Abstract
We have previously shown that extended-access subjects exhibit heightened motivation for cocaine in the runway model, as reflected by reduced number of retreats (Ben-Shahar et al., 2008). This heightened motivation could reflect either an increase in cocaine-induced reward or a decrease in cocaine-induced aversion. The current experiment was therefore devised to assess the cocaine-induced reward and aversion in extended access rats using a place conditioning test. Rats trained to lever-press for IV cocaine (0.25 mg/infusion) were provided 6-h daily access to the drug over 10 days. Lever-pressing in control subjects produced IV infusions of saline. Following drug self-administration, subjects underwent place conditioning for the immediate or delayed effects of cocaine (1.0 mg/kg or 2.5 mg/kg, IV). In control subjects, the immediate effects of the low dose of cocaine produced conditioned places preferences (CPPs) while the delayed effects produced conditioned place aversions (CPAs). In contrast, the 6-h animals receiving the low cocaine dose, exhibited place aversions but not preferences; an effect that was reversed when the dose of cocaine was increased. Additionally, in the 6-h group, delayed conditioning was associated with a reduction in zif-268 immunoreactivity in the medial prefrontal cortex and nucleus accumbens shell while immediate conditioning was associated with an increase in zif-268-positive cells in the central nucleus of the amygdala. Collectively, these data suggest that extended daily access to cocaine produces a shift in the subject’s perceived reward threshold that is paralleled by alterations in the activity of both the reward and stress pathways.
Keywords: allostatic theory, conditioned place aversions, conditioned place preferences, cocaine, drug self-administration, immunohistochemistry
Introduction
Cocaine is an extremely powerful reinforcer, inducing strong euphoria and addiction in humans (e.g. Fischman et al., 1976; Gawin, 1991; Resnick & Resnick, 1984). However, addicts also describe profound levels of anxiety, agitation, depression, and fatigue (commonly known as the “crash”) once the euphoric effects of the drug have subsided (Gawin, 1991; Resnick & Resnick, 1984; Smith, 1986; Washton, Gold, & Pottash, 1983; Williamson et al., 1987). These aversive effects of cocaine, however, are not restricted to the experiences associated with drug withdrawal. For example, the acute effects of cocaine have been shown to elicit panic attacks (Aronson & Craig, 1986; Roy-Byrne & Udhe, 1988) and exacerbate post-traumatic stress disorder symptoms in war veterans (Hamner, 1993). Such positive and negative effects of cocaine have also been demonstrated in laboratory animals. While rodents readily selfadminister the drug (sometimes even ingesting a lethal overdose) (e.g. Deneau, Yanagita, & Seevers, 1969; Johanson, Balster, Bonese, 1976) and exhibit robust cocaine-induced conditioned place preferences (CPPs) (Mucha et al., 1982; Mueller & Stewart 2000; Spyraki, Fibiger, & Phillips, 1982), cocaine has been reported to potentiate the avoidance of inherently aversive stimuli in several different behavioral tests (Costall et al., 1989; Erb, Kayyali, & Romero, 2006; Fontana & Commissaris, 1989; Rogerio & Takahashi, 1992; Simon, Dupuis, & Costentin, 1994; Yang et al., 1992).
These dual and opposing actions of acute cocaine administration have been further examined in a drug self-administration runway task. In this model, animals trained to traverse a straight-alley and enter a goal box for a single daily infusion of cocaine, develop an approachavoidance conflict behavior (described as “retreats”) about entering the cocaine-paired goal-box (Ettenberg & Geist, 1991). These retreats provide a reliable index of the subject’s ambivalence about entering an environment paired with a mixed positive and negative reinforcer (for review, see Ettenberg, 2004; 2009). Pretreatment with anxiolytics, or reversible lidocaine-induced lesions of brain areas involved in fear and anxiety (i.e., the central nucleus of the amygdala [CeA], bed nucleus of the stria terminalis [BNST], or the dorsal raphé) were found to effectively reduce the frequency of this conflict behavior (Ettenberg & Geist, 1991; Ettenberg et al., 2011; Wenzel et al., 2011a). In other studies, both the positive and aversive effects of acute cocaine have also been demonstrated using place conditioning methodology. Drug naïve rats were found to develop preferences (CPPs) for environments paired with the immediate and presumably rewarding effects of cocaine, but conditioned place aversions (CPAs) for environments coupled with the delayed and presumably aversive effects of cocaine (Ettenberg et al., 1999). Consistent with the results obtained in the self-administration runway, CPAs were similarly attenuated by pretreatment with the administration of anxiolytic drugs (e.g., buspirone; Ettenberg & Bernardi, 2007) and disruption of neuronal function within the CeA (Su et al., 2010; Wenzel et al., 2012).
To understand the dynamics between the opposing positive and negative effects of cocaine in a “dependent” animal, the runway model was employed in subjects that were previously exposed to extended daily access to cocaine self-administration (i.e., an animal model of “addiction” developed by Ahmed & Koob, 1998). Extended-access subjects exhibited significantly fewer approach-avoidance retreat behaviors in the runway and entered the goal box significantly faster than saline control groups — suggesting an enhanced motivation to seek the drug in putatively “dependent” subjects. However, compared to controls, both “dependent” and “non-dependent” subjects (those respectively having had prior extended daily access or limited daily access to cocaine self-administration) exhibited comparable heightened cocaine-induced anxiety in the elevated plus maze (Ben-Shahar et al., 2008). Thus, the heightened motivation to seek cocaine in subjects with a prior history of extended drug access was not accompanied by a change in responsiveness to the unconditioned anxiogenic effects of the drug.
It remains unclear, however, whether or not the conditioned aversive effects of cocaine are similarly unaltered in the extended access animal. The current experiment was therefore devised to assess the sensitivities of such animals to the dual and opposing effects of the drug using a conditioned place test. Additionally, tissue from extended access subjects was collected and stained with zif-268 antibodies, an indirect marker of neuronal activity (Sheng & Greenberg, 1990), to identify changes in the function of brain regions thought to be involved in producing the positive and negative effects of cocaine on the basis of prior runway and place conditioning results (e.g., Guzman et al., 2009; Su et al., 2010; Wenzel et al., 2011a; 2011b; 2012).
Materials and Methods
Subjects
Adult male Sprague-Dawley rats (n=72), weighing 330–360g at the time of surgery, served as subjects (Charles River Laboratories, Wilmington, MA). Subjects were group housed in plastic cages (two rats/cage) within a temperature-controlled (23° C) vivarium maintained on a reverse 12-hour light-dark cycle (lights off at 0800 h). Unless otherwise stated, animals were provided ad libitum access to food (Purina rat chow) and water throughout the experiment. All animal handling and experimental procedures adhered to the NIH Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the University of California at Santa Barbara’s Institutional Animal Care and Use Committee.
Surgery
Catheters consisted of a stainless steel guide cannula (Item 313G; Plastics One, Roanoke, VA, USA) that was affixed to polyethylene tubing, which was encased in a thicker tubing to provide additional support (13 mm long, 0.3 mm inner tubing and 0.64 mm outer tubing diameters; Dow Corning Corp, Midland, MI, USA). The cannula was in turn affixed with dental cement to a 2 cm square of Mersilene surgical mesh (Bard; Warwick, RI). To implant the catheter, each rat was deeply anesthetized via isoflurane gas inhalation (4% for induction and 1.5–2.5% for maintenance). To prevent respiratory congestion and reduce post-surgical pain, rats were treated with atropine (0.04 mg/kg, intramuscularly) and the non-opiate analgesic flunixin meglumine (Phoenix Pharmaceuticals, Belmont, California, USA; 2 mg/kg, subcutaneously). Each catheter was inserted into the right jugular vein and secured in place by silk sutures. The other end of the catheter (with the guide cannula attached) was passed subcutaneously to a 2mm hole located on the midline of the animal’s back so that the guide cannula protruded through the hole and the mesh laid flat against the subdermal tissue. Following surgery, rats received the antibiotic, ticarcillin disodium/clavulanate potassium (Timentin; 50 mg, i.v.) and 0.1 ml of heparin (6.0 IU/ 0.1 ml prepared in 0.9% physiological saline, i.v.) to maintain catheter patency.
Subjects were given 1-week to recover from surgery during which the catheters were flushed daily with 0.1 ml of 0.9% physiological saline containing the antibiotic Timentin (20 mg/0.1 ml) and 0.1 ml of saline containing 6 units of heparin. Once a week, catheter patency was confirmed by observing the behavioral impact of an i.v. injection of the fast-acting barbiturate, methohexital sodium (Brevital; 2.0 mg/kg/0.1 ml). Animals that did not lose their righting reflex in response to Brevital were re-implanted with a new catheter using the left jugular vein and given additional days for recovery.
Drugs
Cocaine hydrochloride was dissolved in sterile 0.9% physiological saline. Animals were trained to self-administered cocaine (0.25 mg/infusion i.v. delivered in a volume of 0.1 ml delivered over 4.6 sec) and were non-contingently (experimenter) delivered cocaine during the place conditioning portion of the experiment (1.0 mg/kg or 2.5 mg/kg i.v. in a volume of 0.1ml delivered over 4.2 sec). The doses selected for this study were based on prior studies showing that these doses result in escalation of drug intake over trials as well as enduring behavioral adaptations (Ahmed & Koob, 1998; Ben-Shahar et al., 2005; 2008; Paterson & Markou, 2003) and in robust conditioned place preferences and aversions (Ettenberg & Bernardi, 2007; Ettenberg et al., 1999; O’Dell et al., 1996; Nomikos & Spirakyi, 1988). Cocaine was generously provided by the National Institute of Drug Abuse.
Operant Self-Administration Chambers
Ten operant chambers enclosed in sound attenuating boxes (29 cm W × 25 cm L × 30 cm H; Med Associates, St. Albans, VT) were employed for food training and self-administration. Each box contained two levers that were positioned 7 cm above the chamber floor and two cue lights (2.8 W) one situated above each lever (note that only the right cue light was used in the current study). A food trough was located 2.0 cm above the floor in-between the two levers. Noyes 45mg food pellets were delivered to the trough via a food dispenser located outside of the box. A liquid swivel (375-22PS, Instech Laboratories Inc., Plymouth Meeting, PA) permitted free movement within each chamber. The swivel was connected on one end by polyethylene tubing (outer diameter 0.127 cm, inner diameter 0.058cm; Plastics One, Wallingford, CT) to a 10 ml syringe that was seated in an automated syringe pump (Med Associates Inc., St. Albans, VT) and on the other end via additional PE tubing (encased in a flexible metal covering) to the guide cannula/catheter on the animal’s back. A desktop computer running Med Associates software (MED-PC for Windows, Version 1.17) executed all behavioral testing and data collection.
Place Conditioning Apparatus
Two identical CPP apparatuses consisted of wood-constructed rectangular enclosures (156 cm long × 34 cm wide × 30 cm high) that could be divided into three compartments: two larger chambers (61 × 30 cm) that were separated by a smaller intermediate chamber (34 × 30 cm). One of the larger compartments was painted black and had an acrylic (Plexiglas ®) flooring that was mildly scented with acetic acid (2% solution, swabbed 25 cm above the compartment floor). The other larger chamber was unscented, painted white and had the floor surface covered with soft gray bedding (Carefresh; Absorption Corp, Ferndale, Washington, USA). The smaller intermediate chamber had a bare wood surface that was painted gray. These procedures yielded two conditioning environments (the white and dark compartments) for which rats exhibit no reliable inherent preferences prior to conditioning. Situated above each apparatus was a digital camera that detected and recorded the precise location of the animal in real time via a desktop computer running Any-Maze software (Stoetling Co, Wood Dale, IL, USA).
Operant Training
In order to facilitate the learning of operant responding for cocaine, all animals were initially trained to respond for food. Subjects were food-deprived 24-hrs prior to the start of food training and maintained on a strict diet (15g of Purina brand chow/day) throughout training. During 2-hr food-training sessions, depressing the right (active) lever resulted in the delivery of a 45 mg Noyes food pellet and the illumination of the right cue light for 1 sec. Once animals met the food-training criterion (at least 100 reinforcers earned across two consecutive sessions), they were again provided ad libitum access to food and allowed 2–3 days before undergoing i.v. catheterization.
Cocaine self-administration was initiated a week after the catheterization surgery. Saline control animals experienced daily 1-hr self-administration sessions over 17 consecutive days, while cocaine-reinforced animals experienced 1-hr self-administration sessions for 7 trials and then transitioned to extended 6-hr sessions for 10 additional trials/days. Lever presses on the active lever resulted in a single injection of saline (0.1 ml) or cocaine (0.25 mg/0.1 ml/infusion) followed by a 20-sec time out-period during which the cue light was illuminated. Responses emitted during this 20-sec window were recorded but did not result in additional reinforcer delivery. Responses on the inactive lever were also recorded, but produced no programmed consequences.
Experiment 1: Cocaine Place Conditioning – 1.0 mg/kg dose
The place conditioning procedure was initiated 24 hrs following the last self-administration session and consisted of a preconditioning preference test (baseline), eight drug-place conditioning trials, and a final preference/avoidance test. For baseline, the interior walls separating each compartment were removed, subjects were placed into the middle gray section of the apparatus, and the time spent in each of the three compartments was recorded over for 15-min. Each rat then completed eight conditioning trials. Subjects each received an injection of either vehicle (0.1 ml physiological saline) or cocaine (1.0 mg/kg, i.v.) followed by placement into either the white or black compartment for 5-mins. On the following day, each rat received the alternate treatment and was placed in the alternate colored environment. This continued until each subject had experienced four cocaine pairings with one compartment and four saline pairings with the second compartment. Half the animals were placed into the conditioning chambers immediately after their i.v. injections and the remaining half were placed into the chambers 15-min post-injection. Following the completion of place conditioning, animals were given a single 15-min preference test identical to that described for the baseline. The order in which subjects received the drug or vehicle, as well as the side of the apparatus paired with a given treatment were counterbalanced across all animals and between groups (i.e., an unbiased place conditioning design was employed; see Carr et al., 1988). Preferences and aversions were defined as a shift from baseline performance and reflected as positive and negative difference score values, respectively. Difference scores were calculated by subtracting time spent in the cocaine-paired compartment on test-day from baseline. Thus, positive scores denoted increased preferences for the drug-paired environment and negative scores reflected decreased preferences or avoidance of the drug-paired environment. An additional group of animals (n=12) served as an immunostaining saline control. These animals received an identical treatment to that of the saline control group described above with two exceptions: they experienced only 10 saline selfadministration sessions, and were only administered saline during place conditioning training.
Experiment 2: Cocaine Place Conditioning – 2.5 mg/kg dose
Koob & LeMoal (1997, 2001) have proposed an “allostatic” theory of cocaine addiction in which a consequence of chronic daily exposure to the drug is a functional dampening of the brain’s reward system and a consequent reduction in the magnitude of the “normal” hedonic response to the drug. Following this theory, we hypothesized that “dependent” animals will not develop CPP to the minimal established dose of cocaine required to induce reliable CPP in naïve rats (i.e. 1.0 mg/kg; exp. 1; Allen et al., 2007; Ettenberg & Bernardi, 2007; Feduccia & Duvauchelle, 2010; Nokimos & Spyraski, 1988; O’Dell et al., 1996), but will require a larger dose (i.e. 2.5 mg/kg; the maximal dose that induces CPP – difference score of 250 sec – in naïve rats and does not cause convulsions or death; O’Dell et al., 1996; Nomikos & Spyraki, 1988) of cocaine to achieve CPP (Exp 2.). Another group of animals was therefore treated identically to the 6-hr extended-access cocaine group described in Experiment 1 above except that for place conditioning a higher 2.5 mg/kg dose was employed.
Immunohistochemistry
One hour after completion of their final preference test, 6-hr extended access animals and saline immunostaining controls were deeply anesthetized with sodium pentobarbital (Euthasol; 40 mg, i.v.) and transcardially perfused with 60 mL of phosphate buffered saline (PBS; 0.1 M) followed by 120 mL of 4% paraformaldehyde in (0.1 M) PBS. The brains of these subjects were removed and a Leica CM1800 cryostat used to collect 40 µm sections of tissue, which were mounted on 1.5% gelatin-coated slides. Using the Paxinos and Watson (1998) brain atlas as a guide, the following areas were sampled: prelimbic prefrontal cortex (2.7 mm anterior to bregma), nucleus accumbens core and shell (1.2 mm anterior to bregma), bed nucleus of stria terminalis (BNST; 0.3 mm posterior to bregma), and central nucleus of the amygdala (CEA; 2.12 mm posterior to bregma). The slides were washed twice with Tris-buffered saline (TBS; 0.05 M, pH 7.6 at room temperature) between the different treatments and were stained using the ABC method. Sections were treated with 0.25% Triton X-100 (Sigma #X-100 St. Louis, MO) and 5% dimethyl sulfoxide (DMSO; Sigma D-5879); and were then incubated for 1 hr in 20% normal goat serum (NGS; Sigma G6767) + 1% bovine serum albumin (BSA-Fract V; Fisher Scientific, Los-Angeles, CA, BP1605-100) to block nonspecific binding. Slides were then incubated for 24 hr in the zif-268 primary antibody 1:1000 (c-19 anti-zif 268; Santa Cruz Biotechnology, Santa Cruz, CA) + 0.5% Triton X-100 + 1% NGS. Next, sections were incubated for 1 hr in the secondary antibody Anti Rabbit IgG (Elite-Anti Rabbit Vector Kit, Vector labs PK6101, Burlingame, CA), and for 30 min in the avidin–biotin–horseradish-peroxidase complex (Elite-Anti Rabbit Vector Kit, Vector labs PK6101). Staining was visualized using the chromogen DAB (Sigma D-5637) + 0.01% H2O2 (Fisher H325–500). Following staining, sections were dehydrated and cover slipped. Zif-268-positive cell counts were recorded by visual inspection under 40× magnification. Cells were counted from two adjacent sections and restricted to an area defined by a 0.25 mm2 grid. Two trained individuals, blind to the treatment condition of the animals, conducted the counts (each from only one of the adjacent sections) and the means of their scores served as the final data points.
Results
Two animals were removed from the study because they did not meet the self-administration criterion of at least 50 earned infusions during at least five of the 6-hr sessions. Following place conditioning, one additional animal was removed from the high dose group because its difference score was more than two standard deviations away from the group mean. This produced final sample sizes of n=12 (saline SA) and n=10 (cocaine SA) for the 0-min delay (1.0 mg/kg cocaine group); n=12 (saline SA) and n=13 (cocaine SA) for the 15-min delay (1.0 mg/kg group); n=10 (0-min delay) and n=6 (15-min delay) for the 2.5 mg/kg cocaine group. An additional vehicle control group was utilized to serve as immunostaining controls (n=12). Twotailed repeated measures t-tests performed on the baseline data confirmed that in each group subjects did not exhibit an initial bias for either the cocaine- or saline-paired chambers prior to drug-place conditioning (p > .05).
Cocaine Self-Administration
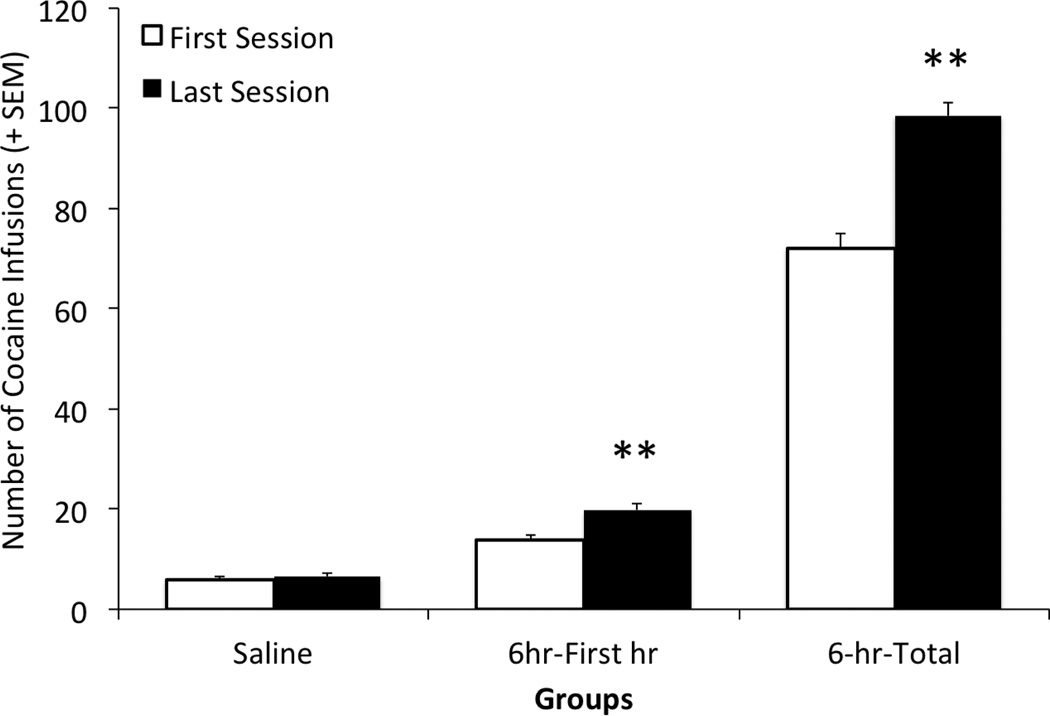
The drug intake of subjects in the self-administration phase of the study is depicted in Figure 1. A two-factor Trial × Group analysis of variance (ANOVA) computed on these data yielded main effects for both Trial (F(1, 61) = 17.065, p < .01) and Group (F(1, 61) = 500.096, p < .01), as well as a Trial × Group interaction (F(1, 61) = 15.055, p < .01). Cocaine-reinforced subjects produced a pattern of escalated intake across sessions that was not observed in salinereinforced controls (Figure 1). Post-hoc one-tailed paired sample t-tests comparing the infusions earned by animals in the 6-h group during the first day versus the last day of testing confirmed that cocaine-reinforced animals escalated their cocaine intake over days during the 1st hour of the session (i.e., compare the middle two bars of Figure 1; t(63) = −6.853, p < .01) and across the whole session (i.e., compare the two bars on the right side of Figure 1; t(63) = −5.007, p < .01). Saline-reinforced animals, however, did not increase their saline intake across sessions (comparing the two bars on the left side of Figure 1; trial 8 vs 17, p >.05).
Figure 1.
Mean (+SEM) number of cocaine infusions earned during the first day (open bars) and last day (closed bars) of self-administration testing (corresponding to trials 7 and 17; see methods for details) for two groups of subjects – a saline self-administration control group and a cocaine self-administration group. The left two bars reflect the total responses/saline infusions during a one-hour session for the saline control subjects on the first and last day of testing; the middle two bars depict responses/cocaine infusions during the first hour of a 6-hr session on the first and last day of testing; and the right-most bars reflect the total number of responses/infusions during full 6-hr sessions for the cocaine-reinforced group on the first and last day of testing ** denotes a significant escalation in responding between the first and last day of testing p < .01.
Experiment 1: Place Conditioning (1.0 mg/kg dose)
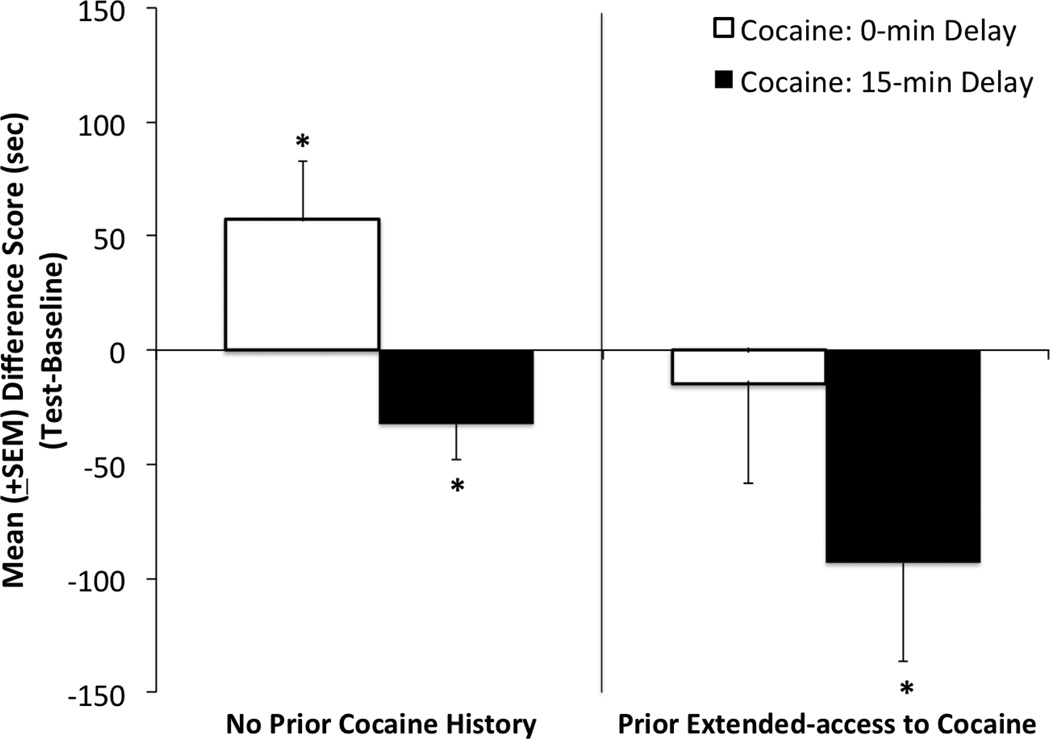
Following self-administration, all animals proceeded to the place conditioning portion of the experiment to assess the effects of prior drug history on the magnitude of CPPs and CPAs (see Fig 2). Difference scores were computed by subtracting time spent in the cocainepaired chamber on test-day from that spent there prior to conditioning on baseline; thus positive values indicate CPPs (shifts toward the cocaine-paired side) whereas negative values denote CPAs (shifts away from the cocaine-paired side). Pre-planned one-sample t- tests to directly assesses whether the magnitude of each group’s shift from baseline was significantly different from zero confirmed that, subjects’ without a history of prior cocaine self-administration history (see the left side of Figure 2) developed preferences for the compartment paired with the immediate effects of cocaine (t(10) = 2.233, p < .05) and aversions for the side paired with the effects present 15-min post-injection (t(11) = −2.031, p < .05). In contrast, animals with extended prior access to cocaine (see the right side of Figure 2) the positive effects of the drug were no longer present (p > .05) but the negative effects remained intact (t(12) = −2.148, p < .05).
Figure 2.
Conditioned place test results: Mean (+SEM) difference scores (time in cocaine-paired environment on test day less time spent there during preconditioning baseline) for four groups of animals: two groups having had no prior cocaine self-administration history (left panel) and two having had prior 6-hr daily access to cocaine (right panel). All animals were exposed to a distinct environment paired with either the immediate positive (open bars) or delayed negative (closed bars) effects of a 1.0 mg/kg dose of i.v. cocaine. Positive values indicate preferences for the cocaine-paired side while negative values reflect aversions to the cocaine-paired side. * denotes a difference score that is statistically different from “zero” p < .05.
Experiment 2: Place Conditioning (2.5 mg/kg dose)
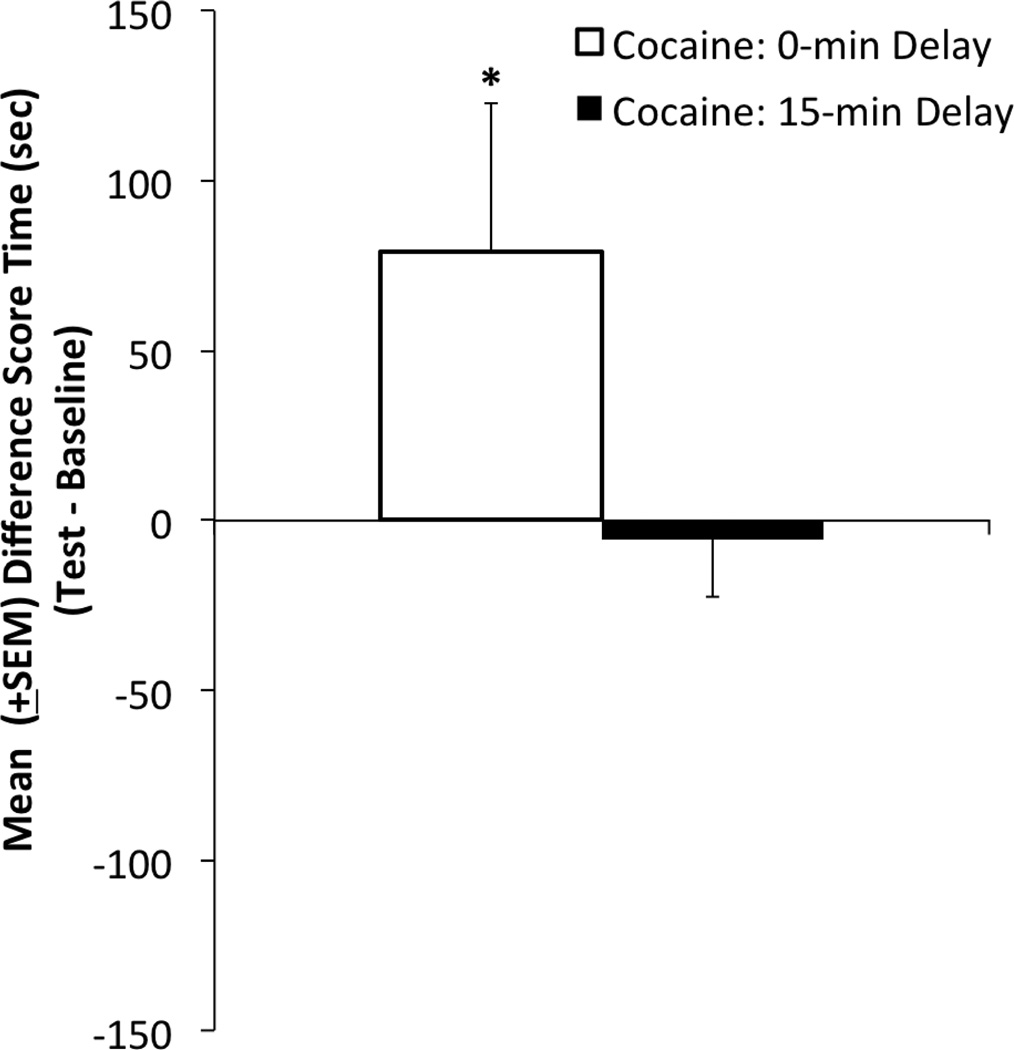
In subjects with a history of prior extended daily access to cocaine, place conditioning with a higher dose (2.5 mg/kg) of cocaine re-asserted the positive effects of the drug while eliminating its negative effects (Figure 3). One-sample one-tailed t-tests confirmed that the difference score (i.e., the shift from baseline) was significantly greater than zero in the immediate cocaine group (t(9) = 1.855, p < .05), but not in the 15-min delay group. Thus, the 2.5 mg/kg dose produced CPPs, but not CPAs, in animals with an extensive cocaine history.
Figure 3.
Conditioned place test results: Mean (+SEM) difference scores (time in cocaine-paired environment on test day less time spent there during preconditioning baseline) for two groups of animals having had prior 6-hr daily access to cocaine. All animals were exposed to a distinct environment paired with either the immediate positive (open bars) or delayed negative (closed bars) effects of a 2.5 mg/kg dose of i.v. cocaine. Positive values indicate preferences for the cocaine-paired side while negative values reflect aversions to the cocaine-paired side. * denotes a difference score that is statistically different from “zero” p < .05.
Zif-268 Immunohistochemistry Results
An additional group of animals (n=12) was employed to establish baseline zif-268 activity in animals without any prior cocaine experience. As expected, these zif-268 saline control subjects did not exhibit CPPs or CPAs and there were no differences between animals that were placed into the CPP apparatus immediately or following a 15-min delay (data not shown, p > .05). Hence, the saline subjects were combined into one group for data analysis.
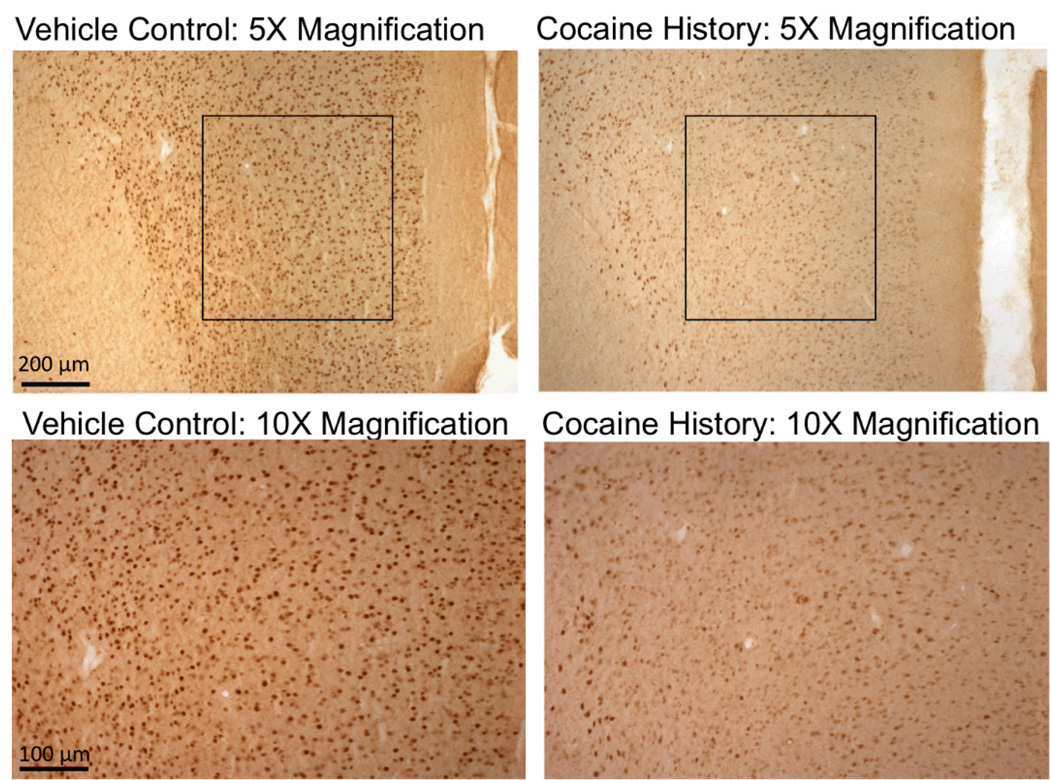
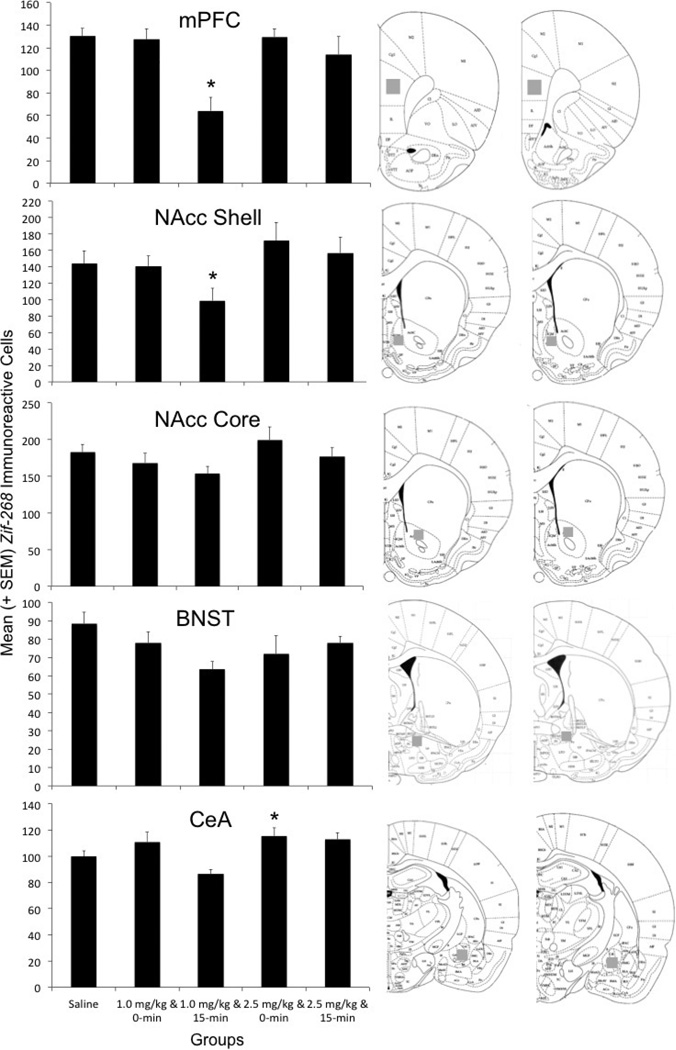
A separate one-way ANOVA was conducted on mean zif-268 cell counts for each of the five anatomical areas of interest (mPFC, NAcc core and shell, BNST and CeA) across each of the following groups 1-hr after the completion of the CPP/CPA test: saline controls (0 mg/kg cocaine dose), 0-min delay (1.0 mg/kg cocaine dose), 15-min delay (1.0 mg/kg dose), 0-min delay (2.5 mg/kg dose), 15-min delay (2.5 mg/kg dose). The statistical analyses identified significant group differences in zif-268 accounts for the prelimbic PFC (F(4, 41) = 9.316; p < .01), NAcc shell (F(4, 38) = 5.095; p < .01), and CeA (F(4, 39) = 5.372; p < .01), but not for the NAcc core or BNST (p > .05) (Figure 3). Compared to the saline control group, LSD post-hoc tests confirmed that subjects expressing CPAs when conditioned with the low dose of cocaine (i.e., 15-min delay conditioned with the 1.0 mg/kg dose) exhibited decreased activity in the prelimbic PFC (p < .01) and NAcc shell (p < .01). In contrast, cocaine-induced CPPs in the high dose group (i.e., 0-min delay subjects conditioned with the 2.5 mg/kg dose) were associated with an elevation of zif-268 immunoreactivity in the CeA (p < .05). Sample pictures of group differences in zif-268 immunostaining are shown in Figure 5.
Figure 5.
Sample zif-268 immunoreactivity in the mPFC of two representative animals: a drugnaïve vehicle control animal (left panels) and a subject with prior extended-access to cocaine (right panels) under 5x and 10x magnification. The darkened cells in all panels represent zif-268 positive neurons.
Discussion
The current experiment was designed and executed to assess the effects of prior 6-hr cocaine access on the sensitivities to the rewarding and anxiogenic actions of cocaine in a conditioned place test. As previously reported, animals provided extended access to cocaine self-administration developed over trials a pattern of escalated cocaine intake (see Figure 1) (Ahmed & Koob, 1998; Ben-Shahar et al., 2005, 2008). When subjects transitioned to the place conditioning portion of the study, the data confirmed previous findings that animals without this history of extended daily access to cocaine spent more time in an environment paired with the immediate rewarding effects of cocaine and less time in a compartment associated with the delayed anxiogenic effects of the drug (Figure 2, left panel; Ettenberg et. al., 1999). A novel and interesting aspect of the current project was the demonstration that prior 6-hr cocaine access attenuated the resulting CPPs to the immediate effects of the drug without affecting the delayed negative effects of the drug (Figure 2, right panel). It would seem then, that the positive but not the negative effects of cocaine were compromised in animals with a strong prior history of cocaine use. However, CPPs were re-established when extended access subjects were administered a higher 2.5 mg/kg dose during place conditioning (see Figure 3). While the administration of the 2.5 mg/kg dose in animals without a prior history of extended cocaine self-administration was not executed in the current study, others have previously reported that this dose produces robust CPPs (O’Dell et al., 1996; Nomikos & Spyraki, 1988). Lastly, expression of CPAs was accompanied by a reduction in zif-268 immunoreactive cells in the mPFC and the NAcc shell while zif-positive cell counts in subjects expressing preferences did not differ from saline control subjects. In contrast, the re-established CPPs observed in the high-dose condition was paralleled by increases in immunoreactivity in the CeA relative to that observed in those subjects exhibiting CPAs (Figure 4).
Figure 4.
Average (+SEM) zif-268 positive cells in five select brain areas collected following a final place preference test for each of five groups of animals: a no-drug saline control group, two groups that experienced place conditioning with the immediate (0-min) or delayed (15-min) effects of 1.0mg/kg i.v. cocaine, and two groups that experienced drug-place pairings involving the immediate or delayed effects of a larger 2.5mg/kg dose of i.v. cocaine. To the right of each panel is a schematic diagram (reproduced from Paxinos and Watson, 1998) identifying the sampling location for each region. *denotes a statistically significant difference compared to saline controls p < .05.
Collectively, the behavioral results of the current experiment suggest that prior extended access to cocaine self-administration diminished the reward value of the drug and thus a higher dose of cocaine was needed to re-establish CPPs. These data align nicely with Koob and LeMoal’s (1997, 2001) “Allostatic Theory”, which postulates that repeated and extensive cocaine exposure produces neuroadaptations that act to elevate the hedonic threshold in the brain’s reward system while concurrently activating an opposing “antireward” system that is associated with anxiety, drug cravings and other negative effects of cocaine. Hence, neuroadaptations that occur within both of these systems induce a negative affective state that fuels the motivation to seek cocaine to relieve these aversive symptoms (i.e., negative reinforcement). Animals attempt to compensate for this diminished reward threshold by taking more drug, as indicated by the escalated pattern of drug intake over successive trials (Ahmed & Koob, 1998). Furthermore, extended-access subjects are more motivated to work for cocaine under a progressive ratio schedule of reinforcement (Paterson & Markou, 2003), to seek cocaine in a self-administration runway task (Ben-Shahar et al. 2008), and to endure aversive stimuli in order to obtain the drug (Vanderschuren & Everitt, 2004; Deroche-Gemonet, Belin, & Piazza, 2004). In addition, 6-hr cocaine access renders the animal tolerant to the locomotor effects of an intraperitoneal cocaine challenge at 60 days of withdrawal (Ben-Shahar et al. 2005).
The shift in the sensitivities to the positive and negative effects of cocaine in extended access animals was accompanied by specific neural alterations in both the reward and stress circuitries (see Figure 4). Converging lines of evidence have acknowledged that the mPFC and NAcc are important components of the reward pathway. For example, rats readily lever press for electrical stimulation of the mPFC (Corbett, Laferriere, & Milner, 1982; Mora & Cobo, 1990; Routtenberg & Sloan, 1972) and self-administer cocaine directly into the region (Goeders & Smith, 1983; 1986; Guzman et al., 2009), while mPFC lesions attenuated cocaine-induced CPPs (Issac et al., 1989). Similarly, intra-NAcc infusions of the selective catecholamine toxin 6-hydroxydopamine (6-OHDA) or dopamine (DA) antagonists impair cocaine self-administration (McGregor & Roberts, 1993; Pettit et al., 1984; Roberts et al., 1980) and attenuate cocaineinduced CPPs (Baker et al., 1998; Morency & Beninger, 1986; Sellings, McQuade, & Clarke, 2006; Spyraki et al., 1982). Moreover, a number of investigators have demonstrated lower levels of c-fos immunoreactivity in the mPFC and NAcc shell occurs in response to negative stimuli, including social defeat, restraint stress, and foot shock (Covington et al., 2005; Jiao et al., 2011; Kerfoot et al., 2007; Shoji & Mizoguchi, 2010). Hence, the current mPFC and NAcc zif-268 data are consistent with these findings and corroborate the involvement of the mPFC and NAcc in cocaine-induced CPA.
Unlike the mPFC and NAcc, the CeA is regarded as a neural substrate implicated in fear and anxiety states (c.f. de la Mora et al. 2010; Paré, Quirk, & Ledoux, 2004; Tanimoto et al. 2003; Walker, Toufexis, & Davis, 2003). Lesions of this region attenuate fear responses (e.g. Hitchcock & Davis, 1986; LaBar & LeDoux, 1996) and naloxone-precipitated morphine withdrawal induced an increase in c-fos expression in the CeA (Lucas et al., 2008; Jin et al., 2005; Veinante et al. 2003). The current CeA immunostaining results seem contradictory to the wealth of evidence demonstrating the observation that enhanced CeA activition should produce more stress and anxiety. However, positive stimuli were also shown to increase synaptic engagement within the CeA. Thus, subjects exposed to positive social interaction (Varlinskaya, Vogt, & Spear, 2012), a positively-valenced nicotine-paired environment (Shram et al., 2007), and the administration of anxiolytic compounds (Blackshear et al., 2007), all produced elevations in CeA c-fos expression. Additionally, immunohistochemical procedures do not identify the type of cells being stained, For example, the zif-268 positive cells within the CeA may be inhibitory GABA-ergic inter-neurons (Pitkanen & Amaral, 1994) whose action could result in a decrease in resident corticotropin-releasing factor (CRF) neurons (Palkovits et al., 1983) or NE neurons, either or both of which would be expected to produce a reduction in fear/anxiety (Cecchi et al., 2002; Leri et al., 2002). Alternatively, the elevated zif-268 cell counts within the CeA could reflect enhanced activity of CRF-containing neurons known to project to the VTA where they in turn stimulate dopamine (DA) neurons that project to the NAcc and are presumably required for cocaine reward (Rodaros et al., 2007; Tagllaferro & Morales, 2008).
Of course, zif-268 expression in the current experiment occurred following exposure to the cocaine-, neutral-, and saline-paired compartments of the conditioning apparatus during the 15-min place preference test. However, since the saline-paired compartment and the intermediate chamber were both operationally neutral to the animal (i.e., neither was associated with the positive or negative effects of the drug), the cocaine-paired side of the apparatus should have dictated the overall emotional valence of the apparatus on test day. Hence, subjects exposed to the immediate positive effects of cocaine would be expected to develop an overall net positive association with the conditioning apparatus while those animals conditioned to the delayed anxiogenic properties of cocaine would be expected to perceive the apparatus in a more negative light.
It should also be noted that in some cases, the current results conflict with those reported by others. For example, exposure to food deprivation or restricted feeding (Moscarello, Ben-Shahar & Ettenberg, 2009; Mendoza, Angeles-Castellanos & Escobar, 2005), aversive foot-shock (Duncan, Knapp, & Breese, 1996), psychosocial stress (Singewald et al., 2009), and drug withdrawal (Harris et al., 2007) have each been associated with increased Fos activity in the mPFC, NAcc shell, and CeA. In each of these instances, however, the negative condition involved exposure to an unlearned aversive situation (i.e., foot shock, fearful places, food deprivation, drug withdrawal, the drug itself). Conversely, the current experiment is unique in that the observed changes in neural activity (i.e., zif-268 cell counts) were triggered by positive or negative associative cues and measured in undrugged animals. Indeed, when one examines the impact of conditioned cues on neuronal activity, the results are more closely aligned with those reported here. For example, animals exposed to external stimuli associated with lithium chloride (a compound known to produce conditioned taste aversions) produced decreased levels of c-fos activity in the mPFC (Mickley et al, 2005) and NAcc shell (Kerfoot et al., 2007). Additionally, animals expressing cocaine-induced CPPs that were then isolated in the cocainepaired chamber, exhibited elevated c-fos levels in the CeA (Fritz et al., 2011).
Taken together, these data demonstrate that subjects without a prior and extensive history of cocaine self-administration display “normal” responding to the positive and negative effects of cocaine, while repeated prior exposure (via 6-hr of daily cocaine access) induces an elevation in the reward threshold that necessitated a higher dose of cocaine for animals to experience the rewarding properties of the drug. Since re-exposure to contextual cues induces insatiable craving in human addicts (Ehrman et al., 1992; Foltin & Haney, 2000) and reinstates drug-seeking behaviors in animals (Alleweireldt, Weber, & Neisewander, 2001; Fuchs et al., 2005; for review, see Crombag et al., 2008), understanding how the rewarding and anxiogenic properties of cocaine may shift as a consequence of drug history is important for our understanding of the precise means by which drug-paired cues induce relapse. The current data suggest that while positive reinforcement processes may underlie the motivation to re-engage in drug-seeking behavior in the “non-dependent” organism, negative reinforcement processes become more prominent in motivating drug-seeking behavior in “addicts.” This transition from positive to negative reinforcement mechanisms is accompanied by perturbations within the brain’s reward and stress systems.
Acknowledgements
The authors acknowledge, with gratitude, Amy Eisenberg, Michelle Gaynor, Michael Ghermezi, Michelle Hanna, and Vera Fomenko for their assistance with various aspects of the project. We extend our special thanks to Skirmantas Janusonis for his assistance in obtaining zif-268 images for the current project. This research was funded by NIDA grant DA05041 awarded to A.E.
Footnotes
Authors Contribution
ZIS, AE, and OBS all contributed to the concept and design of the current study. ZIS and JW were responsible for data collection. ZIS, AE, and OBS participated in the data analysis and interpretation of the findings. ZIS drafted the manuscript, which was critically edited by AE and OBS. All authors critically reviewed the content and approve the final version for publication.
References
- Ahmed SH, Koob GS. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Allen RM, Everett CV, Nelson AM, Gulley JM, Zahniser NR. Low and high locomotor responsiveness to cocaine predicts intravenous cocaine conditioned place preference in male Sprague-Dawley rats. Pharmacol Biochem Behav. 2007;86:37–44. doi: 10.1016/j.pbb.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleweireldt AT, Weber SM, Neisewander JL. Passive exposure to a contextual discriminative stimulus reinstates cocaine-seeking behavior in rats. Pharmacol Biochem Behav. 2001;69:555–560. doi: 10.1016/s0091-3057(01)00573-1. [DOI] [PubMed] [Google Scholar]
- Aronson TA, Craig TJ. Cocaine precipitation of panic disorder. Amer J Psychiatry. 1986;143:643–645. doi: 10.1176/ajp.143.5.643. [DOI] [PubMed] [Google Scholar]
- Baker DA, Fuchs RA, Specio SE, Khroyan TV, Neisewander JL. Effects of intraaccumbens administration of SCH-23390 on cocaine-induced locomotion and conditioned place preference. Synapse. 1998;30:181–193. doi: 10.1002/(SICI)1098-2396(199810)30:2<181::AID-SYN8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Moscarello JM, Jacob B, Roarty MP, Ettenberg A. Prolonged daily exposure to IV cocaine results in tolerance to its stimulant effects. Pharmacol Biochem Behav. 2005;82:411–416. doi: 10.1016/j.pbb.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Posthumus EJ, Waldroup SA, Ettenberg A. Heighted drug-seeking motivation following extended daily access to self-administered cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:863–869. doi: 10.1016/j.pnpbp.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear A, Yamamoto M, Anderson BJ, Holmes PV, Lundström L, Langel U, Robinson JK. Intracerebroventricular administration of galanin or galanin receptor subtype 1 agonist M617 induces c-Fos activation in central amygdala and dorsomedial hypothalamus. Peptides. 2007;28:1120–1124. doi: 10.1016/j.peptides.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Corbett D, Laferriere A, Milner PM. Plasticity of the medial prefrontal cortex: facilitated acquisition of intracranial self-stimulation by pretreating stimulation. Phys Behav. 1982;28:531–534. doi: 10.1016/0031-9384(82)90151-2. [DOI] [PubMed] [Google Scholar]
- Costall B, Kelly ME, Naylor RJ, Onaivi ES. The actions of nicotine and cocaine in a mouse model of anxiety. Pharmacol Biochem Behav. 1989;33:197–203. doi: 10.1016/0091-3057(89)90450-4. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Jr, Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y, Review Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Mora MP, Gallegos-Cari A, Arizmendi-García Y, Marcellino D, Fuxe K. Role of dopamine receptor mechanisms in the amygdaloid modulation of fear and anxiety: Structural and functional analysis. Prog Neurobiol. 2010;90:198–216. doi: 10.1016/j.pneurobio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Deneau G, Yanagita T, Seevers MH. Self-administration of psychoactive substances by the monkey. Psychopharmacology. 1969;16:30–48. doi: 10.1007/BF00405254. [DOI] [PubMed] [Google Scholar]
- Deroche-Garmonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;13:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Knapp DJ, Breese GR. Neuroanatomical characterization of Fos induction in rat behavioral models of anxiety. Brain Res. 1996;713:79–91. doi: 10.1016/0006-8993(95)01486-1. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O'Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology. 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Erb S, Kayyali H, Romero K. A study of the lasting effects of cocaine pre-exposure on anxiety-like behaviors under baseline conditions and in response to central injections of corticotropin-releasing factor. Pharm Biochem Behav. 2006;85:206–213. doi: 10.1016/j.pbb.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. An animal model for investigating the anxiogenic effects of self-administered cocaine. Psychopharmacology. 1991;103:455–461. doi: 10.1007/BF02244244. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Raven MA, Danluck DA, Necessary BD. Evidence for opponent-process actions of intravenous cocaine. Pharmacol Biochem Behav. 1999;64:507–512. doi: 10.1016/s0091-3057(99)00109-4. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. Opponent processes properties of self-administered cocaine. Neurosci Biobehav Rev. 2004;27:721–728. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Bernardi RE. Effects of buspirone on the immediate positive and delayed negative properties of intravenous cocaine as measured in the conditioned place preference test. Pharmacol Biochem Behav. 2007;8:171–178. doi: 10.1016/j.pbb.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A. The runway model of drug self-administration. Pharmacol Biochem Behav. 2009;91:271–277. doi: 10.1016/j.pbb.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Ofer OA, Mueller CL, Waldroup S, Cohen A, Ben-Shahar O. Inactivation of the dorsal raphe nucleus reduces the anxiogenic response of rats running an alley for intravenous cocaine. Pharmacol Biochem Behav. 2011;97:632–639. doi: 10.1016/j.pbb.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feduccia AA, Duvauchelle CL. Novel apparatus and method for drug reinforcement. J Vis Exp. 2010:42. doi: 10.3791/1998. doi:pii: 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman MW, Schuster CR, Resnekov L, Shick JF, Krasnegor NA, Fennell W, Freedman DX. Cardiovascular and subjective effects of intravenous cocaine administration in humans. Arch Gen Psychiatry. 1976;33:983–989. doi: 10.1001/archpsyc.1976.01770080101010. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M. Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology (Berl.) 2000;149:24–33. doi: 10.1007/s002139900340. [DOI] [PubMed] [Google Scholar]
- Fontana DJ, Commissaris RL. Effects of cocaine on conflict behavior in the rat. Life Sci. 1989;45:819–827. doi: 10.1016/0024-3205(89)90175-6. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Druhan JP. Expression of Fos-related antigens in the nucleus accumbens and associated regions following exposure to a cocaine-paired environment. Eur J Neurosci. 2000;12:2097–2106. doi: 10.1046/j.1460-9568.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- Fritz M, El Rawas R, Salti A, Klement S, Bardo MT, Kemmler G, Dechant G, Saria A, Zernig G. Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addict Biol. 2011;16:273–284. doi: 10.1111/j.1369-1600.2010.00285.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Funk D, Li Z, Fletcher PJ, Lê AD. Effects of injections of 8-hydroxy-2-(di-n-propylamino) tetralin or muscimol in the median raphe nucleus on c-fos mRNA in the rat brain. Neuroscience. 2005;131:475–479. doi: 10.1016/j.neuroscience.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science. 1983;221:773–775. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Reinforcing properties of cocaine in the medial prefrontal cortex: primary action on presynaptic dopaminergic terminals. Pharmacol Biochem Behav. 1986;25:191–199. doi: 10.1016/0091-3057(86)90252-2. [DOI] [PubMed] [Google Scholar]
- Guzman D, Moscarello JM, Ettenberg A. The effects of medial prefrontal cortex infusions of cocaine in a runway model of drug self-administration: evidence of reinforcing but not anxiogenic actions. E J Pharmacol. 2009;605:117–122. doi: 10.1016/j.ejphar.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamner MB. PTSD and cocaine abuse. Hosp Community Psychiatry. 1993;44:591–592. doi: 10.1176/ps.44.6.591. [DOI] [PubMed] [Google Scholar]
- Harris GC, Hummel M, Wimmer M, Mague SD, Aston-Jones G. Elevations of FosB in the nucleus accumbens during forced cocaine abstinence correlate with divergent changes in reward function. Neuroscience. 2007;147:583–591. doi: 10.1016/j.neuroscience.2007.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M. Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behav Neurosci. 1986;100:11–22. doi: 10.1037//0735-7044.100.1.11. [DOI] [PubMed] [Google Scholar]
- Issac WL, Nonneman AJ, Neisewander JL, Landers T, Bardo MT. Prefrontal cortex lesions differentially disrupt cocaine-reinforced conditioned place preference but not conditioned taste aversion. Behav Neurosci. 1989;103:345–355. doi: 10.1037//0735-7044.103.2.345. [DOI] [PubMed] [Google Scholar]
- Jiao X, Pang KC, Beck KD, Minor TR, Servatius RJ. Avoidance perseveration during extinction training in Wistar-Kyoto rats: an interaction of innate vulnerability and stressor intensity. Behav Brain Res. 2011;221:98–107. doi: 10.1016/j.bbr.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Araki H, Nagata M, Shimosaka R, Shibata K, Suemaru K, Kawasaki H, Gomita Y. Expression of c-Fos in the rat central amygdala accompanies the acquisition but not expression of conditioned place aversion induced by withdrawal from acute morphine dependence. Behav Brain Res. 2005;16:107–112. doi: 10.1016/j.bbr.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Balster RL, Bonese K. Self-administration of psychomotor stimulant drugs: the effects of unlimited access. Pharmacol Biochem Behav. 1976;4:45–51. doi: 10.1016/0091-3057(76)90174-x. [DOI] [PubMed] [Google Scholar]
- Johnson ZV, Revis AA, Burdick MA, Rhodes JS. A similar pattern of neuronal Fos activation in 10 brain regions following exposure to reward- or aversion-associated contextual cues in mice. Physiol Behav. 2010;99:412–418. doi: 10.1016/j.physbeh.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerfoot EC, Agarwal I, Lee HJ, Holland PC. Control of appetitive and aversive taste- reactivity responses by an auditory conditioned stimulus in a devaluation task: a FOS and behavioral analysis. Learn Mem. 2007;14:581–589. doi: 10.1101/lm.627007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug Abuse: Hedonic Homeostatic Dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Lucas M, Frenois F, Vouillac C, Stinus L, Cador M, Le Moine C. Reactivity and plasticity in the amygdala nuclei during opiate withdrawal conditioning: differential expression of c-fos and arc immediate early genes. Neuroscience. 2008;154:1021–1033. doi: 10.1016/j.neuroscience.2008.04.006. [DOI] [PubMed] [Google Scholar]
- McGregor A, Roberts DC. Dopaminergic antagonism within the nucleus accumbens or the amygdala produces differential effects on intravenous cocaine self-administration under fixed and progressive ratio schedules of reinforcement. Brain Res. 1993;624:245–252. doi: 10.1016/0006-8993(93)90084-z. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Angeles-Castellanos M, Escobar C. Differential role of the accumbens Shell and Core subterritories in food-entrained rhythms of rats. Behav Brain Res. 2005;158:133–142. doi: 10.1016/j.bbr.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Mickley GA, Kenmuir CL, Yocom AM, Wellman JA, Biada JM. A role for prefrontal cortex in the extinction of a conditioned taste aversion. Brain Res. 2005;1051:176–182. doi: 10.1016/j.brainres.2005.05.033. [DOI] [PubMed] [Google Scholar]
- Mickley GA, Hoxha Z, Bacik S, Kenmuir CL, Wellman JA, Biada JM, DiSorbo A. Spontaneous recovery of a conditioned taste aversion differentially alters extinction-induced changes in c-Fos protein expression in rat amygdala and neocortex. Brain Res. 2007;1152:139–157. doi: 10.1016/j.brainres.2007.03.050. [DOI] [PubMed] [Google Scholar]
- Mora F, Cobo M. The neurobiological basis of prefrontal cortex self-stimulation: a review and an integrative hypothesis. In: Uylings HMB, Van Eden CG, De Bruin JPC, Corner MA, Freenstra MPG, editors. Progress in brain research. Elsevier: Amsterdam, Netherlands; 1990. pp. 419–431. [DOI] [PubMed] [Google Scholar]
- Morency MA, Beninger RJ. Dopaminergic substrates of cocaine-induced place conditioning. Brain Res. 1986;399:33–41. doi: 10.1016/0006-8993(86)90598-6. [DOI] [PubMed] [Google Scholar]
- Moscarello JM, Ben-Shahar O, Ettenberg A. Effects of food deprivation on goal-directed behavior, spontaneous locomotion, and c-Fos immunoreactivity in the amygdala. Behav Brain Res. 2009;197:9–15. doi: 10.1016/j.bbr.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha RF, van der Kooy D, O'Shaughnessy M, Bucenieks P. Drug reinforcement studied by the use of place conditioning in rat. Brain Res. 1982;243:91–105. doi: 10.1016/0006-8993(82)91123-4. [DOI] [PubMed] [Google Scholar]
- Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Spyraki C. Cocaine-induced place conditioning: importance of route of administration and other procedural variables. Psychopharmacology. 1988;94:119–125. doi: 10.1007/BF00735892. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Khroyan TV, Neisewander JL. Dose-dependent characterization of the rewarding and stimulant properties of cocaine following intraperitoneal and intravenous administration in rats. Psychopharmacology (Berl) 1996;123:144–153. doi: 10.1007/BF02246171. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased motivation for self-administration cocaine after escalated cocaine intake. Neuroreport. 2003;14:2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5th ed. Academic Press; New York, NY: 1998. [Google Scholar]
- Paré D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl) 1984;84:167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Amaral DG. The distribution of GABAergic cells, fibers, and terminals in the monkey amygdaloid complex: an immunohistochemical and in situ hybridization study. J Neurosci. 1994;14:2200–2224. doi: 10.1523/JNEUROSCI.14-04-02200.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick RB, Resnick EB. Cocaine abuse and its treatment. Psychiatric Clinics North Am. 1984;7:713–728. [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self- administration following 6-hydroxydopamine lesions in the nucleus accumbens. Pharmacol Biochem Behav. 1980;12:781–787. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- Rogerio R, Takahashi RN. Anxiogenic properties of cocaine in the rat evaluated with the elevated plus maze. Pharmacol Biochem Behav. 1992;43:631–633. doi: 10.1016/0091-3057(92)90203-r. [DOI] [PubMed] [Google Scholar]
- Routtenberg A, Sloan M. Self-stimulation in the frontal cortex of Rattus norvegicus. Behav Biol. 1972;7:567–572. doi: 10.1016/s0091-6773(72)80218-9. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne PP, Uhde TW. Exogenous factors in panic disorder: clinical and research implications. J Clin Psychiatry. 1988;49:56–61. [PubMed] [Google Scholar]
- Sellings LH, McQuade LE, Clarke PB. Evidence for multiple sites within rat ventral striatum mediating cocaine-conditioned place preference and locomotor activation. J Pharmacol Exp Ther. 2006;317:1178–1187. doi: 10.1124/jpet.105.100339. [DOI] [PubMed] [Google Scholar]
- Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Lê AD. Acute nicotine enhances c-fos mRNA expression differentially in reward-related substrates of adolescent and adult rat brain. Neurosci Lett. 2007;418:286–291. doi: 10.1016/j.neulet.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Shoji H, Mizoguchi K. Acute and repeated stress differentially regulates behavioral, endocrine, neural parameters relevant to emotional and stress response in young and aged rats. Behav Brain Res. 2010;211:169–177. doi: 10.1016/j.bbr.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice: influence of dopaminergic transmissions. Behav Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Singewald GM, Nguyen NK, Neumann ID, Singewald N, Reber SO. Effect of chronic psychosocial stress-induced by subordinate colony (CSC) housing on brain neuronal activity patterns in mice. Stress. 2009;12:58–69. doi: 10.1080/10253890802042082. [DOI] [PubMed] [Google Scholar]
- Smith DE. Cocaine-alcohol abuse: epidemiological, diagnostic and treatment considerations. J Psychoactive Drugs. 1986;18:117–129. doi: 10.1080/02791072.1986.10471392. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Fibiger HC, Phillips AG. Cocaine-induced place preference conditioning: lack of effects of neuroleptics and 6-hydroxydopamine lesions. Brain Res. 1982;253:195–203. doi: 10.1016/0006-8993(82)90686-2. [DOI] [PubMed] [Google Scholar]
- Su Z-I, Wenzel JM, Santoostaroam A, Kichaev G, Hammond H, Ettenberg A. Society for Neuroscience. Washington, DC: Nov, 2010. Inactivation of the central nucleus of the amygdala blocks the anxiogenic effects of IV cocaine. Poster presented at the. [Google Scholar]
- Tagliaferro P, Morales M. Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic. J Comp Neurol. 2008;506:616–626. doi: 10.1002/cne.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LA, Xu K, Vaccarino FJ, Lovejoy DA, Rotzinger S. Teneurin C-terminal associated peptide (TCAP)-1 attenuates corticotropin-releasing factor (CRF)-induced c-Fos expression in the limbic system and modulates anxiety behavior in male Wistar rats. Behav Brain Res. 2009;201:198–206. doi: 10.1016/j.bbr.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Tanimoto S, Nakagawa T, Yamauchi Y, Minami M, Satoh M. Differential contributions of the basolateral and central nuclei of the amygdala in the negative affective component of chemical somatic and visceral pains in rats. Eur J Neurosci. 2003;18:2343–2350. doi: 10.1046/j.1460-9568.2003.02952.x. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Vogt BA, Spear LP. Dev Psychobiol. Epub ahead of print; 2012. Social context induces two unique patterns of c-Fos expression in adolescent and adult rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Stoeckel ME, Lasbennes F, Freund-Mercier MJ. c-Fos and peptide immunoreactivities in the central extended amygdala of morphine-dependent rats after naloxone-precipitated withdrawal. Eur J Neurosci. 2003;18:1295–1305. doi: 10.1046/j.1460-9568.2003.02837.x. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Washton AM, Gold MS, Pottash AC. Intranasal cocaine addiction. Lancet. 1983;22:1374. doi: 10.1016/s0140-6736(83)91143-1. [DOI] [PubMed] [Google Scholar]
- Wenzel JM, Waldroup SA, Haber ZM, Su Z-I, Ben-Shahar O, Ettenberg A. Effects of lidocaine-induced inactivation of the bed nucleus of the stria terminalis, the central or the basolateral nucleus of the amygdala on the opponent-process actions of self-administered cocaine in rats. Psychopharmacology (Berl) 2011a;100:458–463. doi: 10.1007/s00213-011-2267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel JM, Su Z-I, Haber Z, Ettenberg A. Society for Neuroscience. Washington, DC: Nov, 2011b. Noradrenergic antagonism within the extended amygdala attenuates the anxiogenic effects of cocaine in a self-administration runway model. Poster presented at the. [Google Scholar]
- Wenzel JM, Su Z-I, Haber Z, Ettenberg A. Society for Neuroscience. New Orleans, LA: 2012. Oct, Norepinephrine antagonism in the central nucleus of the amygdala blocks the development of conditioned place aversions stemming from the delayed negative effects of IV cocaine in rats. Poster presented at the. [Google Scholar]
- Williamson S, Gossop M, Powis B, Griffiths P, Fountain J, Strang J. Adverse effects of stimulant drugs in a community sample of drug users. Drug Alcohol Depend. 1987;44:87–94. doi: 10.1016/s0376-8716(96)01324-5. [DOI] [PubMed] [Google Scholar]
- Yang XM, Gorman AL, Dunn AJ, Goeders NE. Anxiogenic effects of acute and chronic cocaine administration: neurochemical and behavioral studies. Pharmacol Biochem Behav. 1992;41:643–650. doi: 10.1016/0091-3057(92)90386-t. [DOI] [PubMed] [Google Scholar]