Abstract
Purpose
MR-based quantification of liver magnetic susceptibility may enable field strength-independent measurement of liver iron concentration (LIC). However, susceptibility quantification is challenging, due to non-local effects of susceptibility on the B0 field. The purpose of this work is to demonstrate feasibility of susceptibility-based LIC quantification using a fat-referenced approach.
Methods
Phantoms consisting of vials with increasing iron concentrations immersed between oil/water layers, and twenty-seven subjects (9 controls/18 subjects with liver iron overload) were scanned. Ferriscan (1.5T) provided R2-based reference LIC. Multi-echo 3D-SPGR (1.5T/3T) enabled fat-water, B0- and R2*-mapping. Phantom iron concentration (mg Fe/l) was estimated from B0 differences (ΔB0) between vials and neighboring oil. Liver susceptibility and LIC (mg Fe/g dry tissue) was estimated from ΔB0 between the lateral right lobe of the liver and adjacent subcutaneous adipose tissue (SAT).
Results
Estimated phantom iron concentrations had good correlation with true iron concentrations (1.5T:slope=0.86, intercept=0.72, r2=0.98; 3T:slope=0.85, intercept=1.73, r2=0.98). In liver, ΔB0 correlated strongly with R2* (1.5T:r2=0.86; 3T:r2=0.93) and B0-LIC had good agreement with Ferriscan-LIC (slopes/intercepts nearly 1.0/0.0, 1.5T:r2=0.67, slope=0.93±0.13, p≈0.50, intercept=1.93±0.78, p≈0.02; 3T:r2=0.84, slope=1.01±0.09, p≈0.90, intercept=0.23±0.52, p≈0.68).
Discussion
Fat-referenced, susceptibility-based LIC estimation is feasible at both field strengths. This approach may enable improved susceptibility mapping in the abdomen.
Keywords: susceptibility, iron overload, chemical shift encoded imaging, field map, magnetic resonance imaging, liver
Introduction
Excessive accumulation of iron in the body can result from abnormal intestinal absorption in hereditary hemochromatosis or repeated intravenous blood transfusions (ie: transfusional hemosiderosis) (1–3). Excess body iron is toxic, and requires treatment aimed at reducing body iron stores (4). Measurement of body iron stores is critical for detection of iron overload, staging its severity and monitoring of iron-reducing therapies that are often extremely expensive and carry their own toxicities (5–8).
The simplest method available to assess body iron is the use of serum markers of iron overload: measuring serum ferritin or serum transferrin receptor concentration from a blood sample. Unfortunately, it is well known that serum biochemical tests are often confounded by a number of factors such as inflammatory states and do not accurately reflect body iron levels (9).
Liver iron content (LIC) is widely considered the best reference for assessing total body iron stores, because the amount of iron in the liver is closely correlated to total body iron (8). Currently, chemical analysis of liver biopsy samples is the best available reference standard to measure LIC. However, biopsy is limited because it is invasive, expensive, and has poor sampling variability (8,10). In addition, biopsy is contraindicated in patients who have thrombocytopenia or coagulopathies, which commonly occur in patients with anemia receiving blood transfusions, due to the risk of uncontrolled bleeding.
Iron is the only non-trace element in the body that impacts tissue magnetic susceptibility, which is proportional to iron concentration (8,11). Liver iron susceptometry using superconducting quantum interference devices (SQUID) provides an alternative non-invasive method for measuring LIC (12–15). Although SQUID has been calibrated, validated and used for clinical studies, its complexity, high cost and limited availability have precluded its widespread use (8).
MRI is widely available and accessible, has been shown to be very sensitive to the presence of iron, and is capable of measuring magnetic susceptibility in some regions of the body, such as the brain (16,17). However, MR-based susceptibility mapping in the abdomen is a challenging problem, due to the lack of focal sources of susceptibility, the presence of fat and air, as well as motion related artifacts (18). Instead, current methods to measure iron using MRI are indirect and include signal intensity ratio (19), R2 relaxometry (20,21), and R2* relaxometry (22–27). R2- or R2*-based liver iron quantification is performed using empirically-derived calibration curves relating the relaxation parameter (R2 or R2*) to LIC (20–28). Although these methods have been shown to work well in practice, there is significant interest in the quantification of iron concentration based on measurement of magnetic susceptibility, a fundamental parameter of tissue.
The purpose of this work is to demonstrate the feasibility of susceptibility as a biomarker for liver iron concentration measurements. In previous work by other groups, it has been shown that MRI-based liver susceptometry is feasible using “boundary” B0 field measurements (29–31), by measuring B0 in the liver and adjacent muscle, resulting in “muscle-referenced” liver iron measurements that correlate well with R2*-derived LIC at 1.5T (18). This important work demonstrated the feasibility of liver susceptometry using a boundary approach, however it has several limitations: 1) the use of muscle as a reference for boundary measurements may not easily lead to automated methods for liver susceptometry (ie: without the need for manually tracing regions of interest), and 2) validation at only 1.5T. In this work, we expand on previous studies by performing fat-referenced liver susceptibility quantification with subcutaneous adipose tissue (SAT) as reference for B0 measurements (32). Further, we test this approach at both 1.5T and 3T, and validate our technique with an FDA-approved R2-based measure of liver iron (Ferriscan) (20,33). The inherent ability of chemical shift-encoded imaging to perform fat-water separation in addition to B0 mapping provides automated and co-registered mapping of adipose tissue locations (34,35). Thus, the locations and magnetic susceptibility of adipose tissue may provide a useful constraint to enable the development of automated quantitative susceptibility mapping techniques in the abdomen. The main purpose of this manuscript is to demonstrate that the fundamental information contained in B0 field maps allows susceptibility measurement in the liver at both 1.5T and 3T. Based on these results, we aim to provide a foundation for future development of comprehensive quantitative susceptibility mapping techniques in the liver as a fundamental biomarker of liver iron overload.
Theory
The susceptibility difference between two adjacent tissues (Δχ, in ppm) is related to the difference in magnetic field (ΔB0, in ppm) at both sides of the boundary, based on the boundary conditions for Maxwell’s equations. Including the sphere of Lorentz effects and hyperfine shift the susceptibility difference and magnetic field difference can be written (29,30,32):
| [1] |
where ΔB0=B0,liver − B0,SAT is the magnetic field difference (in ppm) between the liver and the adjacent SAT, θ is the angle between the vector perpendicular to the boundary and the main B0 field, and Shf is the hyperfine shift assumed to be −0.133 from previous works (18). In this work, boundaries parallel to the main magnetic field were selected such that θ ≈ 90° (and thus cos θ ≈ 0), by measuring B0 fields in the lateral aspect of the right lobe of the liver (figure 1). With this constraint, Eq. 1 can be simplified as:
| [2] |
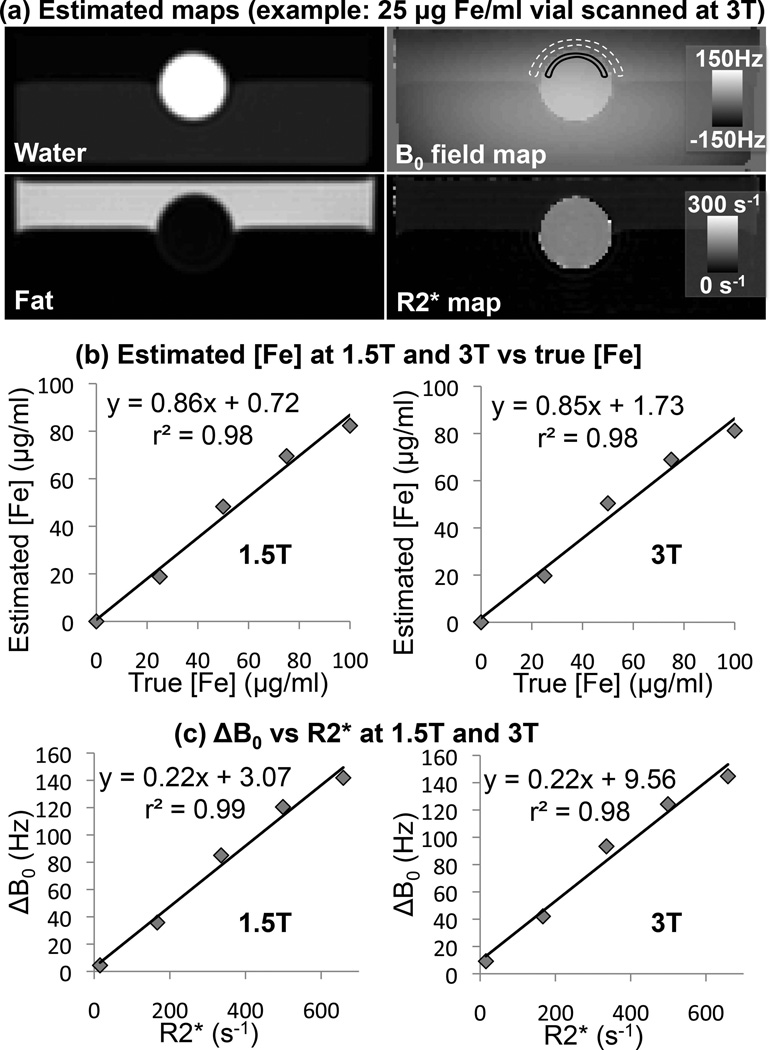
Figure 1.
Geometry of proposed boundary B0 measurements. The boundary between liver and subcutaneous adipose tissue (SAT) is nearly parallel to the main B0 field (thus θ≈90° and cos(θ)≈0 in Eq. 1), simplifying the analysis and estimation of liver susceptibility. Note that the measurements are obtained from axial slices, but a coronal schematic view is shown here for clarity.
In this work, we focus on susceptibility differences between liver tissue and nearby subcutaneous adipose tissue (SAT). Note that even if SAT and liver are not in direct contact, SAT can be used as a reference for susceptibility measurements due to the fact that the distance to the liver is usually small. In addition, we assume that SAT is homogeneous with no significant iron accumulation since it is not part of the reticuloendothelial system (RES) that accumulates iron tissue in Kupffer cells and other phagocytic white blood cells.
In this setting, we can decompose the susceptibility difference between liver and SAT into two components, as follows:
| [3] |
where Δχbase is the baseline susceptibility difference between both tissues (in the absence of liver iron), and ΔχFe is the additional paramagnetic contribution of iron to the susceptibility of liver (11,36). Equation 3 is an approximation, by assuming that iron is the only factor affecting the magnetic susceptibility of liver tissue (eg: it ignores the potential presence of liver fat).
Based on the paramagnetic properties of storage iron, the paramagnetic contribution of iron to susceptibility can be described as (36):
| [4] |
where N is the number of iron particles per m3, μ0=4π×10−7 V·s/(A·m) is the vacuum permeability, μeff is the effective number of Bohr magnetons per atom, approximately =3.78 as in (36), μB=9.274×10−24 is the Bohr magneton, k=1.38×10−23 J·K−1 is the Boltzmann constant, and T≈310 K is body temperature. Based on Eq. 4, and using the approximated density 1.05 g/cm3 for liver tissue (11), the iron concentration (in mg Fe/g wet tissue) can be related to the paramagnetic contribution of iron to the susceptibility, as follows (11,36):
| [5] |
Finally, combining Eqs [2] and [4], we can express the iron concentration in terms of the measured magnetic field difference ΔB0 as follows:
| [6] |
Note that liver iron concentration is typically expressed in mg Fe/g dry tissue, ie: [Fe]dry instead of [Fe]wet (in this manuscript LIC is equivalent to [Fe]dry unless explicitly stated otherwise). The relationship between these two measures of iron concentration is not completely understood. Recent works have suggested a conversion factor of 5.5+/−1.0 (37). Using the conversion factor [Fe]dry = 5.5[Fe]wet, we arrive at the final expression for iron concentration in mg Fe/g dry tissue:
| [7] |
where [Fe]dry (mg Fe/g dry tissue) is related directly to ΔB0 (ppm) and Δχbase (ppm). In this work, the baseline susceptibility difference Δχbase is calibrated from a set of control subjects, by assigning them an average [Fe]dry = 0.99 mg Fe/g dry tissue, which is the mean of LIC measured in control subjects in this study (33,38). Aside from this baseline, this approach requires no calibration, in contrast with R2- or R2*-based iron quantification techniques.
Methods
Phantoms
Iron concentration phantoms were constructed using super-paramagnetic iron oxides (SPIOs, Feridex, Bayer Inc, Wayne, NJ). Five cylindrical 20ml vials were constructed with iron concentrations of 0, 25, 50, 75 and 100 mg/l suspended in agar gel, following the technique described by Hines et al (39).
Subjects
All subjects were scanned in accordance to the local Institutional Review Board, and in compliance with HIPAA. 9 healthy volunteers were recruited as controls (5 men aged 24–44 years, and 4 women aged 33–62 years), and 18 patients with known or suspected iron overload (11 men aged 11–79 years, and 7 women aged 12–70 years) were scanned. Patients had a variety of transfusion-dependent anemias, leukemia, myelodysplastic syndrome. One of the patients was heterozygotic with the HFE gene for hemochromatosis.
Imaging Protocol
Data were acquired at 1.5T (Signa HDxt, GE Healthcare, Waukesha, WI) and 3T (MR750, v22.0, GE Healthcare, Waukesha, WI), using investigational versions of a 3D multiecho SPGR sequence with monopolar readouts and flyback gradients (40).
Phantoms were scanned one at a time at both 1.5T and 3T using a single-channel head coil, by placing them in a holder within a plastic container with a layer of water and a layer of peanut oil. This setup enabled a fat-referenced assessment of iron concentration in the vials, similarly to the in vivo case. Phantoms were placed in the magnet with the long axis of the cylindrical vials parallel to the main magnetic field. Acquisition parameters at 1.5T included: axial plane, readout direction R/L, 28 × 22cm field of view, 160×160 matrix, 4mm slice thickness, 32 slices, 10° flip angle, ±125 kHz receiver bandwidth, TR = 20.4 ms, and 12 echoes (2 interleaved echo trains) with TEinit=1.1 ms and ΔTE=0.9 ms. Acquisition parameters at 3T included: axial plane, readout direction R/L, 28 × 20cm field of view, 160×160 matrix, 4mm slice thickness, 32 slices, 10° flip angle, ±125 kHz receiver bandwidth, TR = 9.7 ms, and 8 echoes (two interleaved echo trains) with TEinit=1.1 ms and ΔTE=0.9 ms.
In vivo liver acquisitions included a spin-echo sequence for R2-based liver iron quantification (Ferriscan) at 1.5T. This was performed using a 2D multi-slice spin-echo sequence. Acquisition parameters included: 18 minutes free-breathing, axial plane, readout direction R/L, 44cm field of view, 256×256 matrix, 6mm slice thickness, 11 slices, ±62.5 kHz receiver bandwidth, TR = 1000 ms, five separate echo times (6, 9, 12, 15, 18 ms).
Chemical shift encoded acquisitions for R2* quantification and Bo field mapping were performed at both 1.5T and 3T, using similar acquisitions at both field strengths:
Acquisition parameters at 1.5T included: 19s breath-hold, axial plane, readout direction R/L, 40×36cm field of view, 256×160 matrix, 8mm slice thickness, 32 slices, 5° flip angle, ±125 kHz readout bandwidth, self-calibrated parallel imaging with acceleration factor 2×2 (effective acceleration = 3.8 accounting for auto-calibration lines and k-space corner-cutting) (41), TR = 14.1 ms. An echo train was collected for each TR, consisting of 6 TEs with TEinit=1.2 ms, ΔTE=2.0 ms.
Acquisition parameters at 3T included: 22s breath-hold, axial plane, readout direction R/L, 40×32cm field of view, 256×144 matrix, 8mm slice thickness, 32 slices, 3° flip angle, ±125 kHz readout bandwidth, self-calibrated parallel imaging with acceleration factor 2×2 (effective acceleration = 3.6 accounting for auto-calibration lines and k-space corner-cutting) (41), TR = 8.6 ms. Two interleaved echo trains (3 echoes/TR) were collected to achieve shorter echo spacing, resulting in 6 TEs with TEinit=1.2 ms, ΔTE=1.0 ms. Additionally, the 3T SPGR sequence included baseline phase correction, obtained by acquiring additional low-resolution phase encodes with opposite polarity, in order to remove residual phase errors between the interleaved echo trains (42).
Image Reconstruction
Multi-coil chemical shift encoded data were combined before fat-water separation using the eigenvector filter method described by Walsh et al. (43). The chemical shift-encoded complex signal for echo time tn at a voxel containing water and fat in the presence of R2* decay and B0 field offset can be described as (44,45):
| [8] |
where ρW and ρF are the amplitudes of water and fat signals, respectively, R2* = 1/T2*, fB is the frequency shift due to local magnetic B0 field inhomogeneities, fF,p are the known frequencies for the multiple spectral peaks of the fat signal relative to the water peak, and αP are the relative amplitudes of the fat signal (44,46). The unknown parameters (ρW, ρF, R2*, fB) are estimated in this work by nonlinear least-squares fitting at each voxel using a Levenberg-Marquardt algorithm from the GNU Scientific Library (GSL) in MEX under Matlab (The Mathworks, Natwick, MA). In order to provide a good initialization for the fitting procedure (particularly to avoid fat-water swaps in regions of large field inhomogeneity), an initial guess for the unknown parameters is obtained by regularized estimation from the complex data using a graph-cut algorithm (47). Using the estimated water and fat images, and proton-density fat-fraction (PDFF) maps are generated, including correction for noise bias (48).
Data Analysis (Phantoms)
In each of the phantom datasets, ΔB0 measurements were obtained from a single slice (chosen to have minimum slice-to-slice variation in B0 within the vial, to minimize susceptibility artifacts from the top and bottom of the cylindrical vial) by placing regions of interest (ROIs) inside the vial and in the adjacent oil (figure 2), and subtracting the B0 measurement in the oil from that in the vial to obtain ΔB0. Note that the analysis of B0 field measurements in SPIO phantoms is conveniently performed in units of Hz rather than ppm due to the magnetization saturation of SPIOs. The measured ΔB0 (in Hz) was converted to iron concentration based on the saturation magnetization (Ms) of ferumoxides, assuming Ms=93.6 Am2/kg (49) (including sphere of Lorentz effects as in Eq. 1, but assuming hyperfine shift Shf=0). The conversion was expressed as [Fe] = aΔB0+b, where a ≈ 0.6 µg Fe/ml/Hz and b (in µg Fe/ml) is a “baseline” constant (analogously to the in vivo case) to account for the ΔB0 between agar and peanut oil, chosen to impose that the vial with no iron has [Fe]=0. Additionally, R2* measurements were obtained in each vial by placing an ROI over the vial in one slice. For each field strength, estimated [Fe] (in µg Fe/ml) was compared to true [Fe] using linear regression, and measured ΔB0 (in Hz) was compared to measured R2* (in s−1) using linear regression.
Figure 2.
Phantom results at both 1.5T and 3T (example images in (a)) show that boundary B0 (ΔB0)-based iron concentration estimates correlate well with true iron concentration (b), and ΔB0 measurements correlate well with measured R2* (c). ΔB0 field measurements were obtained by measuring B0 at both sides of the boundary of cylindrical vials containing iron in increasing concentrations. R2* was measured by placing one ROI inside the vial for each iron concentration.
Data Analysis (In Vivo)
Ferriscan is an FDA-approved R2-based method for measurement of LIC, based on previously published calibration data described by St. Pierre et al (21). Ferriscan spin-echo acquisitions were uploaded to Resonance Health (Claremont, Australia), where they were processed to generate R2 maps (50). An R2 measurement was made by Resonance Health (Claremont, Australia) in an ROI over a slice free of severe motion artifacts, while avoiding large vessels and bile ducts. The R2 measurement was converted to an LIC measurement (“Ferriscan-LIC”, with units of mg Fe/g dry tissue), based on a previously validated calibration (21).
In all 27 subjects, an ROI was chosen in segments 5 or 6, in the inferior aspect of the right lobe of the liver, at a level where there were no obvious background B0 field gradients visualized on the field map (mid-liver, away from the liver/lung boundary). An oval region of interest, measuring approximately 3 cm2 in area was chosen near the lateral aspect of the liver, avoiding large vessels and bile ducts. The average value and standard deviation of the B0 field at this location were recorded. In addition, a similar size ROI was placed in the SAT on the PDFF map and copied into the B0 field map, where the average value and standard deviation of the B0 field were recorded. These ROIs were placed avoiding regions of rapid background B0 field variation. Using Eq. 7, the estimated iron concentration was calculated.
Additionally, muscle-referenced liver iron estimates were calculated for comparison. This was performed by using analogous measurements of B0 in liver (inferior aspect of the right lobe of the liver) and adjacent muscle, similarly to the approach described in Ref. (18). From these measurements, LIC was estimated using Eq. 7 as in the fat-referenced case.
Further, a larger circular ROI (approximate area 11 cm2) was chosen in the same or adjacent slice on the R2* map, in either Couinaud segment 5 or 6, avoiding large blood vessels and bile ducts. This ROI was copied to the PDFF map, and the averages and standard deviations of R2* and PDFF were recorded.
Statistical Analysis
Linear regression analysis was performed in phantoms to compare the measured ΔB0 to R2* for each vial, and the estimated iron concentration to the true iron concentration. Linear regression was performed with the in vivo measurements to compare ΔB0 to R2*, and B0-LIC to Ferriscan-LIC. Additionally, Bland-Altman analysis was performed to compare in vivo B0-LIC (fat- and muscle-referenced at each field strength) to Ferriscan-LIC, as well as to compare B0-LIC across field strengths.
Results
Figure 2 shows phantom results, comparing the measured ΔB0 with the true iron concentration, as well as with the measured R2* inside each vial. Phantom iron estimation resulted in a constant offset b=−2.66 µg Fe/ml (1.5T), and b=−5.50 µg Fe/ml (3T). A strong correlation was observed between the true and estimated iron concentration (figure 2b, 1.5T: r2=0.98, 3T: r2=0.98), as well as between ΔB0 and R2* (figure 2c, 1.5T: r2=0.99, 3T: r2=0.98). Regression between estimated and true iron concentration in the phantom resulted in a slope smaller (although not significantly different) than 1.0 (1.5T: 0.86 ± 0.06, p≈0.11, 3T: 0.85 ± 0.07, p≈0.12), and intercept close to 0 (1.5T: 0.72 ± 3.76, p≈0.86, 3T: 1.73 ± 4.34, p≈0.72).
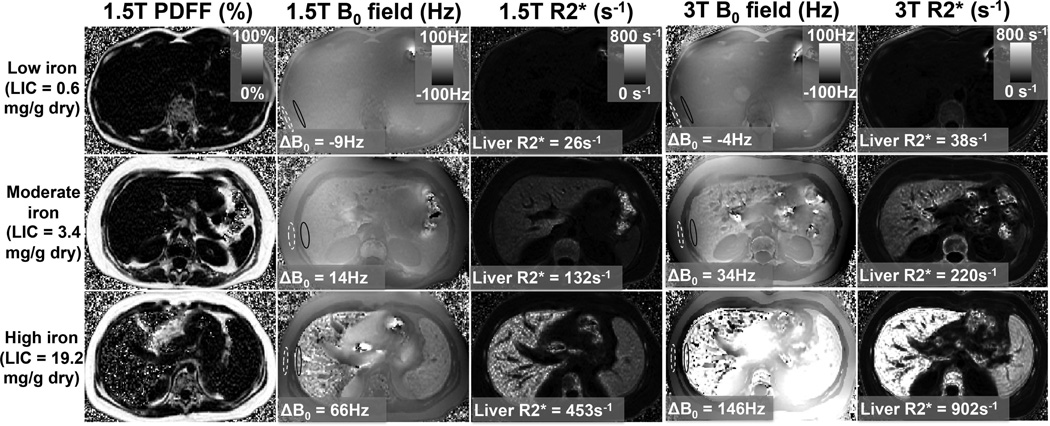
Figure 3 shows examples of the B0 field maps and R2* maps in three subjects with no iron overload, moderate iron overload and more severe iron overload at both 1.5T and 3.0T. The R2* maps show a definite increase with increasing levels of iron overload. Further, the B0 field maps show an apparent increase in the “step-off” difference in the B0 field between the liver and the SAT as iron overload in the liver increases. Note that there are potential complications for B0 field mapping in the presence of very high iron concentrations (figure 3, bottom row). In these cases, the R2* decay of the measured multi-echo signal is very rapid, thus complicating the measurement of B0 fields. This problem may be addressed by acquiring shorter and more closely spaced echoes.
Figure 3.
Example results at 1.5T and 3T: Proton Density Fat-Fraction (PDFF), B0 field maps, and R2* maps from subjects with no iron overload, moderate iron overload and high iron overload. Note the increase in ΔB0 (as well as R2*) with increasing liver iron (Ferriscan-LIC).
In control subjects, the measured ΔB0 between liver and SAT was 3.9 ± 7.3 Hz (1.5T), and 9.0 ± 10.1 Hz (3T). Figure 4 compares the calculated iron concentration B0-LIC (mg Fe/g dry) against Ferriscan-LIC (determined by Resonance Health from 1.5T R2 mapping) at 1.5T (4a) and 3.0T (4b). In addition to good correlation (r2=0.67 at 1.5T, r2=0.84 at 3T), good agreement between the predicted iron concentration and measured iron concentration is also observed evidenced by a slope close to 1.0 (1.5T: slope =0.93 ± 0.13, 95% confidence interval=[0.67,1.18], p≈0.5; 3T: slope =1.01 ± 0.09, 95% confidence interval=[0.84,1.18], p≈0.9) and an intercept close to 0.0 (1.5T: intercept = 1.93 ± 0.78, 95% confidence interval=[0.40,3.47], p≈0.02 (significantly different from 0); 3T: intercept = 0.23 ± 0.52, 95% confidence interval=[−0.80,1.24], p≈0.6). Note that estimation of LIC using the boundary B0 measurement approach leads to relatively large variability (particularly at 1.5T), including some negative LIC values. This is likely due in part to the presence of uncorrected background B0 field variations, and possibly also to imperfect alignment of the boundary with the B0 field (ie: θ≠90° in Eq. 1). Comparison of B0-LIC at 1.5 and 3T (figure 4c) results in a correlation r2 = 0.84 with slope = 0.89 ± 0.08 (95% confidence interval=[0.70,1.04], p=0.16), and intercept = −0.77 ± 0.59 (95% confidence interval=[−1.92,0.38], p=0.20).
Figure 4.
Good correlation and agreement was observed between LIC estimated from susceptibility measurements (at a) 1.5T and b) 3.0T), and LIC estimated from R2 measurements (Ferriscan, measured at 1.5T). Good correlation and agreement was observed between LIC estimated from susceptibility measurements at 1.5T vs 3T (c).
Muscle-referenced ΔB0-based LIC estimation produced results similar to fat-referenced estimation, including correlation (r2=0.61 at 1.5T, r2=0.83 at 3T), slope (1.5T: slope =0.74 ± 0.12, 95% confidence interval=[0.51,0.97], p≈0.04 (significantly different from 1); 3T: slope =0.89 ± 0.08, 95% confidence interval=[0.75,1.05], p≈0.20) and intercept (1.5T: intercept = 1.35 ± 0.71, 95% confidence interval=[−0.80,1.24], p≈0.07; 3T: intercept = −0.02 ± 0.49, 95% confidence interval=[−0.98,0.94], p≈0.96). Bland-Altman analysis results for fat- and muscle-referenced B0-LIC at both field strengths are summarized in Table 1.
Table 1.
Bland-Altman analysis results comparing different LIC estimation techniques.
| 1.5T B0-LIC vs Ferriscan-LIC |
3T B0-LIC vs Ferriscan-LIC |
1.5T B0-LIC vs 3T B0-LIC |
|
|---|---|---|---|
| Fat-referenced | 1.66 ± 3.07 mg Fe/g dry | 0.26 ± 2.02 mg Fe/g dry | −1.40 ± 2.13 mg Fe/g dry |
| 95% CI = [−4.35,7.67] | 95% CI = [−3.70,4.22] | 95% CI = [−5.58,2.78] | |
| Muscle-referenced | 0.34 ± 3.01 mg Fe/g dry | 0.44 ± 1.96 mg Fe/g dry | −0.77 ± 2.38 mg Fe/g dry |
| 95% CI = [−5.57,6.25] | 95% CI = [−4.29,3.41] | 95% CI = [−5.43,3.89] | |
In addition, scatter plots comparing ΔB0 and R2* values measured at 1.5T and 3.0T are shown in figure 5, demonstrating good correlation (r2=0.86 at 1.5T and r2=0.93 at 3.0T). Finally, it should be noted that the ratio of the slope of the R2* measured at 3.0T compared to 1.5T is 1.91 ± 0.04 (with very strong correlation r2=0.99), demonstrating a strong field strength dependence of R2* measurements for iron overload. This slope is close to the expected value of 2.00 (51).
Figure 5.
Good correlation was observed between magnetic field offset measurements ΔB0 and R2* measurements at a) 1.5T and b) 3.0T.
Discussion
In this work, we have demonstrated the feasibility of a fat-referenced approach for measuring magnetic susceptibility in the liver as a biomarker of liver iron overload, using an R2-based measure of liver iron concentration (LIC) as reference standard. Although full dipole inversion of the B0 field map was not performed, the results of this work demonstrate that the fundamental information of susceptibility measurements can be ascertained from B0 field maps derived from multi-echo chemical shift based fat-water separation methods.
This work builds on previous studies (18,30,31) suggesting that susceptibility, which is a fundamental property of tissue with a well-understood relationship to iron concentration, may serve as a useful biomarker for tissue iron concentration. Further, this work illustrates the potential of susceptibility measurement techniques based on 3D chemical shift-encoded acquisitions, in combination with complex reconstructions. These techniques allow simultaneous measurement of PDFF (with 0–100% dynamic range), R2* maps and B0 field maps. In this way, PDFF (and potentially R2*) can be used to enable or improve susceptibility measurements based on the B0 field map.
The main technical drawback of MRI susceptibility-based iron quantification methods (compared to relaxivity-based methods) is the inability to directly estimate susceptibility at every voxel in the liver by considering only the measured signal at that particular voxel. Magnetic susceptibility affects the observed B0 field map in a non-local manner. In this work, susceptibility was obtained from nearby values in the B0 field using boundary conditions (30). This approach is based on techniques recently demonstrated in phantoms (29), heart (31), and liver (18,29).
Another technical difficulty is the fact that B0 field measurements are noisy at very high iron concentrations (where the signal decays very rapidly with increasing echo time). This rapid signal decay may limit the range of LIC values that can be reliably measured, particularly at 3T. Note that in this work we use SPGR acquisitions with small flip angles in order to provide T1-independent measurements of liver fat-fraction (PDFF) jointly with measurements of liver iron. These small flip angles result in reduced SNR, and alternatively data could be obtained with higher flip angles at the cost of potential T1 bias in fat-fraction quantification.
A previous technique used the adjacent muscle as a reference for Bo field measurements (18). In this work, we have chosen to use subcutaneous adipose tissue (SAT) as a reference. This may enable constrained quantitative susceptibility mapping (QSM) by imposing the known locations and susceptibility of fat tissues. Note that fat regions (eg: subcutaneous and visceral adipose tissue) can be easily segmented automatically from fat-water separated images, obtained in the same acquisitions. This work is a feasibility study towards the overall goal of liver QSM using fat as a constraint to regularize the ill-posed QSM inversion problem.
If uncorrected for fat, phase variations due to the presence of fat in tissue (particularly in subcutaneous and visceral adipose tissue) will confound the B0 measurements. A possible alternative approach to avoid this confounder is to use “in-phase” echoes, where the relative phase of water and the main methylene fat peak is constant for all echoes (32). However, the long echo spacing imposed by “in-phase” acquisitions will likely complicate B0 field map estimation (and thus susceptibility measurements) in cases of high liver iron where the signal decays very rapidly (eg: T2*=1–2ms with an echo spacing of 4.6ms). In contrast, chemical shift encoded fat-water separation techniques are well suited for mapping of B0 using closely spaced echo times. The B0 field maps measured in this work are corrected for the presence of liver fat, similarly to the R2* measurements (note that liver R2* has been shown to be unaffected by liver fat, as long as the spectral complexity of the fat signal is included in the estimation (52)). This fat-corrected B0 mapping also enables fat-corrected susceptibility measurements. The presence of liver fat may have an effect on the conversion between susceptibility and LIC (given that the susceptibility of fat is different from that of water). However, this effect was not included in this manuscript as it resulted in a small correction of LIC values upon preliminary evaluation, and required further assumptions about the fraction of MR-visible material in liver.
This study included susceptibility and liver iron quantification at both 1.5T and 3T. In this work, the correlation between B0-LIC and Ferriscan-LIC were superior at 3T compared to 1.5T. The reason for this difference is currently under investigation. A possible reason may be the larger B0 field offsets (in Hz) observed at 3T, which may be relatively less affected by low-level artifacts and noise.
There is some uncertainty in the conversion of iron concentration units from [Fe]wet to [Fe]dry. A factor of 3.3 has been employed in the past, although this value had not been validated (53) and more recent results have suggested that a factor of 5.5 +/− 1.0 may be more appropriate (37). The uncertainty present in this conversion results in uncertainty in the accuracy validation performed in this work. However, the strong correlation between measured ΔB0 and Ferriscan-LIC suggests that the measured B0 field map contains the necessary information to assess LIC.
The main theory and results of this work were originally presented as a conference abstract in Ref. (54). Subsequently, in an erratum published by Taylor et al (18), B0-LIC ([Fe]wet, mg Fe/mL wet) measurements were compared to R2*-LIC based on Hankins’ calibration (27) ([Fe]dry, mg Fe/g dry), resulting in a slope of 5.17 and intercept of −3.31. In this work, this calculation results in a slope of 3.44 and an intercept of −0.29. The remaining discrepancy with the results reported by Taylor et al and our work may be due to differences in the techniques used to calculate R2* or B0 maps, as well as slight differences in the value of μeff (4 vs 3.78), and possibly due to differences in the patient groups.
Phantom results seemed to underestimate the true iron concentration (although the slope was not statistically different from 1.0). This may be due to residual background B0 variations from the top and bottom of the vials, as well as uncertainty in the saturation magnetization of Feridex: we have used a value of 93.6 Am2/kg (49), although recent reports have measured lower values, eg: 80–82 Am2/kg (55). Using Ms=80 Am2/kg would lead to a slope nearly 1.0 in phantom iron concentration estimation (1.5T: 1.00 ± 0.07, 3T: 0.99 ± 0.08).
In the proposed technique (as in previous susceptibility-based techniques) we have made several assumptions in converting measured susceptibility values to iron concentration. Specifically, the relation between susceptibility and iron concentration may depend on the type of iron deposition (eg: ferritin vs hemosiderin), which may in turn be dependent on the type of iron overload and give rise to inter-organ, inter-patient or inter-disease variability (56). Further, we have assumed that liver iron is the only factor affecting magnetic susceptibility. In principle, the presence of liver fat may also affect liver susceptibility. It may be possible to include this in the formulation, as fat quantification (measurement of PDFF) can be performed using the proposed chemical shift-encoded technique. However, this approach may in turn require further approximations, as the measured PDFF includes only MR-visible proton signals. A thorough characterization of the effects of liver fat on liver susceptibility is beyond the scope of this work.
Another limitation of this study is the lack of exact co-localization between B0, R2* and Ferriscan measurements. B0 measurements need to be performed near the interface liver-muscle on the right side of the liver. R2 and R2* measurements could be co-localized, but the R2 maps contain substantial motion artifacts. This work assumed that iron concentration does not vary substantially throughout the liver in the scanned subjects.
The major limitation of this study is that a complete dipole inversion of the B0 field map (ie: QSM) was not performed to determine values of susceptibility over the entire liver. Dipole inversion is a mathematically challenging, ill-posed problem (16,29). The majority of QSM techniques have been applied in the brain (17), where a number of filtering and regularization techniques have been developed to estimate the susceptibility distribution from the measured B0 field map. However, it is unclear whether these techniques are appropriate for abdominal susceptibility mapping. Adipose tissue, however, is unaffected by iron overload and, as we have demonstrated, can serve as an excellent reference to regularize the inversion problem and help determine the absolute susceptibility (and hence iron concentration) within the tissue of interest (eg. liver). The main purpose of this manuscript was to demonstrate that the fundamental information contained in the B0 field maps will in principle facilitate QSM in the liver as an alternative to R2* and R2 mapping.
In conclusion, we have demonstrated the feasibility of using B0 field maps derived from multi-echo chemical shift encoded imaging at 1.5T or 3T to quantity iron in the liver. In addition, this work demonstrates the use of adipose tissue as a possible baseline reference that is required for all QSM related methods. Although further work is needed for dipole inversion of the B0 field map to measure iron concentration over the entire abdomen, these results are promising and demonstrate the feasibility of this approach. Finally, these results could be extended into quantification of iron in other abdominal tissues, such as the pancreas and spleen.
Acknowledgements
We acknowledge the support of the NIH (R01 DK083380, R01 DK088925, RC1 EB010384, and R01 DK096169), the Wisconsin Alumni Research Foundation (WARF) Accelerator Program, and the University of Wisconsin Institute for Clinical and Translational Research (ICTR). We also thank GE Healthcare for their support.
References
- 1.Gordeuk VR, Bacon BR, Brittenham GM. Iron overload: causes and consequences. Annu Rev Nutr. 1987;7:485–508. doi: 10.1146/annurev.nu.07.070187.002413. [DOI] [PubMed] [Google Scholar]
- 2.Siegelman ES, Mitchell DG, Semelka RC. Abdominal iron deposition: metabolism, MR findings, and clinical importance. Radiology. 1996;199:13–22. doi: 10.1148/radiology.199.1.8633135. [DOI] [PubMed] [Google Scholar]
- 3.Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997;89:739–761. [PubMed] [Google Scholar]
- 4.Alustiza JM, Castiella A, De Juan MD, Emparanza JI, Artetxe J, Uranga M. Iron overload in the liver diagnostic and quantification. Eur J Radiol. 2007;61:499–506. doi: 10.1016/j.ejrad.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 5.McLeod C, Fleeman N, Kirkham J, Bagust A, Boland A, Chu P, Dickson R, Dundar Y, Greenhalgh J, Modell B, Olujohungbe A, Telfer P, Walley T. Deferasirox for the treatment of iron overload associated with regular blood transfusions (transfusional haemosiderosis) in patients suffering with chronic anaemia: a systematic review and economic evaluation. Health Technol Assess. 2009;13:iii–iv. ix–xi, 1–121. doi: 10.3310/hta13010. [DOI] [PubMed] [Google Scholar]
- 6.Barry M, Sherlock S. Measurement of liver-iron concentration in needle-biopsy specimens. Lancet. 1971;1:100–103. doi: 10.1016/s0140-6736(71)90838-5. [DOI] [PubMed] [Google Scholar]
- 7.Carneiro AA, Baffa O, Fernandes JP, Zago MA. Theoretical evaluation of the susceptometric measurement of iron in human liver by four different susceptometers. Physiol Meas. 2002;23:683–693. doi: 10.1088/0967-3334/23/4/308. [DOI] [PubMed] [Google Scholar]
- 8.Brittenham GM, Badman DG. Noninvasive measurement of iron: report of an NIDDK workshop. Blood. 2003;101:15–19. doi: 10.1182/blood-2002-06-1723. [DOI] [PubMed] [Google Scholar]
- 9.Lee MH, Means RT., Jr Extremely elevated serum ferritin levels in a university hospital: associated diseases and clinical significance. Am J Med. 1995;98:566–571. doi: 10.1016/s0002-9343(99)80015-1. [DOI] [PubMed] [Google Scholar]
- 10.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 11.Schenck JF. The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatibility of the first and second kinds. Med Phys. 1996;23:815–850. doi: 10.1118/1.597854. [DOI] [PubMed] [Google Scholar]
- 12.Bauman JH, Hoffman RW. Magnetic susceptibility meter for in vivo estimation of hepatic iron stores. IEEE Trans Biomed Eng. 1967;14:239–243. doi: 10.1109/tbme.1967.4502511. [DOI] [PubMed] [Google Scholar]
- 13.Bauman JH, Harris JW. Estimation of hepatic iron stores by vivo measurement of magnetic susceptibility. J Lab Clin Med. 1967;70:246–257. [PubMed] [Google Scholar]
- 14.Paulson DN, Fagaly RL, Toussaint RM. Biomagnetic Susceptometer with SQUID Instrumentation. IEEE Trans Magnetics. 1991;27:3249–3252. [Google Scholar]
- 15.Sheth S. SQUID biosusceptometry in the measurement of hepatic iron. Pediatr Radiol. 2003;33:373–377. doi: 10.1007/s00247-003-0877-x. [DOI] [PubMed] [Google Scholar]
- 16.de Rochefort L, Brown R, Prince MR, Wang Y. Quantitative MR susceptibility mapping using piece-wise constant regularized inversion of the magnetic field. Magn Reson Med. 2008;60:1003–1009. doi: 10.1002/mrm.21710. [DOI] [PubMed] [Google Scholar]
- 17.Langkammer C, Schweser F, Krebs N, Deistung A, Goessler W, Scheurer E, Sommer K, Reishofer G, Yen K, Fazekas F, Ropele S, Reichenbach JR. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. Neuroimage. 2012;62:1593–1599. doi: 10.1016/j.neuroimage.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor BA, Loeffler RB, Song R, McCarville MB, Hankins JS, Hillenbrand CM. Simultaneous field and R2* mapping to quantify liver iron content using autoregressive moving average modeling. J Magn Reson Imaging. 2012;35:1125–1132. doi: 10.1002/jmri.23545. [published erratum appears in J Magn Reson Imaging, early view, doi 1110.1002/jmri.24135] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandon Y, Guyader D, Heautot JF, Reda MI, Yaouanq J, Buhe T, Brissot P, Carsin M, Deugnier Y. Hemochromatosis: diagnosis and quantification of liver iron with gradient-echo MR imaging. Radiology. 1994;193:533–538. doi: 10.1148/radiology.193.2.7972774. [DOI] [PubMed] [Google Scholar]
- 20.St Pierre TG, Clark PR, Chua-Anusorn W. Single spin-echo proton transverse relaxometry of iron-loaded liver. NMR Biomed. 2004;17:446–458. doi: 10.1002/nbm.905. [DOI] [PubMed] [Google Scholar]
- 21.St Pierre TG, Clark PR, Chua-anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK, Pootrakul P, Robins E, Lindeman R. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105:855–861. doi: 10.1182/blood-2004-01-0177. [DOI] [PubMed] [Google Scholar]
- 22.Wood JC, Ghugre N. Magnetic resonance imaging assessment of excess iron in thalassemia, sickle cell disease and other iron overload diseases. Hemoglobin. 2008;32:85–96. doi: 10.1080/03630260701699912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, Nelson MD, Coates TD. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106:1460–1465. doi: 10.1182/blood-2004-10-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood JC. Magnetic resonance imaging measurement of iron overload. Curr Opin Hematol. 2007;14:183–190. doi: 10.1097/MOH.0b013e3280d2b76b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghugre NR, Coates TD, Nelson MD, Wood JC. Mechanisms of tissue-iron relaxivity: nuclear magnetic resonance studies of human liver biopsy specimens. Magn Reson Med. 2005;54:1185–1193. doi: 10.1002/mrm.20697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, Firmin DN, Wonke B, Porter J, Walker JM, Pennell DJ. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–2179. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 27.Hankins JS, McCarville MB, Loeffler RB, Smeltzer MP, Onciu M, Hoffer FA, Li CS, Wang WC, Ware RE, Hillenbrand CM. R2* magnetic resonance imaging of the liver in patients with iron overload. Blood. 2009;113:4853–4855. doi: 10.1182/blood-2008-12-191643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghugre NR, Wood JC. Relaxivity-iron calibration in hepatic iron overload: probing underlying biophysical mechanisms using a Monte Carlo model. Magn Reson Med. 2011;65:837–847. doi: 10.1002/mrm.22657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu Z, Cohen AR, Muthupillai R, Chung T, Wang ZJ. MRI measurement of hepatic magnetic susceptibility-phantom validation and normal subject studies. Magn Reson Med. 2004;52:1318–1327. doi: 10.1002/mrm.20305. [DOI] [PubMed] [Google Scholar]
- 30.Wang ZJ, Li S, Haselgrove JC. Magnetic resonance imaging measurement of volume magnetic susceptibility using a boundary condition. J Magn Reson. 1999;140:477–481. doi: 10.1006/jmre.1999.1873. [DOI] [PubMed] [Google Scholar]
- 31.Wang ZJ, Fischer R, Chu Z, Mahoney DH, Jr, Mueller BU, Muthupillai R, James EB, Krishnamurthy R, Chung T, Padua E, Vichinsky E, Harmatz P. Assessment of cardiac iron by MRI susceptometry and R2* in patients with thalassemia. Magn Reson Imaging. 2010;28:363–371. doi: 10.1016/j.mri.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang ZJ, Chu Z, Chung T, Muthupillai R. Proceedings of the 13th Annual Meeting of ISMRM. Miami, FL: 2005. Quantification of the magnetic susceptibility of the heart using MRI: demonstration on normal subjects; p. 1666. [Google Scholar]
- 33.St Pierre TG, Clark PR, Chua-Anusorn W. Measurement and mapping of liver iron concentrations using magnetic resonance imaging. Ann N Y Acad Sci. 2005;1054:379–385. doi: 10.1196/annals.1345.046. [DOI] [PubMed] [Google Scholar]
- 34.Alabousi A, Al-Attar S, Joy TR, Hegele RA, McKenzie CA. Evaluation of adipose tissue volume quantification with IDEAL fat-water separation. J Magn Reson Imaging. 2011;34:474–479. doi: 10.1002/jmri.22603. [DOI] [PubMed] [Google Scholar]
- 35.Poonawalla AH, Sjoberg BP, Rehm JL, Hernando D, Hines CD, Irarrazaval P, Reeder SB. Adipose tissue MRI for quantitative measurement of central obesity. J Magn Reson Imaging. 2013;37:707–716. doi: 10.1002/jmri.23846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schenck JF. Health and physiological effects of human exposure to whole-body four-tesla magnetic fields during MRI. Ann N Y Acad Sci. 1992;649:285–301. doi: 10.1111/j.1749-6632.1992.tb49617.x. [DOI] [PubMed] [Google Scholar]
- 37.Fischer R, Piga A, Harmatz P, Nielsen P. Monitoring long-term efficacy of iron chelation treatment with biomagnetic liver susceptometry. Ann N Y Acad Sci. 2005;1054:350–357. doi: 10.1196/annals.1345.043. [DOI] [PubMed] [Google Scholar]
- 38.Bassett ML, Halliday JW, Powell LW. Value of hepatic iron measurements in early hemochromatosis and determination of the critical iron level associated with fibrosis. Hepatology. 1986;6:24–29. doi: 10.1002/hep.1840060106. [DOI] [PubMed] [Google Scholar]
- 39.Hines CD, Yu H, Shimakawa A, McKenzie CA, Brittain JH, Reeder SB. T1 independent, T2* corrected MRI with accurate spectral modeling for quantification of fat: validation in a fat-water-SPIO phantom. J Magn Reson Imaging. 2009;30:1215–1222. doi: 10.1002/jmri.21957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meisamy S, Hines CD, Hamilton G, Sirlin CB, McKenzie CA, Yu H, Brittain JH, Reeder SB. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology. 2011;258:767–775. doi: 10.1148/radiol.10100708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brau AC, Beatty PJ, Skare S, Bammer R. Comparison of reconstruction accuracy and efficiency among autocalibrating data-driven parallel imaging methods. Magn Reson Med. 2008;59:382–395. doi: 10.1002/mrm.21481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu H, Shimakawa A, McKenzie CA, Lu W, Reeder SB, Hinks RS, Brittain JH. Phase and amplitude correction for multi-echo water-fat separation with bipolar acquisitions. J Magn Reson Imaging. 2010;31:1264–1271. doi: 10.1002/jmri.22111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magn Reson Med. 2000;43:682–690. doi: 10.1002/(sici)1522-2594(200005)43:5<682::aid-mrm10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 44.Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med. 2008;60:1122–1134. doi: 10.1002/mrm.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hernando D, Liang ZP, Kellman P. Chemical shift-based water/fat separation: a comparison of signal models. Magn Reson Med. 2010;64:811–822. doi: 10.1002/mrm.22455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamilton G, Yokoo T, Bydder M, Cruite I, Schroeder ME, Sirlin CB, Middleton MS. In vivo characterization of the liver fat (1)H MR spectrum. NMR Biomed. 2011;24:784–790. doi: 10.1002/nbm.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernando D, Kellman P, Haldar JP, Liang ZP. Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm. Magn Reson Med. 2010;63:79–90. doi: 10.1002/mrm.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med. 2007;58:354–364. doi: 10.1002/mrm.21301. [DOI] [PubMed] [Google Scholar]
- 49.Jung CW, Jacobs P. Physical and chemical properties of superparamagnetic iron oxide MR contrast agents: ferumoxides, ferumoxtran, ferumoxsil. Magn Reson Imaging. 1995;13:661–674. doi: 10.1016/0730-725x(95)00024-b. [DOI] [PubMed] [Google Scholar]
- 50.Clark PR, Chua-anusorn W, St Pierre TG. Bi-exponential proton transverse relaxation rate (R2) image analysis using RF field intensity-weighted spin density projection: potential for R2 measurement of iron-loaded liver. Magn Reson Imaging. 2003;21:519–530. doi: 10.1016/s0730-725x(03)00080-8. [DOI] [PubMed] [Google Scholar]
- 51.Storey P, Thompson AA, Carqueville CL, Wood JC, de Freitas RA, Rigsby CK. R2* imaging of transfusional iron burden at 3T and comparison with 1.5T. J Magn Reson Imaging. 2007;25:540–547. doi: 10.1002/jmri.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuhn JP, Hernando D, Munoz Del Rio A, Evert M, Kannengiesser S, Volzke H, Mensel B, Puls R, Hosten N, Reeder SB. Effect of Multipeak Spectral Modeling of Fat for Liver Iron and Fat Quantification: Correlation of Biopsy with MR Imaging Results. Radiology. 2012;265:133–142. doi: 10.1148/radiol.12112520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fischer R, Harmatz PR. Non-invasive assessment of tissue iron overload. Hematology Am Soc Hematol Educ Program. 2009:215–221. doi: 10.1182/asheducation-2009.1.215. [DOI] [PubMed] [Google Scholar]
- 54.Hernando D, Reeder SB. Proceedings of the 21st Annual Meeting of ISMRM. Salt Lake City, UT: 2013. Magnetic Susceptibility as a Field-Independent MRI Biomarker of Liver Iron Overload; p. 3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cantillon-Murphy P, Wald LL, Zahn M, Adalsteinsson E. Measuring SPIO and Gd contrast agent magnetization using 3 T MRI. NMR Biomed. 2009;22:891–897. doi: 10.1002/nbm.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hackett S, Chua-anusorn W, Pootrakul P, St Pierre TG. The magnetic susceptibilities of iron deposits in thalassaemic spleen tissue. Biochim Biophys Acta. 2007;1772:330–337. doi: 10.1016/j.bbadis.2006.12.007. [DOI] [PubMed] [Google Scholar]