Abstract
Objective
The long-term changes in insulin sensitivity and β-cell function in morbidly obese patients with type 2 diabetes (T2DM) who undergo RYGB surgery or standard medical care remain unclear. We prospectively studied longitudinal changes of glucostatic parameters in morbidly obese patients with T2DM undergoing RYGB surgery or Diabetes Support and Education (DSE).
Research Methods and Design
61 morbidly obese subjects (41.7±0.6 kg/m2) with T2DM were assigned to RYGB surgery (n=30) or DSE (n=31). They were matched for sex, age and body weight. Insulin sensitivity index (Si) and acute insulin response (AIR) were derived from frequently sampled intravenous glucose tolerance test. Body composition was measured using dual-emission absorptiometry X-ray. General linear model with repeated measures were used to examine the longitudinal changes (baseline, 6-month, 12-month) in these parameters.
Results
At 12-month follow-up, significant improvement in obesity measures, body composition, glucose homeostasis, Si and AIR were observed following RYGB surgery and weight loss. These outcomes were not influenced by pre-operative insulin use. Although there were no significant changes in the body composition amongst DSE subjects, they experienced a decline in the Si and AIR, along with an increase in fasting glucose and HbA1c. The between-group differences in Si and AIR at 12-month follow-up were completely attenuated with adjustment to changes in body weight.
Conclusions
The long-term effects of RYGB surgery on glucostatic parameters are partly dependent on weight loss. In morbidly obese diabetic patients who were offered DSE, a progressive decline in the glucose homeostasis and glucostatic parameters is observed despite absence of weight gain. (NCT00787670)
Keywords: Roux-en-Y gastric bypass, Diabetes Support and Education, insulin sensitivity, β-cell function, body composition, morbid obesity, type 2 diabetes
People with morbid obesity (i.e. body mass index [BMI] >40 kg/m2) constitute about 6% of all US adults aged 20 years and older1. The doubling of its prevalence rate over the past decade poses a worrying trend. In addition to the associated high risk for cardiometabolic diseases, studies have shown that the co-existence of morbid obesity and type 2 diabetes mellitus (T2DM) exponentially increases the risk of premature death2. Although lifestyle intervention and medications remain the cornerstone treatment in the public health effort in fighting both obesity and T2DM, they are less effective for most people with morbid obesity. In recent years, Roux-en-Y gastric bypass (RYGB) surgery has been used widely and with great success to facilitate weight loss in morbidly obese individuals. RYGB is also effective in providing high rate of remission of T2DM and other cardiometabolic risk factors3. In addition, several studies have shown that RYGB surgery is a very cost-effective treatment for T2DM compared with standard medical therapy4. For these reasons, the American Diabetes Association has recommended that bariatric surgery should be considered in patients with T2DM who have a BMI of or greater than 35 kg/m2, 4.
However, there remain several unresolved issues that might deter a wider adoption of bariatric strategy in managing obese diabetic individuals in clinical practice. Firstly, reversal of hyperglycemia following RYGB surgery and weight loss is often accompanied by an improvement in insulin secretion and insulin sensitivity which persists over time.5, 6 However, these studies are limited by the inclusion of a small number of patients with T2DM. In addition, the preoperative use of insulin therapy has been associated with a lower rate of diabetes remission after bariatric surgery but there was no long-term systematic assessment of the glucostatic parameters7, 8. Finally, there is a large population of morbidly obese individuals who are offered standard medical care with diabetes support and education (DSE) due to prohibitive cost of bariatric surgery or lack of insurance coverage. The Look AHEAD study has recently reported favorable metabolic changes amongst morbidly obese T2DM individuals randomized to intensive lifestyle intervention9, but the metabolic and glucostatic changes in those who were offered DSE is largely unknown.
Herein, in this study we test the hypothesis that the longitudinal changes in the glucostatic parameters in patients with T2DM are partly dependent on the amount of weight loss induced by RYGB surgery. In addition, we systematically compare the long-term changes in the glucostatic parameters amongst insulin users with non-insulin users at baseline. Given the progressive nature of T2DM, we hypothesize that those who are offered DSE may exhibit deterioration in the glucostatic parameters due to increasing adiposity. The findings of this study may provide further impetus for health care decision makers in making intensive lifestyle intervention, if not RYGB surgery more easily accessible for those with morbidly obese T2DM.
Research design and methods
Patients and study design
This was a prospective cohort study aimed to enroll obese subjects with T2DM. Participants approved for surgical weight loss treatment by their health insurance carrier were recruited for the RYGB surgery group. Participants who were eligible for RYGB surgery but did not have surgery due to insurance company refusal to cover the cost of surgery were enrolled into the DSE Program. The RYGB group was matched with the DSE group using a matching patient’s algorithm according to age, sex and body weight. The matching process was verified every 10 enrollments in the RYGB group, to allow targeted recruitment in the DSE group. Inclusion criteria were clinical diagnosis of T2DM diabetes mellitus according to the American Diabetes Association criteria 10, body mass index ≥ 35 kg/m2 in accord with the 1991 NIH obesity surgery consensus conference criteria, stable weight for the previous 3 months, and age between 18-60 years old. Exclusion criteria included history of cardiovascular heart disease (previous myocardial infarction, stroke, and peripheral artery disease), malignancy, uncontrolled hypertension, previous esophageal, gastric, pancreatic, small bowel, or large bowel surgery, tobacco use, or significant psychiatric disorder. All subjects provided informed consent before participating in the study, and ethics approval was obtained from the Duke University Institutional Review Board.
Study investigations
All hypolipidemic medications were stopped four weeks, oral anti-diabetic and anti-hypertensive agents were withheld three days, and insulin therapy was stopped 24 hours prior to the study procedures. Participants were refrained from strenuous exercise for at least 24 hours prior to the study procedures. Participants were instructed to follow a weight-maintaining daily diet containing 35 kcal/kg of body weight and consisting of 55% carbohydrates, 25% fat and 20% protein one-week prior to the initial examination. All participants were examined in the early morning (8 am) following 12-hour overnight fast. Anthropometric measures were taken with the participant in light weight clothing with shoes removed. Height and weight were measured to the nearest 0.1 cm and 0.5 kg respectively. Body mass index (BMI) was calculated as body weight (in kg) divided by the square of height (in m). Waist circumference was measured with a non-elastic tape at the mid-point between the costal margin and the iliac crest in the mid-axillary line to the nearest 0.1 cm. Body composition analysis (percent body fat, fat-free mass [FFM] and percent trunk fat) was estimated from dual-energy X-ray absorptiometry (DEXA) using a Hologic system (Hologic Discovery QDR Wi).
A 3-hour frequently sampled intravenous glucose tolerance test (IVGTT) was performed to estimate acute insulin response to glucose (AIRg) and insulin sensitivity (Si). Briefly, a bolus of glucose (0.3g/kg body weight in a 50% solution) was given within 60 sec into the antecubital vein. Regular insulin was administered as a bolus injection at 20 min (0.03 unit/kg body weight Actrapid; NovoNordisk, Copenhagen, Denmark). Blood was sampled from the contralateral antecubital vein at −15,−10,−5, 0, 3, 4, 5, 6, 7, 9, 11, 15, 20, 23, 25, 30, 50, 70, 100, 140 and 180 min for assessment of the plasma glucose and insulin. Samples were placed in chilled tubes, and plasma was separated within 20 min and stored at −80°C.
The anthropometric measurements, DEXA and IVGTT were repeated at 6-month and 12-month of follow-up.
Laboratory procedures
Serum samples were stored at −80°C until assayed. All samples were analyzed at the same time. Plasma glucose concentrations were determined by the glucose oxidase method on a Beckman-Coulter Unicel DxC600 analyzer. Immunoreactive insulin was determined in plasma with a double-antibody immunoassay method (Linco Research, St. Louis, MO). Non-esterified free fatty acids (FFA) levels were measured using an enzymatic colorimetric method on a Beckman-Coulter DxC600 analyzer. HbA1C was measured using an ion exchange chromatography method (Variant II, Bio-Rad Laboratories). Serum highly-sensitive C-reactive protein (hsCRP) was measured using immunoassay implemented on Beckman-Coulter Synchron Systems. The lowest concentration that can be measured with an inter-assay CV of 20% is ≤0.18 mg/L.
Study interventions
Roux-en-Y gastric bypass surgery
The procedure consisted of a laparoscopic approach with two 10-mm ports and four 5-mm ports. The technique included the creation of an isolated 10-15-ml proximal gastric pouch, an ante-colic Roux-en-Y gastrojejunostomy with linear stapler technique, a 100-cm Roux-limb, a 50-cm biliopancreatic limb, and a stapled end-side enteroenterostomy. All operations included endoscopy at the conclusion of the procedure to check for anastomotic leaks. There were no post-operative complications and all subjects were discharged between post-operative day 1 and 3.
Diabetes support and education
Diabetes Support and Education Program included three group educational and social support sessions per year after enrollment. The educational sessions included one seminar on diet and nutrition counseling, and one seminar related to exercise. These sessions were informational and did not teach behavioral self-regulation skills. Sessions were conducted by a certified diabetic educator and a nutritionist with a background in diabetes education, exercise, and nutrition. Support groups were offered to provide an opportunity for participants to discuss issues related to living with diabetes.
For the entire duration of the study, all participants (RYGB and DSE) continued to receive comprehensive management of diabetes and other cardiovascular risk factors. The medications were adjusted or withdrawn by the treating physician on the follow-up examination to meet the recommended goal for glycemic control (HbA1c <7%), blood pressure <130/80 mmHg, LDL-C <100 mg/dl and triglycerides <200mg/dl.
Statistical analyses
AIRg and Si were estimated using mathematical modeling methods (MINMOD Millennium, ver. 6.02). AIRg was used as index of first-phase insulin secretion in response to glucose. Si represents an index of the ability of insulin to promote the disposal of glucose; a higher Si indicates enhanced insulin sensitivity. The disposition index (DI) was expressed as DI=SI x AIRg. Fat-to-FFM ratio was calculated as total body fat divided by FFM, and represents the relative amount of body fat to lean mass.
All values were given as means ± standard error (SE) unless otherwise stated. The statistical analyses were carried out using the SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA). We used univariate analysis to compare the magnitude of change at 6-month and 12-month from baseline between the two intervention groups, with and without adjustment for changes in body weight, and to examine the trend in the variables from baseline to 12-month of follow-up. General linear model with repeated measures were used to examine the changes in the variables with follow-up period. Interaction term “interventions*follow-up period” was used to examine the difference in the trend between RYGB and DSE interventions. Comparison for the changes from baseline to 12-month follow-up in body composition, glucose homeostasis and glucostatic parameters between insulin users and non-insulin users at baseline in the RYGB group were conducted using independent student t-test. All statistical tests were two-sided and a P-value < 0.05 was considered significant.
Results
The study enrolled 67 obese participants with T2DM from January 2008 to June 2010. Thirty-two participants approved for surgical weight loss treatment by their health insurance carrier were recruited for RYGB surgery. Two patients of the RYGB group were withdrawn from the trial according to study’s protocol because did not undergo RYGB.
Another 35 participants were enrolled into the DSE Program group. Four DSE patients were withdrawn from the study because failed to show for the baseline testing (n=2) and had fasting blood glucose >300 mg/dl on the morning of the study’s testing (n=2). The DSE group included 22 patients recruited among individuals who were eligible for RYGB surgery but did not have surgery due to insurance company refusal to cover the cost of surgery. Another 13 patients were enrolled from the Diabetes Clinic in order to ensure adequate matching with the RYGB group. Table 1 shows the baseline characteristics of the study participants. The mean (SE) age was 48.5 (1.0) years and body weight 117.2 (2.2) kg. Although there was no significant difference in the body weight, participants in the RYGB group had significantly greater BMI and waist circumference than those in the DSE group. The percent trunk fat was also significantly greater in the RYGB compared to DSE group. However, the percent body fat did not differ significantly between the two groups. Other metabolic profiles (fasting glucose, HbA1c, fasting insulin, insulin sensitivity, insulin secretion, FFA and hsCRP) were similar between the two groups. There was no statistical difference in the diabetes therapy between two groups.
Table 1.
Baseline characteristics of study participants. Values are shown as mean ± SE. P-value shown in the right column refers to differences between the Diabetes Support and Education (DSE) and RYGB group at baseline.
| Variable | DSE | Roux-en-Y Bypass | P-value |
|---|---|---|---|
| n | 31 | 30 | |
| Female, % | 67.7 | 66.7 | 0.929 |
| Age, years | 47.4 ± 1.5 | 49.6 ± 1.4 | 0.292 |
| Weight, kg | 114.3 ± 3.1 | 120.1 ± 3.1 | 0.186 |
| BMI, kg/m2 | 40.1 ± 0.9 | 43.4 ± 0.8 | 0.006 |
| Waist circumference, cm | 122.7 ± 2.3 | 130.3 ± 2.2 | 0.018 |
| Percent body fat,% | 42.0 ± 1.1 | 44.4 ± 1.1 | 0.159 |
| Fat free mass, kg | 63.6 ± 15.7 | 63.0 ± 20.5 | 0.798 |
| Trunk fat, % | 43.7 ± 1.2 | 47.4 ± 1.1 | 0.030 |
| Fat-to-FFM ratio | 0.73 ± 0.03 | 1.07 ± 0.25 | 0.159 |
| HbA1c, % | 7.51 ± 0.23 | 7.53 ± 0.23 | 0.943 |
| Fasting glucose, mg/dL | 149.1 ± 9.2 | 155.6 ± 8.6 | 0.607 |
| Fasting insulin, uU/mL | 16.9 ± 2.6 | 21.9 ± 5.4 | 0.401 |
| Insulin sensitivity, mU/L-1.min−1 | 1.39 ± 0.21 | 1.48 ± 0.22 | 0.753 |
| Acute insulin response, mU−1.min | 93.3 ± 28.6 | 71.7 ± 26.8 | 0.684 |
| Disposition index | 95.2 ± 27.7 | 77.6 ± 61.0 | 0.796 |
| Glucose effectiveness, | 11.6 ± 1.0 | 12.3 ± 1.0 | 0.626 |
| Free fatty acid, umol/L | 700.4 ± 31.4 | 757.6 ± 48.4 | 0.486 |
| hsCRP, mg/dL | 1.20 ± 0.40 | 0.86 ± 0.19 | 0.448 |
| Anti-diabetes medication, % | 0.875 | ||
| Oral agents | 71 | 73 | |
| Insulin therapy | 29 | 27 |
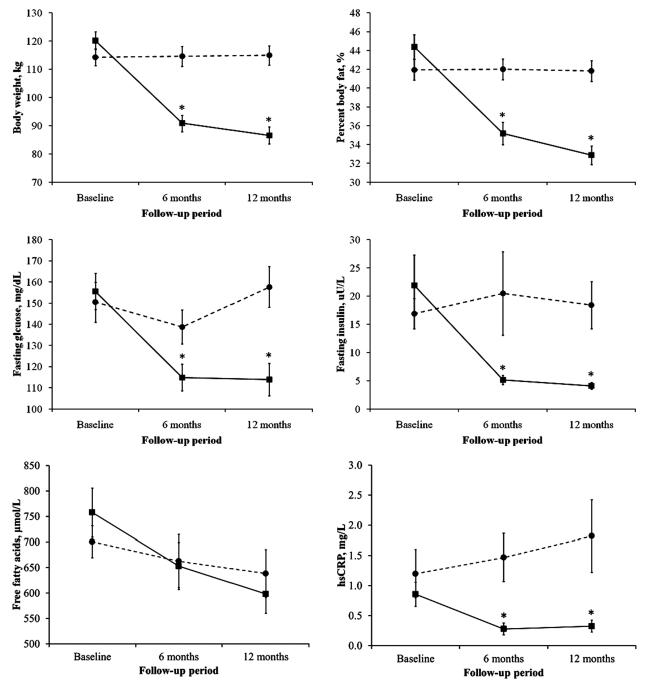
Table 2 shows the magnitude change in body composition, glucose homeostasis and glucostatic parameters from baseline to 6-month and 12-month of follow-up for both intervention groups. The outcome data were collected for 97% subjects in the RYGB group (n=30) and 87% in the DSE (n=31) group at 6-month, and 100% in both groups at 12-month of follow-up. The hsCRP concentrations were significantly reduced in the RYGB group (P-trend<0.001 from baseline to 12-month follow-up). The FFA levels were reduced in both intervention groups, with a greater decline was seen in the RYGB group over 12-month follow-up (P-trend=0.042) (Figure 1).
Table 2.
Changes from baseline in glucose homeostasis, lipoprotein profiles, body composition at 6-month and 12-month follow-up for Diabetes Support and Education (DSE) and RYGB surgery. Values are shown as mean ± SE. 1P and 2P refers to between-group differences for the changes from baseline to 6-month and 12-month respectively. Ptrend refers to temporal changes in the variable of interest from baseline to 12-month of follow-up.
| DSE | RYGB surgery | DSE vs RYGB | P trend from baseline to 12 months |
|||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Baseline to 6 months |
Baseline to 12 months |
Baseline to 6 months |
Baseline to 12 months |
1p | 2p | DSE | RYGB | |
| Body weight, kg | 2.1 ± 2.6 | 0.6 ± 1.0 | −29.5 ± 1.3 | −33.6 ± 2.0 | <0.001 | <0.001 | 0.655 | <0.001 |
| Body mass index, kg/m2 | 0.1 ± 0.3 | 0.3 ± 0.4 | −10.5 ± 0.6 | −12.2 ± 0.8 | <0.001 | <0.001 | 0.446 | <0.001 |
| Waist circumference, cm | −2.0 ± 1.2 | −1.0 ± 1.3 | −23.3 ± 1.5 | −26.6 ± 2.0 | <0.001 | <0.001 | 0.469 | <0.001 |
| Percent body Fat,% | 0.1 ± 0.3 | −0.1 ± 0.3 | −8.7 ± 0.7 | −11.2 ± 1.1 | <0.001 | <0.001 | 0.851 | <0.001 |
| Fat free mass, kg | 0.4 ± 0.5 | 0.6 ± 0.4 | −7.8 ± 0.6 | −8.2 ± 0.7 | <0.001 | <0.001 | 0.384 | <0.001 |
| Trunk fat, % | 1.0 ± 0.7 | 0.0 ± 0.6 | −10.7 ± 1.0 | −13.4 ± 1.5 | <0.001 | <0.001 | 0.189 | <0.001 |
| Total fat-to-FFM ratio | 0.00 ± 0.05 | −0.01 ± 0.01 | −0.51 ± 0.26 | −0.56 ± 0.25 | 0.047 | 0.028 | 0.322 | 0.006 |
| HbA1c, % | −0.1 ± 0.2 | 0.4 ± 0.3 | −1.2 ± 0.2 | −1.2 ± 0.2 | <0.001 | <0.001 | 0.136 | <0.001 |
| Fasting glucose, mg/dL | −5.3 ± 6.3 | 6.4 ± 8.4 | −42.3 ± 7.3 | −42.1 ± 8.7 | <0.001 | <0.001 | 0.224 | <0.001 |
| Fasting insulin, uU/mL | 4.7 ± 5.8 | 1.2 ± 3.1 | −17.2 ± 5.2 | −18.3 ± 5.1 | 0.009 | 0.002 | 0.607 | <0.001 |
| Insulin sensitivity, mU/L−1.min−1 |
1.9 ± 1.0 | −0.4 ± 0.2 | 1.8 ± 0.6 | 2.5 ± 0.5 | 0.357 | 0.001 | 0.606 | <0.001 |
| Acute insulin response, mU−1.min |
−5.5 ± 20.3 | −35.9 ± 19.8 | 49.0 ± 17.3 | 32.5 ± 17.0 | 0.073 | 0.020 | 0.163 | 0.020 |
| Disposition index | 28.1 ± 79.8 | 6.6 ± 58.2 | 158.4 ± 57.3 | 218.9 ± 60.6 | 0.188 | 0.014 | 0.893 | <0.001 |
| hsCRP, mg/L | 0.11 ± 0.16 | 0.26 ± 0.22 | −0.58 ± 0.18 | −0.53 ± 0.18 | 0.615 | 0.016 | 0.170 | <0.001 |
| Free fatty acid, umol/L | −37.8 ± 62.1 | −62.1 ± 60.9 | −104.6 ± 62.5 | −159.5 ± 63.1 | 0.351 | 0.216 | 0.591 | 0.042 |
Figure 1.
Mean (SE) changes in body weight, percent body fat, fasting glucose and fasting insulin from baseline to 12-month follow-up. *p<0.05 is for between-group differences at the indicated follow-up time-point. Interaction terms interventions*period were significant for body weight (p<0.001), percent body fat (p<0.001), fasting glucose (<0.001), fasting insulin (p=0.001) but did not reach statistical significance for free fatty acids (p=0.525) and hsCRP (p=0.223). ■ RYGB surgery, • Diabetes Support and Education (DSE)
Changes in the body composition
There was a marked reduction in the body weight, BMI, waist circumference percent body fat and percent trunk fat from baseline to 6-month and 12-month of follow-up in the RYGB group (all P values <0.001) (Table 2 and Figure 1), with most robust improvement observed at 6-month of follow-up. There was no significant change in the body composition in the DSE group over 12-month of follow-up. The interaction term “interventions*follow-up period” was significant for changes in BMI, body weight, waist circumference, percent body fat and percent trunk fat (all P-values <0.01).
There was a significant reduction in the FFM by an estimate of 12% at 6-month and 13% at 12-month of follow-up in the RYGB group (Supplemental Figure S1). However, total fat-to-FFM ratio was significantly reduced following RYGB surgery indicates that the weight loss was accompanied by a greater loss in the total body fat than in lean mass (Table 2). Indeed, by using linear regression analysis, we found that changes in the total body fat explained 92.7% of the variance in body weight loss at 12-month of follow-up, whereas FFM explained only 3.0%.
Changes in glucose homeostasis
Significant improvement was observed for fasting glucose, fasting insulin and HbA1c at 6- and 12-month of follow-up in the RYGB group (Table 2 and Figure 1). At 12-month post-RYGB surgery, fasting insulin was significantly reduced by an estimate of 71% and fasting glucose by 24%. In contrast, at 12-month of follow-up in the DSE group, we observed a deterioration in the fasting glucose, fasting insulin and HbA1c from baseline, although they did not reach statistical significance (Table 2). The interaction term “interventions*follow-up period” was significant for fasting glucose (p<0.001), fasting insulin (p=0.001) and HbA1c (p<0.001), indicating that while overall glucose homeostasis improved following RYGB surgery and weight loss, a deterioration in the glucose homeostasis is seen in the DSE group. The between-group differences in fasting glucose and fasting insulin remained significant at each follow-up time point, although attenuated with adjustment to the changes in body weight. The between-group differences in HbA1c during follow-up were no longer significant after adjustment for changes in body weight. At 1-year in the RYGB group, 77% patients stopped all diabetes medications, including all patients who were on insulin therapy at baseline. The remaining 23% patients experienced reduction in the anti-diabetes medication. On the contrary, in the DSE group, there were no changes in the diabetes medications in 71%, increased in 26% and reduced in 3% patients.
Changes in the glucostatic parameters
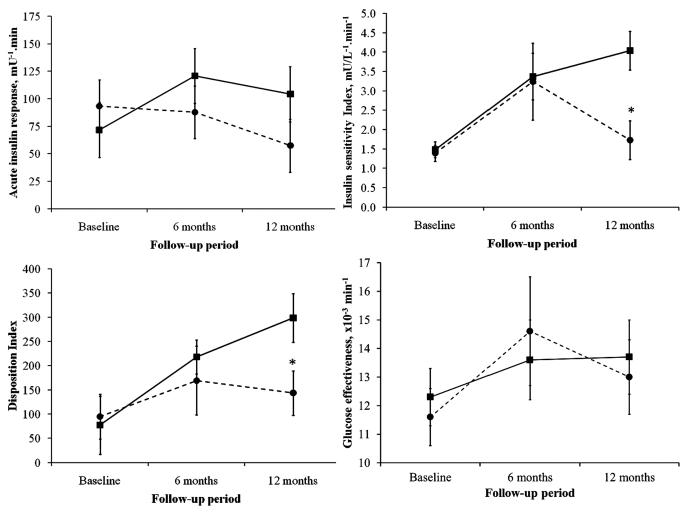
Changes in the key indices of glucose-insulin dynamics estimated using the Minimal Model from frequently sampled IVGTT test are shown in Table 2. In the RYGB group, Si, AIR to glucose, and DI improved significantly at each follow-up time point compared to baseline (Figure 2). In contrast, in the DSE group, there was deterioration in Si, AIRg and DI over 12-month of follow-up, but did not reach a statistical significance (Table 2, P-trend>0.05). The interaction term “interventions*follow-up period” was significant for AIRg (p=0.036) and Si (p=0.037), but did not reach statistical significance for DI (p=0.053) (Figure 2). The between-group differences in these minimal model indices were no longer significant with adjustment to the changes in body weight. We did not observe significant changes in the glucose effectiveness with either intervention groups.
Figure 2.
Mean (SE) changes in key indices from frequently sampled intravenous glucose tolerance test derived from minimal model analyses from baseline to 12-month follow-up. *p<0.05 is for between-group differences at the indicated follow-up time-point. Interaction terms “interventions*follow-up period” were significant for acute insulin response to glucose (p=0.036) and insulin sensitivity index (p=0.037), but did reach significance for disposition index (p=0.053) and glucose effectiveness (p=0.741). ■ RYGB surgery • Diabetes Support and Education (DSE)
The glucose plot during IVGTT is shown in Supplemental Figure S2. The glucose excursion were significantly altered after RYGB surgery and weight loss, and between-period differences were significant from baseline to 6-month (p<0.001) and from baseline to 12-month follow-up (p<0.001). The interaction term “interventions*follow-up period” was significant (p=0.006), indicating that the kinetic in the glucose profiles during IVGTT were significantly altered following RYGB surgery and weight loss at 12-month of follow-up.
Changes in body composition, glucose homeostasis and glucostatic parameters between pre-operative insulin and non-insulin users among RYGB participants
The changes in the body composition, glucose homeostasis and glucostatic parameters over 12-month of follow-up between pre-operative insulin and non-insulin users were similar among RYGB participants (Table 3). At 12-month follow-up, the changes in the glucose homeostasis and glucostatic parameters trended higher in insulin users compared to non-insulin users at baseline, however did not reach statistical significance.
Table 3.
Changes from baseline to 12-month of follow-up in glucose homeostasis, lipoprotein profiles, body composition following RYGB surgery between insulin and non-insulin users at baseline. Values are shown as mean ± SE. P-values refer to the comparison between insulin and non-insulin users.
| Non-insulin users | Insulin users | P value | |
|---|---|---|---|
| N | 22 | 8 | |
| Age, years | 50.2 ± 1.8 | 48.0 ± 1.6 | 0.342 |
| Body weight, kg | −33.5 ± 2.2 | −33.7 ± 5.1 | 0.969 |
| Body mass index, kg/m2 | −12.5 ± 0.2 | −11.5 ± 1.5 | 0.525 |
| Waist circumference, cm | −26.4 ± 2.3 | −27.4 ± 4.5 | 0.837 |
| Percent body fat,% | −11.5 ± 1.2 | −10.3 ± 2.5 | 0.619 |
| Fat free mass, kg | −8.32 ± 0.87 | −7.76 ± 1.13 | 0.738 |
| Trunk fat, % | −13.5 ± 1.7 | −12.9 ± 3.4 | 0.858 |
| Total fat-to-FFM ratio | −0.66 ± 0.33 | −0.27 ± 0.08 | 0.521 |
| HbA1c, % | −1.01 ± 0.29 | −1.79 ± 0.39 | 0.181 |
| Fasting glucose, mg/dL | −39.5 ± 10.3 | −52. 0 ± 15.8 | 0.572 |
| Fasting insulin, uU/L | −16.1 ± 3.6 | −26.7 ± 21.6 | 0.408 |
| Free fatty acids, umol/L | −167.3 ± 81.1 | −115.3 ± 123.6 | 0.745 |
| hsCRP, mg/L | −0.56 ± 0.23 | −0.46 ± 0.21 | 0.821 |
| Insulin sensitivity, mU/L−1.min−1 | 2.54 ± 0.62 | 2.45 ± 0.45 | 0.939 |
| Acute insulin response, mU−1.min | 29.0 ± 19.5 | 49.9 ± 43.0 | 0.640 |
| Disposition index | 246.6 ± 73.1 | 112.7 ± 80.1 | 0.381 |
Conclusions
Two recent randomized controlled trial (RCT) studies have prospectively compared the effectiveness of bariatric surgery versus medical therapy in the management of obese T2DM patients11, 12. Mingrone et al. showed that patients who had undergone either RYGB or biliarypancreatic diversion had greater weight loss, and better glucose and lipids profiles compared to medical therapy11. Similar metabolic results were shown by Schauer et al. with significant reduction in the use of cardiovascular medications at one year of post-bariatric surgery12. Although both studies showed a greater attainment in glycemic goals with bariatric surgery, the changes in the glucose parameters in relation to insulin secretion and insulin sensitivity over the follow-up period are not known. In this study, we showed that in morbidly obese patients with T2DM, 1) RYGB surgery resulted in a marked improvement in body composition, glucose homeostasis and glucostatic parameters at 1-year follow-up, where the most robust improvement was seen at 6-month post-RYGB surgery; 2) baseline insulin users improved to a similar extent in all aforementioned parameters compared to non-insulin users at 12-month post-RYGB surgery; and 3) in the DSE group, there was deterioration in glucose homeostasis and glucostatic parameters over 12-month follow-up despite no significant changes in the body composition.
Studies have demonstrated that in morbidly obese patients, RYGB surgery results in significant changes in tissue insulin sensitivity and substrate utilization. In the early phase of RYGB surgery, many have attributed changes in the insulin sensitivity predominantly as a result of caloric restriction13. Only few studies have systematically documented the longitudinal effect of RYGB surgery on insulin sensitivity and insulin secretion at longer follow-up. However, these studies involved only few patients with T2DM5, 6, 14. The changes in these glucostatic parameters are important, as the fundamental defect in the progression to T2DM is a mismatch between insulin resistance and insulin secretion. In one study, complete remission in diabetes in 13 non-insulin-treated T2DM subjects after RYGB surgery at 1-year follow-up was accompanied by a marked improvement in peripheral insulin sensitivity (measured by hyperinsulinemic clamp study), AIRg and substrate utilization6. In addition, the findings of this study imply that the aforementioned effects could be explained by energy intake restriction early after surgery and by weight loss in the longer follow-up term. Lin E et al further showed that in 11 severely obese patients with hyperglycemia who underwent RYGB surgery, β-cell function improved early following surgery due largely to increase in insulin secretion, and stabilized over 2 years of follow-up due to improved insulin sensitivity associated with reduced adiposity5. Our findings are in line with these reports, but with the advantage of having a larger population of T2DM patients with inclusion of subjects on insulin therapy pre-operatively, and using insulin sensitivity and β-cell function indices from minimal model analyses. We showed that the improvement in the glucose homeostasis and glucostatic parameters at 1-year post-RYGB surgery was in part mediated by the amount of weight loss. When we adjusted for the amount of body weight loss at one-year follow-up, the between-group differences for glucostatic parameters between RYGB surgery and DSE disappeared.
Potential modulators for the postoperative improvement in insulin secretion and insulin sensitivity and weight loss include reversal of glucotoxicity and lipotoxicity. Both glucotoxicity and lipotoxicity have been shown to impaired glucose-stimulated insulin secretion15-17. The exact mechanism responsible for impaired insulin secretion is still unclear. However, the use of intensive insulin therapy to reverse glucotoxicity has been examined in few studies18. Elevated FFA concentrations may affect posttranslational insulin modification, and induce β-cell apoptosis 19. Elevation in the plasma FFA also causes hepatic and skeletal muscle insulin resistance in healthy individuals17, 20. In our study, we observed a marked reduction in fasting glycemia following RYGB surgery. Although we did not measure postprandial glucose, the reduction in the HbA1c concentrations indicates an improvement in overall glycemia and the postprandial glucose excursion. In addition, we found a significant reduction in the serum FFA concentrations following RYGB surgery. A reduction in the FFA concentrations has been shown to improve insulin secretion and insulin sensitivity in T2DM patients17, 21. Obesity, in particular central adiposity is also strongly associated with low-grade systemic inflammation, as measured by hsCRP22. It is well-documented that hsCRP is an independent predictor of insulin sensitivity23. A recent systematic review suggests that weight loss, regardless of whether it occurs through lifestyle, dietary and/or exercise intervention, is an effective strategy for lowering hsCRP levels24. In this study, we found that the hsCRP levels were significantly reduced following RYGB surgery and weight loss. In line with a recent study by Monte SV et al, improvement in insulin sensitivity and resolution of T2DM after RYGB may be attributable, at least in part, to the reduction of endotoxemia and associated proinflammatory mediators25. However, it needs further clarification whether improvement in the systemic inflammation might have an independent impact on insulin secretory function following RYGB surgery. DSE is a nationwide program that aims to optimize metabolic control and quality of life in patients with diabetes. Several studies have shown that DSE improved glycemic control and reduced body weight26. The Look AHEAD study is the largest to date that compared DSE and intensive lifestyle intervention in individuals with T2DM27. In that study, 37.8% of participants in the intensive lifestyle intervention group achieved >10% weight lost compared to 3.2% of DSE participants at 1-year follow-up. Nevertheless, the participants in the DSE group experienced some improvement (not worsening) in fitness and cardiovascular risk factors. In this study, we showed that while DSE program might help to curb further increase in body weight over 12-month of follow-up, it did not prevent further decline in all objective measures of glucostatic parameters. We observed further deterioration in glucose homeostasis in the DSE group at 12-month follow-up. Our findings are in line with UKPDS and the Insulin Resistance Atherosclerosis Study that demonstrate progressive loss of β-cell function as key determinant of deterioration of glycemic control28, 29. The mechanisms underlying the decline in the glucostatic parameters in the DSE group are not clear. Longer and sustained exposure to hyperglycemia, continuing insult from lipotoxicity and possible amyloid accumulation might further undermine β-cell function and worsen glycemic control16.
Several studies have suggested that pre-operative use of insulin therapy confers poorer outcomes (i.e. diabetes remission) after RYGB surgery7, 8. Insulin therapy has been used as a proxy measure for significant β-cell function loss since we do not have a good and reliable measure of β-cell function in routine clinical practice. Thus, insulin therapy would indicate a more advanced stage of diabetes which is associated with longer duration of diabetes. In a recent report of 32 morbidly obese T2DM patients who underwent RYGB surgery, β-cell glucose sensitivity was the only significant predictors of diabetes remission30. Diabetes duration, antidiabetic therapy, and baseline HbA1c were not predictors of remission. In our study, we found that insulin users at baseline had similar improvement in all obesity measures and body composition than non-insulin users. The improvement in glucostatic parameters tended higher in the insulin users during 1-year follow-up after RYGB surgery. By implication, impairment in the AIRg is potentially reversible and insulin use may not preclude the improvement in glucose homeostasis after RYGB surgery. However, the number of insulin users in our study is small, and this finding will require further validation from a larger study with longer follow-up.
The major strength of this study is that we provided a comprehensive assessment on the body composition measurement using DEXA, and insulin sensitivity and β-cell function using minimal model analysis over 12-month follow-up period in morbidly obese T2DM patients. There are several limitations of this study. Our study is a non-randomized study and prone to inherent biases, but it offers a practical means of answering important clinical questions pertaining to bariatric surgery (i.e. long-term changes in insulin secretion and insulin sensitivity). We had a priori clinical question, collected all information in identical fashion from both groups, and only drew specific conclusions based on the data. While a RCT would have been ideal, it poses several practical challenges in particular to the amount of funding to cover the cost of surgical procedure, DSE program, T2DM medications and follow-ups. Secondly, the lack of information on the gut hormones precludes us from examining the factors that might impact on the changes in insulin sensitivity and β-cell function. However, the difference in the temporal changes in these glucostatic parameters disappeared with adjustment to difference in weight loss indicates that it is likely weight loss is the key determinant in the improvement of these glucostatic parameters. We did not report on the duration of diabetes which might impact on the prospect of improvement in the glucostatic parameters after intervention. However, we showed that pre-operative insulin users improved comparably to non-insulin users in regards to insulin sensitivity, AIRg and body composition. Finally, we reported the derivative disposition index from IVGTT which has been used widely to account for the changes in insulin secretion in response to insulin resistance in maintaining normoglycemia. However, assessment of insulin secretion in humans is more complex as pancreatic β-cell function is related to β-cell mass, β-cell sensitivity to glucose, integrity of entero-insular axis (incretin response to nutrient or neural stimulation to β-cell) and hepatic insulin extraction29. Thus, IVGTT is insufficient to characterize β-cell function satisfactorily. Furthermore, acute insulin response also reflects hepatic insulin extraction29, and by using plasma C-peptide instead of plasma insulin levels in the minimal model would have minimized the issue of hepatic insulin extraction.
In summary, our study and other investigators suggest that clinical remission in morbidly obese patients with T2DM is achievable with RYGB surgery and weight loss. A marked reduction in adiposity decreases glucogenic, lipogenic and inflammatory stresses that result in a marked improvement in insulin secretion and insulin sensitivity. However, in morbidly obese T2DM patients who elect standard diabetes care, we observed further deterioration in the glucostatic parameters and glucose homeostasis over 12-month follow-up, despite minimal changes in body composition. In addition, pre-operative insulin users improved to similar extent as non-insulin users in regard to body composition, glucose homeostasis and glucostatic parameters following RYGB surgery and weight loss. Further study is required to ascertain if these changes are sustainable for longer follow-up period.
Supplementary Material
Supplementary Figure S1. Mean (SE) changes in waist circumference, percent trunk fat, fat-free mass, HbA1c, free fatty acids and hsCRP from baseline to 12-month follow-up. *p<0.05 is for between-group differences at the indicated follow-up time-point. Interaction terms “interventions*follow-up period” were significant for waist circumference (p<0.001), percent trunk fat (p<0.001) and HbA1c (p=0.006) but did not reach statistical significance for fat-free mass (p=0.354). ■ RYGB surgery • Diabetes Support and Education
Supplemental Figure S2. Mean changes in the plasma glucose during frequently sampled intravenous glucose tolerance test from baseline to 12-month follow-up. The changes in the plasma glucose was significant for follow-up period in the RYGB group only (p<0.001).
We prospectively studied longitudinal changes of glucostatic parameters in morbidly obese patients with T2DM undergoing RYGB surgery or Diabetes Support and Education (DSE). At 12-month follow-up, significant improvement in obesity measures, body composition, glucose homeostasis, Si and AIR were observed following RYGB surgery but not after DSE.
Acknowledgements
This study was supported by NIH Grant K23 DK075907 to AT.
The authors would like to thank all of the volunteer participants and the study clinical coordinators (Dr Xiong Yuan, Dr Ning Jiang and Joan Kaiser RN).
Footnotes
Authors’ contributions CMK wrote the manuscript and performed statistical analysis of the data. ZP and JC collected the data and reviewed the manuscript. AT designed and implemented the study, reviewed and edited the manuscript.
The authors declare that there is no duality of interest associated with this manuscript.
Editorial assistance Nil
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999-2008. Jama. 303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Rogers RG, Hummer RA, Krueger PM. The effect of obesity on overall, circulatory disease- and diabetes-specific mortality. J Biosoc Sci. 2003;35(1):107–29. [PubMed] [Google Scholar]
- 3.Powell MS, Fernandez AZ., Jr Surgical Treatment for Morbid Obesity: The Laparoscopic Roux-en-Y Gastric Bypass. Surg Clin North Am. 91(6):1203–24. doi: 10.1016/j.suc.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Dixon JB, Zimmet P, Alberti KG, et al. Bariatric surgery: an IDF statement for obese Type 2 diabetes. Arq Bras Endocrinol Metabol. 55(6):367–82. doi: 10.1590/s0004-27302011000600003. [DOI] [PubMed] [Google Scholar]
- 5.Lin E, Liang Z, Frediani J, et al. Improvement in ss-cell function in patients with normal and hyperglycemia following Roux-en-Y gastric bypass surgery. Am J Physiol Endocrinol Metab. 299(5):E706–12. doi: 10.1152/ajpendo.00405.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camastra S, Gastaldelli A, Mari A, et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia. 54(8):2093–102. doi: 10.1007/s00125-011-2193-6. [DOI] [PubMed] [Google Scholar]
- 7.Kadera BE, Lum K, Grant J, et al. Remission of type 2 diabetes after Roux-en-Y gastric bypass is associated with greater weight loss. Surg Obes Relat Dis. 2009;5(3):305–9. doi: 10.1016/j.soard.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Torquati A, Lutfi R, Abumrad N, et al. Is Roux-en-Y gastric bypass surgery the most effective treatment for type 2 diabetes mellitus in morbidly obese patients? J Gastrointest Surg. 2005;9(8):1112–6. doi: 10.1016/j.gassur.2005.07.016. discussion 1117-8. [DOI] [PubMed] [Google Scholar]
- 9.Unick JL, Beavers D, Jakicic JM, et al. Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes: results from the Look AHEAD trial. Diabetes Care. 34(10):2152–7. doi: 10.2337/dc11-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–7. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 11.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 366(17):1577–85. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 12.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 366(17):1567–76. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isbell JM, Tamboli RA, Hansen EN, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 33(7):1438–42. doi: 10.2337/dc09-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plum L, Ahmed L, Febres G, et al. Comparison of glucostatic parameters after hypocaloric diet or bariatric surgery and equivalent weight loss. Obesity (Silver Spring) 19(11):2149–57. doi: 10.1038/oby.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S151–6. doi: 10.2337/dc09-S301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finegood DT, Topp BG. beta-cell deterioration - prospects for reversal or prevention. Diabetes Obes Metab. 2001;3(Suppl 1):20–27. [PubMed] [Google Scholar]
- 17.Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab. 2004;89(2):463–78. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- 18.Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371(9626):1753–60. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- 19.Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14(3):281–7. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46(1):3–10. [PubMed] [Google Scholar]
- 21.Santomauro AT, Boden G, Silva ME, et al. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes. 1999;48(9):1836–41. doi: 10.2337/diabetes.48.9.1836. [DOI] [PubMed] [Google Scholar]
- 22.Visser M, Bouter LM, McQuillan GM, et al. Elevated C-reactive protein levels in overweight and obese adults. Jama. 1999;282(22):2131–5. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 23.Festa A, D’Agostino R, Jr., Howard G, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102(1):42–7. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 24.Selvin E, Paynter NP, Erlinger TP. The effect of weight loss on C-reactive protein: a systematic review. Arch Intern Med. 2007;167(1):31–9. doi: 10.1001/archinte.167.1.31. [DOI] [PubMed] [Google Scholar]
- 25.Monte SV, Caruana JA, Ghanim H, et al. Reduction in endotoxemia, oxidative and inflammatory stress, and insulin resistance after Roux-en-Y gastric bypass surgery in patients with morbid obesity and type 2 diabetes mellitus. Surgery. doi: 10.1016/j.surg.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 26.Funnell MM, Brown TL, Childs BP, et al. National standards for diabetes self-management education. Diabetes Care. 2009;32(Suppl 1):S87–94. doi: 10.2337/dc08-S087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes. 1995;44(11):1249–58. [PubMed] [Google Scholar]
- 29.Festa A, Williams K, D’Agostino R, Jr., et al. The natural course of beta-cell function in nondiabetic and diabetic individuals: the Insulin Resistance Atherosclerosis Study. Diabetes. 2006;55(4):1114–20. doi: 10.2337/diabetes.55.04.06.db05-1100. [DOI] [PubMed] [Google Scholar]
- 30.Nannipieri M, Mari A, Anselmino M, et al. The role of beta-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery. J Clin Endocrinol Metab. 96(9):E1372–9. doi: 10.1210/jc.2011-0446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Mean (SE) changes in waist circumference, percent trunk fat, fat-free mass, HbA1c, free fatty acids and hsCRP from baseline to 12-month follow-up. *p<0.05 is for between-group differences at the indicated follow-up time-point. Interaction terms “interventions*follow-up period” were significant for waist circumference (p<0.001), percent trunk fat (p<0.001) and HbA1c (p=0.006) but did not reach statistical significance for fat-free mass (p=0.354). ■ RYGB surgery • Diabetes Support and Education
Supplemental Figure S2. Mean changes in the plasma glucose during frequently sampled intravenous glucose tolerance test from baseline to 12-month follow-up. The changes in the plasma glucose was significant for follow-up period in the RYGB group only (p<0.001).