Abstract
Posttraumatic stress disorder (PTSD), a pathologic response to severe stress, is a common comorbid disorder in substance dependent individuals. Evidence from twin studies suggests PTSD is moderately heritable. Genetic association studies to date have reported a limited number of replicated findings. We conducted a candidate gene association study in trauma-exposed individuals within the Comorbidity and Trauma Study’s sample (1343 heroin dependent cases and 406 controls from economically-disadvantaged neighborhoods). After data cleaning, the 1430 SNPs retained for analyses provided coverage of 72 candidate genes and included additional SNPs for which association was previously reported as well as 30 ancestry informative markers. We found a functional DRD2 promoter polymorphism (rs12364283) to be most highly associated with PTSD liability [OR 1.65 (1.27–2.15); p= 1.58 × 10−4]; however, this association was not significant with a stringent Bonferroni correction for multiple comparisons. The top hits include SNPs from other dopaminergic system genes: DRD2 DRD3, TH, and DBH. Additional analyses revealed the association involving rs12364283 is largely limited to amphetamine dependent individuals. Substantial risk is observed in amphetamine dependent individuals with at least one copy of this SNP [OR 2.86 (1.92–4.27); p=2.6 × 10−7]. Further analyses do not support extensive mediation of PTSD risk via self-reported impulsivity (BIS total score). These findings suggest roles for impairment in inhibitory control in the pathophysiology of PTSD and raise questions about stimulant use in certain populations (e.g., those in combat).
Keywords: amphetamine dependence, association study, DRD2, PTSD
INTRODUCTION
Posttraumatic stress disorder (PTSD) is a severe and persistent pathologic response to trauma that occurs in some, but not all, exposed individuals. Twin studies (True et al., 1993; Sartor et al., 2011; Sartor et al., 2012) have consistently found PTSD to be moderately heritable (e.g., h2=0.46) (Sartor et al., 2012). Genetic factors thus likely play an important role in determining susceptibility among those who have experienced trauma.
Attempts to identify genes contributing to liability for PTSD are complicated by the necessity of trauma exposure for the disorder’s symptoms to manifest. Most genetic studies have focused on populations who have experienced specific forms of trauma [e.g., child abuse (Binder et al., 2008), combat (Lawford et al., 2006; Voisey et al., 2009)]. The gene with the strongest evidence reported to date of association with PTSD is FKBP5. Several reports have found evidence of gene-environment interactions involving FKBP5 SNPs and history of childhood abuse (Binder et al., 2008; Xie et al., 2010). Other investigations have found that lower expression of this gene, either measured before (van Zuiden et al., 2012) or after (Yehuda et al., 2009; Mehta et al., 2011) trauma exposure, is associated with greater PTSD risk. The results of association studies of PTSD that examined other genes have been more equivocal [e.g., SLC6A4 (Xie et al., 2009; Goenjian et al., 2012), DRD2 (Gelernter et al., 1999; Lawford et al., 2006; Voisey et al., 2009; Bailey et al., 2010)] or, in some cases, consistently negative [e.g., NPY (Lappalainen et al., 2002)].
The current report examines the association of single nucleotide polymorphisms (SNPs) with PTSD in the Comorbidity and Trauma Study (CATS), a case-control genetic association study of heroin dependence (Maloney et al., 2009; Shand et al., 2010; Nelson et al., 2012; Nelson et al., 2013,). The study’s cases [heroin dependent individuals ascertained from opioid replacement therapy (ORT) clinics] and controls (ascertained from economically-disadvantaged neighborhoods in proximity to the clinics) both report high prevalence of childhood and adult trauma exposure and lifetime PTSD. Association studies of PTSD have not commonly focused on drug dependent samples. Investigations (Driessen et al., 2008; Villagonzalo et al., 2011) in samples of alcohol and drug dependent individuals have observed very high rates of trauma exposure and PTSD including one report (Villagonzalo et al., 2011) that found 52.7% of methadone maintenance patients screened positive for PTSD. Conversely, in a recent study (Seal et al., 2012) of U.S. veterans of the Iraq and Afghanistan conflicts, those with PTSD were more likely to be prescribed opioids and to display behaviors suggestive of opioid misuse (i.e., prescriptions for multiple opioids; early refill requests).
Our investigation initially examined association for 1430 SNPs, observing the strongest association with rs12364283, a functional D2 dopamine receptor gene (DRD2) promoter polymorphism (Zhang et al., 2007) that had not been examined previously in this context. Prior studies have observed that the rs12364283 minor (G) allele significantly impacts performance in behavioral tasks pertinent to PTSD (Frank and Hutchison, 2009; Hamidovic et al., 2009). One report (Hamidovic et al., 2009) observed that administration of amphetamines worsened the performance of rs12364283 minor (G) allele carriers on a behavioral inhibition task while improving the performance of non-carriers. We thus conducted additional analyses to examine whether greater risk is observed with the rs12364283 minor (G) allele in amphetamine dependent individuals.
MATERIALS AND METHODS
The methods for CATS have been described in detail elsewhere (Maloney et al., 2009; Shand et al., 2010; Nelson et al., 2012; Nelson et al., 2013). A brief summary is provided below.
Sample
Cases were recruited from opioid replacement therapy (ORT) clinics in the greater Sydney region and required to be age 18 or older, understand English, and have participated in ORT for opioid dependence. Those who reported recent suicidal intent or current psychosis were excluded. Individuals recruited from geographic areas in proximity to ORT clinics, termed “neighborhood controls”, were excluded for having used opioids recreationally more than five times over their lifetime (data are included from 23 controls who denied this level of opioid use at screening, but reported greater use with no dependence symptoms at interview). All other inclusion and exclusion criteria were identical to cases. Institutional review board (IRB) approval was obtained from University of New South Wales, Washington University School of Medicine, Queensland Institute of Medical Research, and all New South Wales area health service ethics committees governing participating clinics. Data were collected between 2004 and 2008. Participants provided written informed consent and were reimbursed AU$50.00. Data reported here include only those individuals (N=1749) who endorsed at least one form of trauma exposure in the interview’s PTSD section; those denying all assessed forms of trauma exposure were skipped out of the section with a PTSD diagnosis coded as missing. The sample for the current report includes 1343 heroin dependent cases [61.1% male; mean age 36.6 (SD 8.5)] and 406 neighborhood controls [47.5% male; mean age 35.5 (SD 10.5)].
Assessment
All interviews were conducted in person by experienced trained interviewers. Diagnostic sections on illicit drug and alcohol dependence, and major depressive disorder were modified from the Semi-Structured Assessment for the Genetics of Alcoholism - Australia (SSAGA-OZ) (Bucholz et al., 1994; Hesselbrock et al., 1999). The assessment of lifetime DSM-IV PTSD was modified from the National Comorbidity Survey (NCS) (Kessler et al., 1995) interview which itself was derived from the Revised Diagnostic Interview Schedule (Breslau et al., 1991). The NCS assessment, for which excellent psychometric properties have been reported (Kessler et al., 1995), first asks respondents whether they had ever experienced a series of traumatic events. Respondents are then asked which event was most disturbing and the assessment of lifetime PTSD focuses on the identified event. Additional non-diagnostic sections of the interview included SSAGA-OZ demographics and suicidal thoughts and behavior sections and a screening instrument for borderline personality, adopted from the International Personality Disorder Examination (IPDE) (Loranger et al., 1994). The Barratt Impulsiveness Scale (BIS), a 30-item self-report questionnaire (Patton et al., 1995; Maloney et al., 2009), was added to the assessment protocol after data collection had begun and is thus only available on a subset of participants (N=1315).
Marker selection
The pair-wise option of Tagger (de Bakker et al., 2005) (implemented in Haploview: Barrett et al., 2005) with a threshold of r2≥0.8 for most genes was used to select a custom set of 1536 SNPs. The set, selected on the basis of relevance for heroin dependence, provided coverage of 72 candidate genes (see Supplementary Table 1), 47 additional SNPs for which association was previously reported, and 30 ancestry-informative markers (AIMs). The set of 30 ancestry-informative markers (AIMs), distributed physically across the genome, was selected from SNPs for which the greatest allele frequency differences were found between populations with European and East Asian ancestry in Hapmap2 data for use in principal components analyses (AIMs are indicated as such in Supplementary Table 2).
Genotyping
Genotyping was performed on an Illumina BeadStation using GoldenGate technology (Peters et al., 2008). DNA samples from CEPH trio 1334 obtained from the Coriell Cell Repository served as internal quality controls for clustering and reproducibility. Primary genotypic data analyses with Illumina BeadStudio software were followed by visual inspection and assessment of data quality and clustering.
Statistical Analyses
Data Cleaning
Details of data cleaning have been reported previously (Nelson et al., 2012; Nelson et al., 2013). In brief, SNPs were excluded due to genotyping failure ( 23), call rate less than 95% (9), minor allele frequency less than 2% (47), and Hardy-Weinberg deviations (27). The mean call rate for SNPs remaining after data cleaning was 99.9%. All 1430 SNPs (shown in Supplementary Table 2) that remained after data cleaning were examined for association with PTSD. Data from samples were excluded due to phenotypic-genotypic gender mismatch, duplication due to participation in the project multiple times, and cryptic relatedness with identity by descent greater than 0.5.
Admixture
Principal components analysis (PCA) was conducted using the smartpca program in the Eigensoft 3.0 package (Patterson et al., 2006) to determine whether correction for admixture was necessary. The kill r2 setting of 0.8 was used to remove 307 SNPs in high LD with others in the panel for the examination of admixture. PCA using data from the remaining 1123 SNPs found that comparisons of cases to neighborhood controls did not require inclusion of principal components as covariates. The breakdown of self-reported ancestry, available for 590 individuals with PTSD, is 68.6% European, 5.1% Asian, 1.4% Aboriginal, and 24.9% mixed. The similar breakdown available for 1011 individuals without PTSD is 76.1% European, 7.0% Asian, 1.8% Aboriginal, and 15.1% mixed.
Data Analyses
All analyses were limited to trauma-exposed individuals (N=1749). The relationships between PTSD and control variables [i.e., case status (heroin dependence) and sex] and descriptive analyses characterizing separately by case status those with, and without, PTSD were performed using SAS 9.2. Association analyses with PTSD as the dependent variable, and including sex and heroin dependence case status as independent variables, were performed using logistic regression in PLINK (Purcell et al., 2007) to examine the log-additive effects of the minor allele dosage separately for each of the 1430 remaining SNPs. An adjusted significance threshold of 3.50 × 10−5 (i.e.,.05/1430 SNPs) was used for these association analyses; this value was obtained by applying a strict Bonferroni correction for multiple testing which assumes all SNPs are statistically independent measures. Dummy variables were coded to represent jointly lifetime amphetamine dependence (missing in one individual who was dropped from these analyses) and the presence of at least one rs12364283 G allele as follows: both amphetamine dependence and the rs12364283 G allele present; amphetamine dependence alone, and the rs12364283 G allele alone (those with neither amphetamine dependence nor a copy of the rs12364283 G allele served as the comparison group). Post-hoc analyses that included sex and heroin dependence case status as independent (control) variables were performed in SAS 9.2 to examine risk for PTSD (dependent variable) associated with these dummy variables. These analyses were repeated with inclusion of BIS total score as a covariate to determine whether self-reported impulsivity mediated these relationships in the sub-sample (N=1315) who had this measure available.
RESULTS
As expected, PTSD is more prevalent in heroin dependent cases than controls [41.0% vs. 24.6%; OR 2.13 (1.66–2.74)]. Controlling for case status, greater risk for PTSD is observed in women than men [OR 2.30 (1.88–2.82)]. Lifetime substance dependence and depression diagnoses were generally more common in both cases and controls with PTSD than those without the disorder (Table 1). Cases and controls with PTSD had higher total BIS scores than their counterparts without PTSD.
Table 1.
Characteristics of those with, and without, PTSD shown separately by case status
| Descriptive variables | Heroin Dependent Cases | Socially-disadvantaged controls | ||
|---|---|---|---|---|
| PTSD + N=551 |
PTSD − N=792 |
PTSD + N=100 |
PTSD − N=306 |
|
| Male | 49.0% | 69.6% | 35.0% | 51.6% |
| Mean age (SD) | 35.7 (8.2) | 37.2 (8.7) | 36.5 (10.3) | 35.2 (10.6) |
| Major depressive disorder | 75.6% | 51.9% | 79.0% | 45.9% |
| Amphetamine dependence | 57.6% | 48.0% | 16.0% | 16.3% |
| Cannabis dependence | 63.3% | 51.3% | 39.0% | 29.1% |
| Sedative dependence | 46.3% | 32.3% | 2.0% | 1.0% |
| Cocaine dependence | 37.6% | 29.9% | 5.0% | 2.9% |
| Alcohol dependence | 46.3% | 37.7% | 40.0% | 28.8% |
| Nicotine dependence | 73.2% | 62.1% | 50.0% | 43.5% |
| BIS total score mean (SD)* | 65.6 (9.7) | 62.7 (9.7) | 60.8 (10.4) | 58.7 (10.1) |
Data collected for 1056 cases and 259 controls
The SNP most strongly associated (Table 2) with PTSD is rs12364283 [OR 1.65 (1.27–2.15); unadjusted p= 1.58 × 10−4], a functional DRD2 promoter polymorphism. Consistent with an additive model (see Supplementary Table 3), the prevalence of PTSD increases with the number of G allele copies: 35.6% (0 copies); 45.6% (1 copy); 70.0% (2 copies). However, the relatively low minor allele frequency (7.7%) precludes definitive determination of mode of inheritance. The association observed for rs12805897 is likely due to its high linkage disequilibrium with rs12364283 (r2=.96). The only other SNP with an association within an order of magnitude of the strongest observed signal is rs10840491 located in the tyrosine hydroxylase (TH) gene. Although the associations of these additional SNPs are of considerably lower magnitude, it is interesting to note that included among them are polymorphisms from genes encoding dopamine (DA) receptors (DRD2, DRD3), an enzyme (TH) involved in DA synthesis, and an enzyme (DBH) that converts DA to norepinephrine.
Table 2.
SNPs most strongly associated (top 20 shown) with PTSD in trauma-exposed individuals (N=1749) – additive models controlling for case status and sex
| Gene | SNP | Chr | SNP Location* | MA | p Value^ | Odds Ratio |
|---|---|---|---|---|---|---|
| DRD2 | rs12364283 | 11 | 112852165 | G | .00014 | 1.66 |
| DRD2 | rs12805897 | 11 | 112829503 | A | .00029 | 1.63 |
| TH | rs10840491 | 11 | 2150966 | A | .0012 | 1.39 |
| TH | rs10840490 | 11 | 2150393 | G | .0029 | 1.35 |
| ARRB1 | rs7929974 | 11 | 74706815 | A | .0054 | 0.82 |
| ITGA6 | rs7604404 | 2 | 173016564 | A | .0063 | 0.76 |
| SLC6A3 | rs6869645 | 5 | 1457548 | GA | .0076 | 1.22 |
| DRD3 | rs6787134 | 3 | 115385439 | C | .0088 | 0.80 |
| NCAM1 | rs6589363 | 11 | 112559644 | A | .0097 | 1.25 |
| NCAM1 | rs1245119 | 11 | 112506871 | C | .011 | 1.22 |
| GABRG3 | rs12592749 | 15 | 25415996 | A | .012 | 1.39 |
| GRIN2A | rs3859123 | 16 | 9978396 | G | .014 | 1.20 |
| GRIN2A | rs7200719 | 16 | 10002047 | A | .014 | 1.35 |
| intergenic | rs265983 | 5 | 174767723 | T | .015 | 0.82 |
| GRIN2A | rs9933111 | 16 | 10072100 | G | .015 | 1.35 |
| DBH | rs2519154 | 9 | 135502096 | G | .016 | 0.84 |
| TPH1 | rs17794760 | 11 | 18012496 | A | .017 | 1.25 |
| GRIN2B | rs10845847 | 12 | 13912004 | A | .017 | 0.84 |
| DRD2 | rs4648317 | 11 | 112836742 | A | .018 | 1.26 |
| NRXN3 | rs12147956 | 14 | 79232192 | G | .019 | 0.75 |
NCBI build 37.2; Chr = chromosome; MA = minor allele;
not adjusted for multiple testing
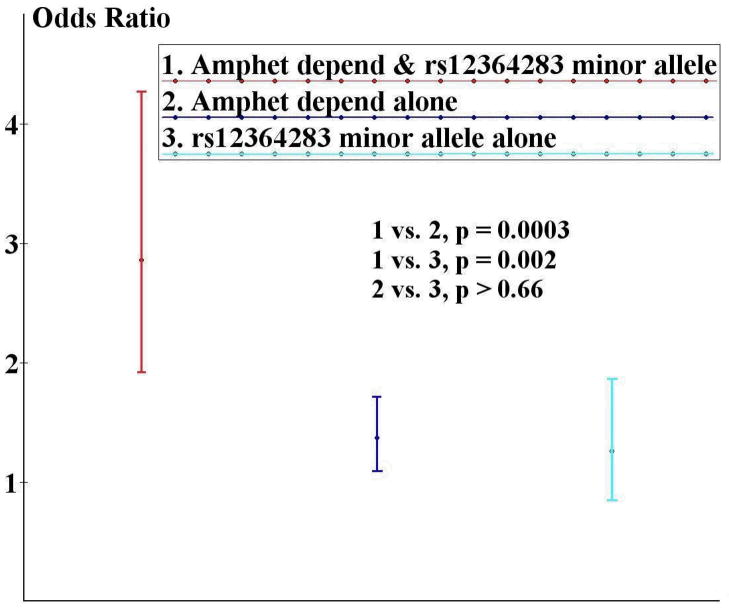
Because a prior report (Hamidovic et al., 2009) found amphetamines had differing effects on performance based on rs12364283 genotype, we conducted post-hoc analyses to examine whether differential risk is associated with this SNP based on amphetamine dependence status. We first confirmed that a higher prevalence of lifetime amphetamine dependence is found among heroin dependent cases [51.9% (N=697)] than controls [16.3% (N=66)]. We then observed significantly greater PTSD risk (Table 3) in those with one or more copy of the rs12364283 G allele who were also amphetamine dependent [OR 2.86 (1.92–4.27); p=2.6 × 10−7], than those who were either amphetamine dependent without a copy of the rs12364283 G allele or those with a copy of the rs12364283 G allele, but not amphetamine dependent (see Figure 1). Thus, in our sample, PTSD risk associated with rs12364283 occurs primarily among amphetamine dependent individuals. When these analyses were repeated in those individuals (N=1315) for whom BIS total score was available for inclusion as a covariate to control for self-reported impulsivity, results remained significant with risk only mildly reduced [OR 2.43 (1.56–3.81); p<.0001].
Table 3.
Risks for PTSD associated with amphetamine dependence and the presence of one or more rs12364283 G allele
| Independent variables | PTSD Risk N=1748 |
PTSD Risk Adjusted* N=1315 |
|---|---|---|
| Amphetamine dependence +, any rs12364283 G allele + | 2.86ab 1.92–4.27 |
2.43cd 1.56–3.81 |
| Amphetamine dependence +, any rs12364283 G allele − | 1.37a 1.09–1.72 |
1.31c 1.00–1.70 |
| Amphetamine dependence −, any rs12364283 G allele + | 1.26b 0.85–1.87 |
1.34d 0.83–2.17 |
| Heroin dependence | 2.13 1.63–2.79 |
1.94 1.39–2.70 |
| Male | 2.43 1.98–2.99 |
2.40 1.89–3.04 |
| BIS Total Score | --- | 1.02 1.01–1.04 |
Controlling for BIS total score; Difference in risks associated with indicated independent variables:
p=0.0003;
p=0.002;
p=0.006;
p=0.056
Figure 1.
Greater risk for PTSD, controlling for sex and case status, is observed for individuals with amphetamine dependence and a copy of the rs12364283 G allele (N=124) than those with either AD alone (N=639) or a copy of the rs12364283 G allele alone (N=133). The odds ratios shown are for comparison to those with neither amphetamine dependence nor a copy of the rs12364283 G allele (N=852).
DISCUSSION
We observed a functional DRD2 promoter polymorphism (rs12364283) (Zhang et al., 2007) to be the SNP most highly associated with PTSD in our sample. However, the p value for this association was not significant when a stringent Bonferroni correction for multiple comparisons was applied. Further analyses revealed that this association is largely limited to amphetamine dependent individuals. We found that controlling for self-reported impulsivity resulted in only a mild reduction in estimated risk.
Prior genetic association studies (Gelernter et al., 1999; Lawford et al., 2006; Voisey et al., 2009; Bailey et al., 2010) of PTSD have included DRD2 SNPs, but failed to examine risk associated with either rs12364283 or SNPs with which it is in high LD. These investigations (Gelernter et al., 1999; Lawford et al., 2006; Voisey et al., 2009; Bailey et al., 2010) include both positive and negative reports; however, evidence of association based on this literature is not compelling. It is important to note that prior studies also differed from the current report in that none had a sample with a high prevalence of amphetamine dependent individuals.
Prior studies offer strong evidence that rs12364283 is a functional polymorphism. Those with a copy of the minor (G) allele have significantly greater DRD2 expression (Zhang et al., 2007). Minor allele carriers have been reported to differ significantly in their performance on behavioral tasks pertinent to PTSD (Frank and Hutchison, 2009; Hamidovic et al., 2009). On a probabilistic selection reinforcement learning task, rs12364283 G allele heterozygotes demonstrated an intact ability to select a stimulus that was previously highly reinforced (Frank and Hutchison, 2009); however, they were significantly less likely to refrain from choosing a stimulus that was previously less reinforced. Some trauma exposure (e.g., natural disaster) is unavoidable; deficits in avoidance learning would likely increase risk for other forms (i.e., involving components of repeated risk-taking). Another report (Hamidovic et al., 2009) noted that young adults heterozygous for the rs12364283 G allele had lower levels of self-reported impulsivity and significantly faster stop signal reaction times on a behavioral inhibition task. Administration of d-amphetamine improved stop signal reaction times at all doses in non-carriers of the rs12364283 G allele. In contrast, the stop signal reaction times of G allele heterozygotes worsened at all doses (significantly so at the 10mg dose).
Deficits in inhibitory control have been observed using response inhibition tasks in PTSD patients (Falconer et al., 2008; Wu et al., 2010) and in methamphetamine dependent individuals (Monterosso et al., 2005; Tabibnia et al., 2011). Reductions in striatal D2/D3 receptor availability have been reported in the methamphetamine dependent (Volkow et al., 2001; Lee et al., 2009) including one report that observed a significant correlation with self-reported impulsivity (Lee et al., 2009). Animal studies (Nader et al., 2006; Dalley et al., 2007) have found that baseline D2-like receptor availability is predictive of impulsive behavior and cocaine self-administration. A recent report (Ghahremani et al., 2012) that examined behavioral inhibition in healthy controls found stop signal reaction time was significantly correlated (negatively) with D2/D3 availability in the caudate and putamen (i.e., those with greater receptor availability demonstrated better response inhibition). Using fMRI, they also found inhibition-related activation in frontostriatal circuits to be highly correlated (positively) with D2/D3 receptor availability. Reductions in inhibition-related frontal activation have been reported in those with PTSD (Falconer et al., 2008). Consistent with these reports, Hamidovic et al.’s (2009) findings suggest that the rs12364283 G allele, while associated with better response inhibition and lower impulsivity at baseline, increases carriers’ sensitivity to amphetamine’s effects on inhibitory control and perhaps PTSD liability. The findings in amphetamine dependent individuals in our sample, which do not appear to be primarily mediated via impulsivity, may thus be viewed as the results of a naturalistic experiment that could provide insight into the pathophysiology of PTSD.
A number of limitations must be considered when interpreting our findings. First, enthusiasm must be tempered until our results are replicated in an independent sample; doing so is particularly important given that the significance of the association with PTSD in the sample as a whole failed to meet the conservative Bonferroni correction for multiple testing. Our decision to apply this threshold rather other less conservative options may be viewed as overly stringent given that a number of the SNPs are in LD and thus are not entirely independent measures. Similarly, it is possible that by opting to include case status (i.e., heroin dependence) as a covariate in the genetic analyses, we may have underestimated the association for SNPs contributing to the shared genetic variance with PTSD. In addition, post-hoc analyses confirmed the lack of a significant association of rs12364283 with either heroin or amphetamine dependence. Separate analyses of case and control data found significant effects in both groups, demonstrating that our findings were not limited to heroin dependent cases. BIS data were not available on all participants and this could have introduced some bias into analyses examining mediation. An examination of risk for missing BIS data using logistic regression found a significant effect for case status (66.1% of those missing BIS data versus 80.3% with data; OR 0.48; 95%CI 0.38–0.61), but not for PTSD. Including case status as a covariate in an examination of missing BIS data, no significant effects were found for the various +/− amphetamine dependence, +/− rs12364283 G allele status combinations. Thus, these missing data likely resulted only in reduced power which likely contributed to the comparison of risk associated with the amphetamine dependence +, any rs12364283 G allele + versus amphetamine dependence −, any rs12364283 G allele + falling just below statistical significance. Another potential limitation, population admixture, was addressed by observing that the inclusion of two principal components as covariates in analyses did not change our findings. Regardless, generalizability of our findings to samples from other regions and of differing ethnicity will need to be demonstrated.
Our findings may have important, immediate relevance. The wide-ranging prescription of stimulant medications includes their use to maintain alertness in military personnel (Gore et al., 2010), a practice reportedly more frequently employed by the United States military in recent conflicts (Friedman, 2012). In fact, a recent New York Times column (Friedman, 2012) raised the question of whether the surge in PTSD prevalence could be attributed to the increase in stimulant use. Given the decreased performance reported (Hamidovic et al., 2009) with administration of amphetamines in rs12364283 G allele carriers, and our current findings of increased PTSD risk, additional research will be necessary to determine whether this practice, and perhaps all stimulant prescriptions, are best avoided in individuals with this polymorphism.
Supplementary Material
Acknowledgments
This work was supported by the National Institute on Drug Abuse (R01 DA017305 to ECN); support was also received from the National Drug and Alcohol Research Centre and the Australian National Health and Medical Research Council (to LD). The authors would like to thank the NDARC research team (Fiona Shand, Elizabeth Conroy, Michelle Torok, Caitlin McCue, Cherie Kam, and Greg French), Anthony Caracella for his work in sample receipt and preparation, Megan Campbell for project coordination, and Sara Smith and Leanne Wallace for their efforts in sample genotyping.
Footnotes
Author Contributions
ECN, ACH, MTL, LD, NGM, and GWM were responsible for the study concept and design. AA assisted with data analysis and interpretation of findings. ECN drafted the manuscript. ACH, MTL, AA, AKH, AAT, PAFM, EM, LD, NGM, and GWM provided critical revision of the manuscript and contributed to its intellectual content. All authors critically reviewed content and approved the final version of the manuscript.
Conflicts of Interest
None of the authors have a financial or personal conflict of interest.
References
- Bailey JN, Goenjian AK, Noble EP, Walling DP, Ritchie T, Goenjian HA. PTSD and dopaminergic genes, DRD2 and DAT, in multigenerational families exposed to the Spitak earthquake. Psychiatry Res. 2010;178:507–510. doi: 10.1016/j.psychres.2010.04.043. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry. 1991;48:216–222. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bakker PI, Yelensky R, Pe’er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–1223. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- Driessen M, Schulte S, Luedecke C, Schaefer I, Sutmann F, Ohlmeier M, Kemper U, Koesters G, Chodzinski C, Schneider U, Broese T, Dette C, Havemann-Reinicke U TRAUMAB-Study Group. Trauma and PTSD in patients with alcohol, drug, or dual dependence: a multi-center study. Alcohol Clin Exp Res. 2008;32:481–488. doi: 10.1111/j.1530-0277.2007.00591.x. [DOI] [PubMed] [Google Scholar]
- Falconer E, Bryant R, Felmingham KL, Kemp AH, Gordon E, Peduto A, Olivieri G, Williams LM. The neural networks of inhibitory control in posttraumatic stress disorder. J Psychiatry Neurosci. 2008;33:413–422. [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Hutchison K. Genetic contributions to avoidance-based decisions: striatal D2 receptor polymorphisms. Neuroscience. 2009;164:131–140. doi: 10.1016/j.neuroscience.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RA. The New York Times. New York: Apr 22, 2012. Why are we drugging our soldiers? p. SR5. [Google Scholar]
- Gelernter J, Southwick S, Goodson S, Morgan A, Nagy L, Charney DS. No association between D2 dopamine receptor (DRD2) “A” system alleles, or DRD2 haplotypes, and posttraumatic stress disorder. Biol Psychiatry. 1999;45:620–625. doi: 10.1016/s0006-3223(98)00087-0. [DOI] [PubMed] [Google Scholar]
- Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, Brown AK, Monterosso JR, Aron AR, Mandelkern MA, Poldrack RA, London ED. Striatal dopamine D2/D3 receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. J Neurosci. 2012;32:7316–7324. doi: 10.1523/JNEUROSCI.4284-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goenjian AK, Bailey JN, Walling DP, Steinberg AM, Schmidt D, Dandekar U, Noble EP. Association of TPH1, TPH2, and 5HTTLPR with PTSD and depressive symptoms. J Affect Disord. 2012;140:244–252. doi: 10.1016/j.jad.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Gore RK, Webb TS, Hermes ED. Fatigue and stimulant use in military fighter aircrew during combat operations. Aviat Space Environ Med. 2010;81:719–727. doi: 10.3357/asem.2755.2010. [DOI] [PubMed] [Google Scholar]
- Hamidovic A, Dlugos A, Skol A, Palmer AA, de Wit H. Evaluation of genetic variability in the dopamine receptor D2 in relation to behavioral inhibition and impulsivity/sensation seeking: an exploratory study with d-amphetamine in healthy participants. Exp Clin Psychopharmacol. 2009;17:374–383. doi: 10.1037/a0017840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Kranzler HR, Malison R, Price LH, Dyck CV, Rosenheck RA, Cramer J, Southwick SM, Charney DS, Krystal J, Gelernter J. A functional neuropeptide Y Leu7Pro polymorphism associated with alcohol dependence in a large population sample from the United States. Arch Gen Psychiatry. 2002;59:825–831. doi: 10.1001/archpsyc.59.9.825. [DOI] [PubMed] [Google Scholar]
- Lawford BR, Young R, Noble EP, Kann B, Ritchie T. The D2 dopamine receptor (DRD2) gene is associated with co-morbid depression, anxiety and social dysfunction in untreated veterans with post-traumatic stress disorder. Eur Psychiatry. 2006;21:180–185. doi: 10.1016/j.eurpsy.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loranger AW, Sartorius N, Andreoli A, Berger P, Buchheim P, Channabasavanna SM, Coid B, Dahl A, Diekstra R, Ferguson B, Jacobsberg L, Mombour W, Pull C, Ono Y, Regier D. The International Personality Disorder Examination: The World Health Organization/Alcohol, Drug Abuse, and Mental Health Administration international pilot study of personality disorders. Arch Gen Psychiatry. 1994;51:215–224. doi: 10.1001/archpsyc.1994.03950030051005. [DOI] [PubMed] [Google Scholar]
- Maloney E, Degenhardt L, Darke S, Nelson EC. Impulsivity and borderline personality as risk factors for suicide attempts among opioid-dependent individuals. Psychiatry Res. 2009;169:16–21. doi: 10.1016/j.psychres.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Gonik M, Klengel T, Rex-Haffner M, Menke A, Rubel J, Mercer KB, Pütz B, Bradley B, Holsboer F, Ressler KJ, Müller-Myhsok B, Binder EB. Using polymorphisms in FKBP5 to define biologically distinct subtypes of posttraumatic stress disorder: evidence from endocrine and gene expression studies. Arch Gen Psychiatry. 2011;68:901–910. doi: 10.1001/archgenpsychiatry.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Nelson EC, Lynskey MT, Heath AC, Wray N, Agrawal A, Shand F, Henders AK, Wallace L, Todorov AA, Schrage AJ, Saccone NL, Madden PAF, Degenhardt L, Martin NG, Montgomery GW. ANKK1, TTC12, and NCAM1 polymorphisms are associated with heroin dependence – importance of considering drug exposure. JAMA Psychiatry. 2013 Mar;70(3):325–33. doi: 10.1001/jamapsychiatry.2013.282. doi: 10.1001/jamapsychiatry.2013.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EC, Lynskey MT, Heath AC, Wray N, Agrawal A, Shand FL, Henders AK, Wallace L, Todorov AA, Schrage AJ, Madden PAF, Degenhardt L, Martin NG, Montgomery GW. Association of OPRD1 polymorphisms with heroin dependence in a large case-control series. Addict Biol. 2012 Apr 13; doi: 10.1111/j.1369-1600.2012.00445.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2:e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Peters K, Wiltshire S, Henders AK, Dragovic M, Badcock JC, Chandler D, Howell S, Ellis C, Bouwer S, Montgomery GW, Palmer LJ, Kalaydjieva L, Jablensky A. Comprehensive analysis of tagging sequence variants in DTNBP1 shows no association with schizophrenia or with its composite neurocognitive endophenotypes. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1159–1166. doi: 10.1002/ajmg.b.30741. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Grant JD, Lynskey MT, McCutcheon VV, Waldron M, Statham DJ, Bucholz KK, Madden PAF, Heath AC, Martin NG, Nelson EC. Common heritable contributions to low-risk trauma, high-risk trauma, posttraumatic stress disorder, and major depression. Arch Gen Psychiatry. 2012;69:293–299. doi: 10.1001/archgenpsychiatry.2011.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, McCutcheon VV, Pommer NE, Nelson EC, Grant JD, Duncan AE, Waldron M, Bucholz KK, Madden PAF, Heath AC. Common genetic and environmental contributions to post-traumatic stress disorder and alcohol dependence in young women. Psychol Med. 2011;41:1497–1505. doi: 10.1017/S0033291710002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal KH, Shi Y, Cohen G, Cohen BE, Maguen S, Krebs EE, Neylan TC. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940–947. doi: 10.1001/jama.2012.234. Erratum in: JAMA 2012; 2307:2489. [DOI] [PubMed] [Google Scholar]
- Shand FL, Degenhardt L, Nelson EC, Mattick RP. Predictors of social anxiety in an opioid dependent sample and a control sample. J Anxiety Disord. 2010;24:49–54. doi: 10.1016/j.janxdis.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibnia G, Monterosso JR, Baicy K, Aron AR, Poldrack RA, Chakrapani S, Lee B, London ED. Different forms of self-control share a neurocognitive substrate. J Neurosci. 2011;31:4805–4810. doi: 10.1523/JNEUROSCI.2859-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True WR, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, Nowak J. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50:257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- van Zuiden M, Geuze E, Willemen HL, Vermetten E, Maas M, Amarouchi K, Kavelaars A, Heijnen CJ. Glucocorticoid receptor pathway components predict posttraumatic stress disorder symptom development: a prospective study. Biol Psychiatry. 2012;71:309–316. doi: 10.1016/j.biopsych.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Villagonzalo KA, Dodd S, Ng F, Mihaly S, Langbein A, Berk M. The relationship between substance use and posttraumatic stress disorder in a methadone maintenance treatment program. Compr Psychiatry. 2011;52:562–566. doi: 10.1016/j.comppsych.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Voisey J, Swagell CD, Hughes IP, Morris CP, van Daal A, Noble EP, Kann B, Heslop KA, Young RM, Lawford BR. The DRD2 gene 957C>T polymorphism is associated with posttraumatic stress disorder in war veterans. Depress Anxiety. 2009;26:28–33. doi: 10.1002/da.20517. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Wu J, Ge Y, Shi Z, Duan X, Wang L, Sun X, Zhang K. Response inhibition in adolescent earthquake survivors with and without posttraumatic stress disorder: a combined behavioral and ERP study. Neurosci Lett. 2010;486:117–121. doi: 10.1016/j.neulet.2010.07.040. [DOI] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Brady K, Weiss RD, Farrer L, Gelernter J. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Arch Gen Psychiatry. 2009;66:1201–1209. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, Gelernter J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35:1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Cai G, Golier JA, Sarapas C, Galea S, Ising M, Rein T, Schmeidler J, Müller-Myhsok B, Holsboer F, Buxbaum JD. Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biol Psychiatry. 2009;66:708–711. doi: 10.1016/j.biopsych.2009.02.034. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R, Lee MLT, Xiao T, Papp A, Wang D, Sadée W. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci U S A. 2007;104:20552–20557. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.