Abstract
Objectives
IL-23 has been implicated in the pathogenesis of Ankylosing Spondylitis (AS). Aim of the study was to clarify the mechanisms underlying the increased IL-23 expression in the gut of AS patients.
Methods
Consecutive gut biopsies from 30 HLA-B27+ AS patients, 15 Crohn’s disease (CD) patients and 10 normal subjects were obtained. Evidence for HLA-B27 misfolding was studied. Unfolded protein response (UPR) and autophagy were assessed by rt-PCR and immunohistochemistry. The contribution of UPR and autophagy in the regulation of IL-23 expression was evaluated in in vitro experiments on isolated lamina propria mononuclear cells (LPMCs).
Results
Intracellular co-localization of SYVN1 and FHCs but not a significant over-expression of UPR genes was observed in the gut of AS patients. Conversely, up-regulation of the genes involved in the autophagy pathway was observed in the gut of AS and CD patients. Immunohistochemistry showed an increased expression of LC3II, ATG5 and ATG12 but not of SQSTM1 in the ileum of AS and CD patients. LC3II was expressed among infiltrating mononuclear cells and epithelial cells resembling Paneth cells and co-localized with ATG5 in AS and CD. Autophagy but not UPR was required to modulate the expression of IL-23 in isolated LPMCs of AS patients with chronic gut inflammation, CD patients and controls.
Conclusions
Our data suggest that HLA-B27 misfolding occurs in the gut of AS patients and is accompanied by activation of autophagy rather than an unfolded protein response. Autophagy appears to be associated with intestinal modulation of IL-23 in AS.
Keywords: Ankylosing spondylitis, subclinical gut inflammation, unfolded protein response, autophagy, interleukin-23
Introduction
Subclinical ileal inflammation, resembling Crohn’s disease (CD), has been demonstrated in up to 70% of Ankylosing Spondylitis (AS) patients apparently never resolving, suggesting the presence of chronic alterations in host-microbe interactions in the gut.[1–3] The terminal ileum of normal subjects basically produces IL-23 in the presence of commensal microbes [4] and its local excessive production has been found in the gut of AS patients with subclinical gut inflammation,[5] suggesting that intestinal mucosa may be a key site of IL-23 production in AS in AS. IL-23 pathway has been hypothesized to be strongly involved in the pathogenesis of AS based on the cumulating genetic evidence from several GWAS studies.[6–8]
Although increased concentrations of IL-23 have been demonstrated in the peripheral blood [9–11] and tissues of AS patients the exact mechanism involved in IL-23 up-regulation has not been definitively defined. In particular, it is not clear whether or not the over-production of IL-23 could be linked to the misfolding of HLA-B27 through the induction of the unfolded protein response (UPR).[12] On the other hand, the demonstration that IL-23 is active at intestinal mucosal surfaces [4] and evidence that certain types of bacterial stimuli may influence its intestinal expression,[13] could suggest a role for microbes in the IL-23 over-expression observed in AS.[14]
A key role in the intestinal innate immune response against bacterial infection is played by macroautophagy [15] (hereafter referred as autophagy), a basic cellular machinery responsible in eukaryotic cells for bulk degradation of cellular constituents [16] that also acts as an effector of pattern recognition receptor (PRR) response to pathogens,[17–19] directly eliminating intracellular microbes or their products. Another function of autophagy is connected to its ability to target improperly folded proteins for degradation in close connection with the endoplasmic reticulum stress response known as the UPR.[20–22] In this study we present data on autophagy as a probable regulator of IL-23 production in the gut of patients with subclinical gut inflammation. We also provide the first evidence that HLA-B27 misfolding occurs in the gut of HLA-B27+ AS patients.
Methods
Patients
Multiple adjacent ileal mucosal biopsies from patients with AS (diagnosed according to the modified New York classification criteria),[31] Crohn’s disease (CD) and healthy subjects (HS) were consecutively obtained (baseline characteristics of patients and controls are specified in Table I). As a control group, 10 normal subjects undergoing to ileocolonscopy for diagnostic purposes but without evidence of underlying disease, were also considered. Collection of ileal biopsies was approved by the ethical committee and the institutional review board of the University of Palermo and informed consent was obtained from each patient and control.
Table 1.
Baseline characteristics of the patients and controls*
| AS patients no inflammation N=6 |
AS patients acute N=9 |
AS patients chronic N=15 |
CD patients N=15 |
Controls N=10 |
|
|---|---|---|---|---|---|
| Age, mean (range) years | 32 (22–45) | 31 (20–50) | 33 (20–53) | 38 (18–51) | 60 (35–70) |
| Sex, no. (%) male | 5 (83) | 7 (78) | 13 (87) | 9 (60) | 7 (70) |
| HLA-B27, no. (%) | 6 (100) | 9 (100) | 15 (100) | 1 (6) | - |
| Disease duration, mean (range) months | 26 (18–36) | 28 (11–40) | 20 (8–38) | 15 (4–46) | NA |
| CRP (mg/l), mean (range) | 1 (0.5–2) | 1.2 (0.5–3) | 1.9 (0.5–3) | 6.5 (2–13)* | NA |
| Axial involvement, no. (%) | 6 (100) | 9 (100) | 15 (100) | 1 | NA |
| Peripheral arthritis, no. (%) | - | 3 (33) | 4 (27) | - | NA |
| Enthesitis/dactylitis, no. (%) | 1 (16) | 2 (22) | 2 (13) | - | NA |
| Uveitis, no. (%) | - | 2 (13) | - | NA | |
| Concomitant medical treatment, no. (%) NSAIDs Biological agentsImmunosuppressan ts |
3 (50) - - |
5 (55) - - |
9 (60) - - |
- - - |
NA NA NA |
| BASDAI score, mean (range)§ | 5.45 (4.2–8) | 6 (4.4–9) | 6.33 (5–9) | NA | NA |
| CDAI score, mean (range)¶ | - | - | - | 234 (170–334) | - |
AS=ankylosing spondylitis; CD=Crohn’s disease; NA=not applicable; CRP=C-reactive protein; NSAIDs=nonsteroidal antiinflammatory drugs.
p<0.0001
Scores for the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) range from 0 to 10, with higher scores indicating more severe disease.
Scores for the Crohn’s Disease Activity Index (CDAI) range from 150 to 450, with higher scores indicating more severe disease
RNA extraction and quantitative TaqMan reverse transcriptase–polymerase chain reaction (RT-PCR) for human epithelial cell lines and ileal biopsies
Ileal biopsies, immediately after removal were stored in RNAlater® solution (Applied Biosystem, Foster City, CA, USA) and processed as previously described.[23] For quantitative TaqMan real-time PCR, sets of primers and probes were obtained from Applied Biosystems (Foster City, CA) or (see Supplementary Table II). Samples were run in triplicate using the Step-One Real-Time PCR system (Applied Biosystem, Foster City, CA, USA). Relative changes in gene expression between controls and patients were determined using the ΔΔCt method as previously described.[23] Final values were expressed as fold of induction (FOI).
Histomorphologic grading and immunohistochemistry
Specimens from patients with AS were divided into 3 subgroups,[5] those with normal gut histology, those with acute inflammation, and those with chronic inflammation. Immunohistochemistry was performed as previously described.[23] The primary and secondary antibodies used are listed in Table II. The number of immunoreactive cells was determined by counting the reactive cells on microphotographs obtained from 3 randomly selected high-power microscopic fields (original magnification × 400). To specifically address the presence of heavy chains (HCs)/HRD1 complexes, as a marker of misfolding,[24] a double staining was performed on paraffin-embedded sections of human ileum and the sections were treated with FITC- or Rhodamine Red–conjugated anti-mouse or anti-rabbit antibodies plus RNasi (200 ng/mL) and counterstained using Toto-3 iodide (642/660; Invitrogen). Confocal analysis was used to acquire fluorescence staining.
Isolation of LPMCs and flow cytometry analysis of surface and intracellular antigens
LPMCs were isolated from gut biopsy specimens of twelve patients with AS and chronic gut inflammation, 10 CD patients and 10 healthy controls as previously described. [23, 25] In order to evaluate the role of UPR and autophagy in regulating the production of IL-23p19 by lamina propria macrophages and dendritic cells, isolated cells were stimulated with thapsigargin (1 µM) (to activate UPR), 3-methyl-adenine (100ug/ml, to inhibit autophagy) and anisomycin (10 µg/ml, to inhibit CAM) with or without LPS. All cultures were set up in triplicate and cells were used for rt-PCR and flow-cytometric analyses. Before intracellular staining, cells were stimulated with PMA (1µg/ml) plus ionomycin (0.5 µg/ml) for 4 hours. After 2 h Brefeldin A (10 µg/ml; Sigma Aldrich, St Louis, MO) was added. After simulation, the cells were stained with PerCP-labeled CD11c (Becton Dickinson, NJ, USA), fixed in 4% paraformaldehyde, permeabilized with 0.1% saponin (Sigma Aldrich, St Louis, MO), and then stained with PE-conjugated mouse anti-human IL-23p19 antibody (R&D systems, MN, USA). Three-colour flow cytometric analysis was performed using a FACSCalibur (Becton Dickinson, NJ, USA) and cell death was assessed by trypan blue exclusion. At least 50.000 cells (events) were acquired for each sample. LPMCs were expressed as percentage of cells within the lymphocytes gate. The acquired data were analysed using the CellQuest software program (Becton Dickinson, NJ, USA).
Statistical analysis
Statistical analysis of quantitative variables was performed using the Mann-Whitney rank-sum test. Spearman’s correlation analysis was utilized to quantify the expression associations between the genes of interest. P values less than 0.05 were considered significant.
Results
Patients
No significant differences between BASDAI score, clinical manifestations and NSAIDs assumption were observed in the different subgroups of AS patients (Table I). Representative images of the intestinal biopsies of patients with AS enrolled are shown in supplemental figure 1.
Autophagy is differentially regulated in the gut of AS patients and CD patients
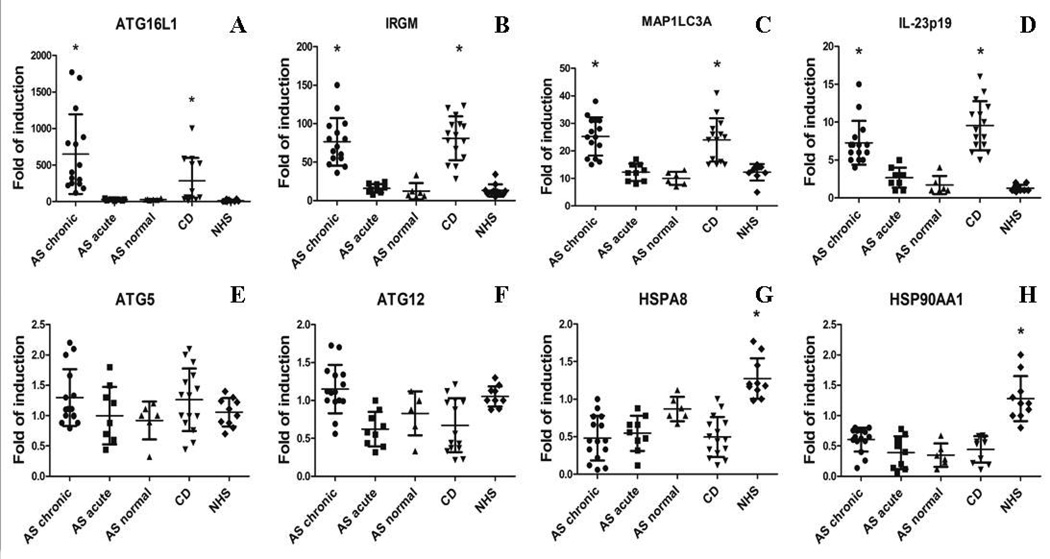
We first studied the gene expression of proteins involved in autophagy and assessed their correlation with the expression of IL-23p19 gene. As shown in Figure 1 a strong and significant up-regulation of ATG16L1 (Figure 1A), IRGM (Figure 1B), and MAP1LC3A (Figure 1C) together with increased IL-23p19 levels (Figure 1D) was observed in the ileum of CD patients and in the chronic inflamed ileum of AS patients compared to the other AS patients and controls. Expression levels of ATG16L1, IRGM and MAP1LC3A were correlated with the IL-23p19 levels only in AS patients with chronic gut inflammation (r2=0.78, 0.67 and 0.83, respectively, p<0.0001; data not shown) and CD patients (r2=0.66, 0.68, 0.77, respectively, p<0.0001; data not shown). Differently from the other autophagy genes evaluated, ATG5 and ATG12 transcripts levels were only modestly and not significantly increased in the ileum of patients with CD and AS with chronic gut inflammation (Figure 1E and 1F, respectively). Interestingly, HSPA8 (Figure 1G) and HSP90AA1 (Figure 1H) that are markers of chaperone-mediated autophagy (CMA) were significantly down-regulated in the gut of all AS and CD patients compared to controls (p<0.0001), and were inversely correlated with the IL-23p19 levels in both chronically inflamed AS patients (r2=−0.63 and −0.58, respectively, p<0.05, data not shown) and CD patients (r2= −0.71 and −0.64, respectively, p<0.005, data not shown) suggesting differential level of regulation of intestinal autophagy machinery.
Figure 1. Macroautophagy and chaperone-mediated autophagy in the gut in the gut of AS patients.
A–H: Relative m-RNA quantification of ATG16L1 (A), IRGM (B), MAP1LC3A (C), IL-23p19 (D), ATG5 (E), ATG12 (F), HSPA8 (G) and HSP90AA1 (H) was assessed by quantitative rt-PCR in ileal biopsy specimens obtained from 30 AS, 15 CD patients and 10 controls. Patients with AS were further divided into 3 groups: those with normal histologic findings, those with acute inflammation and those with chronic inflammation. Data are shown as mean (SD). *p<0.0001
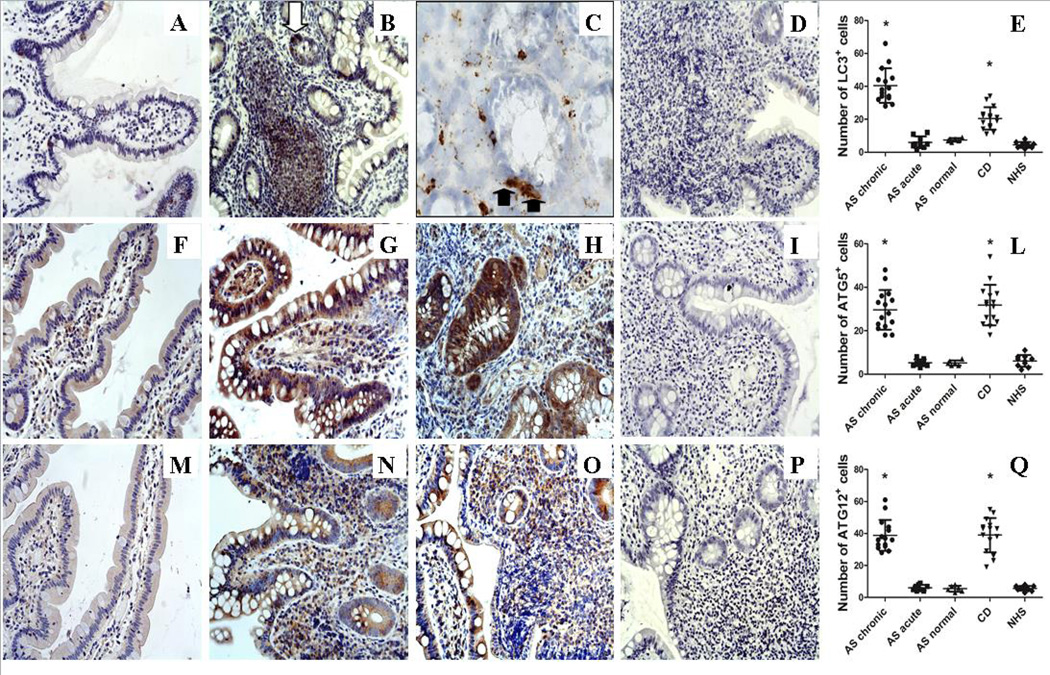
In light of the differential expression of autophagy genes we evaluated by immunohistochemistry the protein expression of ATG5, ATG12 and LC3. A strong diffuse expression of LC3 was found only in the paraffin-embedded AS ileal samples with chronic gut inflammation (Figure 2A–D) and CD patients (data not shown). The classic punctate LC3 staining, characteristic of autophagosome buildup, was clearly observed only in the frozen ileal samples (Figure 2C) among infiltrating mononuclear cells and among some epithelial cells that we hypothesized to be Paneth cells (PC) because of their pyramidal shape (Figure 2A–C, E). Despite the absence of a clear up-regulation at transcript levels, immunohistochemical analysis showed that ATG5 (Figure 2F–H, L) and ATG12 proteins (Figure 2M–O, Q) were strongly up-regulated only in the gut of CD and AS patients with chronic gut inflammation. Accordingly to the increased activation of autophagy pathway, expression of SQSTM1 was observed only in few epithelial cells in normal controls (Supplemental Figure 2A) whereas no SQSTM1 immunoreactivity was detectable in the gut of AS patients with chronic gut inflammation and CD (2B and C respectively). In order to confirm that the immunoreactivity for LC3-II observed was associated with autophagosomes, we also examined the co-localization of LC3 with ATG5. As shown in supplemental figure 2 a significant co-localization of LC3II and ATG5 was observed in the gut of AS patients with chronic gut inflammation (2E–G) and CD patients (2H–L) compared to the other AS patients (data not shown) and healthy controls (2M–O).
Figure 2. Autophagy is up-regulated in the gut in the gut of AS patients.
Five-µm–thick paraffin embedded sections of ileal biopsies obtained from AS, CD patients and controls were stained with anti-LC3II, anti-ATG5 and anti-ATG12 antibodies. A–C: Representative microphotographs showing LC3II immunostainings in HC (A), and AS patients with chronic inflammation (B–C). C: representative immunostaining of LC3II staining on frozen samples obtained from AS patients. E–G: Representative microphotographs showing ATG5 immunostainings in HC (E), AS patients with chronic inflammation (F) and CD patients (G). I–M: Representative microphotographs showing ATG12 immunostainings in HC (I), AS patients with chronic inflammation (L) and CD patients (M). Diffuse expression of LC3II, ATG5 and ATG12 was observed in epithelial cells and infiltrating mononuclear cells of AS patients with chronic gut inflammation and CD patients compared to controls. Intense LC3 expression was observed in some epithelial cells of AS patients highly resembling Paneth cells for their pyramidal shape (black arrow) (C). The classic punctate staining was highly evident among infiltrating mononuclear cells and Paneth cells (arrows). A–B, E–G, I–M original magnification ×250; C original magnification × 630. D, H, N: Number of LC3+, ATG5+, ATG12+ cells in the ileal mucosa; *p<0.0001
UPR is not up-regulated in the inflamed ileum of AS
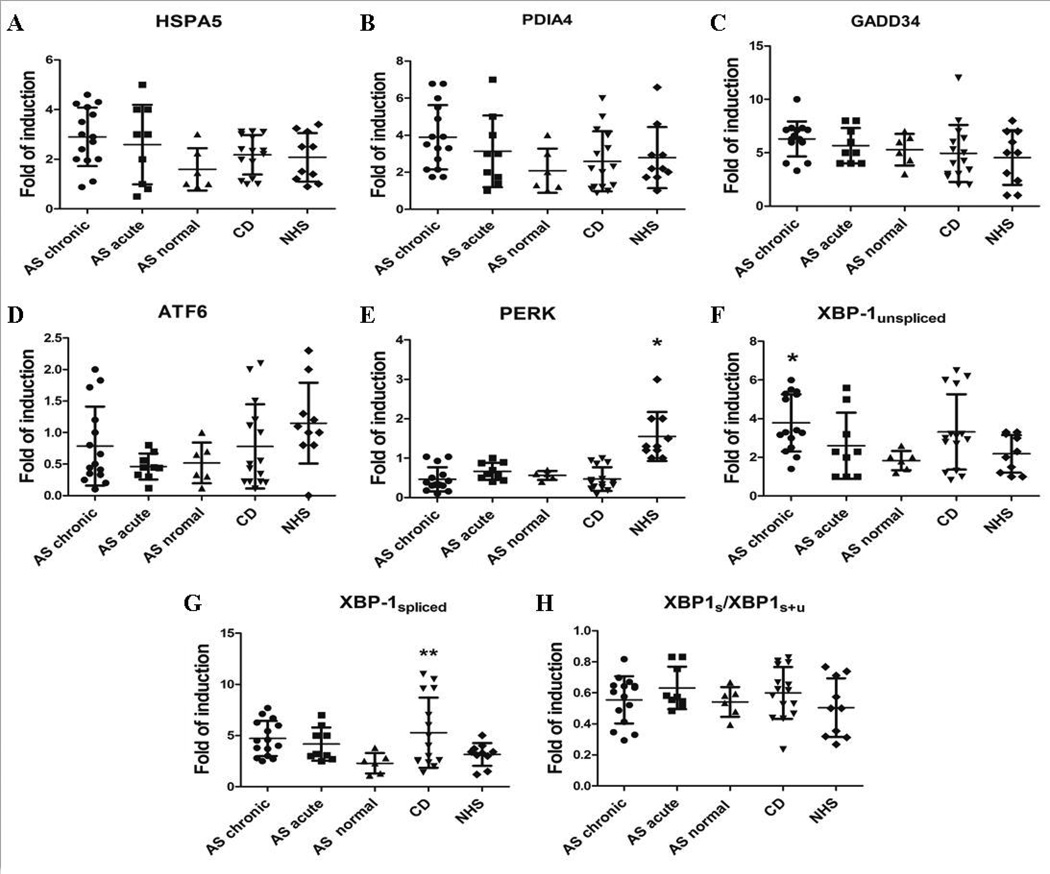
Since there is a close relationship between autophagy and UPR we also analyzed the UPR response in the gut of AS patients and controls. The expression of the heat shock 70 kDa protein 5 (HSPA5) was assessed as a marker of global UPR.[26] The three main UPR pathways were also evaluated through the measure of the expression of PDIA4, GADD34, ATF6, PERK and XBP1. The expression levels of HSPA5 (Figure 3A), PDIA4 (Figure 3B), GADD34 (Figure 3C), and ATF6 (Figure 3D) were similar in the ileal samples from patients and controls, whereas PERK expression was significantly less in the gut of all AS and CD patients (Figure 3E). Different regulation of XBP-1 was observed. Unspliced XBP-1 (Figure 3F) was in fact up-regulated in the gut of AS patients with chronic intestinal inflammation and CD patients compared to controls. Conversely, XBP-1 spliced (Figure 3G) levels were significantly up-regulated only in the gut of CD patients, whereas XBP-1 splicing, evaluated as the ratio of XBP1s(XBP-1s+XBP-1u) [27] did not significantly differ between patients and controls (Figure 3H). The protein levels of HSPA5 and XBP-1 were also evaluated by immunohistochemistry. As showed in supplemental figure 3, no significant differential expression of HSPA5 (A–D)) and XBP-1 (F–H) was observed in AS and CD patients compared to controls.
Figure 3. Unfolded protein response in the gut of AS and CD patients and healthy subjects.
A–H: Relative m-RNA quantification of HSPA5 (A), PDIA4 (B), GADD34 (C), PERK (D), ATF6 (E), XBP1-unspliced (F), XBP1-spliced (G) and XBP1 ratio (H) was assessed by quantitative rt-PCR in ileal biopsy specimens from AS patients, CD patients and controls. Data are shown as mean (SD). *p<0.0001; **p<0.05
Heavy chain misfolding occurs in the gut of AS patients
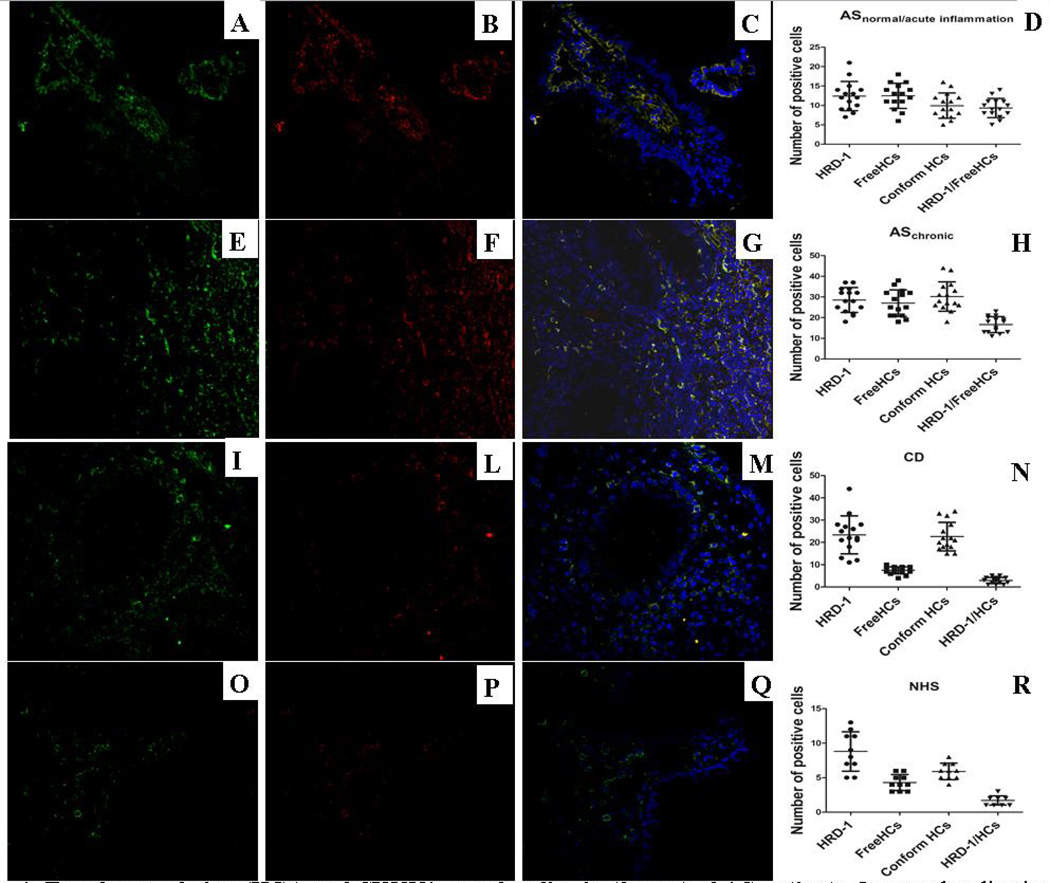
The absence of a clear UP activation prompted us to investigate the occurrence of HC accumulation and possible misfolding. Since SYVN1 is involved in the degradation of non-β2m bound misfolded MHC class I heavy chains but not properly conformed MHC I-β2m-peptide heterotrimers,[28] double immunofluorescent confocal microscopy analysis was performed by using two monoclonal antibodies for HCs (the W6/32, specific for fully assembled MHC class I molecules and the mAb HC10, specific for β2m-free HLA-B and C HCs (FHCs), including misfolded forms of HLA-B27) and the anti-SYVN1 antibody. Properly folded (data not shown) and SYVN1 (were both over-expressed in the gut of AS (Figure 4 A,D and E,H) and CD (Figure 4 I, N) patients compared to controls (Figure 4 O, R). Conversely, a significantly higher amount of free HCs was detected intra-cellularly only in the gut of AS patients independently by the presence/absence of intestinal inflammation (Figure 4B,D, and F,H) and in the HLA-B27+ CD patient (data not shown); rare co-localization of properly-folded HCs and SYVN1 was observed in either patients and controls (data not shown). Interestingly in both group of AS patients (independent of gut inflammation) (Figure 4C–D and G–H) and in the HLA-B27+ CD patient (data not shown) a strong co-localization of free HCs and SYVN1 was observed, providing the first evidence of HLA-B27 misfolding in AS patients.
Figure 4. Free heavy chains (HCs) and SYVN1 co-colocalize in the gut of AS patients.
Immunolocalization by confocal microscopy of SYVN1 (green staining) and HCs (red staining) in AS, CD and control ileal biopsies. Paraffin-embedded section from patients and controls were stained with rabbit anti-human SYVN-1 and mouse anti-human free HCs (HC10) antibodies and treated with FITC-conjugated anti-rabbit antibody and Rhodamine Red–conjugated anti-mouse antibody. SYVN1 and free HCs expression was significantly increased in the gut of AS patients independently by the degree of intestinal inflammation (A–B, D and E–F, H) compared to controls (O–P, R). Significant co-localization of SYVN1 and free HCs was detected in the gut of AS patients independently by the degree of intestinal inflammation (C–D, G–H) compared to CD (M–N) and controls (Q–R). (A–C, E–G, I–M, O–Q: original magnification × 250).
Autophagy but not UPR regulates IL-23p19 production in LPMC
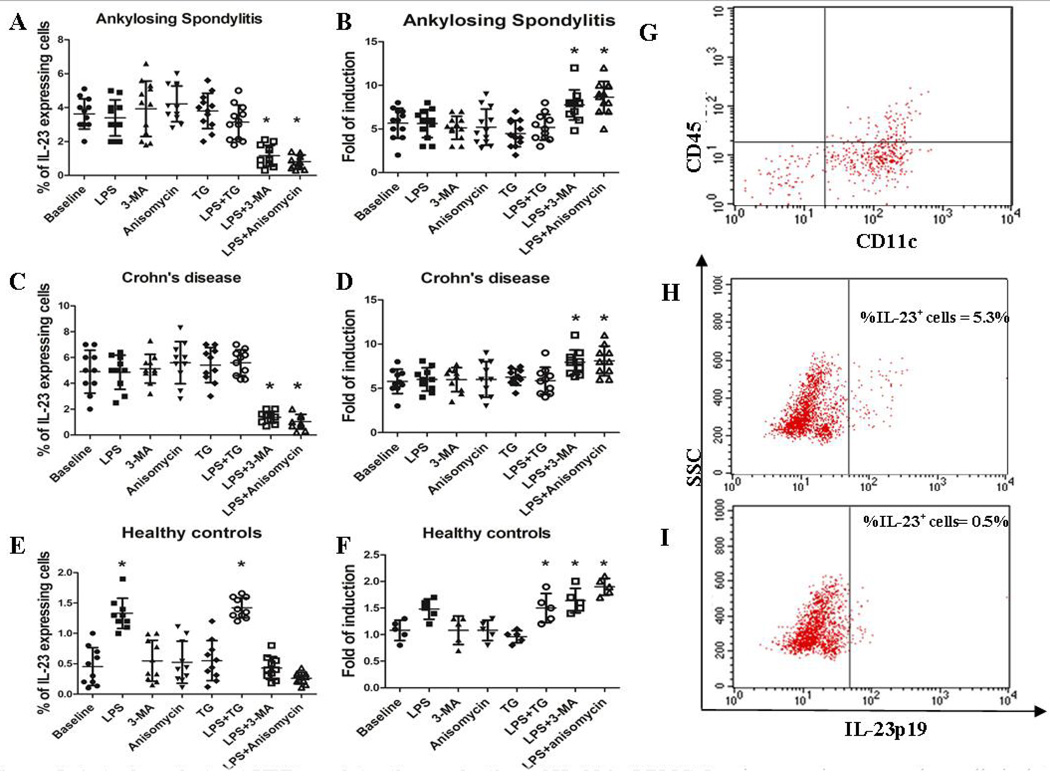
We next examined the contribution of UPR and autophagy to the regulation of IL-23 expression. In this regard, isolated mononuclear cells from 12 AS, 10 CD patients and 10 controls were analyzed for the IL-23p19 expression before and after stimulation with LPS, in the presence or absence of TG (to induce UPR) and 3-MA (to block autophagy). Unstimulated LPMC from AS patients with chronic gut inflammation (Figure 5 A) and CD patients (Figure 5 C) produced significantly higher amounts of IL-23p19 compared to controls (Figure 5E). Incubation with LPS significantly increased the number of IL-23p19 producing LPMC from control subjects (Figure 5F) but not from AS and CD patients (Figure 5B and D respectively), suggesting a maximal pre-activation of LPMC to produce IL-23p19. Treatment and/or pre-treatment of LPMC with thapsigargin did not result in greater LPS-induced up-regulation of IL-23p19-producing cells either in AS, CD or normal controls (Figure 5A–B), arguing against a role for UPR in the modulation of IL-23 production in LPMC from patients and controls. Finally, only pre-treatment with the autophagy inhibitor 3-MA and CMA-inhibitor anisomycin similarly reduced the number of IL-23p19-expressing cells in patients and controls. Modulation of IL-23 m-RNA levels by UPR and autophagy was also evaluated by RT-PCR. IL-23p-19 mRNA levels in AS and CD patients were significantly increased only by the combination of 3-MA+LPS and anisomycin+LPS (Figure 5B, D). In healthy controls LPMCs, LPS increased IL-23p19 expression and the pre-incubation with 3-MA/anisomycin further enhance this effect. Altogether these experiments suggest that autophagy process but not the UPR activation may be involved in regulating IL-23 in the gut of AS patients.
Figure 5. Autophagy but not UPR regulates the production of IL-23 in LPMC.
Lamina propria mononuclear cells isolated from patients with AS, CD and HCs were cultured in the presence of LPS, 3-MA/anisomycin (to block autophagy) and TG (to induce UPR) alone and with LPS+TG and LPS+3-MA. The percentage of IL-23-producing cells and the m-RNA levels of IL-23p19 were evaluated by flow cytometry and RT-PCR respectively. The percentage of IL-23 producing cells and the m-RNA levels were modified in AS (A) and CD (C) only by the combination of 3MA/anisomycin and LPS. In normal controls LPS alone increased the percentage of IL-23-producing cells and the levels of IL-23 that were not further increased by the co-incubation with TG. Block of autophagy and chaperone mediated autophagy significantly reduced the number of IL-23-producing cells in AS and CD and restrained the LPS-dependent IL-23 expression in LPMC from controls, also increasing the IL-23p19 m-RNA levels (B, D, F) *p<0.05. G: representative dot plot of CD45+ versus CD11c+ cells among LPMC from AS patients. H–I: representative dot plot showing the percentage of IL-23 expressing cells before (H) and after (I) 3-MA/LPS incubation.
Discussion
In this study we confirm our previous results that showed that IL-23 is over-expressed in the gut of AS patients [5] and demonstrate for the first time that autophagy but not the UPR is differentially regulated and may be modulating production of IL-23p19 in the lamina propria mononuclear cells. Finally, we provide the first evidence that HLA-B27 misfolding is occurring in the gut of AS patients.
A considerable amount of genetic, immunological and therapeutic studies have demonstrated that IL-23 may be an important driver in AS aetiopathogenesis. The identification of the IL-23 pathway as a key regulator of entheseal pro-inflammatory responses [29] has added new arguments in favor of a pathophysiological role of this cytokine in AS. [5, 9–11] Why IL-23 is over-expressed in the gut of AS is not still known, however. The discovery that the HLA-B27 heavy chain is prone to misfold inducing ER stress and activating the so called Unfolded Protein Response (UPR), and the demonstration in a murine model that UPR activation in macrophages can promote IL-23p19 induction in response to lipopolysaccharide (LPS) provides a possible explanation.[12, 30] However, UPR activation was not observed in human macrophages from AS patients where IL-23 production was substantially greater than controls [9] rendering the exact role of misfolding unclear.
MHC I HCs are retained intra-cellularly, becoming associated with the endoplasmic-reticulum-associated protein degradation-associated E3 ubiquitin-protein ligase (SYVN1) and targeted for endoplasmic-reticulum-associated protein degradation (ERAD).[28] In the absence of SYVN1, misfolded HLA-B27 accumulates in the cells.[28] Thus the co-colocalization of SYVN1 together with non-β2m bound (HC-10-reactive) MHC class I heavy chains discriminates misfolded MHC class I from properly folded MHC I-β2m-peptide heterotrimers.[28] In our study, in the chronically inflamed gut of AS, both conformational and free HCs were over-expressed but only the unbound HCs significantly co-localized with SYVN1, strongly suggesting the occurrence of HLA-B27 misfolding in the intestine of HLA-B27+ AS patients.
IL-23 is constitutively produced in the terminal ileum in the presence of commensal microbes and plays an important role in the host defense against bacterial infection.[4] Subclinical gut inflammation have been demonstrated in a considerable percentage of AS patients and it seems to be immunologically characterized by the increased expression of IL-23 [5][5] perhaps as the result of chronic alterations in host-microbe interactions. In the gut of AS patients the main source of IL-23 is represented by infiltrating inflammatory mononuclear cells and Paneth cells (PC),[5] a subset of specialized epithelial cells that is considered to have a role in innate immune function through the secretion of antimicrobial peptides.[31] This secretory function seems to be closely related to the autophagy pathway, as suggested by the PC granule abnormalities observed in Crohn’s disease patients who have polymorphisms in the autophagy gene Atg16L1.[32]
Autophagy is a mechanism of controlled digestion of damaged organelles within a cell that may be differentiated in macroautophagy, in which entire regions of cytosol are sequestered in vesicular compartments and chaperone-mediated autophagy (CMA), that is exclusively dedicated to degradation of soluble proteins.[33] During infection, autophagy may be also induced as an innate defense mechanism to eliminate bacteria or toxins [20, 21] and activation of autophagy has been demonstrated in PC of patients with Crohn’s disease independently of disease-associated variants of ATG16L1 or IRGM.[34]
In our study in the inflamed gut of AS and CD patients, a different regulation of autophagy and CMA was observed. The expression of macro-autophagy genes was in fact significantly up-regulated in comparison with healthy subjects whereas chaperone-mediated autophagy appears to be importantly down-regulated. The macro-autophagy activation was confirmed by the immunohistochemistry experiments as recommended by the recently published Guidelines for the use and interpretation of assays for monitoring autophagy.[35] LC3II, a marker of global autophagy, was demonstrated to be in fact up-regulated in the gut of AS and CD patients and associated with autophagosome formation, as suggested by the strong co-localization of with ATG5. Two additional markers of autophagy, ATG5 and ATG12, were also found to be increased at protein level in the gut of AS and CD patients. Finally, SQSTM1 a ubiquitin binding protein that accumulates when autophagy is inhibited, and is reduced when autophagy is induced, was not detectable in the gut of CD patients and AS patients with chronic gut inflammation.
Interestingly, increased autophagy gene expression was correlated with augmented IL-23p19 levels. In this regard, numerous cytokines have been shown to regulate autophagy in macrophages and dendritic cells and, obviously, we cannot exclude that the increased autophagy expression in the AS gut may be the consequence rather than the cause of IL-23 over-expression. Since the important role of PC in the defense against microorganisms and their activation to release antimicrobial peptides in both AS and CD ileal samples,[36] we can also speculate that specific microbiota alterations could drive activation of autophagy in CD and AS.
However, our data suggest that autophagy may be one of the immunological factors regulating the production and secretion of IL-23 in AS. Specifically, inhibition of autophagy and CMA (with 3-MA and anisomycin) was sufficient, in the presence of LPS, to reduce the percentage of IL-23 expressing-cells at the same time increasing the mRNA levels for IL-23p19 in the LPMCs isolated from the gut of patients and controls. The increased expression of IL-23p19 mRNA after LPS/3-MA and LPS/anisomycin stimuli could suggest that the reduced percentage of IL-23-producing cells observed in flow-cytometry may be attributable to the active cellular release of IL-23. However, the significant down-regulation of chaperone-mediated autophagy observed in inflamed gut and its negative correlation with IL-23 levels could suggest a role of specific branch of autophagy pathway in modulating IL-23 response. Nevertheless, the in vitro activation of LPMCs with PMA/ionomycin before the incubation with 3-MA is an experimental artifice that could limit the strength of our results. In agreement with this result is however the recent demonstration that autophagy regulates IL-23 secretion in dendritic cells [37] and our immunohistochemical observation that autophagy is localized to the two main cellular sources of IL-23 in the gut of AS, the infiltrating inflammatory mononuclear cells and in those that we consider to be Paneth cells
Our study aimed also to investigate the UPR activation in the gut of AS patients. Similar to a previous study in macrophages,[9] other than increased total (unspliced) XBP1 mRNA, we found no evidence for UPR activation playing a role in IL-23 production. UPR signaling is initiated by the activation of at least three distinct stress sensors located at the ER membrane known as the ER-resident transmembrane protein kinase-endoribonucleases (RNase) (ERN1/IRE1α), the protein kinase PERK EIF2AK3/PERK and a family of type II transmembrane transcription factors, whose most prominent member is ATF6α. When unfolded proteins accumulate in the ER, eukaryotic cells can up-regulate the expressions of the chaperone protein HSPA5 (which is used as a marker of global UPR).[38] Activation of the IRE1 kinase activity was assayed indirectly in our study by measuring the levels of unspliced and spliced XBP1 mRNA (XBP1u and XBPs, respectively), the latter of which is generated by removal of a 26-nucleotides and results in the generation of a functional protein (sXBP1). Activation of the PERK and ATF6 branches were evaluated by assessing the expression levels of transcriptionally activated target genes, GADD34 and PDIA4, respectively. No increased expression levels of UPR genes was observed in the ileal mucosa of AS and CD patients compared to healthy controls. Since our results support the presence of HLA-B27 misfolding, we cannot obviously exclude that low levels of ER stress caused by HLA-B27 in the gut of AS patients contribute to the intense activation of autophagy, which in turn may further limit the full UPR activation by enhancing the removal of misfolded protein. Consistent with this concept, ER stress has been shown to activate autophagy through PERK [39] and/or XBP1.[40] Interestingly, misfolded monomeric proteins have been demonstrated to be degraded by CMA [41, 42] and the down-regulation of CMA could account, at least in part, for the accumulation of misfolded proteins observed in AS gut.
In conclusion we provide evidence that autophagy, rather than robust UPR activation, may be an important regulator of IL-23 production in the gut of AS patients. Since autophagy plays an important role in the intestinal immune response against microorganisms, our results could suggest a fundamental role of AS intestinal microbiota in regulating intestinal IL-23 expression. We also provide the first evidence that HLA-B27 misfolding occurs in the gut of AS. Further studies are required to better study the specific role of autophagy and chaperone-mediated autophagy in regulating the immunology of the gut of AS patients.
Supplementary Material
Acknowledgements
We thank Dr. Francesca Raiata for her excellent assistance in immunohistochemical experiments.
Funding: This study was supported in part by a grant from Ministero della Università e della Ricerca Scientifica of Italy.
Abbreviations
- AS
Ankylosing Spondylitis
- CD
Crohn’s disease
- NSAIDs
non-steroidal anti-inflammatory drugs
- LPMCs
Lamina propria mononuclear cells
- UPR
unfolded protein response
- CMA
chaperone-mediated autophagy
- ATG16L1
autophagy related 16-like 1
- IRGM
immunity-related GTPase family
- MAP1LC3A
microtubule-associated protein 1 light chain 3 alpha
- IL-23
interleukin 23
- ATG5
autophagy related 5
- ATG12
autophagy related 12
- HSPA8
heat shock 70kDa protein 8
- HSP90AA1
heat shock protein 90kDa alpha, class A member 1
- HSPA5
heat shock 70kDa protein 5
- PDIA4
protein disulfide isomerase family A, member 4
- GADD34
Growth arrest and DNA damage-inducible protein
- ATF6
Activating transcription factor 6
- PERK
protein kinase (PKR)-like endoplasmic reticulum kinase
- XBP-1
X-box binding protein 1
- SYVN1
E3 ubiquitinprotein ligase synoviolin
- SQSTM1
sequestosome 1
Footnotes
Competing Interest: None declared
Contributorship: FC, AA-P, AR and GG planned the study, performed laboratory work, participated to data analysis and wrote the manuscript. SR, ARG, AC performed laboratory work, participated to data analysis and patient selection and diagnosis. RAC, RA and GT planned the study, participated to data analysis and wrote the manuscript.
The Corresponding Author, Prof. Giovanni Triolo, has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in ARD and any other BMJPGL products and sublicenses such use and exploit all subsidiary rights, as set out in our license
References
- 1.Mielants H, Veys EM, Cuvelier C, de Vos M. Ileocolonoscopic findings in seronegative spondylarthropathies. Br J Rheumatol. 1988;27:95–105. doi: 10.1093/rheumatology/xxvii.suppl_2.95. [DOI] [PubMed] [Google Scholar]
- 2.Mielants H, Veys EM, De Vos M, et al. The evolution of spondyloarthropathies in relation to gut histology. I. Clinical aspects. J Rheumatol. 1995;22:2266–2272. [PubMed] [Google Scholar]
- 3.Mielants H, Veys EM, Cuvelier C, et al. The evolution of spondyloarthropathies in relation to gut histology. II. Histological aspects. J Rheumatol. 1995;22:2273–2278. [PubMed] [Google Scholar]
- 4.Becker C, Wirtz S, Blessing M, et al. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J Clin Invest. 2003;112:693–706. doi: 10.1172/JCI17464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciccia F, Bombardieri M, Principato A, et al. Overexpression of interleukin-23, but not interleukin-17, as an immunologic signature of subclinical intestinal inflammation in ankylosing spondylitis. Arthritis Rheum. 2009;60:955–965. doi: 10.1002/art.24389. [DOI] [PubMed] [Google Scholar]
- 6.Rahman P, Inman RD, Gladman DD, et al. Association of interleukin-23 receptor variants with ankylosing spondylitis. Arthritis Rheum. 2008;58:1020–1025. doi: 10.1002/art.23389. [DOI] [PubMed] [Google Scholar]
- 7.Rueda B, Orozco G, Raya E, et al. The IL23R Arg381Gln non-synonymous polymorphism confers susceptibility to ankylosing spondylitis. Ann Rheum Dis. 2008;67:1451–1454. doi: 10.1136/ard.2007.080283. [DOI] [PubMed] [Google Scholar]
- 8.Reveille JD, Sims AM, Danoy P, et al. Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat Genet. 2010;42:123–127. doi: 10.1038/ng.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng L, Lindstrom MJ, Smith JA. Ankylosing spondylitis macrophage production of higher levels of interleukin-23 in response to lipopolysaccharide without induction of a significant unfolded protein response. Arthritis Rheum. 2011;63:3807–3817. doi: 10.1002/art.30593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mei Y, Pan F, Gao J, et al. Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin Rheumatol. 2011;30:269–273. doi: 10.1007/s10067-010-1647-4. [DOI] [PubMed] [Google Scholar]
- 11.Melis L, Vandooren B, Kruithof E, et al. Systemic levels of IL-23 are strongly associated with disease activity in rheumatoid arthritis but not spondyloarthritis. Ann Rheum Dis. 2010;69:618–623. doi: 10.1136/ard.2009.107649. [DOI] [PubMed] [Google Scholar]
- 12.DeLay ML, Turner MJ, Klenk EI, et al. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 2009;60:2633–2643. doi: 10.1002/art.24763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinnebrew MA, Buffie CG, Diehl GE, et al. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36:276–287. doi: 10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodall JC, Wu C, Zhang Y, et al. Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc Natl Acad Sci U S A. 2010;107:17698–17703. doi: 10.1073/pnas.1011736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goto Y, Kiyono H. Epithelial barrier: an interface for the cross-communication between gut flora and immune system. Immunol Rev. 2012;245:147–163. doi: 10.1111/j.1600-065X.2011.01078.x. [DOI] [PubMed] [Google Scholar]
- 16.Murrow L, Debnath J. Autophagy as a Stress-Response and Quality-Control Mechanism: Implications for Cell Injury and Human Disease. Annu Rev Pathol. 2013;8:105–137. doi: 10.1146/annurev-pathol-020712-163918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delgado MA, Elmaoued RA, Davis AS, et al. Toll-like receptors control autophagy. EMBO J. 2008;27:1110–1121. doi: 10.1038/emboj.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanjuan MA, Dillon CP, Tait SW, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 19.Yano T, Kurata S. Induction of autophagy via innate bacterial recognition. Autophagy. 2008;4:958–960. doi: 10.4161/auto.6802. [DOI] [PubMed] [Google Scholar]
- 20.Qiu W, Zhang J, Dekker MJ, et al. Hepatic autophagy mediates endoplasmic reticulum stress-induced degradation of misfolded apolipoprotein B. Hepatology. 2011;53:1515–1525. doi: 10.1002/hep.24269. [DOI] [PubMed] [Google Scholar]
- 21.Kruse KB, Brodsky JL, McCracken AA. Autophagy: an ER protein quality control process. Autophagy. 2006;2:135–137. doi: 10.4161/auto.2.2.2388. [DOI] [PubMed] [Google Scholar]
- 22.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 23.Ciccia F, Accardo-Palumbo A, Alessandro R, et al. Interleukin-22 and IL-22-producing NKp44(+) NK cells in the subclinical gut inflammation of patients with ankylosing spondylitis. Arthritis Rheum. 2012;64:1869–1878. doi: 10.1002/art.34355. [DOI] [PubMed] [Google Scholar]
- 24.Stam NJ, Spits H, Ploegh HL. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986;137:2299–2306. [PubMed] [Google Scholar]
- 25.Ciccia F, Accardo-Palumbo A, Giardina A, et al. Expansion of intestinal CD4+CD25(high) Treg cells in patients with ankylosing spondylitis: a putative role for interleukin-10 in preventing intestinal Th17 response. Arthritis Rheum. 2010;62:3625–3634. doi: 10.1002/art.27699. [DOI] [PubMed] [Google Scholar]
- 26.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bogaert S, De Vos M, Olievier K, et al. Involvement of endoplasmic reticulum stress in inflammatory bowel disease: a different implication for colonic and ileal disease? PLoS One. 2011;6:e25589. doi: 10.1371/journal.pone.0025589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burr ML, Cano F, Svobodova S, et al. HRD1 and UBE2J1 target misfolded MHC class I heavy chains for endoplasmic reticulum-associated degradation. Proc Natl Acad Sci U S A. 2011;108:2034–2039. doi: 10.1073/pnas.1016229108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherlock JP, Joyce-Shaikh B, Turner SP, et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4-CD8- entheseal resident T cells. Nat Med. 2012;18:1069–1076. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- 30.Turner MJ, Sowders DP, DeLay ML, et al. HLA-B27 misfolding in transgenic rats is associated with activation of the unfolded protein response. J Immunol. 2005;175:2438–2448. doi: 10.4049/jimmunol.175.4.2438. [DOI] [PubMed] [Google Scholar]
- 31.Keshav S. Paneth cells: leukocyte-like mediators of innate immunity in the intestine. J Leukoc Biol. 2006;80:500–508. doi: 10.1189/jlb.1005556. [DOI] [PubMed] [Google Scholar]
- 32.Cadwell K, Liu JY, Brown SL, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaushik S, Cuervo AM. Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol. 2012;22:407–417. doi: 10.1016/j.tcb.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thachil E, Hugot JP, Arbeille B, et al. Abnormal activation of autophagy-induced crinophagy in Paneth cells from patients with Crohn's disease. Gastroenterology. 2012;142:1097–1099. doi: 10.1053/j.gastro.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 35.Klionsky DJ, Abdalla FC, Abeliovich H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ciccia F, Bombardieri M, Rizzo A, et al. Over-expression of paneth cell-derived anti-microbial peptides in the gut of patients with ankylosing spondylitis and subclinical intestinal inflammation. Rheumatology (Oxford) 2010;49:2076–2083. doi: 10.1093/rheumatology/keq239. [DOI] [PubMed] [Google Scholar]
- 37.Peral de Castro C, Jones SA, Ni Cheallaigh C, et al. Autophagy regulates IL-23 secretion and innate T cell responses through effects on IL-1 secretion. J Immunol. 2012;189:4144–4153. doi: 10.4049/jimmunol.1201946. [DOI] [PubMed] [Google Scholar]
- 38.Diehl JA, Fuchs SY, Koumenis C. The cell biology of the unfolded protein response. Gastroenterology. 2011;141:38–41. doi: 10.1053/j.gastro.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kouroku Y, Fujita E, Tanida I, et al. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- 40.Margariti A, Li H, Chen T, et al. XBP1 mRNA splicing triggers an autophagic response in endothelial cells through BECLIN-1 transcriptional activation. J Biol Chem. 2013;288:859–872. doi: 10.1074/jbc.M112.412783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishida Y, Yamamoto A, Kitamura A, et al. Autophagic elimination of misfolded procollagen aggregates in the endoplasmic reticulum as a means of cell protection. Mol Biol Cell. 2009;20:2744–2754. doi: 10.1091/mbc.E08-11-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali AB, Nin DS, Tam J, Khan M. Role of chaperone mediated autophagy (CMA) in the degradation of misfolded N-CoR protein in non-small cell lung cancer (NSCLC) cells. PLoS One. 2011;6:e25268. doi: 10.1371/journal.pone.0025268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.