Abstract
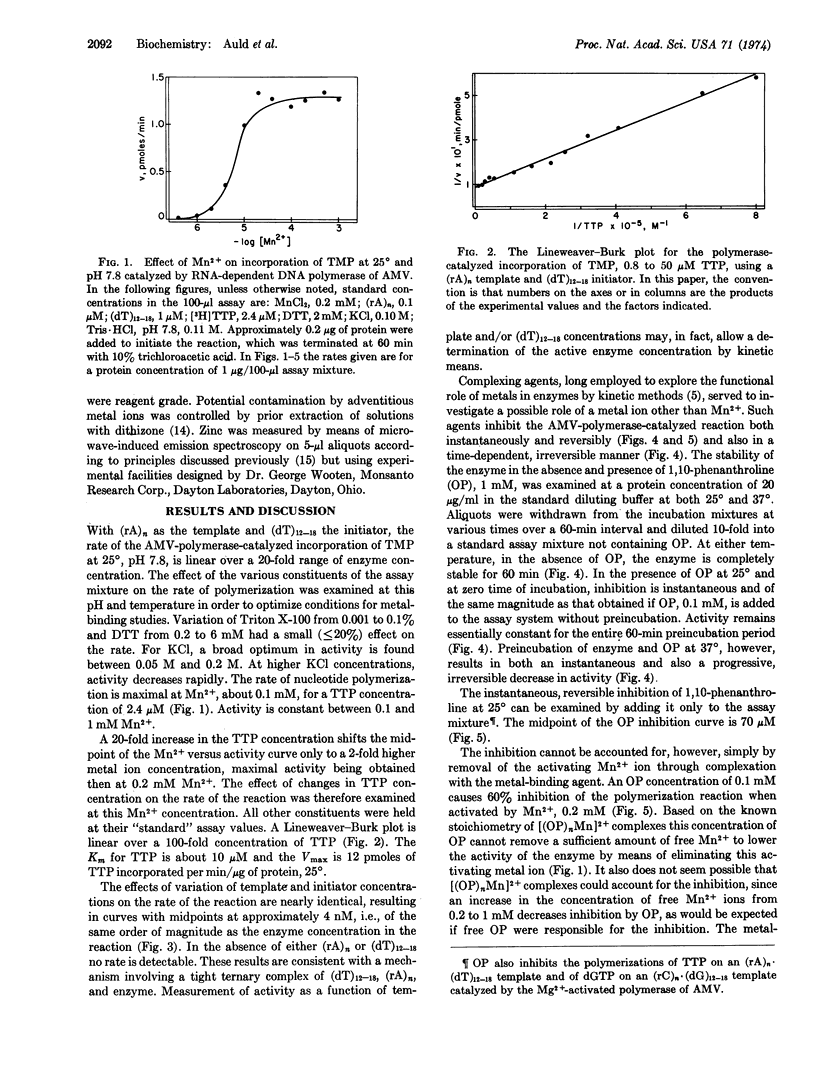
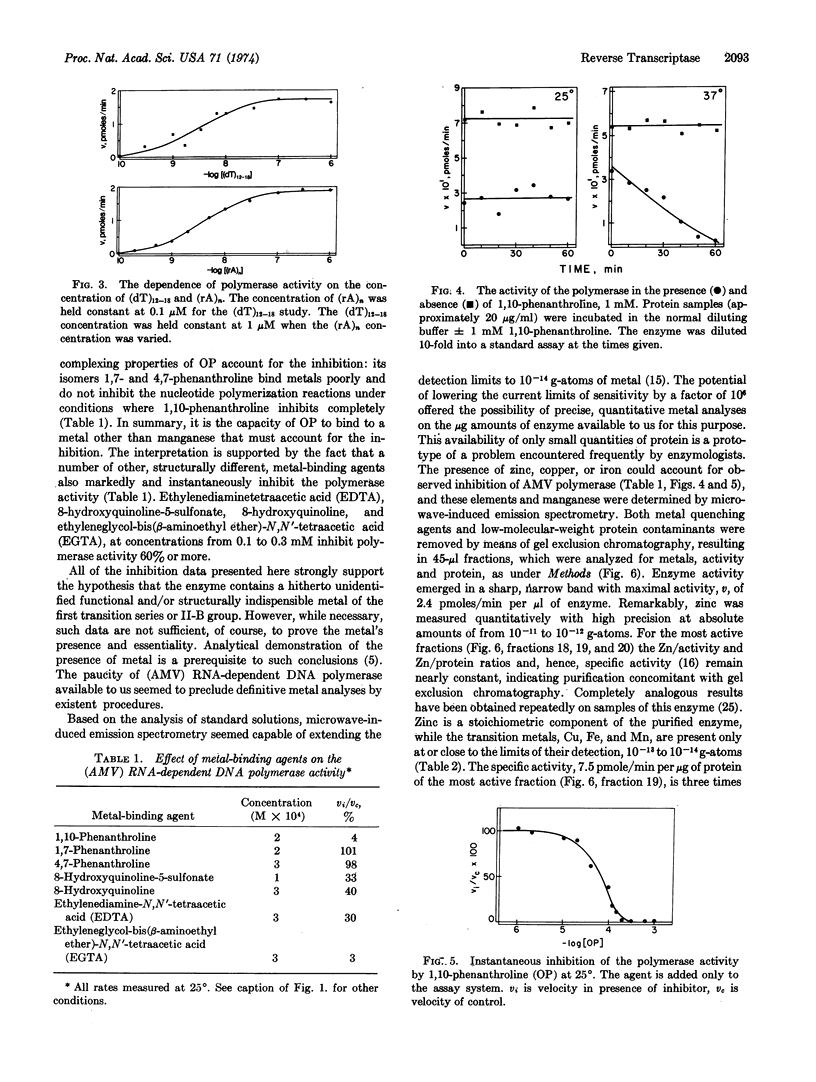
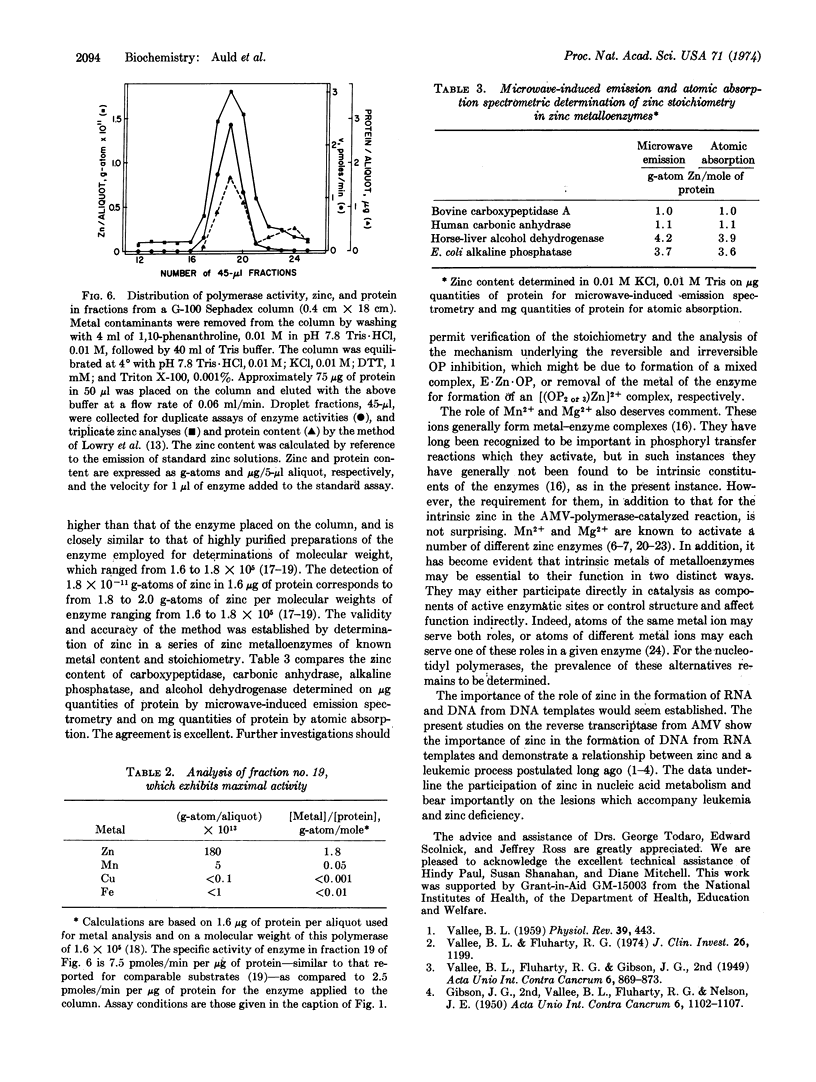
RNA tumor viruses contain a characteristic RNA-dependent DNA polymerase (reverse transcriptase) which has been thought to be related to the induction of leukemia by this virus. A disturbance in a zinc-dependent enzyme system was first postulated to account for the demonstrated differences in zinc metabolism of normal and leukemic leukocytes [Vallee et al. in (1949) Acta Unio. Int. Contra Cancrum 6, 869 and (1950) Acta Unio. Int. Contra Cancrum 6, 1102]. In order to investigate the relationship between zinc and the initiation of leukemia in chickens by avian myeloblastosis virus, we have examined the metalloenzyme nature of its reverse transcriptase. The present data show that this protein is a zinc metalloenzyme demonstrating the postulated relationship between zinc and a leukemic process. Paucity of purified enzyme generated the design of a novel system of analysis incorporating microwave-induced emission spectrometry combined with gel exclusion chromatography. It provides precision, reproducibility, and remarkable limits of detection on μl samples containing 10-12 to 10-14 g-atoms of metal, and is thus orders of magnitude more sensitive than other methods. The chromatographic fraction with highest enzymatic activity contains 1.8 × 10-11 g-atoms of zinc per 1.6 μg of protein, corresponding to either 1.8 or 2.0 g-atoms of zinc per mole of enzyme for a molecular weight previously determined either as 1.6 or 1.8 × 105. Copper, iron and manganese are absent, i.e., at or below the limits of detection, 10-13 to 10-14 g-atoms. Agents known to chelate zinc inhibit the enzyme, while their nonchelating isomers do not. The data underline the participation of zinc in nucleic acid metabolism and bear importantly upon the lesions that accompany leukemia and zinc deficiency.
Keywords: microwave excitation spectrometry, zinc metabolism, leukemia
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auld D. S., Kawaguchi H., Livingston D. M., Vallee B. L. Reverse transcriptase from avian myeloblastosis virus: a zinc metalloenzyme. Biochem Biophys Res Commun. 1974 Apr 23;57(4):967–972. doi: 10.1016/0006-291x(74)90790-6. [DOI] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. Deoxyribo ucleic acid-directed synthesis of ribonucleic acid by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1962 Jan 15;48:81–94. doi: 10.1073/pnas.48.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter F. H., Vahl J. M. Leucine aminopeptidase (Bovine lens). Mechanism of activation by Mg 2+ and Mn 2+ of the zinc metalloenzyme, amino acid composition, and sulfhydryl content. J Biol Chem. 1973 Jan 10;248(1):294–304. [PubMed] [Google Scholar]
- Englund P. T., Huberman J. A., Jovin T. M., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXX. Binding of triphosphates to deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):3038–3044. [PubMed] [Google Scholar]
- Fuwa K., Wacker W. E., Druyan R., Bartholomay A. F., Vallee B. L. NUCLEIC ACIDS AND METALS, II: TRANSITION METALS AS DETERMINANTS OF THE CONFORMATION OF RIBONUCLEIC ACIDS. Proc Natl Acad Sci U S A. 1960 Oct;46(10):1298–1307. doi: 10.1073/pnas.46.10.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgenett D. P., Gerard G. F., Green M. A single subunit from avian myeloblastosis virus with both RNA-directed DNA polymerase and ribonuclease H activity. Proc Natl Acad Sci U S A. 1973 Jan;70(1):230–234. doi: 10.1073/pnas.70.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelhoch S. Leucine aminopeptidase: a zinc metloenzyme. Arch Biochem Biophys. 1969 Nov;134(2):597–602. doi: 10.1016/0003-9861(69)90322-1. [DOI] [PubMed] [Google Scholar]
- Hurwitz J., Leis J. P. RNA-dependent DNA polymerase activity of RNA tumor viruses. I. Directing influence of DNA in the reaction. J Virol. 1972 Jan;9(1):116–129. doi: 10.1128/jvi.9.1.116-129.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacian D. L., Watson K. F., Burny A., Spiegelman S. Purification of the DNA polymerase of avian myeloblastosis virus. Biochim Biophys Acta. 1971 Sep 24;246(3):365–383. doi: 10.1016/0005-2787(71)90773-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ross J., Scolnick E. M., Todaro G. J., Aaronson S. A. Separation of murine cellular and murine leukaemia virus DNA polymerases. Nat New Biol. 1971 Jun 9;231(23):163–167. doi: 10.1038/newbio231163a0. [DOI] [PubMed] [Google Scholar]
- Scrutton M. C., Wu C. W., Goldthwait D. A. The presence and possible role of zinc in RNA polymerase obtained from Escherichia coli. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2497–2501. doi: 10.1073/pnas.68.10.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater J. P., Mildvan A. S., Loeb L. A. Zinc in DNA polymerases. Biochem Biophys Res Commun. 1971 Jul 2;44(1):37–43. doi: 10.1016/s0006-291x(71)80155-9. [DOI] [PubMed] [Google Scholar]
- THIERS R. E. Contamination in trace element analysis and its control. Methods Biochem Anal. 1957;5:273–335. doi: 10.1002/9780470110218.ch6. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- VALLEE B. L. Biochemistry, physiology and pathology of zinc. Physiol Rev. 1959 Jul;39(3):443–490. doi: 10.1152/physrev.1959.39.3.443. [DOI] [PubMed] [Google Scholar]



