1. Introduction
Cystic fibrosis (CF) is a common lethal genetic disease in the North American population, affecting 1:2500 births. There are about 30,000 individuals in the United States with CF, and approximately 1,000 new cases annually. Although declining lung function is the most common cause of mortality in CF [1–3], liver disease is the third leading cause of death, accounting for 2.5% of overall mortality [4,5]. In this review, we will outline proposed definitions of liver disease in CF and review the epidemiology, diagnosis, proposed pathogenesis, management and outcome of liver disease in CF focusing on multilobular cirrhosis with or without portal hypertension.
While the liver is likely involved to some degree in all individuals with CF, most individuals will not develop cirrhosis, which is what most authors consider clinically significant liver disease. CF related liver disease (CFLD) is a nonspecific term without a consistent definition that has been used to refer to several liver disorders often with wide a spectrum of severity and impact on outcome, leading to confusion when comparing disease history, treatment, and outcomes. The spectrum of liver disease in variably included in CFLD has included neonatal cholestasis, elevated aminotransferases, hepatic steatosis, hepatic fibrosis, focal biliary cirrhosis and multilobular cirrhosis with or without portal hypertension. We suggest that uniform definitions would lead to consistently defined clinical and pathologic manifestations and contribute to clarity in research on the prevalence, natural history and treatment of liver disease in CF.
The identification of individuals with CF who are at risk for or who will subsequently develop cirrhosis has been difficult [6]. As new therapies including those that improve the function of the cystic fibrosis transmembrane regulator protein (CFTR) become available, there will be the potential to prevent or reverse clinically significant liver disease in CF. Thus there will be a new impetus to identify individuals at risk for advanced liver disease and those with pre-cirrhotic hepatic fibrosis.
Broad definitions and classifications of CFLD have been traditionally been used. The 1999 CF guidelines defined liver disease as the presence of either clinical or biochemical liver disease [7]. Several recent publications have used the following definition: The presence of at least 2 of the following conditions on 2 or more consecutive examinations over a 6–12 month period [8–10]: 1. hepatomegaly, with liver edge >2 cm below costal margin in the midclavicular line and confirmed by ultrasound. 2. at least 2 of the 3 of AST, ALT and GGTP above the upper limit of normal and 3. William's score of ultrasound abnormalities ≥4 [11] Both definitions include many disparate types of liver disease variable impact on outcome. Recent reports on the prevalence of CFLD using the broader criteria listed above, found a 30–40% prevalence of liver involvement in children and adults with CF [9,10,12]. However, longitudinal studies found that only 20–30% of individuals with CF develop focal biliary cirrhosis and at most 5–10% will develop multilobular cirrhosis [4,5,10]. Thus the broad definitions of CFLD above tend to overestimate the impact of liver disease in CF.
In part due to the variability in definitions, in 2007 the CF Foundation convened a group of hepatologists who proposed the classification of liver involvement in CF shown in Table 1.
Table 1.
Proposed classification of CFLD.
|
This classification separates cirrhosis with or without portal hypertension from other forms of liver disease such as elevated aminotransferases, hepatic steatosis and imaging abnormalities and defines the criteria for considering an individual as having no current clinical evidence for liver involvement in CF, primarily for the purposes of research.
The definitions, clinical presentations and prevalence data for each of the categories are described below.
2. Cystic fibrosis related cirrhosis with and without portal hypertension
2.1. Multilobular cirrhosis
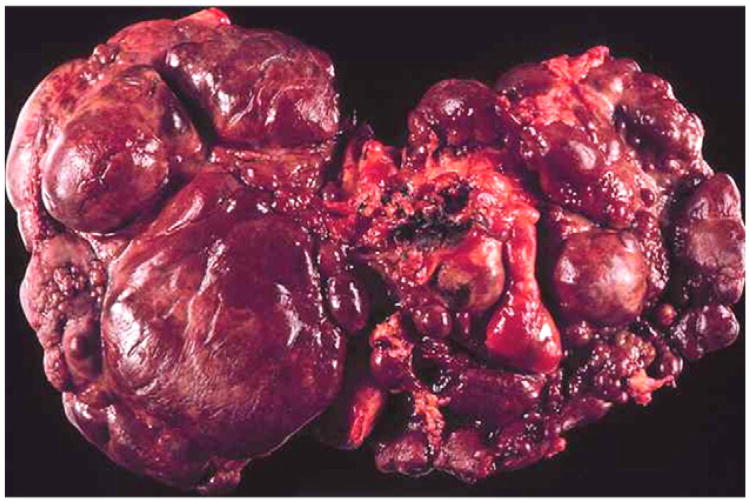
Multilobular cirrhosis differs from focal biliary cirrhosis in the presence of multiple regenerative nodules and diffuse involvement of the liver (Fig. 1). Clinically, multilobular cirrhosis is detected by a hard nodular liver that may or may not be enlarged. On imaging, there is an irregular nodular liver edge and coarse heterogeneous parenchyma. Prior to the development of portal hypertension, there are often no other clinical features. Once portal hypertension is present, splenomegaly, esophageal or gastric varices, or ascites may be the first suggestion of previously unsuspected cirrhosis. Liver biopsy can show features consistent with cirrhosis but may not be sensitive due to the patchy nature of the nodular involvement and the large regenerative nodules that can be mistaken for normal hepatic parenchyma. Multilobular cirrhosis in CF is a pediatric disorder. The median age of discovery is 10 years with very few new cases identified after 20 years of age, and no increased prevalence with increased life span. Our review of 12 reports from the last 20 years involving 4446 subjects from Europe, Canada, Australia and Israel found an average prevalence of multilobular cirrhosis of 5.6% portal hypertension 4.2% and varices 2.4% [4,9,10,12–20].
Fig. 1.
Explant of a liver from an individual with CF related cirrhosis demonstrating involvement of the entire liver with multilobular nodules.
2.2. Portal hypertension without cirrhosis
Interestingly, portal hypertension may predate the onset of cirrhosis with some patients presenting with non-cirrhotic portal hypertension. This has been theorized to involve presinusoidal portal venous hypertension and potentially portal venopathy and myofibroblasts with contractile potential [19,21,22].
2.3. Complications of cirrhosis with portal hypertension
The primary complications of CFLD are restricted to individuals with multilobular cirrhosis with portal hypertension, and include hypersplenism, esophageal or gastric variceal hemorrhage, ascites, and rarely synthetic liver failure with coagulopathy. The inclusion of liver disorders other than cirrhosis in some studies of the complications in CFLD, explains much of the variation in the reported frequency of these complications. While CFLD is listed as the 3rd leading cause of death among CF patients, some studies report no increase in mortality among patients with CF cirrhosis [9,17,18]. Two recent studies report a trend towards younger age of death in those with cirrhosis [12,23]. In a large 18 year retrospective review of 1108 patients with CF, 53 developed cirrhosis, 23 with portal hypertension, 14 with varices, 8 with coagulopathy, 6 with overt liver failure resulting in 3 liver transplants, but only one reported liver related death [14]. The incidence rate of major complications of cirrhosis (bleeding, ascites, encephalopathy) among a cohort of 177 CF patients (17 who developed cirrhosis) followed longitudinally was 0.4%, with an all cause mortality rate of 1.6% among cirrhotic patients [9]. This is in contrast to older reports that showed 11–19% mortality from variceal bleeding or liver failure in CF cirrhosis [24].
Once portal hypertension develops in CFLD, some studies report an increased risk of malnutrition, osteoporosis/hepatic osteodystrophy, and decline in lung function. The decline in lung function secondary to portal hypertension has been variously attributed to intrapulmonary vascular shunting, diaphragmatic splinting due to organomegaly and ascites, and potentially increased infections [25–28]. However, a recent retrospective study of 59 CF patients with cirrhosis and portal hypertension found no decline in lung function associated with portal hypertension as compared to age and gender matched CF specific reference values for lung function [29]. Malnutrition associated with portal hypertension is likely multifactorial with decreased nutrient absorption, increased resting energy expenditure, anorexia and decreased caloric intake. There may be a link between CFLD and increased insulin resistance leading to a higher incidence of CF related diabetes [30]. A single center retrospective case–control study showed an odds ratio of 4.8 (95% CI, 2.49, 9.17) for CF related diabetes in those with cirrhosis and portal hypertension, using a surrogate marker (thrombocytopenia) for cirrhosis with portal hypertension [31]. A case–control study of CF patients with and without CFLD found lower weight, height, and mid upper arm circumference and lower FEV1 scores in CFLD patients [32].
2.3.1. Liver synthetic failure
Only about 10% of individuals with CF and cirrhosis and portal hypertension progress to liver synthetic failure characterized by a high bilirubin and vitamin K resistant coagulopathy [23]. This is in contrast to other causes of cirrhosis where the rate of progression is seemingly much higher.
3. Liver involvement without cirrhosis or portal hypertension
There are a variety of other forms of liver involvement in CF. Some of these may occur together in individual patients.
3.1. Focal biliary cirrhosis
FBC is primarily a histologic diagnosis. Autopsy data have demonstrated liver involvement in 25–72% of adults with CF, with the majority showing focal biliary cirrhosis [33,34]. FBC has been found on postmortem exam in 11% of infants, 27% at 1 year and 25–70% of adults [33,34]. It is the pathognomonic histopathologic liver lesion in CF, and is often clinically silent without abnormalities in AST, ALT or GGT. On ultrasound, FBC is characterized by thickened (>2 mm), hyperechoic periportal tissues. On MRI, there is high intensity signal in the periportal area on T1 weighted imaging [35]. On liver biopsy, FBC is characterized by focal portal fibrosis and inflammation, cholestasis, and bile duct proliferation. It has been suggested that FBC is part of the progression to multilobular cirrhosis, but given the much lower frequency of multilobular cirrhosis compared to FBC, the progression is very rare [4,9,10,13,15–18,20,21].
3.2. Elevations in AST, ALT and/or GGT
Forty to 50% of CF patients have intermittent elevations in AST, ALT or GGT that are not predictive of the development or presence of significant fibrosis. In a longitudinal study of over 250 children identified by newborn screen in Colorado, followed for up to 20 years, 90% had at least one abnormal ALT and 30% had persistently (>6 months) elevated ALT. Persistent elevations of AST, ALT or GGT more than 3 times the upper limit of normal were very rare [36]. Additionally, patients can have cirrhosis with portal hypertension and have normal AST, ALT and GGT. Persistently elevated ALT or GGT (for more than 6 months) are common and warrant further investigation but are not diagnostic of any particular disorder [37].
3.3. Hepatic steatosis
Steatosis is likely the most common hepatic finding in CF with a prevalence of 23–75% of CF patients in all age categories [38]. Steatosis was present in 70% of children undergoing liver biopsy for suspected liver disease [17,21]. Hepatic steatosis has been associated with malnutrition, and deficiencies of essential fatty acid, carnitine and choline. However, steatosis is also found in CF patients with adequate nutritional status [5]. It presents as smooth mild hepatomegaly without signs of portal hypertension. The appearance on ultrasound is typically uniform hyperechogenicity, but it may also have a heterogeneous appearance on ultrasound or as one or several “pseudomasses,” which are lobulated fatty structures 1–2 cm in size [39,40]. In one study, 57% of cases of steatosis detected on ultrasound were associated with elevation in aminotransferases [41]. A note of caution is warranted in regards to this finding as the ultrasound findings may not be specific for steatosis and can be seen in periportal fibrosis [7]. Thus histology is still the gold standard for the diagnosis of hepatic steatosis.
4. Biliary tract disease
4.1. Cholangiopathy
Magnetic resonance cholangiography (MRC) demonstrates abnormalities in the intrahepatic bile ducts in a significant number of CF patients, with structuring, beading, and areas of narrowing and dilatation similar to primary sclerosing cholangitis [39]. MRC detected intrahepatic biliary anomalies were reported in 69% of CF patients regardless of laboratory or clinical evidence of liver disease, with isolated or multiple dilations in the biliary tree noted [42].
4.2. Cholestasis
This is the earliest manifestation of liver involvement in CF and may mimic biliary atresia. Less than 2% of infants with CF present with neonatal cholestasis. Meconium ileus is a known risk factor for the development of cholestasis [5,38,40]. While cholestasis generally resolves within 3 months with no sequelae, some studies have suggested an increased risk for cirrhosis in children with meconium ileus [4,9,10].
4.3. Gallbladder involvement
Gallbladder abnormalities are found in 24–50% of CF patients by US, MRI or MRC. Microgallbladder is reported in 5–45% of CF patients and 3–20% has gallbladder distention and evidence of gallbladder dysfunction. Older studies reported that gallstones develop in 3–25% of pediatric CF patients [38,39,43]. These stones are more commonly calcium bilirubinate stones [44]. Coinheritance of the Gilbert syndrome associated UGT1A1 mutation appears to increase the risk for gallstones in CF [45]. Cholecystectomy is indicated for the management of symptomatic cholelithiasis. Calcium bilirubinate stones do not respond to ursodeoxycholic acid treatment. No treatment is required for microgallbladder.
5. Pathogenesis
The pathogenesis of CF liver disease is largely unknown. In the liver, CFTR is localized to the apical surface of bile duct epithelium and is not found in hepatocytes [46]. CFTR in biliary epithelium increases apical biliary chloride secretion primarily increasing bile acid independent bile flow. In the pancreas and lung, CFTR is similarly located in the apical epithelium. These organ systems are affected to varying degrees by a dysfunctional or absent chloride channel leading to thickened mucus secretions and plugging. A similar pathologic mechanism has been suggested in the liver, with inspissated bile leading to obstruction of small intrahepatic bile ducts. This has been hypothesized to lead to accumulation of toxic bile acids in the liver, depletion of hepatic antioxidants, and liver cell injury. Repeated liver cell injury can activate hepatic stellate cells, which can lead to hepatic fibrosis and in some cases cirrhosis [5–7,47,48]. An alternative theory of pathogenesis is that increased intestinal permeability in CF leads to absorption of pathogen associated molecular patterns that stimulate inflammation and fibrosis [49–53]. It is unknown why only a subset of CF patients develop cirrhosis, while the majority of individuals with a similar CFTR defect do not develop cirrhosis. Cirrhosis occurs predominantly in those individuals with more severe class 1, 2, and 3 mutations and pancreatic insufficiency, but no specific genotype/phenotype correlation exists. In a study of genetic modifiers in CF cirrhosis, the PiZ heterozygote state for alpha-1 antitrypsin (SERPINA1 Z-allele) was associated with an increased risk for cirrhosis and a population attributable risk of 7 percent, but accounted for only 9% of the cirrhotics [54]. Other factors inconsistently associated with cirrhosis have been male sex, meconium ileus and TGF-B1 polymorphisms [9,10,54–57]. Further investigation of the pathogenesis of CF cirrhosis will hopefully aid in the development of targeted therapies.
5.1. Screening for liver disease
The goals of screening for liver disease in CF are two-fold. The first would be to identify individuals at risk for cirrhosis prior to its development in order to institute therapy to prevent or reduce progression to cirrhosis. The second would be to detect patients who have developed clinically silent cirrhosis to allow monitoring and interventions to reduce or mitigate complications.
There are no tests to reliably identify individuals with CF who are at high risk for the development of cirrhosis. In contrast, several methods have shown promise for the detection of clinically silent cirrhosis. A combination of physical examination for hepatomegaly, annual testing of AST/ALT and GGT, and abdominal imaging (ultrasound, CT or MRI/MRC) have been thought to hold the best promise for detecting clinically relevant liver disease in CF (cirrhosis with or without portal hypertension or advanced hepatic fibrosis). However, to date, these tests have not been shown to be sensitive for early stages of hepatic fibrosis or the identification of individuals who are at high risk for cirrhosis. In a prospective cohort study of CF patients with suspected CFLD, dual pass needle core liver biopsy increased the sensitivity of the detection of hepatic fibrosis by 22% compared to a single pass liver biopsy. In this study, the finding of more advanced hepatic fibrosis was the only factor independently associated with the future development of portal hypertension. Clinical exam, ultrasound and ALT were not predictive of development of portal hypertension. Clinical hepatomegaly and ultrasound abnormalities were found to be sensitive, but not specific, and ALT elevation specific but not sensitive for the detection of advanced fibrosis on biopsy [21].
The CF guidelines from 1999 recommend an annual physical exam for liver span and texture, spleen size and annual AST, ALT and GGT determination, but contained no definitive recommendations on screening imaging or liver biopsy. A recent Best Practice guideline paper from the European Cystic Fibrosis Society recommends hepatic ultrasound for patients with persistent elevation of AST, ALT or GGT on 3 consecutive occasions over 12 months, and/or clinical hepatomegaly or splenomegaly. They proposed that abnormalities in ultrasound could be followed by liver biopsy [37].
Liver biopsy remains the “gold standard” for the detection and staging of hepatic fibrosis and the diagnosis of cirrhosis. The risks, cost and lack of ability to perform serial measurements with liver biopsy in large part explain the emphasis on developing reliable and validated noninvasive tests for liver disease in CF.
Routine liver biochemistries are unreliable as indicators of cirrhosis or the risk of development of cirrhosis. Several studies have suggested the use of serum markers of hepatic fibrogenesis for early detection of CF liver disease, such as TIMP-1, collagen type IV, prolyl hydroxylase and glutathione s-transferase [48,58–60]. Composite tests of serum biomarkers for the prediction of hepatic fibrosis have been utilized in adults with hepatitis C. Aspartate aminotransferase to platelet ratio index (APRI) has also been used to predict severe fibrosis and cirrhosis in chronic liver disease such as hepatitis C, but does not predict milder forms of hepatic fibrosis. Neither composite tests, nor APRI have been systematically studied in CFLD.
5.2. Radiologic imaging
The three main imaging modalities: ultrasound, CT and MRI can detect cirrhosis with or without findings of portal hypertension [11]. Ultrasound can demonstrate multilobular nodularity indicative of cirrhosis, but is unreliable at detecting earlier stages of hepatic fibrosis. Steatosis and hepatic fibrosis may be indistinguishable. Children with normal hepatic ultrasounds can have advanced fibrosis [17]. The finding of a normal ultrasound does not indicate low risk for development of cirrhosis in a younger child, as children with a normal US have been shown to progress to cirrhosis, albeit at an unknown rate [16,61]. The presence of abnormalities in ALT or GGT is not predictive of ultrasound findings [16], although abnormal ultrasound findings were associated with abnormalities in ALT or GGT [41]. Ultrasound findings of hyperechogenicity have been intermittent in longitudinal studies [16], and consistency in interpretation of echogenicity and homogeneity may be center dependent leading to increased inter-observer variability [16,41,61]. However some centers perform serial routine abdominal ultrasound to detect early imaging abnormalities such as parenchymal heterogeneity that may indicate an increased risk for progression to cirrhosis [16].
MRI, when combined with MRCP, has the advantage of being able to detect abnormalities in intra and extraheptic bile ducts as well as significant periportal fibrosis and nodularity in the liver parenchyma [39]. Newer MRI modalities are also able to accurately determine the fat content of the liver and may aid in the detection of hepatic steatosis [62]. The role of diffusion weighted MRI imaging in the detection of early fibrosis and the differentiation between mild and severe fibrosis is under study.
Acoustic transient elastography uses a low frequency acoustic wave transmitted through the liver via a probe placed on the skin over the liver. The velocity of the wave propagation is directly proportional to the stiffness of the liver due to its collagen fiber content. It is a well validated test for the detection and quantification of advanced hepatic fibrosis and cirrhosis in adults with hepatocellular disease, primarily hepatitis C, but seems to only reliably detect advanced fibrosis versus normal and mild fibrosis, and is not yet able to differentiate mild fibrosis from no fibrosis [63–67]. Adult studies have shown less intra and inter-observer variability of transient elastography compared to ultrasound, and a faster learning curve when utilized by novice readers [68]. There are limited studies of elastography in CF. Two studies of children and adults with CF found increased liver stiffness in clinical and biochemical CFLD and elastography compared favorably to ultrasound for the detection of advanced fibrosis [60,69]. Two studies in CF found a good correlation between transient elastography values and the presence of esophageal varices [60,70]. It is possible that there will be greater value in serial measurements of elastography, but, further studies are needed to evaluate elastography in CF.
Magnetic resonance elastography (MRE) is a new technology that holds promise for measurement of liver fibrosis and detection of earlier stages of hepatic fibrosis [71,72].
Acoustic radiation forced impulse imaging (ARFI) uses ultrasound to measure liver stiffness and shear wave velocities. A potential advantage is that ARFI may not being influenced by hepatic steatosis [68,72]. Initial studies show a good correlation with transient elastography. One report in a small cohort of CF subjects suggests that ARFI is similar to transient elastography [73].
6. Treatment and outcome
To date, there is no effective therapy in CF to prevent or treat fibrosis. Thus, most efforts are directed at supportive care and management of complications.
6.1. Infants
Infants with cholestasis and prolonged jaundice are at risk for malnutrition, fat-soluble vitamin deficiency, and growth failure. This risk is higher in those infants who have undergone bowel resection. Close monitoring of growth and fat-soluble vitamin status with institution of higher calorie feedings and fat-soluble vitamin supplementation often required. Ursodeoxycholic acid (UCDA) increases bile flow and has been used in this setting despite the lack of published evidence in this age group.
6.2. Children and adults
In older children and adults with CF and cirrhosis, the main issues in care are screening for and management of complications of portal hypertension (splenomegaly, ascites, varices) and optimization of their nutritional status and lung function. In cirrhosis, it is advisable to avoid hepatotoxic medications and alcohol, and immunize against hepatitis A and B. In patients with portal hypertension NSAID's should be avoided due to the increased risk of GI bleeding.
6.2.1. Ursodeoxycholic acid
UDCA at present is the only therapy available that may possibly prevent or delay progression of CFLD. It increases bile flow, may replace potentially toxic bile acids, acts as a cytoprotective agent, and possibly stimulates bicarbonate secretion in the biliary tract [6,7,26]. UDCA at 15–20 mg/kg/day has been shown to improve AST and ALT, bile drainage, liver histology, and nutritional and essential fatty acid status in CF liver disease. However, a 2000 Cochrane review on the use of UCDA in CF found few suitable randomized trials assessing UDCA efficacy and there have not been any randomized studies conducted since that review. They concluded data are insufficient to justify routine use in CF, and no data on impact of UCDA on death or liver transplant are available. A recent study of higher dose UDCA in primary sclerosing cholangitis was terminated early due to an increased risk of death or liver transplantation in the UDCA group [74]. In summary, there is significant disagreement about the use of UDCA in CF [7,37,75]. There remains no well controlled randomized study of UDCA in CF and no strong evidence for benefit or harm in CF. Further study is needed to clarify the potential role of UDCA in CF. Other potential therapies such as essential fatty acid supplementation and antioxidants have not been sufficiently investigated to provide any recommendations.
7. Management of CF cirrhosis with portal hypertension
7.1. Screening
In patients with known cirrhosis, periodic assessment for sequelae of cirrhosis and portal hypertension is important. Abdominal examination should be performed at least annually for splenomegaly and/or ascites that can indicate advancing portal hypertension. Platelet count should be determined at least annually. Platelet counts <150,000 units×103/μL can indicate hypersplenism and has been shown to correlate with an increased risk of development of esophageal or gastric varices [76]. Periodic abdominal ultrasound with Doppler velocity measurements should be performed to screen for hepatocellular carcinoma, measure spleen size, assess ascites, portal venous velocities and presence of varices.
7.2. Esophageal and gastric varices
Esophageal and gastric varices occur as a consequence of portal hypertension. In CF patients with cirrhosis, varices have been reported to be present in anywhere from 30–100% [4,9,10,13,14,18]. In total these studies reported on 3057 patients with CF with 111 patients (3.6%) with cirrhosis identified in whom 54 (48%) had varices. The risk of variceal bleeding in CF cirrhosis has not been well studied. In 2 studies of a total of 67 patients with CF and cirrhosis, 28 (42%) developed variceal hemorrhage [12,24]. Several methods of assessing the risk of bleeding from varices exist. These include the ratio of the spleen size to platelet count, transient elastography, wedged hepatic venous pressure and endoscopy. In adults with other causes of cirrhosis, screening endoscopy is cost effective due to an increased mortality following esophageal variceal hemorrhage. In children this has not been demonstrated. Thus the use of screening endoscopy for varices and primary variceal prophylaxis remains controversial in the care of children and adults with CF with cirrhotic liver disease. It is clear though that once variceal hemorrhage occurs, variceal eradication is indicated. However, the high frequency of variceal hemorrhage in patients with cirrhosis and portal hypertension in CF has led many centers to consider primary variceal prophylaxis.
Primary variceal prophylaxis is the management of varices before a hemorrhage has occurred. The two modalities are pharmacotherapy with non selective β-blockade and endoscopic variceal eradication. CF patients with cirrhosis are often poor candidates for pharmacotherapy due to the pulmonary side effects of β-blockade. In addition, β-blockers have not shown to be effective in preventing variceal bleeding in children [77]. There is no evidence in children that supports primary variceal prophylaxis. There is evidence in adults with cirrhosis and portal hypertension from other causes that supports primary variceal prophylaxis [78]. If a decision is made to pursue primary variceal prophylaxis, endoscopic variceal band ligation is the treatment of choice [77,78].
Secondary variceal prophylaxis is treatment to prevent re-bleeding after a variceal hemorrhage has occurred. In this setting variceal band ligation is the preferred approach. If variceal band ligation is unsuccessful or if gastric varices are present, options include cyanoacrylate glue for control of gastroesophageal variceal bleeding and β blockade [79]. Refractory cases may require radiologic or surgical measures as secondary prophylaxis. These methods include a transjugular intrahepatic portosystemic shunt (TIPS), selective (splenorenal) or non selective (mesocaval, portocaval) portosystemic shunt. Some authors have used partial splenic embolization or partial or total splenectomy in an attempt to reduce portal venous flow and improve thrombocytopenia. Indeed, partial splenectomy with splenorenal shunt had favorable outcome in 15 of 19 CF patients with portal hypertension, with improvement in liver function and portal hypertensive symptoms, significantly delaying or obviating the need for liver transplantation [80].
7.3. Hypersplenism
Hypersplenism can complicate portal hypertension resulting in sequestration of up to 90% of platelets within the spleen. Persistent severe thrombocytopenia may be an indication for partial splenic embolization [81–83] or total splenectomy in some cases [80,84,85]. Risks of total splenectomy and the immune ramifications must be weighed with the potential benefits of this procedure, with many centers not recommending this procedure [86,87].
7.4. Ascites
The development of ascites represents a poor prognostic sign, and is indicative of relative hepatic decompensation and the need for evaluation for possible liver transplant. Diuretics such as furosemide or spironolactone may be utilized as first line therapy in concert with a salt and fluid restricted diet in attempts to decrease the ascitic fluid load. Spironolactone is often preferred as first line therapy because of the potassium sparing effects, but monitoring for hyperkalemia is warranted. Care must be taken to not overly restrict salt in CF, as hypochloridia may result especially in the summer months. Spontaneous bacterial peritonitis can complicate ascites and should be considered in the setting of fever or abdominal pain without a source in a patient with ascites. Significant ascites can lead to respiratory compromise and renal decompensation, and may warrant large volume paracentesis [88].
7.5. Encephalopathy
Hepatic encephalopathy is rare in CF patients with cirrhosis. In most of the reports of encephalopathy, it developed after portosystemic shunting for portal hypertension. Management includes lactulose, oral non-absorbable antibiotics and provision of adequate calories to avoid catabolism.
7.6. Liver transplantation
Which patients with CF and cirrhosis require liver transplant, and optimal timing of transplantation remains a controversial subject. Reserving liver transplant only for those with liver synthetic failure (a relatively rare late event in CF cirrhosis) versus early transplantation following the development of portal hypertensive complications varies by center. The established indications for liver transplant in CF would include cirrhosis with evidence for hepatic decompensation or uncontrollable variceal bleeding. Some authors feel that evaluation and transplantation should occur earlier in the disease course, before lung function is severely compromised when outcomes are likely to be better [25,89]. The relatively preserved hepatic synthetic function in the CF cirrhosis population affects prioritization for liver transplant, and clear guidelines on selection of CF patients for liver transplantation are lacking, although a scoring system has been proposed [90].
Of 203 liver transplants in CF patients performed in the US from 1987–2008, 148 were performed in children. There was a significant survival advantage in both adults and children with CF cirrhosis receiving liver transplant compared to those with cirrhosis who did not receive a transplant, with further survival advantage in children compared to adults [91,92]. Outcomes of liver transplant in CF have generally been favorable with 1 year patient and graft survival (89%/83% respectively), and 5 year patient survival rates (85.8%) not significantly different than non-CF liver transplant patients [87,92]. However, for CF patients without liver synthetic failure or intractable GI bleeding, their one year survival without transplant would likely be higher. In Europe, the median age of CF liver transplant recipients was 12 years and the 3 year survival was approximately 80% [93]. Thus pediatric CF patients in the US and Europe who undergo liver transplant experience survival rates similar to the 5 year unadjusted survival rates of 86% in US non-CF pediatric liver transplant recipients [92].
Relative contraindications to an isolated liver transplant in CF include infection with multi-drug resistant organisms (Pseudo-monas, Burkolderia), poor pre-transplant pulmonary function (FEV1 <50% predicted) and/or elevated resting arterial pCO2, extensive pulmonary fibrosis on imaging, and severe pulmonary hypertension [86,93]. Short term improvements in lung function are reported in pediatric patients following liver transplant, and poorer lung function prior to surgery has been positively associated with mortality risk [86]. Small single center series have reported both favorable [25] and unfavorable pulmonary function outcomes following liver transplantation [94]. A recent analysis of the CF registry shows no difference in the rate of decline in FEV1 in the 3 years following liver transplant in the CF subjects who underwent liver transplant compared to CF controls without liver disease [95]. This suggests that liver transplantation does not lead to a significant long term change in pulmonary outcome. The effect of liver transplant on the nutritional status of CF patients is also uncertain, with reports of improvements [96,97] and reports of no improvement [95].
Combined lung and liver transplant may be considered in patients with cirrhosis and portal hypertension and extensive pulmonary fibrosis, lower FEV1 and higher resting pCO2. Eleven CF patients underwent combined liver–lung transplantation between 1987 and 2004 in the US. The median age was 15 years, and 1, 3 and 5 year patient survival rates were 79%, 63% and 63% respectively [98]. Eleven combined liver–pancreas transplants in CF patients with cirrhosis and CFRD were performed between 1987 and 2010, with 100% five-year survival reported in follow up of 7 patients, with no further requirement for exogenous insulin or pancreatic enzyme replacement therapy [87].
8. Summary
Although some degree of liver involvement in CF is common, cirrhosis and subsequent portal hypertension is a rare but serious complication seen in only 5–10% of CF patients. Efforts are underway to better define and understand the nature of this condition and to identify which patients are at risk for the development of cirrhosis. Early detection has proven to be elusive, with many patients identified after cirrhosis and portal hypertension have already developed. Cirrhosis impacts the nutritional and pulmonary status of affected patients, but pulmonary status remains the primary determinant of outcome. In the rare patient with hepatic decompensation or significant complications that cannot be managed medically, liver transplantation may be an option. Future efforts should focus on developing accurate methods of detecting individuals at risk for the development of cirrhosis before it is present and therapeutic trials of potential interventions to mitigate or prevent/reverse fibrosis prior to development of cirrhosis and portal hypertension.
References
- 1.Corey M, Edwards L, Levison H, Knowles M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J Pediatr. 1997;131:809–14. doi: 10.1016/s0022-3476(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 2.Corey M, Farewell V. Determinants of mortality from cystic fibrosis in Canada, 1970–1989. Am J Epidemiol. 1996;143:1007–17. doi: 10.1093/oxfordjournals.aje.a008664. [DOI] [PubMed] [Google Scholar]
- 3.Kerem E, Reisman J, CoreyM, Canny GJ, Levison H. Prediction ofmortality in patients with cystic fibrosis. N Engl J Med. 1992;326:1187–91. doi: 10.1056/NEJM199204303261804. [DOI] [PubMed] [Google Scholar]
- 4.Efrati O, Barak A, Modan-Moses D, et al. Liver cirrhosis and portal hypertension in cystic fibrosis. Eur J Gastroenterol Hepatol. 2003;15:1073–8. doi: 10.1097/00042737-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Colombo C. Liver disease in cystic fibrosis. Curr Opin Pulm Med. 2007;13:529–36. doi: 10.1097/MCP.0b013e3282f10a16. [DOI] [PubMed] [Google Scholar]
- 6.Moyer K, Balistreri W. Hepatobiliary disease in patients with cystic fibrosis. Curr Opin Gastroenterol. 2009;25:272–8. doi: 10.1097/MOG.0b013e3283298865. [DOI] [PubMed] [Google Scholar]
- 7.Sokol RJ, Durie PR. Recommendations for management of liver and biliary tract disease in cystic fibrosis. Cystic Fibrosis Foundation Hepatobiliary Disease Consensus Group J Pediatr Gastroenterol Nutr. 1999;28(Suppl. 1):S1–S13. doi: 10.1097/00005176-199900001-00001. [DOI] [PubMed] [Google Scholar]
- 8.Bhardwaj S, Canlas K, Kahi C, et al. Hepatobiliary abnormalities and disease in cystic fibrosis: epidemiology and outcomes through adulthood. J Clin Gastroenterol. 2009;43:858–64. doi: 10.1097/MCG.0b013e31819e8bbd. [DOI] [PubMed] [Google Scholar]
- 9.Colombo C, Battezzati PM, Crosignani A, et al. Liver disease in cystic fibrosis: a prospective study on incidence, risk factors, and outcome. Hepatology. 2002;36:1374–82. doi: 10.1053/jhep.2002.37136. [DOI] [PubMed] [Google Scholar]
- 10.Lamireau T, Monnereau S, Martin S, Marcotte JE, Winnock M, Alvarez F. Epidemiology of liver disease in cystic fibrosis: a longitudinal study. J Hepatol. 2004;41:920–5. doi: 10.1016/j.jhep.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Williams SG, Evanson JE, Barrett N, Hodson ME, Boultbee JE, Westaby D. An ultrasound scoring system for the diagnosis of liver disease in cystic fibrosis. J Hepatol. 1995;22:513–21. doi: 10.1016/0168-8278(95)80444-7. [DOI] [PubMed] [Google Scholar]
- 12.Chryssostalis A, Hubert D, Coste J, et al. Liver disease in adult patients with cystic fibrosis: a frequent and independent prognostic factor associated with death or lung transplantation. J Hepatol. 2011;55:1377–82. doi: 10.1016/j.jhep.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 13.Scott-Jupp R, Lama M, Tanner MS. Prevalence of liver disease in cystic fibrosis. Arch Dis Child. 1991;66:698–701. doi: 10.1136/adc.66.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhattacharjee R, Schibli S, Rose J, et al. The natural history of liver disease in cystic fibrosis. J Cyst Fibros. 2006;5:S61. [Google Scholar]
- 15.Williams SM, Goodman R, Thomson A, McHugh K, Lindsell DR. Ultrasound evaluation of liver disease in cystic fibrosis as part of an annual assessment clinic: a 9-year review. Clin Radiol. 2002;57:365–70. doi: 10.1053/crad.2001.0861. [DOI] [PubMed] [Google Scholar]
- 16.Lenaerts C, Lapierre C, Patriquin H, et al. Surveillance for cystic fibrosis-associated hepatobiliary disease: early ultrasound changes and predisposing factors. J Pediatr. 2003;143:343–50. doi: 10.1067/S0022-3476(03)00329-9. [DOI] [PubMed] [Google Scholar]
- 17.Lindblad A, Glaumann H, Strandvik B. Natural history of liver disease in cystic fibrosis. Hepatology. 1999;30:1151–8. doi: 10.1002/hep.510300527. [DOI] [PubMed] [Google Scholar]
- 18.Desmond CP, Wilson J, Bailey M, Clark D, Roberts SK. The benign course of liver disease in adults with cystic fibrosis and the effect of ursodeoxycholic acid. Liver Int. 2007;27:1402–8. doi: 10.1111/j.1478-3231.2007.01570.x. [DOI] [PubMed] [Google Scholar]
- 19.Lewindon PJ, Ramm GA. Cystic fibrosis-cirrhosis, portal hypertension, and liver biopsy: reply. Hepatology. 2011;53:1065–6. doi: 10.1002/hep.24212. [DOI] [PubMed] [Google Scholar]
- 20.Feigelson J, Anagnostopoulos C, Poquet M, Pecau Y, Munck A, Navarro J. Liver cirrhosis in cystic fibrosis—therapeutic implications and long term follow up. Arch Dis Child. 1993;68:653–7. doi: 10.1136/adc.68.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewindon PJ, Shepherd RW, Walsh MJ, et al. Importance of hepatic fibrosis in cystic fibrosis and the predictive value of liver biopsy. Hepatology. 2011;53:193–201. doi: 10.1002/hep.24014. [DOI] [PubMed] [Google Scholar]
- 22.Witters P, Libbrecht L, Roskams T, et al. Noncirrhotic presinusoidal portal hypertension is common in cystic fibrosis-associated liver disease. Hepatology. 2011;53:1064–5. doi: 10.1002/hep.24183. [DOI] [PubMed] [Google Scholar]
- 23.Rowland M, Gallagher CG, O'Laoide R, et al. Outcome in cystic fibrosis liver disease. Am J Gastroenterol. 2011;106:104–9. doi: 10.1038/ajg.2010.316. [DOI] [PubMed] [Google Scholar]
- 24.Debray D, Lykavieris P, Gauthier F, et al. Outcome of cystic fibrosis-associated liver cirrhosis: management of portal hypertension. J Hepatol. 1999;31:77–83. doi: 10.1016/s0168-8278(99)80166-4. [DOI] [PubMed] [Google Scholar]
- 25.Milkiewicz P, Skiba G, Kelly D, et al. Transplantation for cystic fibrosis: outcome following early liver transplantation. J Gastroenterol Hepatol. 2002;17:208–13. doi: 10.1046/j.1440-1746.2002.02671.x. [DOI] [PubMed] [Google Scholar]
- 26.Colombo C, Russo MC, Zazzeron L, Romano G. Liver disease in cystic fibrosis. J Pediatr Gastroenterol Nutr. 2006;43(Suppl. 1):S49–55. doi: 10.1097/01.mpg.0000226390.02355.52. [DOI] [PubMed] [Google Scholar]
- 27.Westaby D. Cystic fibrosis:Liver disease. Prog Respir Res. 2006;34:251–61. [Google Scholar]
- 28.Linnane B, Oliver MR, Robinson PJ. Does splenectomy in cystic fibrosis related liver disease improve lung function and nutritional status? A case series Arch Dis Child. 2006;91:771–3. doi: 10.1136/adc.2006.093773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polineni D, et al. Pulmonary function (FEV1) in cystic fibrosis patients with and without severe liver disease with portal hypertension (CFLD) Am J Respir Crit Care Med. 2009;179 [Google Scholar]
- 30.Minicucci L, Lorini R, Giannattasio A, et al. Liver disease as risk factor for cystic fibrosis-related diabetes development. Acta Paediatr. 2007;96:736–9. doi: 10.1111/j.1651-2227.2007.00250.x. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan KM, Moran A, Schwarzenberg S. Cystic fibrosis related diabetes in CF patients with cirrhosis. Pediatr Pulmonol. 2009;44:414. [Google Scholar]
- 32.Corbett K, Kelleher S, Rowland M, et al. Cystic fibrosis-associated liver disease: a population-based study. J Pediatr. 2004;145:327–32. doi: 10.1016/j.jpeds.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 33.Blanc WA, Di Sant'Agnese PA. A distinctive type of biliary cirrhosis of the liver associated with cystic fibrosis of the pancreas; recognition through signs of portal hypertension. Pediatrics. 1956;18:387–409. [PubMed] [Google Scholar]
- 34.Vawter GF, Shwachman H. Cystic fibrosis in adults: an autopsy study. Pathol Annu. 1979;14(Pt 2):357–82. [PubMed] [Google Scholar]
- 35.Robertson MB, Choe KA, Joseph PM. Review of the abdominal manifestations of cystic fibrosis in the adult patient. Radiographics. 2006;26:679–90. doi: 10.1148/rg.263055101. [DOI] [PubMed] [Google Scholar]
- 36.Woodruff SA, Sontag MK, Accurso F, Sokol RJ, Narkewicz MR. Prevalence of elevated liver function tests in children with cystic fibrosis diagnosed by newborn screen. J Pediatr Gastroenterol Nutr. 2007;45:E27–8. doi: 10.1016/j.jcf.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros. 2011;10(Suppl. 2):S29–36. doi: 10.1016/S1569-1993(11)60006-4. [DOI] [PubMed] [Google Scholar]
- 38.Herrmann U, Dockter G, Lammert F. Cystic fibrosis-associated liver disease. Best Pract Res Clin Gastroenterol. 2010;24:585–92. doi: 10.1016/j.bpg.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Akata D, Akhan O. Liver manifestations of cystic fibrosis. Eur J Radiol. 2007;61:11–7. doi: 10.1016/j.ejrad.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Diwakar V, Pearson L, Beath S. Liver disease in children with cystic fibrosis. Paediatr Respir Rev. 2001;2:340–9. doi: 10.1053/prrv.2001.0170. [DOI] [PubMed] [Google Scholar]
- 41.Patriquin H, Lenaerts C, Smith L, et al. Liver disease in children with cystic fibrosis: US-biochemical comparison in 195 patients. Radiology. 1999;211:229–32. doi: 10.1148/radiology.211.1.r99ap13229. [DOI] [PubMed] [Google Scholar]
- 42.Durieu I, Pellet O, Simonot L, et al. Sclerosing cholangitis in adults with cystic fibrosis: a magnetic resonance cholangiographic prospective study. J Hepatol. 1999;30:1052–6. doi: 10.1016/s0168-8278(99)80259-1. [DOI] [PubMed] [Google Scholar]
- 43.Wilschanski M, Durie PR. Patterns of GI disease in adulthood associated with mutations in the CFTR gene. Gut. 2007;56:1153–63. doi: 10.1136/gut.2004.062786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Angelico M, Gandin C, Canuzzi P, et al. Gallstones in cystic fibrosis: a critical reappraisal. Hepatology. 1991;14:768–75. doi: 10.1002/hep.1840140505. [DOI] [PubMed] [Google Scholar]
- 45.Wasmuth HE, Keppeler H, Herrmann U, Schirin-Sokhan R, Barker M, Lammert F. Coinheritance of Gilbert syndrome-associated UGT1A1 mutation increases gallstone risk in cystic fibrosis. Hepatology. 2006;43:738–41. doi: 10.1002/hep.21105. [DOI] [PubMed] [Google Scholar]
- 46.Cohn JA, Strong TV, Picciotto MR, Nairn AC, Collins FS, Fitz JG. Localization of the cystic fibrosis transmembrane conductance regulator in human bile duct epithelial cells. Gastroenterology. 1993;105:1857–64. doi: 10.1016/0016-5085(93)91085-v. [DOI] [PubMed] [Google Scholar]
- 47.Lewindon PJ, Pereira TN, Hoskins AC, et al. The role of hepatic stellate cells and transforming growth factor-beta(1) in cystic fibrosis liver disease. Am J Pathol. 2002;160:1705–15. doi: 10.1016/s0002-9440(10)61117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pereira TN, Lewindon PJ, Smith JL, et al. Serum markers of hepatic fibrogenesis in cystic fibrosis liver disease. J Hepatol. 2004;41:576–83. doi: 10.1016/j.jhep.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 49.Norman K, Pirlich M. Gastrointestinal tract in liver disease: which organ is sick? Curr Opin Clin Nutr Metab Care. 2008;11:613–9. doi: 10.1097/MCO.0b013e32830a70bc. [DOI] [PubMed] [Google Scholar]
- 50.Zeuzem S. Gut-liver axis. Int J Colorectal Dis. 2000;15:59–82. doi: 10.1007/s003840050236. [DOI] [PubMed] [Google Scholar]
- 51.Aoyama T, Paik YH, Seki E. Toll-like receptor signaling and liver fibrosis. Gastroenterol Res Pract. 2010;2010:1–8. doi: 10.1155/2010/192543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kisseleva T, Brenner DA. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. J Gastroenterol Hepatol. 2007;22(Suppl. 1):S73–8. doi: 10.1111/j.1440-1746.2006.04658.x. [DOI] [PubMed] [Google Scholar]
- 53.Pradere JP, Troeger JS, Dapito DH, Mencin AA, Schwabe RF. Toll-like receptor 4 and hepatic fibrogenesis. Semin Liver Dis. 2010;30:232–44. doi: 10.1055/s-0030-1255353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bartlett JR, Friedman KJ, Ling SC, et al. Genetic modifiers of liver disease in cystic fibrosis. JAMA. 2009;302:1076–83. doi: 10.1001/jama.2009.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Colombo C, Apostolo MG, Ferrari M, et al. Analysis of risk factors for the development of liver disease associated with cystic fibrosis. J Pediatr. 1994;124:393–9. doi: 10.1016/s0022-3476(94)70361-2. [DOI] [PubMed] [Google Scholar]
- 56.Slieker MG, Deckers-Kocken JM, Uiterwaal CS, van der Ent CK, Houwen RH. Risk factors for the development of cystic fibrosis related liver disease. Hepatology. 2003;38:775–6. doi: 10.1053/jhep.2003.50403. [DOI] [PubMed] [Google Scholar]
- 57.Wilschanski M, Rivlin J, Cohen S, et al. Clinical and genetic risk factors for cystic fibrosis-related liver disease. Pediatrics. 1999;103:52–7. doi: 10.1542/peds.103.1.52. [DOI] [PubMed] [Google Scholar]
- 58.Gerling B, Becker M, Staab D, Schuppan D. Prediction of liver fibrosis according to serum collagen VI level in children with cystic fibrosis. N Engl J Med. 1997;336:1611–2. doi: 10.1056/NEJM199705293362217. [DOI] [PubMed] [Google Scholar]
- 59.Sidlova K, Skalicka V, Kotaska K, et al. Serum alpha-glutathione S-transferase as a sensitive marker of hepatocellular damage in patients with cystic fibrosis. Physiol Res. 2003;52:361–5. [PubMed] [Google Scholar]
- 60.Rath T, Menendez KM, Kugler M, et al. TIMP-1/-2 and transient elastography allow non invasive diagnosis of cystic fibrosis associated liver disease. Dig Liver Dis. 2012;44:780–7. doi: 10.1016/j.dld.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 61.Mueller-Abt PR, Frawley KJ, Greer RM, Lewindon PJ. Comparison of ultrasound and biopsy findings in children with cystic fibrosis related liver disease. J Cyst Fibros. 2008;7:215–21. doi: 10.1016/j.jcf.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34:729–49. doi: 10.1002/jmri.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Friedrich-Rust M, Rosenberg W, Parkes J, Herrmann E, Zeuzem S, Sarrazin C. Comparison of ELF, FibroTest and FibroScan for the non-invasive assessment of liver fibrosis. BMC Gastroenterol. 2010;10:103. doi: 10.1186/1471-230X-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol. 2007;102:2589–600. doi: 10.1111/j.1572-0241.2007.01466.x. [DOI] [PubMed] [Google Scholar]
- 65.Stebbing J, Farouk L, Panos G, et al. A meta-analysis of transient elastography for the detection of hepatic fibrosis. J Clin Gastroenterol. 2010;44:214–9. doi: 10.1097/MCG.0b013e3181b4af1f. [DOI] [PubMed] [Google Scholar]
- 66.Pereira TN, Walsh MJ, Lewindon PJ, Ramm GA. Paediatric cholestatic liver disease: diagnosis, assessment of disease progression and mechanisms of fibrogenesis. World J Gastrointest Pathophysiol. 2010;1:69–84. doi: 10.4291/wjgp.v1.i2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroen-terology. 2008;134:960–74. doi: 10.1053/j.gastro.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 68.Boursier J, Isselin G, Fouchard-Hubert I, et al. Acoustic radiation force impulse: a new ultrasonographic technology for the widespread noninvasive diagnosis of liver fibrosis. Eur J Gastroenterol Hepatol. 2010;22:1074–84. doi: 10.1097/MEG.0b013e328339e0a1. [DOI] [PubMed] [Google Scholar]
- 69.Witters P, De Boeck K, Dupont L, et al. Non-invasive liver elastography (Fibroscan) for detection of cystic fibrosis-associated liver disease. J Cyst Fibros. 2009;8:392–9. doi: 10.1016/j.jcf.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 70.Malbrunot-Wagner AC, Bridoux L, Nousbaum JB, et al. Transient elastography and portal hypertension in pediatric patients with cystic fibrosis Transient elastography and cystic fibrosis. J Cyst Fibros. 2011;10:338–42. doi: 10.1016/j.jcf.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 71.Lee VS, Miller FH, Omary RA, et al. Magnetic resonance elastography and biomarkers to assess fibrosis from recurrent hepatitis C in liver transplant recipients. Transplantation. 2011;92:581–6. doi: 10.1097/TP.0b013e31822805fa. [DOI] [PubMed] [Google Scholar]
- 72.Baranova A, Lal P, Birerdinc A, Younossi ZM. Non-invasive markers for hepatic fibrosis. BMC Gastroenterol. 2011;11:91. doi: 10.1186/1471-230X-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manco M, Zupone CL, Alghisi F, D'Andrea ML, Lucidi V, Monti L. Pilot study on the use of acoustic radiation force impulse imaging in the staging of cystic fibrosis associated liver disease. J Cyst Fibros. 2012;11:427–32. doi: 10.1016/j.jcf.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 74.Lindor KD, Kowdley KV, Luketic VA, et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50:808–14. doi: 10.1002/hep.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ooi CY, Nightingale S, Durie PR, Freedman SD. Ursodeoxycholic acid in cystic fibrosis-associated liver disease. J Cyst Fibros. 2012;11:72–3. doi: 10.1016/j.jcf.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 76.Gana JC, Turner D, Roberts EA, Ling SC. Derivation of a clinical prediction rule for the noninvasive diagnosis of varices in children. J Pediatr Gastroenterol Nutr. 2010;50:188–93. doi: 10.1097/MPG.0b013e3181b64437. [DOI] [PubMed] [Google Scholar]
- 77.Mileti E, Rosenthal P. Management of portal hypertension in children. Curr Gastroenterol Rep. 2011;13:10–6. doi: 10.1007/s11894-010-0151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Funakoshi N, Duny Y, Valats JC, et al. Meta-analysis: beta-blockers versus banding ligation for primary prophylaxis of esophageal variceal bleeding. Ann Hepatol. 2012;11:369–83. [PubMed] [Google Scholar]
- 79.Rivet C, Robles-Medranda C, Dumortier J, Le Gall C, Ponchon T, Lachaux A. Endoscopic treatment of gastroesophageal varices in young infants with cyanoacrylate glue: a pilot study. Gastrointest Endosc. 2009;69:1034–8. doi: 10.1016/j.gie.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 80.Louis D, Duc ML, Reix P, et al. Partial splenectomy for portal hypertension in cystic fibrosis related liver disease. Pediatr Pulmonol. 2007;42:1173–80. doi: 10.1002/ppul.20713. [DOI] [PubMed] [Google Scholar]
- 81.Harned RK, II, Thompson HR, Kumpe DA, Narkewicz MR, Sokol RJ. Partial splenic embolization in five children with hypersplenism: effects of reduced-volume embolization on efficacy and morbidity. Radiology. 1998;209:803–6. doi: 10.1148/radiology.209.3.9844678. [DOI] [PubMed] [Google Scholar]
- 82.Brandt CT, Rothbarth LJ, Kumpe D, Karrer FM, Lilly JR. Splenic embolization in children: long-term efficacy. J Pediatr Surg. 1989;24:642–5. doi: 10.1016/s0022-3468(89)80710-9. [DOI] [PubMed] [Google Scholar]
- 83.Kumpe DA, Rumack CM, Pretorius DH, Stoecker TJ, Stellin GP. Partial splenic embolization in children with hypersplenism. Radiology. 1985;155:357–62. doi: 10.1148/radiology.155.2.3885306. [DOI] [PubMed] [Google Scholar]
- 84.Robberecht E, Van Biervliet S, Vanrentergem K, Kerremans I. Outcome of total splenectomy with portosystemic shunt for massive splenomegaly and variceal bleeding in cystic fibrosis. J Pediatr Surg. 2006;41:1561–5. doi: 10.1016/j.jpedsurg.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 85.Van Biervliet S, Robberecht E. Splenectomy in cystic fibrosis. Arch Dis Child. 2007;92:277–8. doi: 10.1136/adc.2006.110197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Genyk YS, Quiros JA, Jabbour N, Selby RR, Thomas DW. Liver transplantation in cystic fibrosis. Curr Opin Pulm Med. 2001;7:441–7. doi: 10.1097/00063198-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 87.Lu BR, Esquivel CO. A review of abdominal organ transplantation in cystic fibrosis. Pediatr Transplant. 2010;14:954–60. doi: 10.1111/j.1399-3046.2010.01412.x. [DOI] [PubMed] [Google Scholar]
- 88.Kramer RE, Sokol RJ, Yerushalmi B, et al. Large-volume paracentesis in the management of ascites in children. J Pediatr Gastroenterol Nutr. 2001;33:245–9. doi: 10.1097/00005176-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 89.Fridell JA, Bond GJ, Mazariegos GV, et al. Liver transplantation in children with cystic fibrosis: a long-term longitudinal review of a single center's experience. J Pediatr Surg. 2003;38:1152–6. doi: 10.1016/s0022-3468(03)00260-4. [DOI] [PubMed] [Google Scholar]
- 90.Noble-Jamieson G, Barnes N, Jamieson N, Friend P, Calne R. Liver transplantation for hepatic cirrhosis in cystic fibrosis. J R Soc Med. 1996;89(Suppl. 27):31–7. [PMC free article] [PubMed] [Google Scholar]
- 91.Gridelli B. Liver: benefit of liver transplantation in patients with cystic fibrosis. Nat Rev Gastroenterol Hepatol. 2011;8:187–8. doi: 10.1038/nrgastro.2011.39. [DOI] [PubMed] [Google Scholar]
- 92.Mendizabal M, Reddy KR, Cassuto J, et al. Liver transplantation in patients with cystic fibrosis: analysis of United Network for Organ Sharing data. Liver Transpl. 2011;17:243–50. doi: 10.1002/lt.22240. [DOI] [PubMed] [Google Scholar]
- 93.Melzi ML, Kelly DA, Colombo C, et al. Liver transplant in cystic fibrosis: a poll among European centers. A study from the European Liver Transplant Registry. Transpl Int. 2006;19:726–31. doi: 10.1111/j.1432-2277.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- 94.Nash KL, Collier JD, French J, et al. Cystic fibrosis liver disease: to transplant or not to transplant? Am J Transplant. 2008;8:162–9. doi: 10.1111/j.1600-6143.2007.02028.x. [DOI] [PubMed] [Google Scholar]
- 95.Miller MR, Sokol RJ, Narkewicz MR, Sontag MK. Pulmonary function in individuals who underwent liver transplantation: from the US cystic fibrosis foundation registry. Liver Transpl. 2012;18:585–93. doi: 10.1002/lt.23389. [DOI] [PubMed] [Google Scholar]
- 96.Colombo C, Costantini D, Rocchi A, et al. Effects of liver transplantation on the nutritional status of patients with cystic fibrosis. Transpl Int. 2005;18:246–55. doi: 10.1111/j.1432-2277.2004.00013.x. [DOI] [PubMed] [Google Scholar]
- 97.Nightingale S, O'Loughlin EV, Dorney SF, et al. Isolated liver transplantation in children with cystic fibrosis–an Australian experience. Pediatr Transplant. 2010;14:779–85. doi: 10.1111/j.1399-3046.2010.01341.x. [DOI] [PubMed] [Google Scholar]
- 98.Barshes NR, DiBardino DJ, McKenzie ED, et al. Combined lung and liver transplantation: the United States experience. Transplantation. 2005;80:1161–7. doi: 10.1097/01.tp.0000165717.23652.09. [DOI] [PubMed] [Google Scholar]