Abstract
Human colorectal cancers are known to possess multiple mutations, though how these mutations interact in tumor development and progression has not been fully investigated. We have previously described the FCPIK3ca* murine colon cancer model which expresses a constitutively activated phosphoinositide-3 kinase (PI3K) in the intestinal epithelium. The expression of this dominantly active form of PI3K results in hyperplasia and invasive mucinous adenocarcinomas. These cancers form via a non-canonical mechanism of tumor initiation that is mediated through activation of PI3K and not through aberrations in WNT signaling. Since the Adenomatous Polyposis Coli (APC) gene is mutated in the vast majority of human colon cancers and often occurs simultaneously with PIK3CA mutations, we sought to better understand the interaction between APC and PIK3CA mutations in the mammalian intestine. In this study, we have generated mice in which the expression of a constitutively active PI3K and the loss of APC occur simultaneously in the distal small intestine and colon. Here we demonstrate that expression of a dominant active PI3K synergizes with loss of APC activity resulting in a dramatic changes in tumor multiplicity, size, morphology, and invasiveness. Activation of the PI3K pathway is not able to directly activate WNT signaling through the nuclear localization of CTNNB1 (β-catenin) in the absence of aberrant WNT signaling. Alterations at the transcriptional level, including increased CCND1, may be the etiology of synergy between these activated pathways.
Keywords: Colon cancer, APC, PI3K, PIK3CA, Mouse models
Introduction
Colorectal adenocarcinoma continues to be a major etiology of morbidity and mortality despite significant advances in the understanding of tumor biology and treatment options.1 The profile of mutations has now been characterized in multiple human cancers, however the role of these mutations in tumorigenesis, progression, and response to therapy has largely yet to be defined.2
APC mutations have been well characterized as important mediators of colorectal tumorigenesis.3 Germline mutations were discovered in patients with familial adenomatous polyposis (FAP), but somatic mutations are also present in 80–90% of sporadic colorectal polyps and cancers.4 These mutations lead to truncation of the APC protein resulting in the loss of function of this tumor suppressor. Loss of APC function results in dysregulation of CTNNB1 (β-catenin), leading to increased WNT pathway signaling through MYC and CCND1 (cyclin D1), among others.5 The ApcMin/+ (Min) mouse carries a germline mutation in Apc, which results in these mice developing many adenomas throughout the intestinal tract.6 This model has been widely used to study the biology of intestinal tumors and as a valuable tool for chemoprevention studies.
Genetic alterations in the PI3K signaling pathway are the most common mutations found in cancer.7 Oncogenic mutations of PIK3CA are important in multiple cancer types, including 20–30% of colorectal cancers.8 Mutations of the PIK3CA gene encode for a dominant active form of the p110α catalytic subunit of PI3K and occur in three hotspots: E454K, E456K, and H1047R.9 PIK3CA mutations have been investigated in numerous cancer cell lines; however, until recently the effect of a dominant active PI3K in the mammalian intestine had not been investigated. Our lab has developed a murine model, FCPIK3ca*, that expresses a dominant active form of PI3K (p110*) in the distal small bowel and colon.10 This model develops sessile large moderately differentiated invasive mucinous adenocarcinomas in the proximal colon through a non-canonical mechanism and without a polypoid luminal component.
APC and PIK3CA mutations are commonly identified together in human colorectal cancers, yet the interaction between these mutations remains to be investigated in the mammalian intestine.11 The potential for cross-talk between these signaling cascades has been an area of interest. These pathways converge on glycogen synthase kinase 3 (GSK3) and potentially other mediators. Prior studies in cell culture models have had contradictory findings when examining the potential for an interaction between these cascades.12–17 To examine what effect mutations in APC and PIK3CA have on tumorigenesis, we have crossed the Min mouse with the FCPIK3ca* (FC13K1) mouse. To examine the what effect mutations in APC and PIK3CA have on tumorigenesis, we have crossed the Min mouse with the FCPIK3ca* (FC13K1) mouse. This cross results in a murine model with the loss of one allele of APC throughout the intestine and the expression of a dominant active PI3K (3K1) in the distal small intestine and colon due to the expression of Cre under the control of the fatty acid binding protein-1 promoter (FC1). Here we demonstrate increased tumor multiplicity, size, and a more aggressive and poorly differentiated phenotype as a consequence of synergy between APC and PIK3CA mutations.
Results
Expression of a dominantly active PI3K in the setting of allelic loss of Apc results in increased tumor multiplicity and increased tumor size
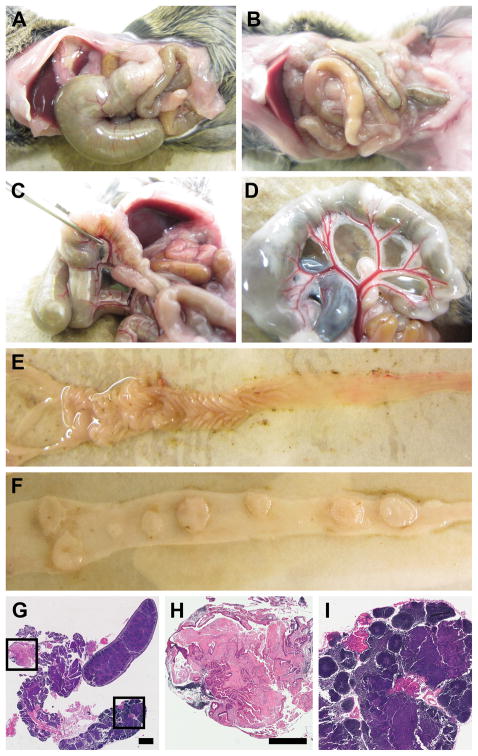
FC13K1ApcMin/+ mice were dissected once moribund with age-matched control littermates (Figure 1; 1 denotes carrier and 0 denotes non-carrier for FC and 3K; + denotes wildtype for Apc). The majority of mice developed massive proximal colon cancers resulting in obstruction of the lumen (Figure 1a). These tumors were associated with an impressive vascular supply and hyperplastic lymphatic tissue (Figures 1c and d). Tumors are seen in the distal small intestine and colon (Figures 1e and f). In some instances, metastatic lesions were noted in the mesenteric lymphatic and adipose tissue (Figures 1g, h and i). No evidence of liver or lung metastases was identified.
Figure 1.
FC13K1ApcMin/+ mice become moribund by 52 days of age on average due obstructive enteropathy or anemia. At necropsy, large proximal colon cancers result in distention of the cecum and small intestine (a) compared to control (b). These tumors are associated with enlarged mesenteric adenopathy (c). In addition, an impressive vascular supply is noted supplying tumors in the colon (c) and distal small intestine (d). The colon was removed and split lengthwise demonstrating a large flat proximal colon cancer and other smaller tumors in the colon (e). The distal small intestine was also resected and split lengthwise revealing multiple large tumors. The mesentery was also examined closely following removal of the colon and small intestine. Hyperplasia of the lymphatic tissue was seen in all mice (c and g). In some mice tumor deposits were seen in the mesenteric adipose tissue (g and h) or within the lymphatic tissue (g and i). Size bars: g = 2mm; h–i = 1mm. h and i are enlargements of the boxes in g.
A total of 61 mice were evaluated for the presence of intestinal tumors, including 18 FC13K1ApcMin/+, 17 FC03K1ApcMin/+ mice, 6 FC13K0ApcMin/+ mice, 9 FC03K0ApcMin/+ mice, and 11 FC13K1Apc+/+ mice (Supplementary Table S1). The average age of FC13K1ApcMin/+ mice at necropsy was 52 days old. Mice appeared well until they became moribund from obstructive enteropathy relating to large obstructing colon cancers or secondary to anemia from bleeding intestinal tumors.
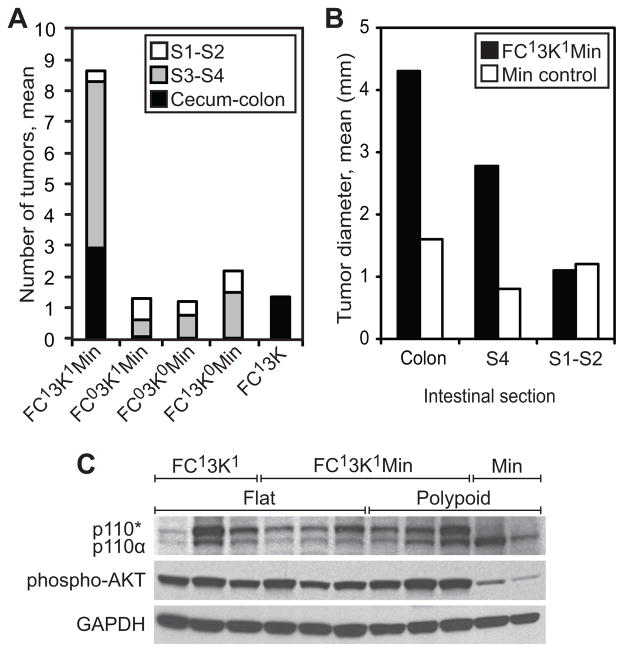
Tumor multiplicity was significantly increased in each section of the intestine where the activated PI3K was expressed in the setting of allelic loss of APC (Figure 2). Overall an average of 8.7 tumors were identified throughout the intestine in FC13K1ApcMin/+ compared to 1.4 in FC13K1Apc+/+ (p<0.001), and 1.4 in ApcMin/+ controls (FC03K1ApcMin/+, FC13K0ApcMin/+, or FC03K0ApcMin/+; p<0.001). Specifically, segment 4 and colon tumors were much more frequent in the FC13K1ApcMin/+ mice. No increase in tumor multiplicity was noted in the proximal most intestinal sections, segments 1 and 2, which lack activated PI3K. This is useful as an internal control and indicates that the increase in tumor multiplicity is related to expression of constitutively active PI3K.
Figure 2.
Activation of the PI3K pathway in the intestine results in increased tumor number and size. In the FC13K1ApcMin/+ the median number of tumors seen was 8.7 compared to the control littermates which averaged 2.2 or less (a). This effect was seen prominently in the colon, where the FC13K1ApcMin/+ mice has a mean of 2.9 tumors per mouse and only 1 colon tumors was seen in 32 ApcMin/+ control littermates (a). In the proximal small intestine no difference was noted in tumor number which is consistent with the activated form of PI3K not being expressed in this area in the FC13K1ApcMin/+ mice. An increase in tumor size was also noted in FC13K1ApcMin/+ (colon: mean 4.3mm, median 3, range 2–10mm; S4: mean 2.8mm, median 2.3mm, range 0.7–7mm) compared to ApcMin/+ (colon: 1.6mm, only one colon tumor was observed in 32 mice; S4: mean 0.8mm, median 0.5mm, range 0.4–2mm, p=0.004). No change in tumor size was noted in the proximal 2 segments of the small intestine, which is consistent with the lack of expression of the activated form of PI3K in this area. The increase in tumor number and size are related to the expression of the activated form of PI3K, p110*, in the FC13K1ApcMin/+ mice, as this can be detected in these tumors and is associated with increased phosphorylation of AKT as compared to ApcMin/+ control tumors (c).
The intestinal sections from FC13K1ApcMin/+ mice and associated controls were also evaluated for tumor size. Fixed tissues were examined under the dissecting microscope. The maximum diameter of each tumor was determined using a measuring reticule. The flat FC13K1 proximal colon tumors, as previously described,10 were not measured as these tumors do not have discrete borders, which are required for reproducible measurements.
An increase in tumor size was noted in segment 4 and the colon (Figure 2b). In segment 4 a total of 66 tumors were measured (61 FC13K1ApcMin/+ and 5 ApcMin/+ controls). The FC13K1ApcMin/+ segment 4 tumors averaged 2.8 mm (range 0.7–7; p=0.004), whereas the control tumors averaged less than 0.8 mm (range 0.4–2 mm) in diameter. These large tumors are forming by just 50 days of age or less. Tumors this large would not be expected in Min mice until closer to 100 days of age or older. A total of 9 FC13K1ApcMin/+ colon tumors were measured and the mean diameter was 4.3 mm (range 2–10 mm). Only one ApcMin/+ control colon tumor was identified in 32 mice. This tumor measured 1.6 mm. A total of 14 tumors were identified in segment 1 and 2 of the small intestine (5 FC13K1ApcMin/+ and 9 ApcMin/+ controls). These tumors were not significantly different in size owing to the lack of constitutively active PI3K in this area (1.2 and 1.1, respectively, p=0.85).
Tumor development and progression are mediated by the expression of the dominant active form of PI3K
Expression of the constitutively active PI3K in the tumors of FC13K1 mice has previously been shown to activate the PI3K signaling cascade.10 In FC13K1ApcMin/+ mice, all tumors in in the distal small and large intestine, regardless of morphology, demonstrate the presence of p110* and activation of the PI3K signaling pathway (Figures 2–4), including elevated phosphorylation of AKT compared to tumors from ApcMin/+ mice (Figure 2C). These observations confirm that the transgene was transcribed and translated in the colon of FC13K1ApcMin/+ mice and that its expression resulted in increased downstream activation of the PI3K/AKT/MTOR pathway.
Figure 4.
Advanced small bowel tumors are seen in FC13K1ApcMin/+ mice. Large, depressed gadenocarcinomas are present in segment 3 and 4 of the small intestine (a and c). Some of these tumors even penetrate through the serosal surface. This is compared to the small adenomas that form less frequently in the ApcMin/+ controls (b and d). CTNNB1 expression is nuclear in both FC13K1ApcMin/+ tumors and controls (e and f). An increase in MKI67 and phospho-AKT IHC staining is noted in the FC13K1ApcMin/+ tumors compared to control (g–j). Scale bars: a–b = 1mm; c–j are 4x enlargements.
Differences in tumor morphology result from the expression of the dominant active form of PI3K in the setting of allelic loss of Apc in the intestine
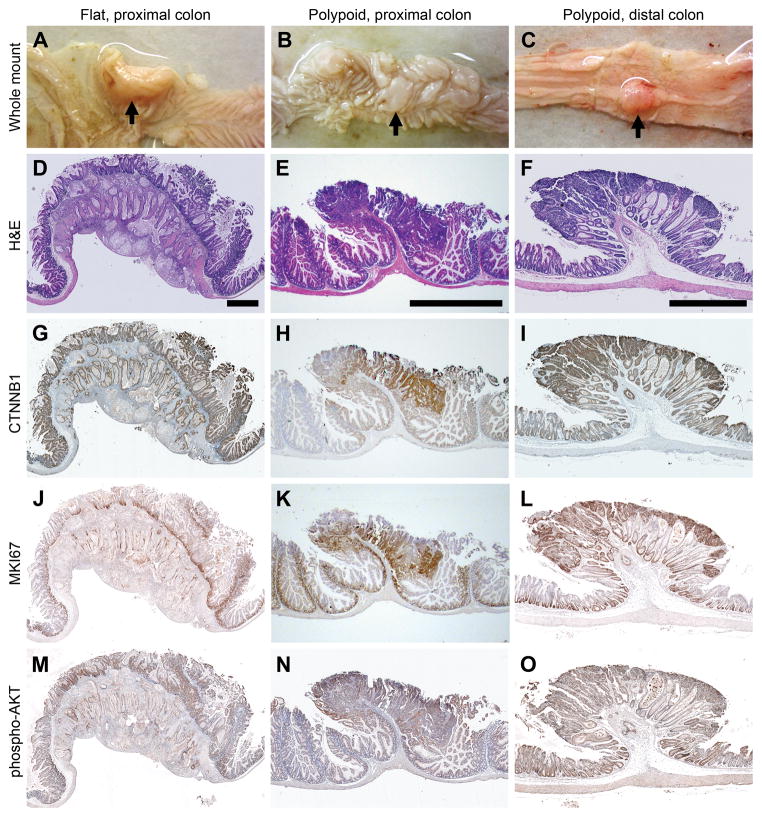
Tumors with multiple distinct morphologies develop in the FC13K1ApcMin/+ mice (Figure 3 and Supplementary Figures. S1 and S2). The flat lesions, previously described in the FC13K1 mice, are present (Figure 3a).10 These flat lesions are moderately differentiated invasive mucinous adenocarcinomas that arise in the setting of villous/serrated hyperplasia in the proximal colon. Extension of these cancers through the serosal layer is usually seen. These lesions are associated with a dramatic desmoplastic reaction and a high degree of tumor budding. No appreciable differences in tumor morphology were noted in the flat FC13K1ApcMin/+ versus the FC13K1Apc+/+ invasive mucinous adenocarcinomas of the proximal colon (Figure 3 and Supplementary Figure S3). In all cases, these tumors formed without significant polypoid lesions extending into the lumen of the colon and invaded through the serosal surface.
Figure 3.
FC13K1ApcMin/+ mice develop multiple tumors with distinct morphologies. Large flat moderately differentiated mucinous adenocarcinomas develop in the proximal colon (a and d). This cancer penetrates through the serosal surface consistent with a T4 lesion. Polypoid intramucosal carcinomas arising in the setting of villous/serrated hyperplasia are seen also in the proximal colon (b and e). In the mid and distal colon, tumors range from small adenomas to large adenomas with high grade dysplasia to adenocarcinomas. A large adenoma with solid growth pattern and a focal area of adenocarcinoma in the submucosa is pictured in c and f. The flat mucinous lesions do not possess nuclear CTNNB1 (g), while the polypoid tumors demonstrate nuclear CTNNB1 IHC staining (h and i). The MKI67 and phospho-AKT IHC staining of all of these tumors indicates that these tumors are highly proliferative and have activated PI3K/AKT signaling. Scale bars: d = 1 mm; e = 500 μm; f = 1 mm. All experiments were completed at least in triplicate.
In addition, polypoid tumors, which are not present in FC13K1 mice,10 are seen in the colon and distal small intestine. A total of 74 lesions were evaluated by a pathologist, and ranged from small adenomas to larger adenomas with high grade dysplasia to invasive adenocarcinomas. The polypoid tumors of the FC13K1ApcMin/+ mice are more representative of ApcMin/+ tumors, but display a greater degree of architectural distortion. Compared to the ApcMin/+ control colon tumor, these are much larger and less differentiated. The polypoid lesions of the FC13K1ApcMin/+ mice demonstrate a high degree of nuclear β-catenin, MKI67, and pAKT staining (Figure 3 and Supplementary Figure S1). A marked difference in tumor morphology was also observed in the exophytic small bowel lesions (Figure 4). Large invasive adenocarcinomas were seen in the small intestine of FC13K1ApcMin/+ mice, whereas only a limited number of small adenomas were seen in control littermates. The lesions of both the small and large intestine exhibited uptake of a novel near-infrared phospholipid ether analog, CLR1502, that selectively accumulates in advanced tumors (Supplementary Figure S4).
FC13K1ApcMin/+ mice develop tumors with and without nuclear localization of CTNNB1
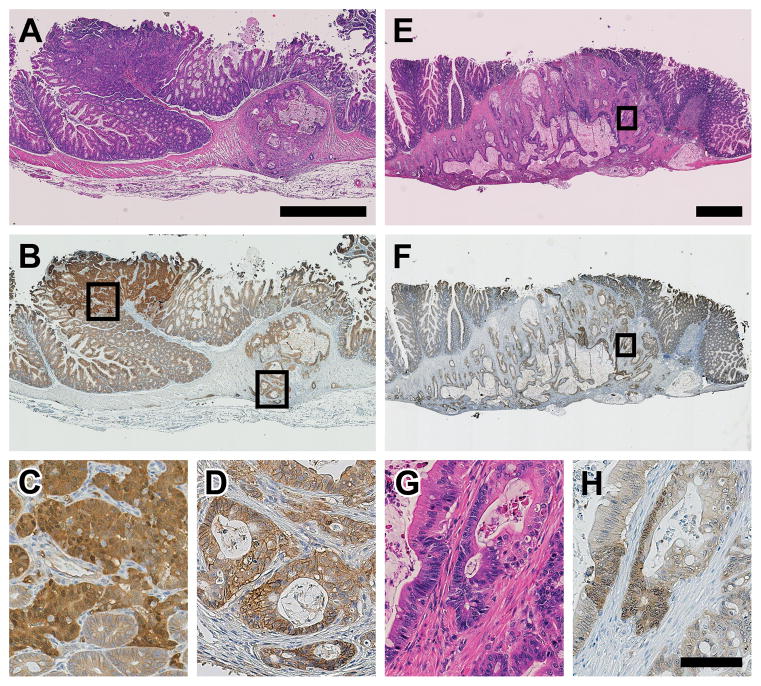
IHC analysis of CTNNB1 has been used for many years as a marker of activated WNT signaling. When functional APC is no longer expressed, CTNNB1 accumulates and translocates to the nucleus. Here we have examined CTNNB1 staining in tumor tissues of FC13K1ApcMin/+ and their control littermates. Similar to what was demonstrated previously, the flat proximal colon mucinous invasive adenocarcinomas of the FC13K1Apc+/+ and FC13K1ApcMin/+ mice largely did not demonstrate nuclear CTNNB1 staining (Figure 5 and Supplementary Figures S3 and S5). However, the polypoid lesions of the colon did demonstrate a high degree of nuclear CTNNB1. Microscopic adenomas are even able to be identified in the colon due to their high degree of nuclear CTNNB1 staining (Supplementary Figure S5).
Figure 5.
The tumors of the FC13K1ApcMin/+ mice develop by both canonical and non-canonical pathways. Exophytic polypoid adenomas with high grade dysplasia (a, left) and mucinous adenocarcinomas (a, right) develop in the proximal colon of FC13K1ApcMin/+ mice. The exophytic polypoid lesions possess a high amount of nuclear CTNNB1 (tumor on the left in b, enlarged in c), while the flat mucinous adenocarcinomas do not possess significant levels of nuclear CTNNB1 (tumor on the right in b, enlarged in d). This indicates that the polypoid lesions are arising secondary to aberrant Wnt signaling, while the flat mucinous lesions form via a non-canonical mechanism. Rarely, the large flat mucinous adenocarcinomas have islands of cells with nuclear CTNNB1 (e, H&E; f, CTNNB1). The cells with nuclear CTNNB1 also have a different morphology (g and h; enlargements of E and F, respectively), with higher nucleus-to-cytoplasm ratio, nuclear enlargement and hyperchromasia, and a more basophilic cytoplasm. Scale bars: a-b = 1mm; e-f = 1mm; c, d, g, h = 100μm.
These data indicate that tumors in the FC13K1ApcMin/+ mice are developing via different mechanisms. In the flat proximal invasive colon adenocarcinomas, the lack of nuclear CTNNB1 indicates that these tumors are not initiated by aberrant WNT signaling. Their non-canonical mechanism of tumorigenesis needs to be further clarified. In the polypoid colonic lesions, where CTNNB1 is nuclear, these tumors are likely initiated by loss of APC and their more aggressive phenotype driven by the presence of the dominantly active PI3K. Interestingly, in two flat mucinous proximal colon cancers, thought to be initiated by PI3K, small foci of nuclear CTNNB1 were identified (Figure 5). The morphology of the cells in these regions also changed. This indicates that the cells with nuclear CTNNB1 likely lost the other allele of Apc after tumor initiation by activated PI3K. The change in morphology indicates that important phenotypic changes in the tumor might occur even if APC is lost after tumor initiation and not just as the initiator of tumorigenesis.
FC13K1ApcMin/+ and FC13K1Apc+/+ tumors are microsatellite stable
Microsatellite instability (MSI) has been demonstrated to be an important marker for prognosis and response to treatment in humans.18 MSI analysis of intestinal tumors from mice with FC13K1ApcMin/+ and FC13K1Apc+/+ mice was performed using mononucleotide repeats, which have been previously shown to be very sensitive for detection of MSI in mouse tumors (Supplementary Figure S6).19 The microsatellite allelic patterns of 18 out of 22 tumor samples and matching normal samples were identical and therefore were considered microsatellite stable. Four tumors contained a novel allele at the mBat-66 locus that was not present in wild type C57BL/6 mice. However, this novel allele was the same in all four tumors and is, therefore, likely due to a polymorphism in these F1 mice. Thus, microsatellite instability does not appear to play a role in intestinal tumorigenesis in FC13K1ApcMin/+ and FC13K1Apc+/+ mice.
Similar tumor multiplicity and phenotypes are seen when Apc is lost only in the epithelial cells of the intestine in the setting of a constitutively active PI3K
In the Min mouse one allele of Apc is lost in all cells. Prior investigations have indicated that the loss of APC in stromal tissue may be important for tumorigenesis.20 To investigate this further, we used a transgenic mouse with a Cre recombinase conditional knockout of Apc (Apcfl/+).21 This mouse contains an allele of Apc that is floxed at exon 14. In the presence of Cre, exon 14 is removed and the APC protein is truncated. These mice were used to generate genetically homogenous FC13K1Apcfl/+ mice. Similar to the FC13K1ApcMin/+ mice, they also developed advanced small intestine and colon cancers (Figure 6). No appreciable significant differences were identified between the FC13K1ApcMin/+ and the FC13K1Apcfl/+ mice, including tumor multiplicity and morphology.
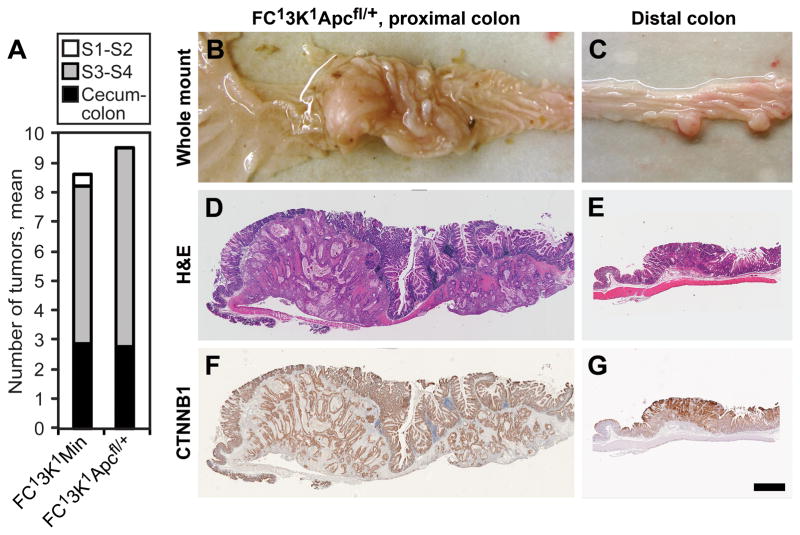
Figure 6.
In ApcMin/+ mice, one functional allele of Apc is lost in all cells, including the epithelial and stromal cells. To examine if a similar phenotype exits when APC is lost only in the epithelial cells, mice possessing an allele of Apc with exon 14 floxed (Apcfl/+) was utilized. In the FC13K1Apcfl/+ mice one allele of Apc was lost only in the epithelial cells of the distal small intestine and colon. No difference in tumor number was noted in FC13K1Apcfl/+ and FC13K1ApcMin/+ mice in the distal small intestine and colon (a). In addition, no difference in tumor morphology or CTNNB1 localization was noted (b–g). Scale bar d–g = 1mm.
FC13K1ApcMin/+ tumors demonstrate downstream activation of the PI3K pathway, MYC expression, and increased CCND1 compared to ApcMin/+ controls
The expression of the dominant active PI3K in the FC13K1ApcMin/+ tumors results in downstream activation of the PI3K pathway (Figures 2c and 7a). RPS6 (ribosomal protein S6) and eukaryotic translation initiation factor 4E binding protein (EIF4EBP1) are phosphorylated in both flat and polypoid FC13K1ApcMin/+ tumors. Interestingly, a significant amount of phosphorylated RPS6 and EIF4EBP1 is also seen in ApcMin/+. Only low levels of phosphorylated ERK1/2 are seen in FC13K1ApcMin/+ and ApcMin/+ controls (Figure 7a).
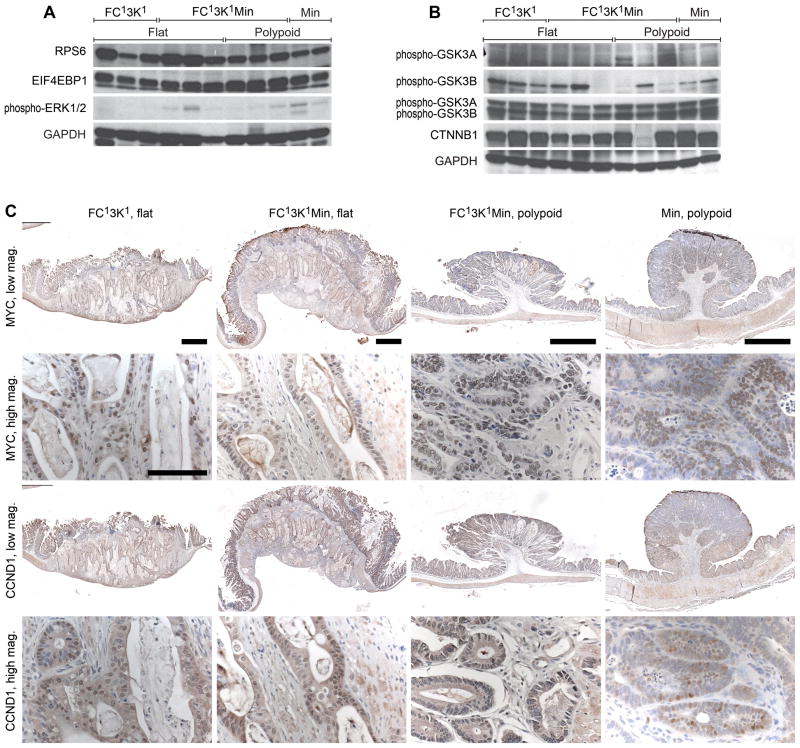
Figure 7.
Several key mediators of PI3K and Wnt signaling are altered in FC13K1ApcMin/+ mice. (a–b) Western blots. Phosphorylation of RPS6 and EIF4EBP1 is observed in the FC13K1ApcMin/+ tumors (a). Only low amounts of phospho-ERK1/2 are seen in these tumors (a). Increased pGSK3B is detected in the majority of flat FC13K1ApcMin/+ cancers with variable phosphorylation noted in the polypoid lesions (b). No change in the levels of total GSK3A or GSK3B were observed. In addition, in the majority of tumors no change in the levels of CTNNB1 was seen (b). Similar levels of MYC were seen in all tumors (c). Increased nuclear CCND1 levels were observed in the FC13K1Apc+/+ and FC13K1ApcMin/+ tumors compared to ApcMin/+ tumors (c). Scale bars low mag 1mm and high mag 100μm. All experiments were completed at least in triplicate.
The PI3K and WNT signaling pathways are known to converge on GSK3, however the role of GSK3 in crosstalk between these two pathways remains controversial. In the majority of flat FC13K1ApcMin/+ cancers, phosphorylation of GSK3B is increased compared to controls. Interestingly, in the polypoid FC13K1ApcMin/+ tumors, GSK3B appears to be phosphorylated less often. Two of these tumors that did not demonstrate phosphorylation of GSK3B did show phosphorylation of GSK3A. Despite the phosphorylation of GSK3B in the flat FC13K1ApcMin/+ cancers nuclear localization of CTNNB1 was not observed (Figures 3 and 5). In addition, no change in total CTNNB1 was observed between the majority of FC13K1ApcMin/+ and ApcMin/+ controls.
The WNT and PI3K pathways also converge on important transcription factors including MYC and CCND1. At necropsy, tumors were excised and prepared for histological analysis. Nuclear MYC was identified in all FC13K1Apc+/+ and FC13K1ApcMin/+ at similar levels to that seen for the ApcMin/+ controls. Nuclear CCND1 was observed in the majority of malignant cells in FC13K1Apc+/+ and FC13K1ApcMin/+ which is increased compared to the ApcMin/+ controls.
Discussion
Human colorectal cancers are a heterogeneous group, with each cancer individualized by its mutation profile. The presence of certain mutations may change with the biological context, such as the possible interaction between PIK3CA mutations and bowel inflammation. This is important clinically as patients with this mutation are more likely to benefit from adjuvant aspirin therapy.22 In addition, certain mutations are often associated with each other. Of colon tumors with PIK3CA mutations, 86% of these cancers also possess mutant APC.11 In addition, PIK3CA mutations have also been associated with KRAS and BRAF mutations, CpG island methylator phenotype (CIMP), and MSI.23–25
Concerted efforts to develop improved and more personalized treatment options for all types of cancer continue. Recent advances include improved targeting of directed therapies against the mutational activation of signaling pathways in cancer. This has lead to the development of classes of exciting anti-neoplastic agents, such as EGFR, BRAF, MEK, EML4-ALK, MET, MTOR, and PI3K inhibitors. To properly develop and utilize these new therapies, a better understanding of how the mutation profile of each cancer affects the tumor biology is required. It is important to recognize the roles of these mutant proteins and how their function is altered by the presence of mutations in other oncogenes or tumor suppressors. Here we report the first in vivo description of the interaction between APC and PIK3CA mutations in mammalian intestinal tumors.
Previously, we have demonstrated that a dominant active PI3K is able to initiate the development of flat mucinous invasive adenocarcinomas in the proximal colon via a non-canonical mechanism of tumorigenesis.10 In addition to these lesions, we now demonstrate that the presence of a dominant active PI3K in the setting of allelic loss of APC results in increased tumor multiplicity, size, and a more aggressive and less differentiated phenotype. We have demonstrated that this is related to increased activation of AKT and phosphorylation of downstream targets including RPS6 and EIF4EBP1.
Interestingly, in the flat proximal colonic mucinous invasive adenocarcinomas, these tumors carry germline mutations in one allele of APC. However, in two tumors we observed the loss of APC activity as a likely later event. In this setting, cells that lost APC after tumor initiation secondary to PI3K had a change in morphology to a less differentiated state. This indicates that, in addition to tumor initiation, the loss of APC can be a late event in tumorigenesis, and could be responsible for clonal outgrowth in some instances, such as was observed here.
The mechanism for the synergy between WNT and PI3K signaling pathways may occur due to a direct interaction between these pathways. Previously studies have aimed to examine this interaction, however they have been contradictory. GSK3 is well known to play an important role in both Wnt and PI3K signaling, and thus has been a target of multiple investigations examining these pathways. GSK3 is a serine-threonine kinase that occurs in two isoforms GSK3A and GSK3B.26 These kinases are naturally constitutively active and are inhibited by N-terminal phosphorylation of Ser-21 for GSK3A and Ser-9 for GSK3B, which results in the formation of a pseudosubstrate motif.27 In Wnt signaling, GSK3 plays an important role in the degradation of CTNNB1. GSK3 is bound by Axis inhibition protein (AXIN1) and phosphorylates CTNNB1.28 This phosphorylation of CTNNB1 targets it for ubiquitination and degradation by proteosomes. If GSK3 is inhibited by WNT signaling, this leads to CTNNB1 accumulation and nuclear translocation, though the mechanism of WNT inhibition of GSK3 remains poorly understood. In PI3K signaling, phosphorylated AKT in turn phosphorylates GSK3 on its inhibitory serines.29
Multiple studies have demonstrated that an interaction between these pathways might exist related to a single pool of GSK3.12–15 Contrary to these findings, other groups have proposed that crosstalk between these two pathways does not exist.16,17 Ng and colleagues demonstrated that a pool of GSK3B is stably bound to AXIN1 and represents only about 3–5% of the total GSK3B.17 They also observed that the phosphorylation level of the AXIN1-bound GSK3B was independent of PI3K pathway activation by insulin or inhibition by wortmannin.
Some evidence indicates that the context in which phospho-AKT signaling is taking place has an effect on its ability to inhibit GSK3B that is bound to AXIN1 and thus acting in WNT signaling. Fukumoto and colleagues demonstrated that phospho-AKT is bound to the AXIN1:GSK3B complex in the presence of Dishevelled, promoting phosphorylation of GSK3B and accumulation of free CTNNB1.13 However, if the WNT cascade was not activated by Dishevelled, phospho-AKT was not found to be bound to the AXIN1:GSK3B complex and free CTNNB1 levels were not elevated. These data indicate that phospho-AKT is not sufficient, but can enhance the activity of WNT signaling. These findings are consistent with those of Ng and colleagues as WNT was not activated in their experiments.
Here we demonstrate synergy between the loss of the tumor suppressor APC and the presence of a dominant active PI3K. FC13K1ApcMin/+ mice demonstrate increased tumor multiplicity, size, and aggressiveness compared to the tumors of control littermates. In the flat mucinous adenocarcinomas an increase in pGSK3B is observed, however this is without change in the total levels or nuclear localization of CTNNB1. This proves that phospho-AKT is insufficient for activation of the WNT signaling pathway in this context. In the polypoid tumors, variable phosphorylation of GSK3B was seen. Again no change in total CTNNB1 levels were seen, but significant nuclear localization of CTNNB1 was noted similar to that seen in ApcMin/+ controls. We cannot exclude the possibility that phospho-AKT signaling is able to enhance CTNNB1 signaling in the presence of an aberrant WNT pathway, such as mutant Apc. Significant levels of nuclear MYC were seen in all the tumors assayed and CCND1 was increased in the FC13K1ApcMin/+ tumors above the level seen in ApcMin/+ tumors, indicating a potential etiology for the synergy between these mutations at the transcriptional level.
In summary, synergy exists between the loss of the tumor suppressor APC and the presence of a dominant active PI3K in this in vivo model of colon cancer. Further studies are required to elucidate to what extent crosstalk between these pathways exists and is responsible for this synergy. In addition, pharmacologic studies using this and similar models will be vital to the development of novel combination therapies for the personalized treatment of patients with colorectal and other cancers.
Methods
Mouse Husbandry and Genotyping
All animal studies were conducted under protocols approved by the Institutional Animal Care and Use Committee at the University of Wisconsin-Madison, following the guidelines of the American Association for the Assessment and Accreditation of Laboratory Animal Care. FC1 mice (FVB/N-Tg(Fabp1-Cre)1Jig; NCI Mouse Repository; Strain number 01XD8), 3K1 mice (C57BL/6-Gt(ROSA)26Sortm7(Pik3ca*,EGFP)Rsky/J; The Jackson Laboratory; Stock Number - 012343), and ApcMin/+ males (C57BL/6J ApcMin/J; The Jackson Laboratory; Stock number 002020) were maintained as previously described.6 ApcMin/+ males were crossed with 3K1 females to generate 3K1ApcMin/+ mice. Male 3K1ApcMin/+ mice were then crossed with FC1 mice to generate F1 FC13K1ApcMin/+ mice and the associated controls. 3K1 mice were also crossed to Apcfl/fl mice (B6.Cg-Apctm2Rak; NCI Mouse Repository; Strain number 01XAA) to generate 3K1Apcfl/+ mice. These mice were then crossed with FC1 mice to generate FC13K1Apcfl/+ mice. Mice were genotyped for FC, 3K, Apcfl/+, and Min as described previously.6, 21, 30, 31 Tumors from ApcMin/+ and (SWRxB6) F1 ApcMin/+ mice treated with 4% dextran sodium sulfate (DSS) were used as controls.
Tumor Fixation and Counts
Mice were euthanized by CO2 asphyxiation. At necropsy the entire intestine was excised. The small intestine was divided into four sections of equal length (numbered 1 through 4 from proximal to distal) and the colon and cecum were kept intact. Each section of the intestine was then split lengthwise, splayed open, and rinsed. Tissues were fixed flat in 10% buffered formalin for 24 to 48 hours, then stored in 70% ethanol. Tumors in each section of the intestine were counted under a dissecting microscope by a single observer who was blinded to the genotypes.
Histology and Immunohistochemistry
Fixed tumors were isolated, embedded in paraffin, and cut into 5 μm sections. Every tenth section was stained with hematoxylin and eosin for histological review. Immunohistochemistry (IHC) was carried out as described previously.10 The primary antibodies included rabbit anti-phospho-AKT (Ser473, 1:100, Cell Signaling Technology, Beverly, MA), mouse anti-CTNNB1 (1:200, BD Biosciences – Clone 14, San Diego, CA), rabbit anti-PCNA (Ki-67) (1:1000, Cell Signaling Technology), and CCND1 (1:25, Cell Signaling Technology). The IHC protocol was modified for the anti-MYC antibody N-262 (1:50, Santa Cruz) as follows. Upon rehydration, slides were incubated in 0.05% sodium borohydride (Aldrich) for 10 min followed by incubation in 0.5% Triton X-100 (Sigma). Slides were blocked for 20 min at room temperature with a fresh blocking solution (2.5ml 1M Tris-HCl pH 8.0, 2 ml 5M NaCl, 2 ml 10% NP-40, 5g nonfat dry milk, distilled deionized water to 100ml).
Western Blot Analysis
Tissue were processed and gels run as previously described.10 The gels were probed with primary antibodies against p110α, phospho-AKT (Ser473), RPS6 (Ser235/236), phosphor-EIF4EBP1 (Thr37/46), pMAPK (Thr202/Tyr 204), pGSK3A, pGSK3B, or Total GSK3 (Cell Signaling Technology) in bovine serum albumin (Sigma-Aldrich) at a 1:1000 ratio for 16 hours. Anti-CTNNB1 (BD Biosciences – Clone 14) was probed at a ratio of 1:5000. Anti-GAPDH antibody (Cell Signaling Technology) was utilized as a loading control at a ratio of 1:5000.
Microsatellite Instability (MSI) Analysis
Tumor DNAs from FC13K1Apc+/+ and FC13K1ApcMin/+ mice were tested for MSI using a multiplex of six mononucleotide repeat loci designed specifically for MSI testing of C57BL/6 mice, including: mBat-56 (GTGTGTATGCTGATTTTATATCCT, GCAAAAATATCATTCGGTATG), mBat-57 (TGAAATCCTTGATGTTCCTCTACTAGGT, GGTCATCCTGTTGTTTCTAAATGATTGT), mBat-58 (CCCCTAAAACTTTCCTTGCTATT, TTCTGAGTTCCAGGGCAGTCTG), mBat-59 (GGTCTTGCCCTGAGGCAAGTAAT, AACCATCCGTAACAAGATCTGACGT), mBat-64 (CAGCCCACACTCCTGAAAACAG, CACTTAGCCAGTTGCGTCACCT) and mBat-66 (TGGGGTGTCTAAAGACAGCTAAG, GCCCACTTCATGCGTAACAG). For MSI analysis, >2ng of DNA was amplified with 1x C57BL/6 multiplex fluorescent primer mix, 1x Gold ST*R Buffer (Promega, Madison, WI) and 0.2U GoTaq®Hot Start Polymerase (Promega) in a GeneAmp PCR System 9700 Thermocycler (Applied Biosystems, Foster City, CA). The amplification conditions used were as follows: 95°C (2 min); 10 cycles of 94°C (30 sec), 58°C (30 sec, 29% ramp), 65°C (1 min, 23% ramp); 20 cycles of 90°C (30 sec), 58°C (30 sec, 29% ramp), 65°C (1 min, 23% ramp); and a final extension of 60°C (30 min). PCR products were denatured in deionized formamide with Internal Lane Standard 600 (Promega) for allele sizing and analyzed on a 3130xl Genetic Analyzer using GeneMapper 4.0 Software (Applied Biosystems). For MSI analysis, the microsatellite allelic patterns of tumor and matching normal samples were compared and classified as microsatellite stable if no loci were unstable, MSI-Low if one locus was unstable, and MSI-High if two or more loci were unstable.32
Supplementary Material
High magnification of histological sections presented in Figure 3. Large flat moderately differentiated mucinous adenocarcinomas develop in the proximal colon and polypoid lesions develop in both the proximal and distal colon with distinct morphologies. The flat mucinous lesions do not possess nuclear CTNNB1 (d), while the polypoid tumors do (e and f). The MKI67 and phospho-AKT staining of all of these tumors indicates that these tumors are highly proliferative and have activated PI3K/AKT signaling (g–l). Scale bar 100μm.
Additional examples of tumors in FC13K1ApcMin/+ tumors. Large flat mucinous adenocarcinomas form in the proximal colon (a, d, and g). Polypoid lesions ranging from small adenomas to adenomas with high grade dysplasia to adenocarcinomas are also found throughout the colon (b, e and h). Invasive cancers are also seen in the small intestine (c, f, and i). Scale bar 1mm.
FC13K1Apc+/+ and ApcMin/+ control colon tumors for comparison to the FC13K1ApcMin/+ tumors pictured in Figure 3. In FC13K1Apc+/+ mice, large flat mucinous adenocarcinomas develop in the proximal colon and cecum. These cancers penetrate through the serosal layer and have an abundance of mucin lakes (a and c). CTNNB1 is not nuclear in these tumors indicating a non-canonical mechanism of tumorigenesis (e). A high rate of proliferation and increased phosphorylation of AKT are noted (g and i). The presented ApcMin/+ control mouse was treated with 4% DSS to increase the number and size of colon tumors to compare to the FC13K1ApcMin/+ colon tumors (b and d). In these tumors CTNNB1 is nuclear (f). Prominent MKI67 staining demonstrates that these tumors are proliferative (h). Minimal phospho-AKT staining is noted (j). Scale bar a–b 1mm. c–j are 4x enlargements.
CLR1502 is a novel near-infrared phospholipid ether analog that selectively accumulates in tumors. FC13K1ApcMin/+ mice were treated with CLR1502 prior to becoming moribund at a dose of 300μg/mouse. After 96 hours, necropsy was performed. The colon was excised and fluorescence was measured using the Spectrum IVIS Caliper Life Science Live Imager. Increased uptake of CLR1502 was observed in the large flat proximal colon cancers (black arrow) and to a lesser extent the smaller polypoid tumors throughout the colon (grey arrows).
CTNNB1 staining can identify microscopic adenomas. Following necropsy the colons were excised, splayed out flat, and rolled. The colon then underwent histological sectioning. Upon H&E staining hyperplasia and dysplasia was noted throughout the proximal colon (a and c). After CTNNB1 staining, small foci of nuclear CTNNB1 could be indentified within the mucosa, consistent with microscopic adenomas (b and d). Scale bar a–b 500μm. c and d are 4x enlargements.
MSI analysis of tumors with activated PI3K. Tumor and normal DNA was amplified using a multiplex of six mononucleotide repeat loci and PCR products were analyzed using a 3130xl Genetic Analyzer. The resulting allelic profiles and sizes (base pairs) for mBat-64 and mBat-66 are shown in blue (a), for mBat-56 and mBat-59 in green (b), and for mBat-57 and mBat-58 in black (c). 18 out of 22 tumor samples and their matching normal samples had the same allelic profile shown above and were therefore considered microsatellite stable. 4 out of 22 samples all had the same novel mBat-66 allele (sized at 235 bp compared to 227 bp for wild type allele), and were therefore attributed to a breeding colony polymorphism and not scored as MSI positive.
Acknowledgments
We thank Ella Ward and Jane Weeks in Experimental Pathology at the UW Carbone Cancer Center for their technical assistance.
Financial support
This project was supported by the Conquer Cancer Foundation of the American Society of Clinical Oncology through A Young Investigator Award (D.A.D.); the National Cancer Institute of the U.S. National Institutes of Health through T32 CA009614 (D.A.D.), P50 CA095103 (Gastrointestinal Specialized Program of Research Excellence Grant, Vanderbilt Ingram Cancer Center), R01 CA123438 (R.B.H), P30 CA014520 (Core Grant, University of Wisconsin Carbone Cancer Center); and start-up funds (R.B.H.) from the UW Division of Gastroenterology and Hepatology, the UW Department of Medicine, and the UW School of Medicine and Public Health.
Footnotes
Conflicts of interest Dr. Jamey Weichert is the founder of Cellectar, Inc. (Madison, WI), which holds the licensing rights to the CLR1404 technology, and therefore has a financial interest in this agent.
Author Contributions D.A.D., A.A.L., and R.B.H. designed, performed and analyzed experiments, and wrote the manuscript. L.N, C.S, M.M, D.A., and J.B. performed and analyzed experiments. M.K.W., L.C., and J.W. analyzed experiments. All authors discussed results and edited the manuscript.
References
- 1.American Cancer Society. Cancer Facts & Figures 2012. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 2.Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinzler KW, Vogelstein B. Colorectal tumors. In: Vogelstein B, Kinzler KW, editors. The genetic basis of human cancer. 2. New York: McGraw-Hill; 2002. pp. 583–612. [Google Scholar]
- 4.Goss KH, Groden J. Biology of the adenomatous polyposis coli tumor suppressor. J Clin Oncol. 2000;18:19671979. doi: 10.1200/JCO.2000.18.9.1967. [DOI] [PubMed] [Google Scholar]
- 5.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 7.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 8.Markowitz SD, Bertagnolli MM. Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao L, Vogt PK. Hot-spot mutations in p110α of phosphatidylinositol 3-kinase (PI3K):differential interactions with the regulatory subunit p85 and with RAS. Cell Cycle. 2010;9:596–600. doi: 10.4161/cc.9.3.10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leystra AA, Deming DA, Zahm CD, Farhoud M, Olson TJ, Hadac JN, et al. Mice Expressing Activated PI3K Rapidly Develop Advanced Colon Cancer. Cancer Res. 2012;72:2931–2936. doi: 10.1158/0008-5472.CAN-11-4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nosho K, Kawasaki T, Ohnishi M, Suemoto Y, Kirkner GJ, Zepf D, et al. PIK3CA mutation in colorectal cancer: Relationship with genetic and epigenetic alterations. Neoplasia. 2008;10:534–541. doi: 10.1593/neo.08336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desbois-Mouthon C, Cadoret A, Blivet-Van Eggelpoel MJ, Bertrand F, Cherqui G, Perret C. Insulin and IGF-1 stimulate the beta-catenin pathway through two signaling cascades involving GSK-3beta inhibition and Ras activation. Oncogene. 2001;20:252–259. doi: 10.1038/sj.onc.1204064. [DOI] [PubMed] [Google Scholar]
- 13.Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH, et al. Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem. 2001;276:17479–17483. doi: 10.1074/jbc.C000880200. [DOI] [PubMed] [Google Scholar]
- 14.Naito AT, Akazawa H, Takano H, Minamino T, Nagai T, Aburatani H, et al. Phosphatidylinositol 3-kinase-Akt pathway plays a critical role in early cardiomyogenesis by regulating canonical Wnt signaling. Circ Res. 2005;97:144–151. doi: 10.1161/01.RES.0000175241.92285.f8. [DOI] [PubMed] [Google Scholar]
- 15.Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding VW, Chen RH, McCormick F. Differential regulation of glycogen synthase kinase 3beta by insulin and Wnt signaling. J Biol Chem. 2000;275:32475–32481. doi: 10.1074/jbc.M005342200. [DOI] [PubMed] [Google Scholar]
- 17.Ng SS, Mahmoudi T, Danenberg E, Bejaoui I, de Lau W, Korswagen HC, et al. Phosphatidylinositol 3-kinase signaling does not activate the Wnt cascade. J Biol Chem. 2009;285:35308–35313. doi: 10.1074/jbc.M109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Des Guetz G, Schischmanoff O, Nicolas P, Perret GY, Morere JF, Uzzan B. Does microsatellite instability predict the efficacy of adjuvant chemotherapy in colorectal cancer? A systemic review with meta-analysis. Eur J Cancer. 2009;45:1890–1896. doi: 10.1016/j.ejca.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Bacher JW, Abdel Megid WM, Kent-First MG, Halberg RB. Use of mononucleotide repeat markers for detection of microsatellite instability in mouse tumors. Mol Carcinog. 2005;44:285–292. doi: 10.1002/mc.20146. [DOI] [PubMed] [Google Scholar]
- 20.Tanwar PS, Zhang L, Roberts DJ, Teixeira JM. Stromal deletion of APC tumor suppressor in mice triggers development of endometrial cancers. Cancer Res. 2011;71:1584–1596. doi: 10.1158/0008-5472.CAN-10-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hung KE, Maricevich MA, Richard LG, Chen WY, Richardson MP, Kunin A, et al. Development of a mouse model for sporadic and metastatic colon tumors and its use in assessing drug treatment. Proc Natl Acad Sci USA. 2010;107:1565–1570. doi: 10.1073/pnas.0908682107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367:1596–1651. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitehall VL, Rickman C, Bond CE, Ramsnes I, Greco SA, Umapathy A, et al. Oncogenic PIK3CA mutations in colorectal cancers and polyps. Int J Cancer. 2012;131:813–820. doi: 10.1002/ijc.26440. [DOI] [PubMed] [Google Scholar]
- 24.Velho S, Moutinho C, Cirnes L, Albuquerque C, Hamelin R, Schmitt F, et al. BRAF, KRAS, and PIK3CA mutations in colorectal serrrated polyps and cancer: primary or secondary genetic events in colorectal carcinogenesis? BMC Cancer. 2008;8:255–260. doi: 10.1186/1471-2407-8-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5:19–27. [PMC free article] [PubMed] [Google Scholar]
- 26.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frame S, Cohen P, Biondi RM. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell. 2001;7:1321–1327. doi: 10.1016/s1097-2765(01)00253-2. [DOI] [PubMed] [Google Scholar]
- 28.Hinoi T, Yamamoto H, Kishida M, Takada S, Kishida S, Kikuchi A. Complex formation of adenomatous polyposis coli gene product and axin facilitates glycogen synthase kinase-3 beta-dependent phosphorylation of beta-catenin and down-regulates beta-catenin. J Biol Chem. 2000;275:34399–34406. doi: 10.1074/jbc.M003997200. [DOI] [PubMed] [Google Scholar]
- 29.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 30.Saam JR, Gordon JI. Inducible gene knockouts in the small intestinal and colonic epithelium. J Biol Chem. 1999;274:38071–38082. doi: 10.1074/jbc.274.53.38071. [DOI] [PubMed] [Google Scholar]
- 31.Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, DePinho RA, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
High magnification of histological sections presented in Figure 3. Large flat moderately differentiated mucinous adenocarcinomas develop in the proximal colon and polypoid lesions develop in both the proximal and distal colon with distinct morphologies. The flat mucinous lesions do not possess nuclear CTNNB1 (d), while the polypoid tumors do (e and f). The MKI67 and phospho-AKT staining of all of these tumors indicates that these tumors are highly proliferative and have activated PI3K/AKT signaling (g–l). Scale bar 100μm.
Additional examples of tumors in FC13K1ApcMin/+ tumors. Large flat mucinous adenocarcinomas form in the proximal colon (a, d, and g). Polypoid lesions ranging from small adenomas to adenomas with high grade dysplasia to adenocarcinomas are also found throughout the colon (b, e and h). Invasive cancers are also seen in the small intestine (c, f, and i). Scale bar 1mm.
FC13K1Apc+/+ and ApcMin/+ control colon tumors for comparison to the FC13K1ApcMin/+ tumors pictured in Figure 3. In FC13K1Apc+/+ mice, large flat mucinous adenocarcinomas develop in the proximal colon and cecum. These cancers penetrate through the serosal layer and have an abundance of mucin lakes (a and c). CTNNB1 is not nuclear in these tumors indicating a non-canonical mechanism of tumorigenesis (e). A high rate of proliferation and increased phosphorylation of AKT are noted (g and i). The presented ApcMin/+ control mouse was treated with 4% DSS to increase the number and size of colon tumors to compare to the FC13K1ApcMin/+ colon tumors (b and d). In these tumors CTNNB1 is nuclear (f). Prominent MKI67 staining demonstrates that these tumors are proliferative (h). Minimal phospho-AKT staining is noted (j). Scale bar a–b 1mm. c–j are 4x enlargements.
CLR1502 is a novel near-infrared phospholipid ether analog that selectively accumulates in tumors. FC13K1ApcMin/+ mice were treated with CLR1502 prior to becoming moribund at a dose of 300μg/mouse. After 96 hours, necropsy was performed. The colon was excised and fluorescence was measured using the Spectrum IVIS Caliper Life Science Live Imager. Increased uptake of CLR1502 was observed in the large flat proximal colon cancers (black arrow) and to a lesser extent the smaller polypoid tumors throughout the colon (grey arrows).
CTNNB1 staining can identify microscopic adenomas. Following necropsy the colons were excised, splayed out flat, and rolled. The colon then underwent histological sectioning. Upon H&E staining hyperplasia and dysplasia was noted throughout the proximal colon (a and c). After CTNNB1 staining, small foci of nuclear CTNNB1 could be indentified within the mucosa, consistent with microscopic adenomas (b and d). Scale bar a–b 500μm. c and d are 4x enlargements.
MSI analysis of tumors with activated PI3K. Tumor and normal DNA was amplified using a multiplex of six mononucleotide repeat loci and PCR products were analyzed using a 3130xl Genetic Analyzer. The resulting allelic profiles and sizes (base pairs) for mBat-64 and mBat-66 are shown in blue (a), for mBat-56 and mBat-59 in green (b), and for mBat-57 and mBat-58 in black (c). 18 out of 22 tumor samples and their matching normal samples had the same allelic profile shown above and were therefore considered microsatellite stable. 4 out of 22 samples all had the same novel mBat-66 allele (sized at 235 bp compared to 227 bp for wild type allele), and were therefore attributed to a breeding colony polymorphism and not scored as MSI positive.