Cardiovasc Interv. 2011;4:104–11. doi: 10.1161/CIRCINTERVENTIONS.110.957332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol. 2012;74:13–40. doi: 10.1146/annurev-physiol-012110-142315. [DOI] [PubMed] [Google Scholar]
- 74.Albinsson S, Suarez Y, Skoura A, Offermanns S, Miano JM, Sessa WC. MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function. Arterioscler Thromb Vasc Biol. 2010;30:1118–26. doi: 10.1161/ATVBAHA.109.200873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Albinsson S, Skoura A, Yu J, DiLorenzo A, Fernandez-Hernando C, Offermanns S, et al. Smooth muscle miRNAs are critical for post-natal regulation of blood pressure and vascular function. PLoS One. 2011;6:e18869. doi: 10.1371/journal.pone.0018869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park C, Yan W, Ward SM, Hwang SJ, Wu Q, Hatton WJ, et al. MicroRNAs dynamically remodel gastrointestinal smooth muscle cells. PLoS One. 2011;6:e18628. doi: 10.1371/journal.pone.0018628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kang H, Hata A. MicroRNA regulation of smooth muscle gene expression and phenotype. Curr Opin Hematol. 2012;19:224–31. doi: 10.1097/MOH.0b013e3283523e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O’Sullivan JF, Martin K, Caplice NM. Microribonucleic acids for prevention of plaque rupture and in-stent restenosis: “a finger in the dam”. J Am Coll Cardiol. 2011;57:383–9. doi: 10.1016/j.jacc.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 79.Albinsson S, Sessa WC. Can microRNAs control vascular smooth muscle phenotypic modulation and the response to injury? Physiol Genomics. 2011;43:529–33. doi: 10.1152/physiolgenomics.00146.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zampetaki A, Mayr M. MicroRNAs in vascular and metabolic disease. Circ Res. 2012;110:508–22. doi: 10.1161/CIRCRESAHA.111.247445. [DOI] [PubMed] [Google Scholar]
- 81.Chen LJ, Lim SH, Yeh YT, Lien SC, Chiu JJ. Roles of microRNAs in atherosclerosis and restenosis. J Biomed Sci. 2012;19:79. doi: 10.1186/1423-0127-19-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–88. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 83.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sarkar J, Gou D, Turaka P, Viktorova E, Ramchandran R, Raj JU. MicroRNA-21 plays a role in hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration. Am J Physiol Lung Cell Mol Physiol. 2010;299:L861–71. doi: 10.1152/ajplung.00201.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang M, Li W, Chang GQ, Ye CS, Ou JS, Li XX, et al. MicroRNA-21 regulates vascular smooth muscle cell function via targeting tropomyosin 1 in arteriosclerosis obliterans of lower extremities. Arterioscler Thromb Vasc Biol. 2011;31:2044–53. doi: 10.1161/ATVBAHA.111.229559. [DOI] [PubMed] [Google Scholar]
- 86.Song JT, Hu B, Qu HY, Bi CL, Huang XZ, Zhang M. Mechanical Stretch Modulates MicroRNA 21 Expression, Participating in Proliferation and Apoptosis in Cultured Human Aortic Smooth Muscle Cells. PLoS One. 2012;7:e47657. doi: 10.1371/journal.pone.0047657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chapman GB, Durante W, Hellums JD, Schafer AI. Physiological cyclic stretch causes cell cycle arrest in cultured vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2000;278:H748–54. doi: 10.1152/ajpheart.2000.278.3.H748. [DOI] [PubMed] [Google Scholar]
- 88.Cheng WP, Wang BW, Chen SC, Chang H, Shyu KG. Mechanical stretch induces the apoptosis regulator PUMA in vascular smooth muscle cells. Cardiovasc Res. 2012;93:181–9. doi: 10.1093/cvr/cvr280. [DOI] [PubMed] [Google Scholar]
- 89.Li C, Wernig F, Leitges M, Hu Y, Xu Q. Mechanical stress-activated PKCdelta regulates smooth muscle cell migration. FASEB J. 2003;17:2106–8. doi: 10.1096/fj.03-0150fje. [DOI] [PubMed] [Google Scholar]
- 90.Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199–227. doi: 10.1146/annurev.pathol.4.110807.092222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jazbutyte V, Thum T. MicroRNA-21: from cancer to cardiovascular disease. Curr Drug Targets. 2010;11:926–35. doi: 10.2174/138945010791591403. [DOI] [PubMed] [Google Scholar]
- 92.Cheng Y, Zhang C. MicroRNA-21 in cardiovascular disease. J Cardiovasc Transl Res. 2010;3:251–5. doi: 10.1007/s12265-010-9169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maegdefessel L, Azuma J, Toh R, Deng A, Merk DR, Raiesdana A, et al. MicroRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Sci Transl Med. 2012;4:122ra22. doi: 10.1126/scitranslmed.3003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119:2634–47. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105:158–66. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–10. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, et al. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009;16:1590–8. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–78. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Long X, Bell RD, Gerthoffer WT, Zlokovic BV, Miano JM. Myocardin is sufficient for a smooth muscle-like contractile phenotype. Arterioscler Thromb Vasc Biol. 2008;28:1505–10. doi: 10.1161/ATVBAHA.108.166066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sun SG, Zheng B, Han M, Fang XM, Li HX, Miao SB, et al. miR-146a and Kruppel-like factor 4 form a feedback loop to participate in vascular smooth muscle cell proliferation. EMBO Rep. 2011;12:56–62. doi: 10.1038/embor.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–58. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 102.Quintavalle M, Elia L, Condorelli G, Courtneidge SA. MicroRNA control of podosome formation in vascular smooth muscle cells in vivo and in vitro. J Cell Biol. 2010;189:13–22. doi: 10.1083/jcb.200912096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta N, et al. MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation. 2012;126:S81–90. doi: 10.1161/CIRCULATIONAHA.111.084186. [DOI] [PubMed] [Google Scholar]
- 104.Dimmeler S, Zeiher AM. Circulating microRNAs: novel biomarkers for cardiovascular diseases? Eur Heart J. 2010;31:2705–7. doi: 10.1093/eurheartj/ehq221. [DOI] [PubMed] [Google Scholar]
- 105.Kosaka N, Iguchi H, Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010;101:2087–92. doi: 10.1111/j.1349-7006.2010.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–84. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 107.Davis-Dusenbery BN, Chan MC, Reno KE, Weisman AS, Layne MD, Lagna G, et al. down-regulation of Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-beta and bone morphogenetic protein 4. J Biol Chem. 2011;286:28097–110. doi: 10.1074/jbc.M111.236950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104:476–87. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem. 2009;284:3728–38. doi: 10.1074/jbc.M808788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Torella D, Iaconetti C, Catalucci D, Ellison GM, Leone A, Waring CD, Bochicchio A, Vicinanza C, Aquila I, Curcio A, Condorelli G, Indolfi C. MicroRNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo. Circ Res. 2011;109:880–93. doi: 10.1161/CIRCRESAHA.111.240150. [DOI] [PubMed] [Google Scholar]
- 111.Boon RA, Seeger T, Heydt S, Fischer A, Hergenreider E, Horrevoets AJ, et al. MicroRNA-29 in aortic dilation: implications for aneurysm formation. Circ Res. 2011;109:1115–9. doi: 10.1161/CIRCRESAHA.111.255737. [DOI] [PubMed] [Google Scholar]
- 112.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–32. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang P, Huang A, Ferruzzi J, Mecham RP, Starcher BC, Tellides G, et al. Inhibition of microRNA-29 enhances elastin levels in cells haploinsufficient for elastin and in bioengineered vessels--brief report. Arterioscler Thromb Vasc Biol. 2012;32:756–9. doi: 10.1161/ATVBAHA.111.238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maegdefessel L, Azuma J, Toh R, Merk DR, Deng A, Chin JT, et al. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J Clin Invest. 2012;122:497–506. doi: 10.1172/JCI61598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Merk DR, Chin JT, Dake BA, Maegdefessel L, Miller MO, Kimura N, et al. miR-29b participates in early aneurysm development in Marfan syndrome. Circ Res. 2012;110:312–24. doi: 10.1161/CIRCRESAHA.111.253740. [DOI] [PubMed] [Google Scholar]
- 116.Chen KC, Liao YC, Hsieh IC, Wang YS, Hu CY, Juo SH. OxLDL causes both epigenetic modification and signaling regulation on the microRNA-29b gene: novel mechanisms for cardiovascular diseases. J Mol Cell Cardiol. 2012;52:587–95. doi: 10.1016/j.yjmcc.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 117.Suarez Y, Fernandez-Hernando C. New insights into microRNA-29 regulation: a new key player in cardiovascular disease. J Mol Cell Cardiol. 2012;52:584–6. doi: 10.1016/j.yjmcc.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 118.Chen KC, Wang YS, Hu CY, Chang WC, Liao YC, Dai CY, et al. OxLDL up-regulates microRNA-29b, leading to epigenetic modifications of MMP-2/MMP-9 genes: a novel mechanism for cardiovascular diseases. FASEB J. 2011;25:1718–28. doi: 10.1096/fj.10-174904. [DOI] [PubMed] [Google Scholar]
- 119.Beadenkopf WG, Daoud AS, Love BM. Calcification in the Coronary Arteries and Its Relationship to Arteriosclerosis and Myocardial Infarction. Am J Roentgenol Radium Ther Nucl Med. 1964;92:865–71. [PubMed] [Google Scholar]
- 120.Fitzgerald PJ, Ports TA, Yock PG. Contribution of localized calcium deposits to dissection after angioplasty. An observational study using intravascular ultrasound. Circulation. 1992;86:64–70. doi: 10.1161/01.cir.86.1.64. [DOI] [PubMed] [Google Scholar]
- 121.Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, et al. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147–54. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 122.Nakano-Kurimoto R, Ikeda K, Uraoka M, Nakagawa Y, Yutaka K, Koide M, et al. Replicative senescence of vascular smooth muscle cells enhances the calcification through initiating the osteoblastic transition. Am J Physiol Heart Circ Physiol. 2009;297:H1673–84. doi: 10.1152/ajpheart.00455.2009. [DOI] [PubMed] [Google Scholar]
- 123.Xiao J, Zhu X, He B, Zhang Y, Kang B, Wang Z, et al. MiR-204 regulates cardiomyocyte autophagy induced by ischemia-reperfusion through LC3-II. J Biomed Sci. 2011;18:35. doi: 10.1186/1423-0127-18-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huang J, Zhao L, Xing L, Chen D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells. 2010;28:357–64. doi: 10.1002/stem.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cui RR, Li SJ, Liu LJ, Yi L, Liang QH, Zhu X, et al. MicroRNA-204 regulates vascular smooth muscle cell calcification in vitro and in vivo. Cardiovasc Res. 2012;96:320–9. doi: 10.1093/cvr/cvs258. [DOI] [PubMed] [Google Scholar]
- 126.Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–75. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 127.Oram JF. Tangier disease and ABCA1. Biochim Biophys Acta. 2000;1529:321–30. doi: 10.1016/s1388-1981(00)00157-8. [DOI] [PubMed] [Google Scholar]
- 128.Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, et al. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–3. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, et al. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–9. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Marquart TJ, Allen RM, Ory DS, Baldan A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci U S A. 2010;107:12228–32. doi: 10.1073/pnas.1005191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gerin I, Clerbaux LA, Haumont O, Lanthier N, Das AK, Burant CF, et al. Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J Biol Chem. 2010 doi: 10.1074/jbc.M110.152090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Horie T, Ono K, Horiguchi M, Nishi H, Nakamura T, Nagao K, et al. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc Natl Acad Sci U S A. 2010;107:17321–6. doi: 10.1073/pnas.1008499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rayner KJ, Esau CC, Hussain FN, McDaniel AL, Marshall SM, van Gils JM, et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011;478:404–7. doi: 10.1038/nature10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121:2921–31. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, et al. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A. 2011;108:7166–71. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ho PC, Chang KC, Chuang YS, Wei LN. Cholesterol regulation of receptor-interacting protein 140 via microRNA-33 in inflammatory cytokine production. FASEB J. 2011;25:1758–66. doi: 10.1096/fj.10-179267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Horie T, Baba O, Kuwabara Y, Chujo T, Watanabe S, Kinoshita M, et al. MicroRNA-33 deficiency reduces the progression of atherosclerotic plaque in apoE−/− mice. J AM Heart Assoc. 2012;1:e003376. doi: 10.1161/JAHA.112.003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Davalos A, Goedeke L, Smibert P, Ramirez CM, Warrier NP, Andreo U, et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc Natl Acad Sci U S A. 2011;108:9232–7. doi: 10.1073/pnas.1102281108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fichtlscherer S, Zeiher AM, Dimmeler S. Circulating microRNAs: biomarkers or mediators of cardiovascular diseases? Arterioscler Thromb Vasc Biol. 2011;31:2383–90. doi: 10.1161/ATVBAHA.111.226696. [DOI] [PubMed] [Google Scholar]
- 140.Bidzhekov K, Gan L, Denecke B, Rostalsky A, Hristov M, Koeppel TA, et al. microRNA expression signatures and parallels between monocyte subsets and atherosclerotic plaque in humans. Thromb Haemost. 2012;107:619–25. doi: 10.1160/TH11-09-0607. [DOI] [PubMed] [Google Scholar]
- 141.Cipollone F, Felicioni L, Sarzani R, Ucchino S, Spigonardo F, Mandolini C, et al. A unique microRNA signature associated with plaque instability in humans. Stroke. 2011;42:2556–63. doi: 10.1161/STROKEAHA.110.597575. [DOI] [PubMed] [Google Scholar]
- 142.Wang S, Wang L, Zhu T, Gao X, Li J, Wu Y, et al. Improvement of tissue preparation for laser capture microdissection: application for cell type-specific miRNA expression profiling in colorectal tumors. BMC Genomics. 2010;11:163. doi: 10.1186/1471-2164-11-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nazari-Jahantigh M, Wei Y, Noels H, Akhtar S, Zhou Z, Koenen RR, et al. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest. 2012;122:4190–202. doi: 10.1172/JCI61716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Donners MM, Wolfs IM, Stoger LJ, van der Vorst EP, Pottgens CC, Heymans S, et al. Hematopoietic miR155 deficiency enhances atherosclerosis and decreases plaque stability in hyperlipidemic mice. PLoS One. 2012;7:e35877. doi: 10.1371/journal.pone.0035877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen T, Huang Z, Wang L, Wang Y, Wu F, Meng S, et al. MicroRNA-125a-5p partly regulates the inflammatory response, lipid uptake, and ORP9 expression in oxLDL-stimulated monocyte/macrophages. Cardiovasc Res. 2009;83:131–9. doi: 10.1093/cvr/cvp121. [DOI] [PubMed] [Google Scholar]
- 146.Guo M, Mao X, Ji Q, Lang M, Li S, Peng Y, et al. miR-146a in PBMCs modulates Th1 function in patients with acute coronary syndrome. Immunol Cell Biol. 2010;88:555–64. doi: 10.1038/icb.2010.16. [DOI] [PubMed] [Google Scholar]
- 147.Wienholds E, Kloosterman WP, Miska E, Alvarez-Saavedra E, Berezikov E, de Bruijn E, et al. MicroRNA expression in zebrafish embryonic development. Science. 2005;309:310–1. doi: 10.1126/science.1114519. [DOI] [PubMed] [Google Scholar]
- 148.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–33. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kutay H, Bai S, Datta J, Motiwala T, Pogribny I, Frankel W, et al. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J Cell Biochem. 2006;99:671–8. doi: 10.1002/jcb.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–81. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 151.van Solingen C, Seghers L, Bijkerk R, Duijs JM, Roeten MK, van Oeveren-Rietdijk AM, et al. Antagomir-mediated silencing of endothelial cell specific microRNA-126 impairs ischemia-induced angiogenesis. J Cell Mol Med. 2009;13:1577–85. doi: 10.1111/j.1582-4934.2008.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hutvagner G, Simard MJ, Mello CC, Zamore PD. Sequence-specific inhibition of small RNA function. PLoS Biol. 2004;2:E98. doi: 10.1371/journal.pbio.0020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.van Rooij E, Marshall WS, Olson EN. Toward microRNA-based therapeutics for heart disease: the sense in antisense. Circ Res. 2008;103:919–28. doi: 10.1161/CIRCRESAHA.108.183426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bader AG, Brown D, Winkler M. The promise of microRNA replacement therapy. Cancer Res. 2010;70:7027–30. doi: 10.1158/0008-5472.CAN-10-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–9. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 156.Krutzfeldt J, Kuwajima S, Braich R, Rajeev KG, Pena J, Tuschl T, et al. Specificity, duplex degradation and subcellular localization of antagomirs. Nucleic Acids Res. 2007;35:2885–92. doi: 10.1093/nar/gkm024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Elmen J, Lindow M, Schutz S, Lawrence M, Petri A, Obad S, et al. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452:896–9. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 158.Worm J, Stenvang J, Petri A, Frederiksen KS, Obad S, Elmen J, et al. Silencing of microRNA-155 in mice during acute inflammatory response leads to derepression of c/ebp Beta and down-regulation of G-CSF. Nucleic Acids Res. 2009;37:5784–92. doi: 10.1093/nar/gkp577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods. 2007;4:721–6. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–8. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 161.Gentner B, Schira G, Giustacchini A, Amendola M, Brown BD, Ponzoni M, et al. Stable knockdown of microRNA in vivo by lentiviral vectors. Nat Methods. 2009;6:63–6. doi: 10.1038/nmeth.1277. [DOI] [PubMed] [Google Scholar]
- 162.Ebert MS, Sharp PA. MicroRNA sponges: progress and possibilities. RNA. 2010;16:2043–50. doi: 10.1261/rna.2414110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–66. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 164.Welch C, Chen Y, Stallings RL. MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene. 2007;26:5017–22. doi: 10.1038/sj.onc.1210293. [DOI] [PubMed] [Google Scholar]
- 165.Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–22. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 166.Chamorro-Jorganes A, Araldi E, Penalva LO, Sandhu D, Fernandez-Hernando C, Suarez Y. MicroRNA-16 and microRNA-424 regulate cell-autonomous angiogenic functions in endothelial cells via targeting vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1. Arterioscler Thromb Vasc Biol. 2011;31:2595–606. doi: 10.1161/ATVBAHA.111.236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sabatel C, Malvaux L, Bovy N, Deroanne C, Lambert V, Gonzalez ML, et al. MicroRNA-21 exhibits antiangiogenic function by targeting RhoB expression in endothelial cells. PLoS One. 2011;6:e16979. doi: 10.1371/journal.pone.0016979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–3. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 169.Takeshita F, Patrawala L, Osaki M, Takahashi RU, Yamamoto Y, Kosaka N, et al. Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via downregulation of multiple cell-cycle genes. Mol Ther. 2010;18:181–7. doi: 10.1038/mt.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–17. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Esquela-Kerscher A, Trang P, Wiggins JF, Patrawala L, Cheng A, Ford L, et al. The let-7 microRNA reduces tumor growth in mouse models of lung cancer. Cell Cycle. 2008;7:759–64. doi: 10.4161/cc.7.6.5834. [DOI] [PubMed] [Google Scholar]
- 172.Wiggins JF, Ruffino L, Kelnar K, Omotola M, Patrawala L, Brown D, et al. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 2010;70:5923–30. doi: 10.1158/0008-5472.CAN-10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 174.Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Reesink HWJH, Zeuzem LAS, Rodriguez-Torres M, Patel K, Chen A, Davis C, et al. Final Results - Randomized, Double-blind, Placebo-controlled Safety, Anti-viral Proof-of-Concept Study of Miravirsen, an Oligonucleotide Targeting miR-122, in Treatment-naive Patients with Genotype 1 Chronic HCV Infection. Infection 47th International Liver Congress (EASL 2012); Barcelona. April 18–22, 2012; 2012. p. Abstract 8. [Google Scholar]