Abstract
Objective
Knowledge of potentially modifiable risk factors for neuropsychiatric symptoms (NPS) in Alzheimer’s disease (AD) is important. This study longitudinally explores modifiable vascular risk factors for NPS in AD.
Methods
Participants enrolled in the Cache County Study on Memory in Aging with no dementia at baseline were subsequently assessed over three additional waves, and those with incident (new onset) dementia were invited to join the Dementia Progression Study for longitudinal follow-up. 327 participants with incident AD were identified and assessed for the following vascular factors: atrial fibrillation, hypertension, diabetes mellitus, angina, coronary artery bypass surgery, myocardial infarction, cerebrovascular accident, and use of antihypertensive or diabetes medicines. A vascular index (VI) was also calculated. Neuropsychiatric symptoms were assessed over time using the Neuropsychiatric Inventory (NPI). Affective and Psychotic symptom clusters were assessed separately. The association between vascular factors and change in NPI total score was analyzed using linear mixed model and in symptom clusters using a random effects model.
Results
No individual vascular risk factors or the VI significantly predicted change in any individual NPS. The use of antihypertensive medications more than four times per week was associated with higher total NPI and Affective cluster scores.
Conclusions
Use of antihypertensive medication was associated with higher total NPI and Affective cluster scores. The results of this study do not otherwise support vascular risk factors as modifiers of longitudinal change in NPS in AD.
Keywords: dementia, Alzheimer’s, neuropsychiatric, vascular
INTRODUCTION
Neuropsychiatric symptoms (NPS) such as depression, delusions, anxiety and agitation affect up to 90% of all patients with Alzheimer’s disease (AD) over the course of their illness (Tariot et al., 1995; Steinberg et al., 2008). NPS have been linked to greater caregiver and patient distress (Rabins et al., 1999; Craig et al., 2005; Gonzalez-Salvador et al., 1999), increased cost of care (Finkel, 2000; Beeri et al., 2002), more rapid cognitive and functional decline (Palmer et al., 2011; Rosenberg et al., 2012), and worse quality of life (Banerjee et al., 2009; Black et al., 2011). Given the high prevalence of NPS, as well as their consequences, understanding what contributes to their occurrence in AD is sorely needed.
Various factors have previously been found to modify risk of NPS (Treiber et al., 2008) Older age, sleep disturbance, white matter disease, and lacunes in the right basal ganglia have been associated with an increased risk of depression (O’Brien et al., 2000; Arbus et al., 2011; Casanova et al., 2011; Palmqvist et al., 2011). Older age and increased cognitive and functional impairment increases risk of apathy (Starkstein et al., 2001; Boyle et al., 2004; Starkstein et al., 2006), and older age, lower premorbid agreeableness, and increased cognitive and functional impairment may increase the risk for agitation and aggression (Holtzer et al., 2003; Copeland et al., 2003 Tsai et al., 1996; Archer et al., 2007). Psychotic symptoms, meanwhile, have been associated with worse cognitive or functional impairment, older age, and lacunes in the left basal ganglia (Harwood et al., 1999; Harwood et al., 2000; Bassiony et al., 2000; Paulsen et al., 2000; Mizrahi et al., 2006; Palmqvist et al., 2011). Anxiety and aberrant motor behavior have been associated with white matter disease (Berlow et al., 2010).
No consistently effective pharmacological treatment is available for any NPS in dementia (Sink et al., 2005; Schneider et al., 2006). Treatment of psychosis and agitation with antipsychotics is associated with significant safety concerns, including mortality (Kales et al., 2007; Gill et al., 2007). Although some previous research has found antidepressants to be effective in treating depression in AD (Lyketsos et al., 2003; Rao et al., 2006), recent larger studies demonstrate no benefit vs. placebo (Weintraub et al., 2010; Banerjee et al., 2011). The efficacy of non-pharmacological treatments (e.g. psychosocial interventions) also remains inconclusive (Jeste et al., 2008). Given the enormous burden of NPS on patients and caregivers and limited treatment options, knowledge of potentially modifiable risk factors for NPS in AD is important. As noted by Treiber et al. (2008), vascular factors, such as hypertension, stroke and hyperlipidemia, are of particular interest because they are common in the elderly and associated with NPS among individuals without AD. In a cross-sectional sample of 254 participants with AD followed in the Cache County Study on Memory in Aging (CCSMA), stroke prior to onset of AD was associated with a 3–4X increased risk for delusions, depression, and apathy; hypertension with 2–3X increased risk for delusions, anxiety, and agitation/aggression (Treiber et al., 2008). The CCSMA and associated Dementia Progression Study (DPS) have followed a cohort of participants with AD for up to 11 years and provide the opportunity to longitudinally examine the interaction between vascular risk factors and NPS. Mielke et al. (2007), for example, studied 135 individuals from this cohort with incident AD and found atrial fibrillation, systolic hypertension, and angina to be associated with a faster rate of AD progression. Our study is, to our knowledge, the first to longitudinally explore modifiable vascular risk factors for NPS in AD.
METHODS
Sampling and screening
The (DPS) (Tschanz et al., 2011) longitudinally followed participants with incident dementia who had been identified through their enrollment in the population-based CCSMA. The methods of the CCSMA have been reported elsewhere (Breitner et al., 1999; Lyketsos et al., 2000). Briefly, we approached all permanent residents of Cache County, Utah, who were 65 years or older (n= 5,677) and enrolled 90% (n= 5,092). Participants were screened using an adaptation of the modified Mini Mental State Examination (3MS) (Tschanz et al., 2002), and those who screened positive, along with a weighted, stratified population subsample, were studied further using an informant based telephone interview (Kawas et al., 1994). Those whose interviews were suggestive of probable or possible dementia underwent a comprehensive assessment including an in depth history with a knowledgeable informant and neuropsychological testing, including Mini-Mental State Examination (Folstein et al., 1975). A psychiatrist and neuropsychologist reviewed assessment data, and those with suspected dementia were asked to undergo neuroimaging, laboratory studies and examination by a psychiatrist. The comprehensive assessment was repeated at 18 months to confirm initial dementia diagnosis with longitudinal data.
Procedure
Dementia diagnoses were assigned by a panel of experts in neurology, geropsychiatry, neuropsychology and cognitive neuroscience who reviewed all available data. AD was diagnosed by National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association criteria (McKhann et al., 1984). The participants with no dementia at baseline were subsequently re-assessed over the course of 3 additional waves, and those incident (new-onset) dementia were invited to join the DPS (Figure 1) for longitudinal follow-up. The studies were approved by the institutional review boards of Duke University Medical Center, Johns Hopkins University, and Utah State University.
Figure 1.
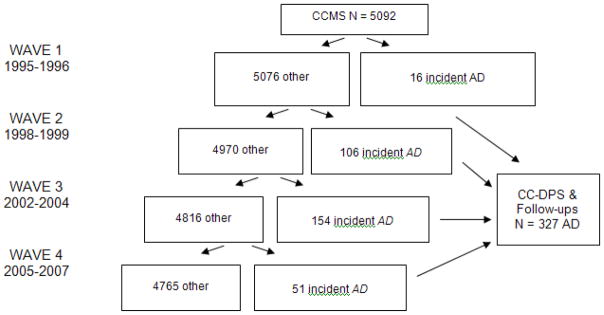
Participants identified with AD in the CCSMA
Assessment of Neuropsychiatric Symptoms
The NPI version used in the CCSMA and DPS (Cummings et al., 1994) assesses ten categories of neuropsychological symptoms: delusions, hallucinations, agitation/aggression, depression, apathy, elation, anxiety, disinhibition, irritability, and abnormal motor behavior (e.g., wandering, pacing). The NPI examines whether symptoms have occurred over the past month. Symptoms are ascertained by a trained examiner via a structured interview with a caregiver. If a symptom is endorsed, the respondent is asked to rate its frequency on a four-point scale and severity on a three-point scale, which when summed across domains produces an NPI total score. Multiplying the frequency and severity scores yields a domain score ranging from 1–12. Based on prior research (Lyketsos et al., 2001), the presence of Affective cluster symptoms was defined here as >0 score on any one of the depression, anxiety or irritability domains, and the presence of Psychotic cluster symptoms as >0 score on either the hallucination or delusion domains.
Assessment of Vascular Factors
Based on information obtained up to the visit at which the subject was diagnosed with dementia (baseline), it was determined whether the participants had a history of the following vascular factors: atrial fibrillation (AF), hypertension (HTN) (defined as lifetime history of diagnosed HTN determined via self-report interview prior to the diagnosis of dementia), diabetes mellitus (DM), angina, coronary artery bypass surgery (CABG), myocardial infarction (MI), and cerebrovascular accident (CVA). Systolic blood pressure (SBP) was assessed as a continuous variable. Participants were assessed for ever having taken antihypertensive medications and diabetes mellitus medications. In addition, a Vascular Index (VI) was calculated. This VI was identical to one used in a previous study of vascular risk factors in the CCSMA and DPS (Mielke et al., 2007), which in turn was adapted from the stroke risk profile of the Copenhagen City Heart Study (CCHS) (Truelsen et al., 1994). As noted by Mielke et al., (2007), two modifications of the VI from the CCHS were made: left ventricular hypertrophy was excluded because this information was not obtained in the CCSMA, and age was excluded to study its effect as a potential confounder or modifier. Using the same point system as the CCHS, the VI includes the following variables: AF, HTN (SBP> 160), DM, smoking, cardiovascular disease (CVD) (defined as history of MI, angina, or CABG), and current antihypertensive use. For nursing home patients, this medication information was obtained from medication administration records. The VI is described in more detail in Mielke et al., 2007. Participants were categorized as anti-hypertensive users if at the baseline visit they took medications from any of the following drug classes >= 4 times weekly: angiotensin converting enzyme inhibitors, beta-blockers, calcium channel blockers, and diuretics.
Data analysis
We used linear mixed effects models to examine the effects of vascular factors and VI on average change in the log of the total NPI score over time. The data were log transformed due to the highly positive skew of the distribution to help meet the assumptions of the linear mixed model. These models included both random intercepts and random effect for time of follow-up. Individual effects were assessed using model-based t and F tests. Due to the nature of the trajectory, a quadratic effect of time (time-squared) was included in models predicting total NPI score (Tschanz et al., 2011), and to examine the association of each predictor with rate of change in NPI, interactions between predictors and time and time-squared were tested. Other potentially confounding factors (including sex, education level, age at onset, dementia duration, and APOE genotype) were likewise included and examined. We examined the presence of cluster symptoms (i.e., presence/absence of affective and psychotic symptoms, respectively) over time using logistic regression models, with random effects for time. Models were evaluated primarily with respect to VI, time, and the interaction between the two in order to assess the differential effects of vascular index on the average odds of affective or psychotic symptoms over time. All variables, including the additional confounding factors mentioned above, were examined using model-based chi-square and likelihood ratio tests for corresponding regression coefficients. Analyses were completed using SAS version 9.2.
RESULTS
The CCSMA identified 327 participants with new-onset AD who were then followed in the DPS. The baseline (i.e., at the time that AD was diagnosed) characteristics of these participants are in Table 1, and baseline NPI scores are in Table 2. Participants completed multiple study visits from 0.7 to 10.5 years from dementia onset. Sixty-three percent died during the study, and 3.3% either refused further follow-up or moved from the area. The mean (SD) duration of AD from onset to the time of last observation was 4.09 (2.87) years. One hundred and five (32%) participants had no follow-up assessments after the baseline visit, of which 60 (57%) were lost due to death, 37 (35%) either refused or died after moving and the rest were pending a subsequent follow-up visit. Compared to those who completed at least one follow-up, these 105 were significantly older and scored lower on the MMSE and NPI total, but years of education and proportion of men and women did not significantly differ.
Table 1.
Demographic and baseline characteristics
| Male N(%) | 113(35) |
|
| |
| Female N(%) | 214(65) |
|
| |
| Age M(SD) | 84.23 (6.46) |
|
| |
| Years of education M(SD) | 13.2 (3.0) |
|
| |
| Caucasian M(SD) | 324 (99) |
|
| |
| Nursing home or locked unit N(%) | 56 (17) |
|
| |
| MMSE M(SD) | 21.97 (4.59) |
|
| |
| CDR M(SD) | 1.05 (0.58) |
|
| |
| GMHR | |
| Excellent N(%) | 39 (12) |
| Good N(%) | 179 (54) |
| Fair N(%) | 107 (33) |
| Poor N(%) | 2 (1) |
|
| |
| Number follow-ups M(SD) | 3.38(2.73) |
|
| |
| Duration follow-ups M(SD) | 5.09 (2.87) |
Table 2.
Baseline Neuropsychiatric Inventory (NPI) scores
| Delusions N(%) | 48(15) |
| Hallucinations N(%) | 17(5) |
| Agitation/aggression N(%) | 32(10) |
| Depression N(%) | 78(25) |
| Apathy N(%) | 54(17) |
| Elation N(%) | 2(1) |
| Anxiety N(%) | 42(13) |
| Disinhibition N(%) | 21(7) |
| Irritability N(%) | 55(17) |
| Aberrant Motor Behavior N(%) | 27(9) |
| NPI any N(%) | 162(51) |
| NPI total M(SD) | 4.22(8,33) |
Fifty-one percent of participants had at least one neuropsychiatric symptom at baseline visit. The most common symptoms were depression (25%), apathy (17%), and irritability (17%); least common were elation (1%), hallucinations (5%), and disinhibition (7%).
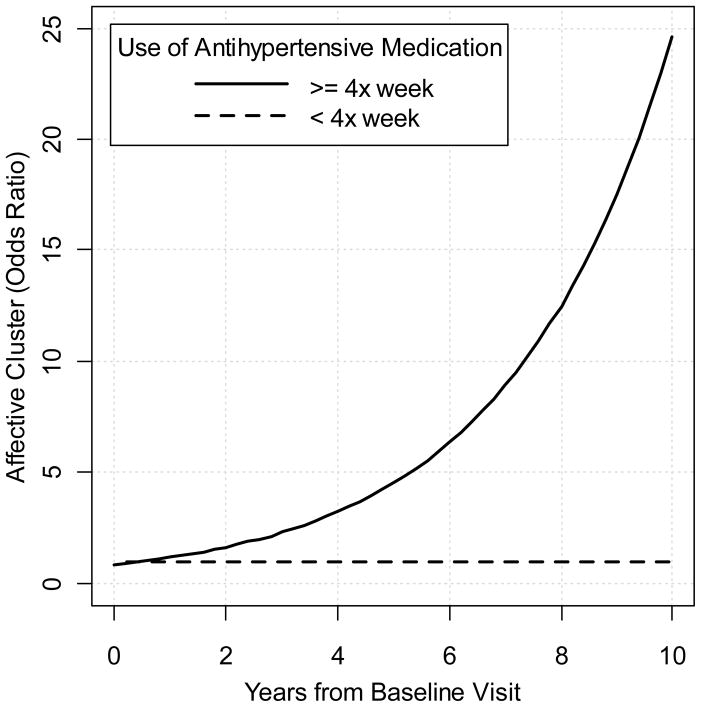
Examining vascular factors as predictors of total NPI score, the following demonstrated no significant interaction with respect to time: VI (p= 0.93), AF (p= 0.70), SBP (p= 0.20), angina (p=0.44), MI (p=0.50), CABG (p= 0.14), diagnosed HTN ever or never (p= 0.96), and use of diabetes medications (p= 0.37). Unexpectedly, the use of antihypertensive medication 4 times per week or more was associated with higher total NPI scores (p= .03) and specifically greater risk of experiencing Affective symptoms (OR= 1.29, p= 0.05) compared to those not taking such medications. The plot of the total NPI score over time by antithypertensive group is displayed in Figure 2. Note that the effect of antihypertensives suggests higher NPI scores in the short term and does not appear to extend to longer follow-up periods. This is an issue that is difficult to examine in this sample due to sparseness of data with longer follow-ups. Note that for ease of interpretation, the raw total (and not transformed total) NPI scores are displayed. The probability plot for the odds ratio of the time*antihypertensive medication interaction for Affective symptoms is displayed in Figure 3. To address the question of whether higher total NPI score in the group using antihypertensives >4X weekly was due to the increase in affective score cluster, the total NPI scores excluding the affective domains were calculated, and the results were nearly identical. Vascular factors did not predict change in any individual NPI symptom over time.
Figure 2.
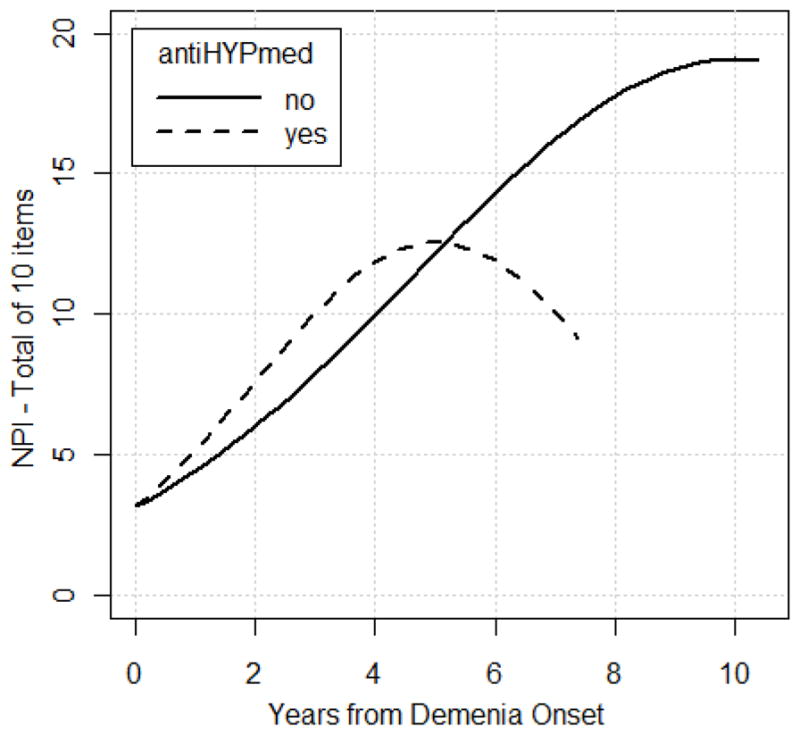
displays the relationship between antihypertensive medication group and total NPI scores over time, plotted to the extent of follow-ups completed in each group. Note that estimates of total NPI scores beyond 7 years after dementia onset are based on 21 or fewer participants and therefore estimates may be less reliable from this point forward.
Figure 3.
displays the time dependant odds ratio comparing the presence of affective symptoms for the group taking antihypertensive medications (at least 4 times per week) compared to those either not taking antihypertensive medications or taking them at a lower frequency. Note that estimates of odds ratios beyond 7 years after dementia onset are based on 21 or fewer participants and therefore estimates may be less reliable from this point forward.
DISCUSSION
In this longitudinal study of the relationship between vascular risk factors and NPS in a community-based AD cohort, we found no association between any individual vascular risk factor, or a cumulative vascular index, and the course of NPS. Our findings stand in contrast to our previous cross-sectional study by Treiber et al., (2008) using CCSMA prevalent and incident AD cases which showed a positive association at baseline between stroke prior to onset of AD and delusion, depression, and apathy, as well as a similar association between hypertension and delusions, anxiety, and agitation/aggression. Longitudinal analysis in the CCSMA (Tschanz et al., 2011), meanwhile, has demonstrated only marginal association between the rate of change of NPS and change in AD severity. Thus while vascular risk factors increase the likelihood of NPS being present (Treiber et al., 2008), as well as modulate the rate of AD progression (Mielke et al., 2007), the course of these NPS appears to occur largely independent of either vascular risk factors or disease progression. The potential benefits of modulating vascular risk factors in those with AD may therefore not extend to NPS.
In the current study, the use of antihypertensive medications more than 4 times per week was associated with higher rate of increase in total NPI score. Based on analyses to date for up to 6.2 years after baseline visit, which encompasses 95% of the collected data, these participants were also more likely to have Affective symptoms but not Psychotic symptoms. Hypertension can increase the risk of cerebrovascular disease, which has in turn been linked to increased incidence of depression in dementia (O’Brien et al., 2000; Lyketsos et al., 2000). However, no association was found in the current sample between the Affective cluster and hypertension itself. These results differ from that of Bassiony et al. (2000) who found an association between antihypertensive use and psychotic symptoms. One possible explanation for our finding is that antidepressant side effects of antihypertensive medications (e.g., beta blockers) may increase the likelihood of depression symptoms. However, the relationship between beta blockers and depression has itself recently been called into question (Verbeek et al., 2011). The model type used in the analyses did not allow comparison between different classes of antihypertensive medications. Furthermore, these findings may represent a Type I error given the many analyses performed. With the application of a stringent Bonferonni correction, the new alpha would be p= .005, and none of the findings would meet that level of significance. The analysis in this study is exploratory, and replication in other samples is needed to determine whether the findings have implications in the clinical setting.
Although the results of this study do not support vascular risk factors as modifiers of NPS in AD, several limitations warrant consideration. First, because incident dementia was only ascertained every three years, participants with more rapidly progressing AD may have died prior to being assessed for NPS. Second, NPI subdomains were examined in terms of their presence or absence, and not by symptom severity. As noted by Treiber et al. (2008), most NPI disturbances in this cohort were in the mild range, limiting the power to assess the relationship between vascular factors and NPS severity. Finally, the CCSMA and DPS cohort is ethnically homogenous, and results may not generalize to other populations.
CONCLUSION
No clear relationship was demonstrated between vascular risk factor and NPS in AD in this longitudinally examined community-based cohort. However, given the severe burden of NPS in AD and limited treatment options available, further study of the effects of antihypertensive medications and modifiable risk factors in AD remains of interest.
Key points.
No individual vascular risk factor, nor a Vascular Index, significantly predicted change in any individual neuropsychiatric symptom in AD.
Use of antihypertensive medications more than four times per week was associated with higher total neuropsychiatric inventory (NPI) and Affective cluster scores in AD
Further study of the effects of antihypertensive medications and modifiable risk factors in AD remains of interest
Acknowledgments
Supported by NIA grants R01AG21136, R01AG11380, K24AG027841
DISCLOSURES
Martin Steinberg:
Grant support from NIMH/NIA
Constantine Lyketsos:
Grant support (research or CME):
--NIMH, NIA, Associated Jewish Federation of Baltimore, Weinberg Foundation, Forest, Glaxo-Smith-Kline, Eisai, Pfizer, Astra-Zeneca, Lilly, Ortho-McNeil, Bristol-Myers, Novartis, National Football League, Elan, Functional Neuromodulation, Inc.
Consultant/Advisor:
--Astra-Zeneca, Glaxo-Smith-Kline, Esai, Novartis, Forest, Supernus, Adlyfe, Takeda, Wyeth, Lundbeck, Merz, Lilly, Pfizer, Genentech, Elan, NFL Players Association, NFL Benefits Office, Avanir, Zinfandel
--Honorarium of travel support:
Pfizer, Forest, Glaxo-Smith-Kline, Health Monitor
References
- Arbus C, Gardette V, Cantet CE, Andrieu S, Nourhashemi F, Schmitt L, Vellas B REAL.FR Group. Incidence and predictive factors of depressive symptoms in Alzheimer’s disease: the REAL.FR study. J Nutr Health Aging. 2011;15:609–617. doi: 10.1007/s12603-011-0061-1. [DOI] [PubMed] [Google Scholar]
- Archer N, Brown RG, Reeves SJ, Boothby H, Nicholas H, Foy C, Williams J, Lovestone S. Premorbid personality and behavioral and psychological symptoms in probable Alzheimer’s disease. Am J Geriatr Psychiatry. 2007;15:202–213. doi: 10.1097/01.JGP.0000232510.77213.10. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Samsi K, Petrie CD, Alvir J, Treglia M, Schwam EM, del Valle M. What do we know about quality of life in dementia? A review of the emerging evidence on the predictive and explanatory value of disease specific measures of health related quality of life in people with dementia. Int J Geriatr Psychiatry. 2009;24:15–24. doi: 10.1002/gps.2090. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Hellier J, Dewey M, Romeo R, Ballard C, Baldwin R, Bentham P, Fox C, Holmes C, Katona C, Knapp M, Lawton C, Lindesay J, Livingston G, McCrae N, Moniz-Cook E, Murray J, Nurock S, Orrell M, O’Brien J, Poppe M, Thomas A, Walwyn R, Wilson K, Burns A. Sertraline or mirtazapine for depression in dementia (HTA-SADD): a randomized, multicenter, double-blind, placebo-controlled trial. Lancet. 2011;378:403–411. doi: 10.1016/S0140-6736(11)60830-1. [DOI] [PubMed] [Google Scholar]
- Bassiony MM, Steinberg M, Warren A, Rosenblatt A, Baker A, Lyketsos C. Delusions and hallucinations in Alzheimer’s disease: prevalence and clinical correlates. Int J Geriatr Psychiatry. 2000;15:99–107. doi: 10.1002/(sici)1099-1166(200002)15:2<99::aid-gps82>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Beeri MS, Werner P, Davidson M, Noy S. The cost of behavioral and psychological symptoms of dementia (BPSD) in community dwelling Alzheimer’s disease patients. Int J Geriatr Psychiatry. 2002;17:403–408. doi: 10.1002/gps.490. [DOI] [PubMed] [Google Scholar]
- Berlow YA, Wells YM, Ellison JM, Sung YH, Renshaw PF, Harper DG. Neuropsychiatric correlates of white matter hyperintensities in Alzheimer’s disease. Int J Geriatr Psychiatry. 2010;25:780–788. doi: 10.1002/gps.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black BS, Johnston D, Morrison A, Rabins PV, Lyketsos CG, Samus QM. Quality of life of community-residing persons with dementia based on self-rated and caregiver-rated measures. Qual Life Res. 2012;21:1379–1389. doi: 10.1007/s11136-011-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PA, Malloy PF. Treating apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2004;17:91–99. doi: 10.1159/000074280. [DOI] [PubMed] [Google Scholar]
- Breitner JC, Wyse BW, Anthony JC, Welsh-Bohmer KA, Steffens DC, Norton MC, Tschanz JT, Plassman BL, Meyer MR, Skoog I, Khachaturian A. APOE-epsilon 4 count predicts age when prevalence of Alzheimer’s disease increases, then declines: the Cache County Study. Neurology. 1999;53:321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Starkstein SE, Jellinger KA. Clinicopathological correlates of behavioral and psychological symptoms of dementia. Acta Neuropath. 2011;122:117–135. doi: 10.1007/s00401-011-0821-3. [DOI] [PubMed] [Google Scholar]
- Copeland MP, Daly E, Hines V, Mastromauro C, Zaitchik D, Gunter J, Albert M. Psychiatric symptomatology and prodromal Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2003;17:1–8. doi: 10.1097/00002093-200301000-00001. [DOI] [PubMed] [Google Scholar]
- Craig D, Mirakhur A, Hart DJ, McIlroy SP, Passmore AP. A cross-sectional study of neuropsychiatric symptoms in 435 patients with Alzheimer’s disease. Am J Geriatr Psychiatry. 2005;13:460–468. doi: 10.1176/appi.ajgp.13.6.460. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Finklel S. Introduction to behavioural and psychological symptoms of dementia (BPSD) Int J Geriatr Psychiatry. 2000;15(Suppl 1):S2–4. doi: 10.1002/(sici)1099-1166(200004)15:1+<s2::aid-gps159>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Salvador MT, Arango C, Lyketsos CG, Barba AC. The stress and psychological morbidity of the Alzheimer patient caregiver. Int J Geriatr Psychiatry. 1999;14:701–710. doi: 10.1002/(sici)1099-1166(199909)14:9<701::aid-gps5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Gill SS, Bronskill SE, Normand SL, Anderson GM, Sykora K, Lam K, Bell CM, Lee PE, Fischer HD, Herrmann N, Gurwitz JH, Rochon PA. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146:775–86. doi: 10.7326/0003-4819-146-11-200706050-00006. [DOI] [PubMed] [Google Scholar]
- Harwood DG, Barker WW, Ownby RL, Duara R. Prevalence and correlates of Capgras syndrome in Alzheimer’s disease. Int J Geriatr Psychiatry. 1999;6:415–420. doi: 10.1002/(sici)1099-1166(199906)14:6<415::aid-gps929>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Harwood DG, Barker WW, Ownby RL, Duara R. Relationship of behavioral and psychological symptoms to cognitive impairment and functional status in Alzheimer’s disease. Int J Geriatr Psychiatry. 2000;15:393–400. doi: 10.1002/(sici)1099-1166(200005)15:5<393::aid-gps120>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Tang MX, Devanand DP, Albert SM, Wegesin DJ, Marder K, Bell K, Albert M, Brandt J, Stern Y. Psychopathological features in Alzheimer’s disease: course and relationship with cognitive status. J Am Geriatr Soc. 2003;51:953–960. doi: 10.1046/j.1365-2389.2003.51308.x. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Blazer D, Casey D, Meeks T, Salzman C, Schneider L, Tariot P, Yaffe K. ANCP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharm. 2008;33:957–970. doi: 10.1038/sj.npp.1301492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kales HC, Valenstein M, Kim HM, McCarthy JF, Ganoczy D, Cunningham F, Blow FC. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164:1568–1576. doi: 10.1176/appi.ajp.2007.06101710. [DOI] [PubMed] [Google Scholar]
- Kawas C, Segal J, Stewart WF, Corrada M, Thal LJ. A validation study of the Dementia Questionnaire. Arch Neurol. 1994;51:901–906. doi: 10.1001/archneur.1994.00540210073015. [DOI] [PubMed] [Google Scholar]
- Lyketsos C, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JCS. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000;157:708–14. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Sheppard JM, Steinberg M, Tschanz JA, Norton MC, Steffens DC, Breitner JC. Neuropsychiatric disturbance in Alzheimer’s disease clusters into three groups: the Cache County study. Int J Geriatr Psychiatry. 2001;16:1043–1053. doi: 10.1002/gps.448. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, DelCampo L, Steinberg M, Miles Q, Steele CD, Munro C, Baker AS, Sheppard JM, Frangakis C, Brandt J, Rabins PV. Treating depression in Alzheimer’s disease: efficacy and safety of sertraline therapy and the benefits of depression reduction: the DIADS. Arch Gen Psychiatry. 2003;7:737–746. doi: 10.1001/archpsyc.60.7.737. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Rosenberg PB, Tschanz J, Cook L, Corcoran C, Hayden KM, Norton M, Rabins PV, Green RC, Welsh-Bohmer KA, Breitner JC, Munger R, Lyketsos CG. Vascular factors predict rate of progression in Alzheimer disease. Neurology. 2007;69:1850–1858. doi: 10.1212/01.wnl.0000279520.59792.fe. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Starkstein SE, Jorge R, Robinson RG. Phenomenology and clinical correlates of delusions in Alzheimer’s disease. Am J Geriatr Psychiatry. 2006;14:573–581. doi: 10.1097/01.JGP.0000214559.61700.1c. [DOI] [PubMed] [Google Scholar]
- O’Brien J, Perry R, Barber R, Gholkar A, Thomas A. The association between white matter lesions on magnetic resonance imaging and noncognitive symptoms. Ann NY Acad Sci. 2000;903:482–489. doi: 10.1111/j.1749-6632.2000.tb06403.x. [DOI] [PubMed] [Google Scholar]
- Palmer K, Lupo F, Perri R, Salamone G, Fadda L, Caltagirone C, Musicco M, Cravello L. Predicting disease progression in Alzheimer’s disease: the role of neuropsychiatric syndromes on functional and cognitive decline. J Alzheimers Dis. 2011;24:35–45. doi: 10.3233/JAD-2010-101836. [DOI] [PubMed] [Google Scholar]
- Palmqvist S, Sarwari A, Wattmo C, Bronge L, Zhang Y, Wahlund LO, Nagga K. Association between subcortical lesions and behavioral and psychological symptoms in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2011;33:417–423. doi: 10.1159/000335778. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Salmon DP, Thal LJ, Romero R, Weisstein-Jenkins C, Galasko D, Hofstetter CR, Thomas R, Grant I, Jeste DV. Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology. 2000;54:1965–1971. doi: 10.1212/wnl.54.10.1965. [DOI] [PubMed] [Google Scholar]
- Rabins PV, Lyketsos CG, Steele CD. Practical Dementia Care. New York: Oxford University Press; 1999. [Google Scholar]
- Rao V, Spiro JR, Rosenberg PB, Lee HB, Rosenblatt A, Lyketsos CG. An open-label study of escitalopram (Lexapro) for the treatment of ‘Depression of Alzheimer’s disease’ (dAD) Int J Geriatr Psychiatry. 2006;21:273–274. doi: 10.1002/gps.1459. [DOI] [PubMed] [Google Scholar]
- Rosenberg PB, Mielke MM, Han D, Leoutsakos JS, Lyketsos CG, Rabins PV, Zandi PP, Breitner JC, Norton MC, Welsh-Bohmer KA, Zukderman IH, Rattinger GB, Green RC, Corcoran C, Tschanz JT. The association of psychotropic medication use with the cognitive, functional, and neuropsychiatric trajectory of Alzheimer’s disease. Int J Geriatr Psychiatry. 2012 doi: 10.1002/gps.3769. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail MS, Lebowitz BD, Lyketsos CG, Ryan JM, Stroup TS, Sultzer DL, Weintraub D, Lieberman JA CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355:1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293:596–608. doi: 10.1001/jama.293.5.596. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Petracca G, Chemerinski E, Kremer J. Syndromic validity of apathy in Alzheimer’s disease. Am J Psychiatry. 2001;158:872–877. doi: 10.1176/appi.ajp.158.6.872. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Jorge R, Mizrahi R, Robinson RG. A prospective longitudinal study of apathy in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006;77:8–11. doi: 10.1136/jnnp.2005.069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M, Shao H, Zandi P, Lyketsos CG, Welsh-Bohmer KA, Norton MC, Breitner JCS, Steffens DC, Tschanz JT. Cache County Investigators. 2008. Point and Five-year Period Prevalence of Neuropsychiatric Symptoms in Dementia: The Cache County Study. Int J Geriatr Psychiatry. 23:170–177. doi: 10.1002/gps.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariot PN, Mack JL, Patterson MB, Edland SB, Weiner MF, Fillenbaum G, Blazina L, Teri L, Rubin E, Mortimer JA. The Behavior Rating Scale for Dementia of the Consortium to Establish a Registry for Alzheimer’s Disease. Am J Psychiatry. 1995;152:1349–1357. doi: 10.1176/ajp.152.9.1349. [DOI] [PubMed] [Google Scholar]
- Treiber KA, Lyketsos CG, Corcoran C, Steinberg M, Norton M, Green RC, Rabins P, Stein DM, Welsh-Bohmer KA, Breitner JC, Tschanz JT. Vascular factors and risk for neuropsychiatric symptoms in Alzheimer’s disease: the Cache County Study. Int Psychogeriatr. 2008;20:538–553. doi: 10.1017/S1041610208006704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truelsen T, Lindenstrom E, Boysen G. Comparison of probability of stroke between the Copenhagen City Heart Study and the Framingham Study. Stroke. 1994;25:802–807. doi: 10.1161/01.str.25.4.802. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Hwang JP, Yang CH, Liu KM. Physical aggression and associated factors in probable Alzheimer’s disease. Azheimer Dis Assoc Disord. 1996;10:82–85. doi: 10.1097/00002093-199601020-00005. [DOI] [PubMed] [Google Scholar]
- Tschanz JT, Welsh-Bohmer KA, Plassman BL, Norton MC, Wyse BW, Breitner JC The Cache County Study Group. An adaptation of the Modified Mini-Mental State Examination: analysis of demographic influences and normative date: The Cache County Study. Neuropsychiatry, Neuropsychol Behav Neurol. 2002;15:28–38. [PubMed] [Google Scholar]
- Tschanz JT, Corcoran CD, Schwartz S, Treiber K, Green RC, Norton MC, Mielke MM, Piercy K, Steinberg M, Rabins P, Leoutsakos JM, Welsh-Bohmer KA, Breitner JC, Lyketsos CG. Progression of cognitive, functional, and neuropsychiatric symptom domains in a population cohort with Alzheimer’s disease: the Cache County Dementia Progression study. 2011;19:532–542. doi: 10.1097/JGP.0b013e3181faec23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek DE, van Riezen J, de Boer RA, van Melle JP, de Jonge P. A review of the putative association between beta-blockers and depression. Heart Fail Clin. 2011;7:89–99. doi: 10.1016/j.hfc.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Rosenberg PB, Drye LT, Martin BK, Frangakis C, Mintzer JE, Porsteinsson AP, Schneider LS, Rabins PV, Munro CA, Meinert CL, Lyketsos CG DIADS-2 Research Group. Sertraline for the treatment of depression in Alzheimer disease: week 24 outcomes. Am J Geriatr Psychiatry. 2010;18:332–340. doi: 10.1097/JGP.0b013e3181cc0333. [DOI] [PMC free article] [PubMed] [Google Scholar]