Abstract
Alcoholic hepatitis is a distinct clinical syndrome amongst people with chronic and active alcohol abuse with a potential for 30–40% mortality at one month amongst those with severe disease. Corticosteroids or pentoxifylline are the current pharmacological treatment options but provide only about 50% survival benefit. These agents are recommended for patients with modified discriminant function (mDF) of ≥32 or model for end-stage disease (MELD) score of ≥18. The Lille score is used to determine response to steroids. Currently, a minimum of 6 months abstinence from alcohol use is required for patients to receive a liver transplant, a requirement that cannot be met by patients with severe alcoholic hepatitis non-responsive to steroids (Lille score ≥0.45). Data are emerging on the benefit of liver transplantation in select patients with first episode of severe alcoholic hepatitis. This review also focuses on recent treatment trials in alcoholic hepatitis including liver transplantation and its associated controversies, as well as possible future targets and pharmacological treatment options for patients with alcoholic hepatitis that are being pursued through upcoming consortium studies.
Keywords: alcoholic hepatitis, apoptosis, MELD, liver injury
Alcoholic cirrhosis is the 8th most common cause of mortality in the US and the second leading cause of mortality among all gastrointestinal diseases.1 This may come as no surprise since the majority of the US population consumes alcohol, with 1 in 10 reporting “heavy” drinking (≥3 drinks/day).2 Fortunately, only a minority of these heavy drinkers develop significant liver disease.2–4 The reasons for this are unclear, although demographic and genetic factors such as gender, ethnicity, binge drinking (5 or more drinks at a time), nutrition status including obesity, coexisting liver diseases such as hepatitis C virus (HCV) infection, and patatin-like phospholipase-3 (PNPLA3) gene polymorphism clearly play a role.2,5–8 A recent study by Becker et al indicates that younger people, females, and binge drinkers are more prone to develop alcoholic hepatitis (AH).4 This clinical decision meeting should take these factors into account, rather than focusing only on a precise level of alcohol consumption. AH is a clinical syndrome amongst subjects with chronic and active alcohol abuse characterized clinically by hepatic decompensation and portal hypertension.9,10 Owing to the high mortality associated with this condition as well as the lack of adequate pharmacological treatments, increasing efforts have been directed towards developing new therapies.11 This review focuses on of the challenges related to management of AH as well as future directions in the field.
Diagnosis of AH
In the absence of confirmatory tests, eliciting an accurate history of alcohol use is one of the major challenges in diagnosing alcoholic hepatitis. In an obese patient who drinks excessively, when it is unclear whether the etiology is alcoholic steatohepatitis or nonalcoholic steatohepatitis (NASH), the Alcohol-non-alcohol index (ANI) may be used to determine the etiology. The ANI uses body mass index, mean corpuscular volume, AST/ALT ratio, and gender to determine whether alcohol is the etiology of liver disease.12 A high MCV, AST/ALT ratio >1, low BMI, and male gender favor alcohol as the etiology, and this is reflected as A positive numerical ANI score; a negative score favors NASH. The ANI calculator is available on-line (http://www.mayoclinic.org/girst/mayomodel10.html) and is helpful in convincing patients who are skeptical that the “minimal amounts” of alcohol they claim they are taking is actually excessive and harmful. Blood alcohol levels can confirm alcohol use only within the previous few hours depending on the amount and rapidity of alcohol consumption (alcohol elimination rate of about 7 g/h). Urinary ethyl glucuronide may be used to detect alcohol use over the preceding 3–4 days.13 Especially provocative is the potential to measure ethyl glucuronide in hair samples as this may correlate with alcohol use over months.14,15 An objective biospecimen based assay to detect longer term, heavy ethanol use is an unmet clinical need.
Patients with chronic and active alcohol abuse usually have some symptoms and signs within 3 weeks prior to presentation.9,10 These may be non-specific or may reflect findings of advanced liver disease.16–18 Neutrophilic leukocytosis with left shift, anemia, hyperbilirubinemia (> 5 mg/dl), elevated AST and ALT (rarely above 400 IU/l) with AST/ALT ratio of >2 are often present in AH.9,10,19 Approximately 8% of patients may have serological markers of HCV infection.11 About 7% of AH patients develop hepatorenal syndrome (HRS) with elevation of serum creatinine.20 The need for a liver biopsy to diagnose AH diagnosis is controversial.21,21 In Europe, the performance of a liver biopsy is commonplace in evaluation of AH. In the US, liver biopsy is limited to cases of diagnostic uncertainty and even then, only when medications with potential toxicity such as corticosteroids are being considered for therapy. A trans-jugular route is generally required to obtain the biopsy specimen in the presence of coagulopathy. Whereas the classical histological changes of AH are well described, recent studies indicate that specific histological findings such as ductular bilirubinostasis, pericellular fibrosis, dense neutrophilic infiltration, and mega mitochondria may indicate worse prognosis.22,23
Assessment of disease severity
Various scores have been developed with the goal of determining which patients are at high risk for mortality. All current scoring systems for evaluation of AH severity have limitations (Table 1), but the Maddrey’s modified discriminant function (mDF) score has been the most widely used.24 Inclusion of the prothrombin time expressed in seconds is a drawback of the calculation of the Maddrey DF score since the prothrombin time can vary greatly depending on the sensitivity of the thromboplastin reagent used for the test. In addition, patients with a DF less than 32 are deemed not to have severe disease, yet are at risk for mortality. The MELD score which uses INR, serum creatinine, and serum bilirubin as variables has the advantage over the Maddrey score of being able to predict probability of survival in the individual patient irrespective of the score. The AASLD guidelines recommends a MELD score >18 threshold to initiate therapy similar to other studies that suggest a score of ≥20.25 Studies have found that both MELD and mDF scores perform with similar accuracy. 26–28,29,30,31 The Glasgow Alcoholic Hepatitis Score (GAHS) and Age Bilirubin INR Creatinine (ABIC) score are limited by lack of international validation, but, may be used to further stratify patient risk.32,33,34 A Lille score (calculated from age, renal function, albumin, prothrombin time, serum bilirubin, and change of serum bilirubin at day seven) of > 0.45 after 1 week of therapy is associated with 75 % mortality at 6 months.35 In clinical practice, Maddrey DF ≥32 or MELD≥20 are used to guide initiation of treatment with steroids, and the Lille score to trigger discontinuation of steroids.9,10,25
Table 1.
Scoring systems to assess severity of alcoholic hepatitis
| Scoring system |
Variables | Severe disease |
Advantages | Limitations |
|---|---|---|---|---|
| mDF24 | 4,6 × (patient’s PT-control PT in seconds) + serum bilirubin |
32 | Simple to use, validated internationally, and guides treatment initiation |
Does not guide treatment response |
| MELD29 | SB, INR, serum creatinine Website: www.mayoclinic.org/meld/mayomodel6.html |
21 | Validated internationally | Variable cut-off across studies |
| GAHS32 | Age, BUN, WBC, SB, and INR each scored 1–3 | 9 | Simple to use and stratifies DF>32 patients for treatment |
Not validated worldwide and needs day 7 laboratory values |
| ABIC33 | Age, SB, INR, serum creatinine | 9 | Stratifies patients to low, intermediate, and high risk |
Only validated in Spain |
| Lille35 | Age, SB, serum albumin, PT, change in SB at day 7 Website: gihep.com/calculators/hepatology/lille-model |
0.45 | Identifies non-responders to steroids | Complex to use and does not guide treatment initiation |
mDF: Modified discriminant function; GAHS: Glasgow alcoholic hepatitis score; ABIC: Age Bilirubin INR Creatinine
SB: Serum bilirubin; PT: Prothrombin time; BUN: Blood urea nitrogen; INR: Institutional normalized ratio
When matched for disease severity, patients with AH and HCV infection have a worse outcome than patients with AH alone.36–38 None of the current survival models incorporate HCV as a variable. In addition, alcohol use > 120 grams/day, infection, and gastrointestinal bleeding are also associated with poor prognosis.
Supportive treatment of alcoholic hepatitis
Abstinence from alcohol
The major focus of attention is prevention of alcohol use Abstinence is the most important factor in predicting long-term outcome of patients surviving the acute AH episode.39,40 Evaluation by a team experienced in the treatment of alcoholism is recommended during the hospitalization for AH, and is helpful in increasing abstinence rates after hospital discharge.41 Tools used to maintain abstinence from alcohol include motivational interviewing and cognitive behavioral therapy. Motivational interviewing is the process of encouraging behavioral change and is aimed at lifestyle modifying. Cognitive behavioral therapy which is a structured goal-directed form of psychotherapy aimed at understanding how the thought process has contributed to unhealthy behaviors, has an important role in maintaining alcohol abstinence. In-patient therapy of alcoholism is recommended in patients with a history of withdrawal complications, in the presence of severe co-existing psychiatric disorders, and if the home situation is unstable. In-patient therapy is also recommended if out-patient therapy of alcoholism fails. Even though in-patient detoxification may be cost-effective, such intervention is usually limited by insurance coverage. The incidence of recidivism after recovery from initial AH episode ranges between 10–70%.42
Regular attendance of meetings at Alcoholics Anonymous (AA), which is a voluntary program based on a spiritual basis for recovery, is also helpful in maintaining abstinence. However, patients who have had their driving license revoked find compliance with this recommendation difficult.
The role of pharmacological agents in maintaining abstinence is unclear and not often recommended. Baclofen, a GABA antagonist, is associated with both efficacy and safety in maintaining higher abstinence rates, longer durations of abstinence, reduced hospital readmission rates, and improved liver function in patients with cirrhosis in randomized studies when compared to placebo treated patients.43,44 However, in one RCT in patients with alcoholic cirrhosis, baclofen and placebo were similar in achieving abstinence.45 Use of intense psychological intervention in both groups and the percent of heavy drinking days rather than any alcohol use as an end point may explain differences between this and other studies.71 Baclofen is not widely used in clinical practice, but may be considered in patients who are unable to comply with behavior modification based interventions. The dose of baclofen is 5 mg three times daily, and if tolerated, the dose is increased to 10 mg three times daily. Since most data on use of baclofen are in patients with alcoholic cirrhosis, studies are required to examine the benefit of baclofen use amongst survivors of acute AH with the primary end point being long-term survival or need for liver transplantation.
Supportive care and nutrition
Patients with AH and a Maddrey DF <32 are currently treated for withdrawal symptoms, and supportive care for complications of portal hypertension. Although a specific prognostic score value does not necessary dictate hospitalization, patients with severe AH as determined by presence of complications that require hospital based therapy; these include especially in the presence of infection, gastrointestinal bleeding, hepatic encephalopathy, and acute kidney injury. Future studies focused on whether specific scoring systems can assist in hospitalization and discharge management would be very topical.
The enteral route is preferred for supplementing nutrition as it is less expensive, maintains gut mucosal integrity, and consequently is associated with a decreased risk of bacterial translocation and infections. Pooled data from 5 randomized controlled trials (RCT) amongst patients with AH have shown improved nutritional status with enteral supplementation compared to standard dietary intake, but without survival benefit.46 Protein intake should not be restricted unless patients are intolerant.47
Pharmacotherapy for alcoholic hepatitis
Corticosteroids
Corticosteroids are the most widely used agents for the treatment of severe AH. However, the 13 RCT evaluating corticosteroids for treating AH reported over the past 40 years have shown mixed results.48 Meta-analysis of individual patient data from five RCT using corticosteroids for severe AH showed an approximately 50% relative survival benefit at 1 month (65% vs. 85% survival amongst untreated vs. treated) with the number needed to treat being 5 patients to reduce one death.49 Despite this documented benefit, practice surveys have shown that physicians in the United States prefer pentoxifylline over steroids for managing AH; there is no consensus on how to manage AH in the presence of concomitant HCV.50,51 When steroids are used, oral prednisolone 40 mg daily or parenteral methylprednisolone (for patients unable to take orally) 32 mg per day is the usual initial dose and is administered for 4 weeks. The steroid is tapered off usually over the next 4 weeks, though the ideal taper has not been studied.
A continued increase in the serum bilirubin in the 5–10 days prior to initiating steroid therapy and a Lille score > 0.45 after one week of steroid therapy are associated with worse outcome.52 However, in another study, only the Lille score but not pre-treatment increase in bilirubin was predictive of survival.53 About 25% of patients with severe AH are infected at presentation. These patients, if appropriately treated with antibiotics, have about 71% survival with steroids, similar to uninfected patients.54 About a quarter of patients develop infection after starting steroids, especially if they are non-responders to steroids (42% vs. 11%; P<0.0001).54 Therefore, discontinuation of steroids is recommended in patients who are non-responders after a week of therapy.35 HRS should be treated prior to initiating steroids as patients in whom HRS is not reversible have a poor response to corticosteroids as compared to patients with reversal of HRS (0% vs. 44%; P<0.001).20 Of note, histological changes in the liver may persist for several months after clinical and biochemical resolution of alcoholic hepatitis.
Pentoxifylline
Pentoxifylline, a phosphodiesterase inhibitor, in a dose of 400 mg three times daily for 28 days was associated with approximately 50% survival benefit in a pivotal study in 101 patients with severe AH,17 and superior to corticosteroids in another study.55 However, a meta-analyses of 5 RCT has failed to show any benefit with pentoxifylline therapy.56 One consistent finding is the beneficial effect of pentoxifylline in prevention of HRS,56,57 but the mechanism of action of pentoxifylline in AH is unclear. Since there is reduction in TNF levels, it is speculated that pentoxifylline acts by neutralizing TNF receptors or regulating cyclic nucleotide levels in mediating its beneficial effects in AH and prevention of HRS.17,57 Pentoxifylline did not show benefit when combined with steroids compared to steroids alone in pooled data from 5 RCT,58 or when used as salvage option in steroid non-responders.59 Currently, pentoxifylline remains an option when corticosteroids are contraindicated, but in many centers, this drug is first line treatment for severe AH patients.9,50,51 A large multicenter clinical trial in the UK entitled Steroids or Pentoxifylline for AH (www.stopah.com) which proposes to recruit 1200 patients (300 each in 4 arms: corticosteroids, pentoxifylline, corticosteroids and pentoxifylline, and no treatment) is currently in progress and results of this study will hopefully determine the status of steroids and pentoxifylline in the management of severe AH.
Tumor necrosis factor-α (TNF-α) inhibitors
Because TNF-α is thought to be a major player in the pathogenesis of AH, two anti-TNF-α agents, infliximab and etanercept were evaluated for treatment of AH, but, did not show benefit.60,61 In fact, the study assessing etanercept had to be closed prematurely due to increasing number of deaths in the treatment arm, mostly from superimposed infections.61 TNF-α, apart from activating inflammatory pathways, also stimulates genes for hepatocyte growth factor and regeneration. Inhibition of these pathways probably makes patient more susceptible to infections and a poor outcome.62 Hence, anti-TNF-α agents are not recommended for the treatment of AH.25
Antioxidants
Alcohol increases reactive oxygen species (ROS) and oxidative stress. Initial studies using an antioxidant cocktail of vitamin E and N-acetylcysteine (NAC) did not show any benefit.63 In a recent RCT on 174 patients with AH, use of NAC in combination with corticosteroids was found to be associated with a better patient survival only at 1 and 2 months when compared with steroids alone. Nonetheless, patients receiving adjuvant NAC died less often from infections. However, the beneficial effect of the combination may be because of the seemingly high mortality rate of 35% in the steroid treated arm as compared to the expected mortality of 15%.64 Further studies are needed before recommending routine addition of NAC to steroids in clinical practice.
Miscellaneous drugs and strategies
Oxandrolone, an anabolic steroid improves muscle strength and nutritional status. In a RCT, oxandrolone was beneficial in improving long-term but not short-term survival in patients with moderate AH.65 Nonetheless, some authors suggest this drug for corticosteroid refractory AH based on their anecdotal experience.66 Granulocytapheresis, a technique that removes up to 60% of activated granulocytes and monocytes from circulating blood, was well tolerated in a series on 6 patients with severe AH (5 of them steroid non-responders).67 Albumin dialysis using the molecular adsorbent recirculating system (MARS) improved hepatic and renal function, hemodynamics, and HVPG but without clear benefit on survival.68,69
Liver transplantation for alcoholic hepatitis
Controversy exists on use of LT for AH that relates to both medical and ethical issues.70 Based on a rationale that AH may improve with medical management and that duration of abstinence can predict abstinence post-transplant, many transplant centers require a period of 6 month abstinence prior to considering transplant in patients with AH.71 However, data on 6 month pre-transplant abstinence and risk of relapse after LT are conflicting. In a pooled analysis of 32 studies, 6 month pre-transplant abstinence did not emerge as a predictor of recidivism and other factors such as patient’s insight, social support, and comorbid psychiatric disorders were stronger predictors.72 Although, recidivism after transplantation occurs in about 60–70% cases, harmful drinking (≥2 drinks/d) is reported in only 15–20%.73 Furthermore, risk of graft loss or recurrent cirrhosis is only about 20% amongst people with harmful drinking (<5% of patients receiving LT).74 A disease complex like alcohol addiction and abuse is unlikely to be defined by a single parameter in regards to relapse, and, therefore, the 6-month rule is overly simplistic. Better insight into the behavioral aspects of this disease is needed to guide transplant policies for this disease.
With this backdrop, French investigators recently challenged the 6 month rule and demonstrated the benefit of LT in a case-control prospective study of 26 patients with severe AH who were not early responders to medical therapy. Patients were selected after very thorough psycho-social evaluation by social workers, transplant hepatologist, anesthesiologist, and transplant surgeon.75 Compared to 52 matched patients, those receiving transplant had better survival at 1 month (96% vs. 85%) and at 1 year (73% vs. 26%; P<0.05). Only 3 patients had self-reported relapse, 2 with social drinking at 45 and 366 days and a third reported harmful drinking at 965 days follow-up. There was no graft loss related to alcohol drinking.75 Additional data supporting the benefit of liver transplantation for severe AH are reported using the United Network of Organ Sharing database. In this study, 55 patients transplanted for AH compared with 165 matched patients transplanted for alcoholic cirrhosis had similar 5 years liver graft survival (85% vs. 87%; P=0.21) and patient survival (91% VS. 89%; P=0.35).76 While these studies indicate that liver transplant may be effective in a highly selected cohort of patients with AH, further studies will be required to validate and refine the criteria by which AH patients should undergo liver transplantation.
Suggested approach for management of alcoholic hepatitis
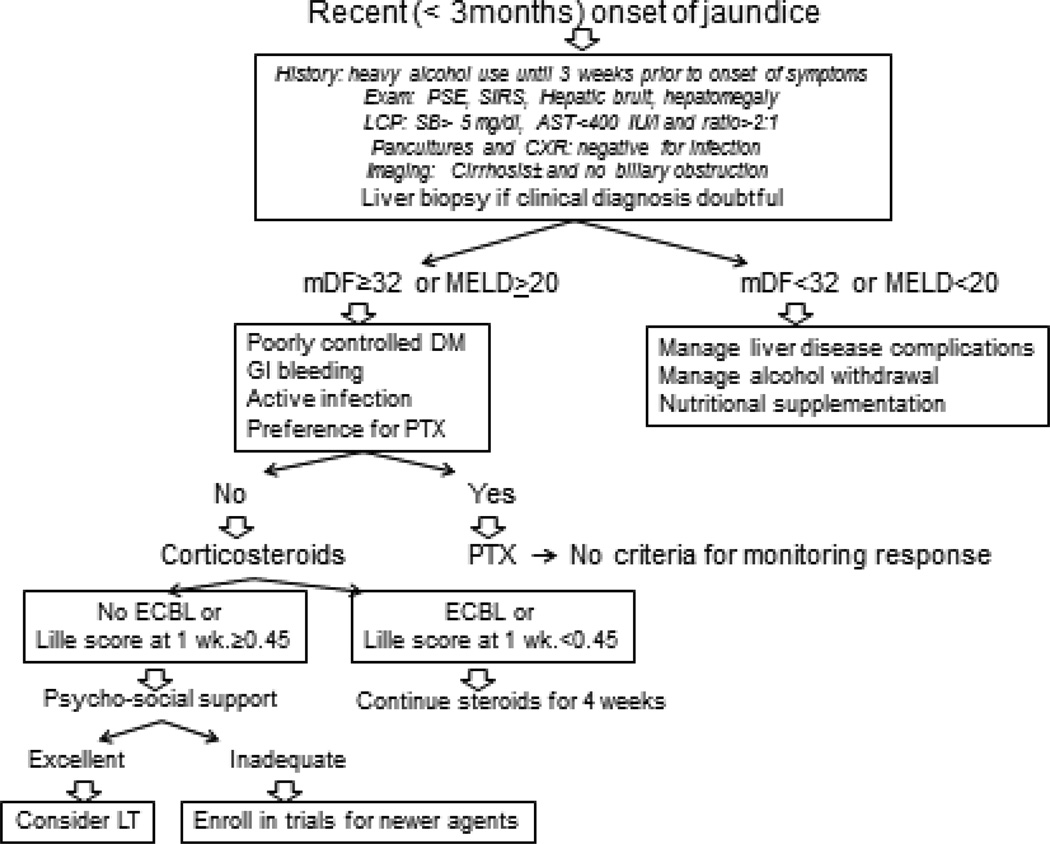
A high index of suspicion is needed for diagnosis of AH amongst patients with chronic and active alcohol abuse and recent onset of jaundice (Figure 1). Diagnosis is often made clinically and liver biopsy is recommended when clinical diagnosis is uncertain. Patients with mild disease are treated with nutritional supplementation with management of alcohol withdrawal, counseling for abstinence and supportive care for liver disease complications. Patients with severe disease (mDF>32 or MELD≥20) should be considered for pharmacotherapy in addition to supportive care. This may include corticosteroids or pentoxifylline depending on patient and provider preference, and presence of contraindications to corticosteroids (Figure 1). Corticosteroids should be discontinued at 1 week in non-responders (Lille score>0.45). These non-responders may be enrolled in clinical trials for newer targets for AH or selected patients may be evaluated for liver transplantation.
Figure 1.
Suggested algorithm in diagnosis and management of alcoholic hepatitis. mDF: Modified discriminant function; LC P: Liver chemistry panel; CXR: Chest X-ray; DM: Diabetes mellitus; PTX: Pentoxifylline; ECBL: Early change in bilirubin level; LT: Liver transplantation
Future Directions
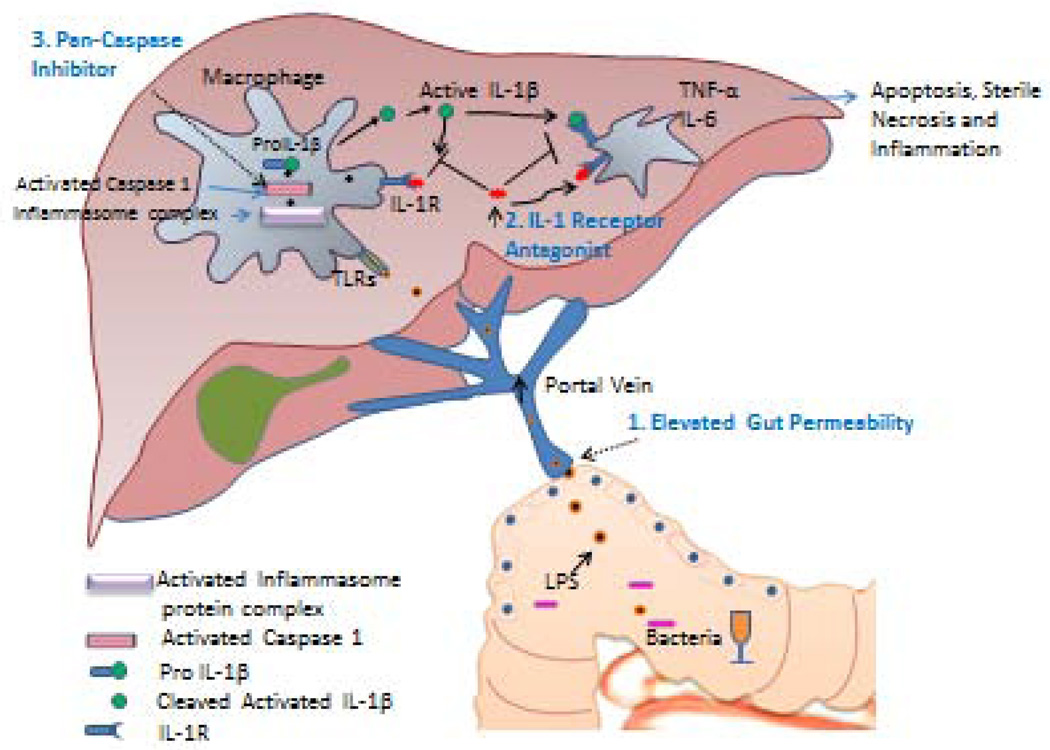
Given the current limitations and therapeutic options for patients with alcoholic hepatitis, there remains a critical need for newer, more effective agents to treat for treating this condition. Until recently, there were scarce clinical resources from government or industry aimed at identifying new therapies for AH. However, more recently, a major initiative from the National Institute on Alcohol Abuse and Alcoholism has spear-headed three large multi-institutional consortia with the task of identifying new therapeutic targets and performing early-phase clinical studies to develop and test new drugs for managing AH (http://projectreporter.nih.gov/reporter.cfm: U01 AA 021883 and U01 AA 021902) (Figure 2). Broadly, these consortia target a number of potentially important pathways in the pathogenesis of AH that fit in three pathophysiological categories: 1) disrupted gut barrier function that leads to bacterial and endotoxin translocation, 2) innate immune system activation in the liver, 3) hepatocellular apoptosis, necrosis, and injury. These are described further below.
Figure 2.
Pathogenesis of alcoholic hepatitis with identification of newer therapeutic targets. 1: Alcohol increases gut permeability and allows translocation of bacterial lipopolysaccharide which stimulates TLR-4 receptors in liver. Antibiotics, probiotics, and immunoglobulin to LPS may attenuate this response. 2: Kupffer cells stimulate inflammatory cascades including production of IL-1 which recruits white blood cells. Anikinra is an antagonist of IL-1 receptor. Macrophage inflammatory factor is another target. 3: TNF-α mediates cell death via caspase-8 (apoptosis) and caspase-1 (sterile necrosis). Caspase inhibitor Emricasan is a potential agent blocking final common pathway without affecting hepatic regeneration as seen with anti-TNF agents, corticosteroids, and other cytokine modulation. FXR agonists may also have hepatoprotective effects through multiple mechanisms. (Figure courtesy of Dr. Vikas Verma).
Intestinal permeability to gut-derived micro-organisms is increased in patients with AH (Figure 2).77 Selective intestinal decontamination with non-absorbable and systemic antibiotics such as rifaximin and norfloxacin respectively decrease plasma endotoxin levels and improve clinical outcomes in patients with alcoholic cirrhosis.78,79–81 Furthermore probiotics such as bifidobacetrium and lactobacillus restore bowel flora, improve neutrophil phagocytic activity, liver enzymes, and prognostic scores amongst alcoholic cirrhotics and patients with mild alcohol related steatohepatitis.82,83,84 With this background in mind, several consortia studies will explore this area in further depth. One will focus on anti-LPS antibody contained within bovine colostrum (Imm 124-E). The test agent will be administered in combination with corticosteroids (compared to or corticosteroids alone) in patients with severe AH. Another consortium study will examine the efficacy of probiotic therapy against placebo in patients with moderate severity AH. Yet a third consortium study will focus on additive effects of zinc, a mineral which improves gut barrier function, on a multidrug cocktail in patients with severe AH. Thus, restoration of gut barrier function and attenuation of the effects of gut endotoxin represent an important future direction of possible therapies in AH.
It is now increasingly recognized that activation of the innate immune response, especially Kupffer cells, is a key step in the process of alcohol induced liver injury. Indeed, inhibition of macrophage function is beneficial in animal models of alcoholic liver injury. The macrophage plays a key role in amplifying LPS induced liver injury since LPS activates Kupffer cells leading to increased production of IL-1β which in turn recruits other inflammatory cells. One of the compounds that will be tested in consortia trials is Anakinra which is an IL-1 receptor antagonist. Recent work showed impressive benefits of this agent in preclinical models of AH. This compound will be tested in combination with pentoxiphylline and zinc in patients with severe AH. An additional approach in consortia investigations will focus on another macrophage regulatory protein, macrophage migration inhibitory factor (MIF). Thus, targeting macrophage activation is a promising trajectory direction for future AH treatment therapies.
Approaches to attenuate ethanol induced hepatocellular injury have represented a longstanding target in AH. Indeed, recent genome-wide association studies have identified several up regulated target genes in this pathway.9,85,86,87 Major initiatives have focused especially on the TNF-α pathway however, a variety of approaches to directly inhibit the TNF-α pathway have not been successful.88,89 With this in mind a number of consortia studies will focus on alternative cell injury pathways. One study focuses on a new class of drugs that inhibit caspases. Caspases are death induction molecules which are situated downstream from TNF-α in the hepatocyte injury signaling cascade. Targeting these molecules should theoretically block alcohol induced hepatocyte injury but avoid blocking the beneficial effects of TNFα on liver regeneration and on immune cell function. Furthermore, since caspases are also important in the stimulation of the sterile necrosis response whereby injured hepatocytes macrophage and other cells recruit inflammatory cells to sites of injury, caspase inhibition has potential to dampen necroinflammation and innate immune cell activation.90 The pancaspase inhibitory compound proposed for future investigation in AH through the consortium is emricasan which will be compared to placebo in patients with severe AH who have contraindications to corticosteroid therapy. Another test compound focused on this pathway is the FXR agonist obeticholic acid. FXR activation has been shown to be potentially beneficial in a number of liver diseases including primary biliary cirrhosis. The mechanism of benefit is not certain but may relate to reduced oxidative stress and/or improved bile salt metabolism. Multiple consortia studies will test this compound in early phase clinical trials including in patients with moderate severity of AH.
Another intriguing molecule for the treatment of alcoholic hepatitis is IL-22. This cytokine is hepatoprotective and exerts potent antioxidant, antiapoptotic, and antisteatotic effects in preclinical models of liver diseases. Its use in the treatment of alcoholic hepatitis has been proposed and may be pursued in one of two additional AH consortia that are soon likely to activate.91 In summary, increasing resources are being allocated to advance management approaches for alcoholic liver disease, especially AH. It is anticipated that these initiatives will lead to treatment advances for this devastating condition in the foreseeable future.
Acknowledgments
Grant Support: NIH: AA021788 and AA021171
Abbreviations
- HCV
hepatitis C virus
- PNPLA3
patatin-like phospholipase-3
- AH
alcoholic hepatitis
- ANI
lcohol-non-alcohol index
- SIRS
systemic inflammatory response syndrome
- HRS
hepatorenal syndrome
- mDF
Maddrey’s modified discriminant function
- GAHS
Glasgow Alcoholic Hepatitis Score
- ABIC
Age Bilirubin INR Creatinine
- NAC
N-acetylcysteine
- MARS
molecular adsorbent recirculating system
- AA
Alcoholics Anonymous
- MIF
migration inhibitory factor
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Authors have no relevant conflicts to disclose
Writing Assistance: N/A
Author Contributions: All authors assisted in drafting and editing the manuscript
REFERENCES
- 1.Asrani SK, Kamath PS, Pedersen R, et al. Liver Related Mortality in the US is Underestimated. Hepatology. 2010;52:408A. [Google Scholar]
- 2.Mandayam S, Jamal MM, Morgan TR. Epidemiology of alcoholic liver disease. Seminars in liver disease. 2004;24:217–232. doi: 10.1055/s-2004-832936. [DOI] [PubMed] [Google Scholar]
- 3.Bellentani S, Saccoccio G, Costa G, et al. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut. 1997;41:845–850. doi: 10.1136/gut.41.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker U, Deis A, Sorensen TI, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23:1025–1029. doi: 10.1002/hep.510230513. [DOI] [PubMed] [Google Scholar]
- 5.Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- 6.Singal AK, Anand BS. Mechanisms of synergy between alcohol and hepatitis C virus. J Clin Gastroenterol. 2007;41:761–772. doi: 10.1097/MCG.0b013e3180381584. [DOI] [PubMed] [Google Scholar]
- 7.Trepo E, Gustot T, Degre D, et al. Common polymorphism in the PNPLA3/adiponutrin gene confers higher risk of cirrhosis and liver damage in alcoholic liver disease. J Hepatol. 2011;55:906–9012. doi: 10.1016/j.jhep.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 8.Raynard B, Balian A, Fallik D, et al. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35:635–638. doi: 10.1053/jhep.2002.31782. [DOI] [PubMed] [Google Scholar]
- 9.Singal AK, Shah VH. Alcoholic hepatitis: prognostic models and treatment. Gastroenterol Clin N Am. 2011;40:611–639. doi: 10.1016/j.gtc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 11.Singal AK, Kuo YF, Anand BS. Hepatitis C virus infection in alcoholic hepatitis: prevalence patterns and impact on in-hospital mortality. Eur J Gastroenterol Hepatol. 2012;24:1178–1184. doi: 10.1097/MEG.0b013e328355cce0. [DOI] [PubMed] [Google Scholar]
- 12.Dunn W, Angulo P, Sanderson S, et al. Utility of a new model to diagnose an alcohol basis for steatohepatitis. Gastroenterology. 2006;131:1057–1063. doi: 10.1053/j.gastro.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piano SMF, Rosi S, Cavallin M, Romano A, Gola E, Marchioro L, Sticca A, Plebani A, Gatta A, Angeli P. Urinary ethyl glucuronide improves the detection of alcohol consumption in liver transplant candidates and recipients. Hepatology. 2012;56:977A. [Google Scholar]
- 14.Sterneck MRG, Andresen G, Graeser C, Schulz K-H, Nashan B. Screening for alcohol consumption in patients after liver transplantation using determination of ethyl glucuronide in urine and hair. Hepatology. 2012;56:281A. [Google Scholar]
- 15.Sterneck MAH, Staufer K, Schulz K-H, Graeser C, Vettorazzi E, Yegles M, Nashan B. Ethyl glucuronide determination in hair for evaluation of long-term alcohol abstention in liver transplant candidates. Hepatology. 2012;56:493A. doi: 10.1111/liv.12243. [DOI] [PubMed] [Google Scholar]
- 16.Mendenhall CL. Alcoholic hepatitis. Clin Gastroenterol. 1981;10:417–441. [PubMed] [Google Scholar]
- 17.Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–1648. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 18.Lischner MW, Alexander JF, Galambos JT. Natural history of alcoholic hepatitis I. The acute disease. Am J Dig Dis. 1971;16:481–494. doi: 10.1007/BF02235538. [DOI] [PubMed] [Google Scholar]
- 19.Ceccanti M, Attili A, Balducci G, et al. Acute alcoholic hepatitis. J Clin Gastroenterol. 2006;40:833–841. doi: 10.1097/01.mcg.0000225570.04773.5d. [DOI] [PubMed] [Google Scholar]
- 20.Louvet AAF, Colin M, Lassailly G, Deltenre P, Canva-Delcambre V, Dharancy S, Mathurin P. Patients with severe alcoholic hepatitis and hepatorenal syndrome need new therapeutic strategy. Hepatology. 2012;56:986A. [Google Scholar]
- 21.Hamid R, Forrest EH. Is Histology Required for the Diagnosis of Alcoholic Hepatitis? A Review of Published Randomised Controlled Trials. Gut. 2011;60:A233–A233. [Google Scholar]
- 22.Lefkowitch JH. Morphology of alcoholic liver disease. Clin Liv Dis. 2005;9:37–53. doi: 10.1016/j.cld.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Altamirano J, Miquel R, Katoonizadeh A, et al. Development and Validation of a Novel Histological Classification with Prognostic Value for Alcoholic Hepatitis. Hepatology. 2011;54:968 A. [Google Scholar]
- 24.Maddrey WC, Boitnott JK, Bedine MS, Weber FL, Jr, Mezey E, White RI., Jr Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193–199. [PubMed] [Google Scholar]
- 25.O'Shea RS, Dasarathy S, McCullough AJ. Practice Guideline Committee of the American Association for the Study of Liver D, Practice Parameters Committee of the American College of G. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 26.Sheth M, Riggs M, Patel T. Utility of the Mayo End-Stage Liver Disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol. 2002;2:2. doi: 10.1186/1471-230X-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srikureja W, Kyulo NL, Runyon BA, Hu KQ. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol. 2005;42:700–706. doi: 10.1016/j.jhep.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 28.Zapata-Irrison L, Jurado-Nunez J, Altamirano-Gomez J. [do MELD or Maddrey?:comparison of 2 forecasting models in patients with hepatitis toxic alcohol.] Revista de Gastroenterol de Mexico. 2008;73:57–62. [PubMed] [Google Scholar]
- 29.Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41:353–358. doi: 10.1002/hep.20503. [DOI] [PubMed] [Google Scholar]
- 30.Verma S, Ajudia K, Mendler M, Redeker A. Prevalence of septic events, type 1 hepatorenal syndrome, and mortality in severe alcoholic hepatitis and utility of discriminant function and MELD score in predicting these adverse events. Dig Dis Sci. 2006;51:1637–1643. doi: 10.1007/s10620-006-9099-z. [DOI] [PubMed] [Google Scholar]
- 31.Jeong JY, Sohn JH, Son BK, et al. [Comparison of model for end-stage liver disease score with discriminant function and child-Turcotte-Pugh scores for predicting short-term mortality in Korean patients with alcoholic hepatitis] Korean J Gastroenterol. 2007;49:93–99. [PubMed] [Google Scholar]
- 32.Forrest EH, Evans CD, Stewart S, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut. 2005;54:1174–1179. doi: 10.1136/gut.2004.050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dominguez M, Rincon D, Abraldes JG, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008;103:2747–2756. doi: 10.1111/j.1572-0241.2008.02104.x. [DOI] [PubMed] [Google Scholar]
- 34.Forrest EH, Morris AJ, Stewart S, et al. The Glasgow alcoholic hepatitis score identifies patients who may benefit from corticosteroids. Gut. 2007;56:1743–1746. doi: 10.1136/gut.2006.099226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45:1348–1354. doi: 10.1002/hep.21607. [DOI] [PubMed] [Google Scholar]
- 36.Singal AK, Sagi S, Kuo YF, Weinman S. Impact of hepatitis C virus infection on the course and outcome of patients with acute alcoholic hepatitis. Eur J Gastroenterol Hepatol. 2011;23:204–209. doi: 10.1097/MEG.0b013e328343b085. [DOI] [PubMed] [Google Scholar]
- 37.Pandya PK, Rao G, Weinman SA. Impact of Hepatitis C Infection on Survival in Alcoholic Hepatitis. Hepatology. 2010;52:672a–673a. [Google Scholar]
- 38.Thuluvath PJ, Nguyen GC. Impact of Hcv and Hepatic Encephalopathy on the Outcome of Patients Admitted with Alcoholic Hepatitis. Hepatology. 2010;52:1114 A. [Google Scholar]
- 39.Potts JGSA, Heneghan MA, Verma S. Long term outcome in severe alcoholic hepatitis. Hepatology. 2012;56:985 A. doi: 10.1111/apt.12427. [DOI] [PubMed] [Google Scholar]
- 40.Altamirano J, Higuera-de la Tijera F, Duarte-Rojo A, et al. The amount of alcohol consumption negatively impacts short-term mortality in Mexican patients with alcoholic hepatitis. Am J Gastroenterol. 2011;106:1472–1480. doi: 10.1038/ajg.2011.141. [DOI] [PubMed] [Google Scholar]
- 41.Yam FGJA, Groessl EJ, Lau JW, Chen W-C, Golshan S, Kuo A, Ho SB. Quality of care and predictors of readmission in hospitalized patients with alcoholic liver disease care in two academic medical centers. Hepatology. 2012;56:947A. [Google Scholar]
- 42.Chung T, Martin CS, Winters KC. Diagnosis, course, and assessment of alcohol abuse and dependence in adolescents. Recent Dev Alcoholism. 2005;17:5–27. doi: 10.1007/0-306-48626-1_1. [DOI] [PubMed] [Google Scholar]
- 43.Addolorato G, Leggio L, Ferrulli A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370:1915–1922. doi: 10.1016/S0140-6736(07)61814-5. [DOI] [PubMed] [Google Scholar]
- 44.Heydtmann M. The GABA-B agonist Baclofen improves alcohol consumption, psychometrics and may have effect on hospital admission rates in patients with alcoholic liver disease. Hepatology. 2012;56:1932 A. [Google Scholar]
- 45.Garbutt JC, Kampov-Polevoy AB, Gallop R, Kalka-Juhl L, Flannery BA. Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind, placebo-controlled trial. Alcohol Clin Exp Res. 2010;34:1849–1857. doi: 10.1111/j.1530-0277.2010.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singal AK, Charlton MR. Nutrition in alcoholic liver disease. Clin Liv Dis. 2012;16:805–826. doi: 10.1016/j.cld.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Cordoba J, Lopez-Hellin J, Planas M, et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41:38–43. doi: 10.1016/j.jhep.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 48.Singal AK, Walia I, Singal A, Soloway RD. Corticosteroids and pentoxifylline for the treatment of alcoholic hepatitis: Current status. World J Hepatol. 2011;3:205–210. doi: 10.4254/wjh.v3.i8.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathurin P, O'Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011;60:255–260. doi: 10.1136/gut.2010.224097. [DOI] [PubMed] [Google Scholar]
- 50.O'Shea R, Kinnard MF, Umar N, Sourianarayanane A, Schraider MD, McCullough AJ. Treatment of Alcoholic Hepatitis (AH) in Clinical Practice. Gastroenterology. 2009;136:830 A. [Google Scholar]
- 51.Singal A, Singal A, Jampana S, Freeman D, Anderson K, Brunder D. Management of Alcoholic Hepatitis in the Presence of Concomitant Hepatitis C Virus Infection: A Survey of Practising Gastroenterologists and Hepatologists. American Journal of Gastroenterology. 2011;106:S109–S109. [Google Scholar]
- 52.Freyssinet MAI, Mosnier J-F, Feray C, Gournay J. Bilirubin evolution before corticosteroid has a major prognostic value in severe alcoholic hepatitis. Hepatology. 2012;56:963 A. [Google Scholar]
- 53.Louvet AAF, Colin M, Lassailly G, Deltenre P, Canva-Delcambre V, Dharancy S, Mathurin P. Spontaneous improvement of liver function is not a strong predictor of outcome in patients with severe alcoholic hepatitis treated with corticosteroids. Hepatology. 2012;56:981 A. [Google Scholar]
- 54.Louvet A, Wartel F, Castel H, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology. 2009;137:541–548. doi: 10.1053/j.gastro.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 55.De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol. 2009;15:1613–1619. doi: 10.3748/wjg.15.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whitfield K, Rambaldi A, Wetterslev J, Gluud C. Pentoxifylline for alcoholic hepatitis. Cochrane database of systematic reviews. 2009:CD007339. doi: 10.1002/14651858.CD007339.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lebrec D, Thabut D, Oberti F, et al. Pentoxifylline does not decrease short-term mortality but does reduce complications in patients with advanced cirrhosis. Gastroenterology. 2010;138:1755–1762. doi: 10.1053/j.gastro.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 58.Thiele MAG, Krag A, Gluud LL. No additional effect of adding pentoxifylline to corticosteroids in severe alcoholic hepatitis: meta-analyses of randomized controlled trials. Hepatology. 2012;56:961A. [Google Scholar]
- 59.Louvet A, Diaz E, Dharancy S, et al. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol. 2008;48:465–470. doi: 10.1016/j.jhep.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 60.Naveau S, Chollet-Martin S, Dharancy S, et al. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39:1390–1397. doi: 10.1002/hep.20206. [DOI] [PubMed] [Google Scholar]
- 61.Boetticher NC, Peine CJ, Kwo P, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953–1960. doi: 10.1053/j.gastro.2008.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akerman P, Cote P, Yang SQ, et al. Antibodies to tumor necrosis factor-alpha inhibit liver regeneration after partial hepatectomy. Am J Physiol. 1992;263:G579–G585. doi: 10.1152/ajpgi.1992.263.4.G579. [DOI] [PubMed] [Google Scholar]
- 63.Singal AK, Jampana SC, Weinman SA. Antioxidants as therapeutic agents for liver disease. Liv Int. 2011;31:1432–1448. doi: 10.1111/j.1478-3231.2011.02604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen-Khac E, Thevenot T, Piquet MA, et al. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med. 2011;365:1781–1789. doi: 10.1056/NEJMoa1101214. [DOI] [PubMed] [Google Scholar]
- 65.Mendenhall CL, Anderson S, Garcia-Pont P, et al. Short-term and long-term survival in patients with alcoholic hepatitis treated with oxandrolone and prednisolone. N Engl J Med. 1984;311:1464–1670. doi: 10.1056/NEJM198412063112302. [DOI] [PubMed] [Google Scholar]
- 66.Choi G, Runyon BA. Alcoholic hepatitis: a clinician's guide. Clin Liv Dis. 2012;16:371–385. doi: 10.1016/j.cld.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 67.Morris JM, Dickson S, Neilson M, Hodgins P, Forrest EH. Granulocytapheresis in the treatment of severe alcoholic hepatitis: a case series. EurJ Gastroenterol Hepatol. 2010;22:457–460. doi: 10.1097/MEG.0b013e328332a360. [DOI] [PubMed] [Google Scholar]
- 68.Jalan R, Sen S, Steiner C, Kapoor D, Alisa A, Williams R. Extracorporeal liver support with molecular adsorbents recirculating system in patients with severe acute alcoholic hepatitis. J Hepatol. 2003;38:24–31. doi: 10.1016/s0168-8278(02)00334-3. [DOI] [PubMed] [Google Scholar]
- 69.Sen S, Mookerjee RP, Cheshire LM, Davies NA, Williams R, Jalan R. Albumin dialysis reduces portal pressure acutely in patients with severe alcoholic hepatitis. J Hepatol. 2005;43:142–148. doi: 10.1016/j.jhep.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 70.Singal AK, Duchini A. Liver transplantation in acute alcoholic hepatitis: Current status and future development. World J Hepatol. 2011;3:215–218. doi: 10.4254/wjh.v3.i8.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vanlemmens C, Di Martino V, Milan C, et al. Immediate listing for liver transplantation versus standard care for Child-Pugh stage B alcoholic cirrhosis: a randomized trial. Ann Int Med. 2009;150:153–161. doi: 10.7326/0003-4819-150-3-200902030-00004. [DOI] [PubMed] [Google Scholar]
- 72.McCallum S, Masterton G. Liver transplantation for alcoholic liver disease: a systematic review of psychosocial selection criteria. Alcohol Alcohol. 2006;41:358–363. doi: 10.1093/alcalc/agl033. [DOI] [PubMed] [Google Scholar]
- 73.DiMartini A, Day N, Dew MA, et al. Alcohol consumption patterns and predictors of use following liver transplantation for alcoholic liver disease. Liv Transpl. 2006;12:813–820. doi: 10.1002/lt.20688. [DOI] [PubMed] [Google Scholar]
- 74.Cannesson ADJ, Pageaux G-P, Boleslawski E, Louvet A, Lassailly , Rolland B, Colin M, Canva V, Declerck N, Mathurin P, René Pruvot F, Dharancy S. Prevalence and natural history of recurrent alcoholic cirrhosis after liver transplantation. Hepatology. 2012;56:506 A. [Google Scholar]
- 75.Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365:1790–1800. doi: 10.1056/NEJMoa1105703. [DOI] [PubMed] [Google Scholar]
- 76.Singal AK, Bashar H, Anand BS, Jampana SC, Singal V, Kuo YF. Outcomes after liver transplantation for alcoholic hepatitis are similar to alcoholic cirrhosis: exploratory analysis from the UNOS database. Hepatology. 2012;55:1398–1405. doi: 10.1002/hep.25544. [DOI] [PubMed] [Google Scholar]
- 77.Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet. 1984;1:179–182. doi: 10.1016/s0140-6736(84)92109-3. [DOI] [PubMed] [Google Scholar]
- 78.Vlachogiannakos J, Saveriadis AS, Viazis N, et al. Intestinal decontamination improves liver haemodynamics in patients with alcohol-related decompensated cirrhosis. Aliment Pharmacol Ther. 2009;29:992–999. doi: 10.1111/j.1365-2036.2009.03958.x. [DOI] [PubMed] [Google Scholar]
- 79.Rasaratnam B, Kaye D, Jennings G, Dudley F, Chin-Dusting J. The effect of selective intestinal decontamination on the hyperdynamic circulatory state in cirrhosis A randomized trial. Ann Int Med. 2003;139:186–193. doi: 10.7326/0003-4819-139-3-200308050-00008. [DOI] [PubMed] [Google Scholar]
- 80.Kalambokis GN, Mouzaki A, Rodi M, Tsianos EV. Rifaximin improves thrombocytopenia in patients with alcoholic cirrhosis in association with reduction of endotoxaemia. Liv Int. 2012;32:467–475. doi: 10.1111/j.1478-3231.2011.02650.x. [DOI] [PubMed] [Google Scholar]
- 81.Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis I, Karamanolis DG, Ladas SD. Long-Term Administration of Rifaximin Improves the Prognosis of Patients with Decompensated Alcoholic Cirrhosis. J Gastroenterol Hepatol. 2013;28:450–455. doi: 10.1111/jgh.12070. [DOI] [PubMed] [Google Scholar]
- 82.Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatol. 2008;48:945–951. doi: 10.1016/j.jhep.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 83.Kirpich IA, Solovieva NV, Leikhter SN, et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675–682. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suk KSKM, Kim YD, Cheon GJ, Choi DH, Kim MY, Baik SK, Kim DJ. Effects of probiotics [cultured lactobacillus subtilis/streptococcus fecium] in the treatment of alcoholic hepatitis: a randomized controlled multicenter study. Hepatology. 2012;56:590 A. doi: 10.1097/MEG.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 85.Patouraux S, Bonnafous S, Voican CS, et al. The osteopontin level in liver, adipose tissue and serum is correlated with fibrosis in patients with alcoholic liver disease. PloS one. 2012;7:e35612. doi: 10.1371/journal.pone.0035612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tirnitz-Parker JE, Viebahn CS, Jakubowski A, et al. Tumor necrosis factor-like weak inducer of apoptosis is a mitogen for liver progenitor cells. Hepatology. 2010;52:291–302. doi: 10.1002/hep.23663. [DOI] [PubMed] [Google Scholar]
- 87.Colmenero J, Bataller R, Sancho-Bru P, et al. Hepatic expression of candidate genes in patients with alcoholic hepatitis: correlation with disease severity. Gastroenterology. 2007;132:687–697. doi: 10.1053/j.gastro.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 88.Kammuller ME. Recombinant human interleukin-6: safety issues of a pleiotropic growth factor. Toxicology. 1995;105:91–107. doi: 10.1016/0300-483x(95)03128-3. [DOI] [PubMed] [Google Scholar]
- 89.Ki SH, Park O, Zheng M, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–1172. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 91.Gao B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. J Gastroenterol Hepatol. 2012;27(Suppl 2):89–93. doi: 10.1111/j.1440-1746.2011.07003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]