Abstract
Irritable bowel syndrome (IBS) is a prevalent functional disorder characterized by abdominal pain and hypervigilance to gastrointestinal sensations. We hypothesized that mindfulness training (MT), which promotes nonreactive awareness of emotional and sensory experience, may target underlying mechanisms of IBS including affective pain processing and catastrophic appraisals of gastrointestinal sensations. Seventy five female IBS patients were randomly assigned to participate in either 8 weeks of MT or a social support group. A theoretically grounded, multivariate path model tested therapeutic mediators of the effect of MT on IBS severity and quality of life. Results suggest that MT exerts significant therapeutic effects on IBS symptoms by promoting nonreactivity to gut-focused anxiety and catastrophic appraisals of the significance of abdominal sensations coupled with a refocusing of attention onto interoceptive data with less emotional interference. Hence, MT appears to target and ameliorate the underlying pathogenic mechanisms of IBS.
Keywords: Mindfulness, irritable bowel syndrome, pain, therapeutic mechanisms, path analysis, interoception
INTRODUCTION
How do psychological interventions ameliorate distressing somatic symptoms? Although several decades of clinical research have demonstrated the efficacy of psychological therapies for mind-body conditions since Engel’s (1977) articulation of the biopsychosocial paradigm, the therapeutic mechanisms of many such treatments remain unspecified. One such condition, irritable bowel syndrome (IBS), remains refractory for a substantial number of patients receiving usual medical care, yet seems to be especially tractable to psychological treatments like cognitive behavior therapy (CBT) (Lackner et al., 2007), clinical hypnosis (Whorwell et al., 1984), and mindfulness-based therapy (Gaylord et al., 2011; Ljotsson et al., 2010). IBS is a functional gastrointestinal (GI) disorder characterized by abdominal pain, heightened visceral sensitivity, and in some cases, altered gastrointestinal motility, and these symptoms are often reciprocally linked to stress, anxiety, catastrophic thinking, and negative affect (Mayer & Tillisch, 2010). Given the established physiological linkages between the enteric and central nervous systems, enhanced responsiveness to psychosocial stressors and perturbations of visceral homeostasis may result in exaggerated autonomic reactions, altered bowel habits, hyperalgesia, and hypervigilance towards interoceptive signals from the gut (Mayer, 1999). Furthermore, selective attention to visceral sensations and heightened threat appraisals may maintain IBS symptoms in lieu of present stressors (Naliboff et al., 2008). Thus, central and peripheral systems interact to produce and preserve the pathogenic processes underpinning IBS.
Given the substantial contribution of psychological factors to IBS symptomatology, the therapeutic benefit of psychological interventions for IBS has been thought to derive from reductions in comorbid psychological distress (Lackner et al., 2007). However, path analyses of CBT as a treatment for IBS conducted by Lackner and colleagues (2007) found no evidence for such a presumed meditational effect. Instead, in their model CBT was directly associated with improvement in GI symptoms which, in turn, was associated with increased quality of life. Enhancements in quality of life were reciprocally linked with reductions in psychological distress, but no evidence was found for a direct effect of CBT on psychological distress. However, a study by Jones et al. (2011) identified an indirect effect of CBT on IBS symptoms, such that improvement in mood led to the perception of improved IBS symptoms. Thus, the therapeutic mechanisms of CBT on IBS severity remain unclear.
Apart from CBT, the other most commonly tested psychological therapy for IBS is clinical hypnosis. In studies of hypnosis, significant reductions in maladaptive IBS-related cognitions, affective symptoms, and somatization are frequently reported along with improvement in gastrointestinal symptoms (Gonsalkorale et al., 2004; Palsson et al., 2002). These psychological changes may mediate the robust therapeutic effects of clinical hypnosis on IBS observed across multiple clinical trials (Miller & Whorwell, 2009).
Like CBT and clinical hypnosis, mindfulness training (MT) may hold promise for IBS sufferers who remain highly symptomatic in spite of the best efforts of conventional medical care. MT, whether provided by secular programs such as Mindfulness-Based Stress Reduction (MBSR) or through more traditional Buddhist practices, involves the cultivation of a state characterized by an attentive and nonjudgmental metacognitive monitoring of moment-by-moment cognition, emotion, perception, and sensation without fixation on thoughts of past and future (Garland, 2007). According to some theoretical accounts (e.g., Chambers, Gullone, & Allen, 2009; Davidson, 2010; Garland et al., 2010) the repeated practice of generating the state of mindfulness leads to the development of trait or dispositional mindfulness, i.e., the propensity towards exhibiting nonjudgmental, nonreactive awareness of one’s thoughts, emotions, experiences, and actions in everyday life (Baer, Smith, Hopkins, Krietemeyer, & Toney, 2006). In support of this notion, Carmody & Baer (2008) found that the therapeutic effects of participation in MBSR are mediated by increases in dispositional mindfulness. Given the growing body of research demonstrating the therapeutic effects of MT on stress and pain symptoms (Grossman et al., 2004; Rosenzweig et al., 2010) in chronic functional disorders such as fibromyalgia and depression (Kaplan et al., 1993; Teasdale et al., 2000), MT may be an effective treatment for IBS, a condition often rendered refractory by stress (Lea & Whorwell, 2004).
Findings from our recent randomized controlled trial (RCT) demonstrate that MT results in statistically and clinically significant improvements in bowel symptoms, affective symptoms, and IBS-related quality-of-life (Gaylord et al., 2011). In this trial, patients receiving MT exhibited significantly larger reductions in IBS severity after treatment (an average 38.2% reduction in symptom scores) and significantly greater improvements in quality of life than those participating in a support group. These findings converge with those of a RCT of an internet-delivered, mindfulness- and exposure-based treatment for IBS, which also identified significant therapeutic effects on IBS symptoms (Ljotsson et al., 2010).
What processes might account for the ameliorative effects of MT on IBS symptomatology? During the development of our recently completed RCT, we theorized that the following therapeutic mechanisms may mediate the effects of MT on IBS symptomatology. First, recurrent mindfulness practice may promote the development of nonreactivity to negative emotions, distressing cognitions, and visceral sensations. Over time, the cultivation of a nonreactive mindset may decrease both visceral sensitivity and pain catastrophizing, the tendency to overemphasize the threat value of the painful stimulus via perseverating on the affective components of pain. Recent research suggests that MT attenuates activation in brain areas (i.e., medial prefrontal cortex) that instantiate self-referential, linguistic processing during negative affective experience while enhancing activation in brain regions subserving interoception (i.e., insula) (Farb et al., 2010). This pattern of activation suggests that MT promotes interoceptive recovery from emotional distress, that is, a refocusing from the affective to viscerosensory aspects of a distressing experience that allows for the emotion to follow its natural time course and return to baseline, rather than being perpetuated through rumination (Farb et al., 2010). If mindfulness promotes interoceptive recovery from negative affective reactions, it may afford a similar interoceptive recovery from stress-induced physiological perturbations of visceral homeostasis often perceived as painful by IBS patients. Indeed, reduced cognitive elaboration of the sensory experience of pain through meditation has been shown to decrease pain unpleasantness (Perlman et al., 2010). Moreover, given recent findings that affective disturbances like anxiety and depression mediate the neural processing of visceral pain in IBS (Elsenbruch et al., 2010), it is reasonable to suppose that cultivating nonreactivity would decrease anxiety over abdominal sensations and thereby lead to diminished abdominal pain. In addition, increasing nonreactivity through MT may reduce pain catastrophizing, which is likely to result in improved quality of life as IBS symptoms are appraised as more manageable and less disruptive to psychosocial functioning.
A second hypothesis to account for the beneficial effects of MT on IBS is that MT may alter the manner in which pain sensations are attended to and processed. MT provides instruction in breaking down the gestalt of an experience into its phenomenological components (Strassman & Galanter, 1980). Rather than confront a monolithic (and overwhelming) experience of “pain,” a practitioner might attend to a sensation of heat localized in the upper right quadrant of the abdomen, or the tightening of one of the abdominal muscles, and then notice how this sensation changes over time. This form of interoceptive processing may attenuate abdominal pain intensity in IBS. Evidence from a number of laboratories suggests that both brief (Zeidan et al., 2009) and long-term mindfulness meditation (Grant & Rainville, 2009; Zeidan et al., in press) are associated with decreased pain and increased pain thresholds during experimental pain stimulation, corroborating findings of decreased chronic pain in clinical research (Grossman et al., 2004; Rosenzweig et al., 2010).
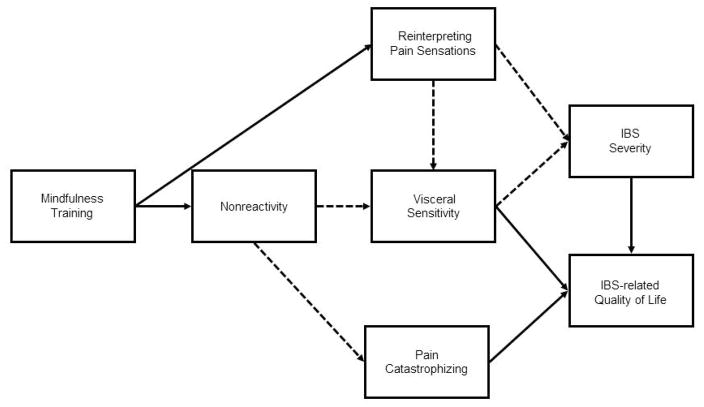
The aim of the present study was to elucidate the therapeutic mechanisms of a MT intervention for women with irritable bowel syndrome. We hypothesized that the reduction in IBS symptoms and improvements in quality of life associated with MT are mediated by reinterpretation of pain sensations and increased nonreactivity, leading to reductions in visceral sensitivity and pain catastrophizing (Figure 1). We sought to test the aforementioned hypotheses in a single multivariate path model that could simultaneously evaluate the multiple causal pathways outlined above.
Figure 1.
Theoretical model of the therapeutic mechanisms of mindfulness training on cognitiveaffective mediators of IBS severity and IBS-related quality of life. Solid arrows represent amplification, whereas dashed arrows represent attenuation.
MATERIALS AND METHODS
A detailed description of the research protocol was described in previous reports (Gaylord et al., 2009; Gaylord et al., 2011) and is presented here more succinctly.
Study Participants
Female patients with IBS were recruited from 2006–2009 through advertisements and an existing registry of IBS patients. Women aged 18–75 years who met Rome II diagnostic criteria for IBS were included in the study. Individuals with a psychotic disorder or a history of psychiatric hospitalization in the past two years were excluded, as were participants with a history of inflammatory bowel disease, celiac disease, gastrointestinal malignancy, liver or pancreatic disease, or abdominal trauma. Subjects continued to receive usual medical care for their IBS.
Study Procedure
Study procedures were approved by the institutional review board of the University of North Carolina at Chapel Hill. Eligible study participants were randomized to either a MT group or support group (SG). Of the 210 women assessed for eligibility, 97 were found to be eligible, and were enrolled. Seventy-five (76.5%) began treatment after randomization - 39 in MT and 36 in the SG (see the CONSORT diagram in Figure 2). Enrolled subjects met in their assigned groups for eight weekly two-hour sessions plus one four-hour retreat. Subjects completed paper-and-pencil questionnaires at baseline and two weeks after the conclusion of the intervention. Nine subjects withdrew from the study during the intervention, 5 in the MT group and 4 in the SG (Figure 2).
Figure 2.
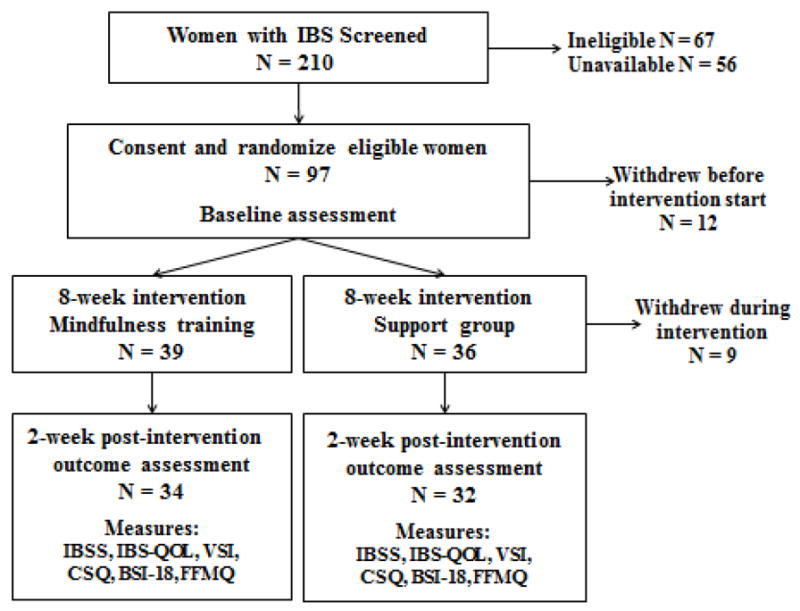
CONSORT diagram.
Mindfulness training intervention
The MT intervention was based on the Mindfulness-Based Stress Reduction (MBSR) program developed by Jon Kabat-Zinn at the University of Massachusetts. The mindfulness instructor was a certified professional health coach with approximately 100 hours of post-baccalaureate education in health coaching, professional training in MBSR from the Center for Mindfulness at University of Massachusetts, and over ten years of experience teaching MBSR in clinical settings. The course was typical in length and content, with instruction in sitting and walking meditations, the body scan technique, and mindful yoga, but was tailored to address IBS-related concerns. For example, participants were instructed to notice any sensations in the abdominal area and distinguish those sensations from thoughts about sensations. In so doing, the mindfulness-training intervention promoted sensory, versus emotional, processing of interoceptive signals and disengagement from pain catastrophizing. Homework assigned each week throughout the course included daily mindfulness practices and psychoeducational readings (Gaylord et al., 2009).
Support Group intervention
For the control intervention, clinical social workers facilitated a support group to control for expectations of benefit and amount of group contact. A previous clinical trial of CBT for IBS used a similar support group as a control condition (Payne & Blanchard, 1995). Weekly sessions focused on IBS-related topics and involved open group discussions about subjects’ experiences with, or reaction to, the topic. Homework assignments consisted of psychoeducational readings (Gaylord et al., 2009).
Measures
IBS Severity
The Irritable Bowel Symptom Severity Scale (IBS-SS) served as the primary outcome variable (Francis et al., 1997). On this scale, responders rate retrospectively, for the past 10 days, abdominal pain severity and frequency (separate ratings), bloating severity, dissatisfaction with bowel habits and life interference from bowel symptoms. These five ratings are totaled to obtain an overall IBS severity score. Internal consistency of the scale in our study sample was acceptable (α = 0.72).
IBS-Related Quality of Life
Changes in physical and psychosocial functioning as a result of IBS were measured with the IBS-Quality of Life (IBS-QOL) scale, a 34-item scale that is responsive to therapeutic change (Drossman et al., 2000), and has high test-retest reliability (Patrick et al., 1998). In the present sample, internal consistency was high (α = 0.95).
Dispositional Mindfulness
We examined the construct of dispositional mindfulness via the Five Facet Mindfulness Questionnaire (FFMQ; Baer et al., 2006), a validated, 39-item Likert-type scale that assesses five internally-consistent factors of mindfulness: nonreactivity to inner experience (tapped by items such as “I watch my feelings without getting lost in them”), observing and attending to experience (“I pay attention to sensations, such as the wind in my hair or the sun on my face”), describing and differentiating experience (“I’m good at finding words to describe my feelings”), nonjudging of experience (reverse coded item: “I tell myself I shouldn’t be feeling the way that I am feeling”), and acting with awareness (reverse coded item: “I find myself doing things without paying attention”). We focused the present analysis on the nonreactivity subscale of the FFMQ (α = 0.86), to test our hypothesis that nonreactivity would mediate the effect of MT on visceral sensitivity and pain catastrophizing.
Pain Catastrophizing
Catastrophizing was assessed with the six-item pain catastrophizing subscale of the Coping Strategy Questionnaire (CSQ) (Rosenstiel & Keefe, 1983), which has been shown to correlate with measures of pain intensity and functional impairment (Robinson et al., 1997). Items on this scale tap agreement with statements like, “I feel I can’t stand it anymore,” and “It’s terrible and I feel it’s never going to get any better.” Internal consistency in this sample was high (α = 0.92).
Visceral Sensitivity
Hypervigilance to visceral sensations and gut-focused anxiety were assessed with the Visceral Sensitivity Index (VSI), a validated 15-item scale (Labus et al., 2004). The VSI has been shown to be a strong predictor of symptom severity (Labus et al., 2004), and evidenced excellent internal consistency in this sample (α = 0.91). For the purposes of the analyses in this paper, the VSI score was calculated as the numerical sum of responses to 15 statements pertaining to anticipatory anxiety with respect to the likely occurrence of distressing gastrointestinal symptoms. As presented in the original scale validation paper, participants were asked to rate the extent to which they could relate to these statements using a six-point scale that ranged from 1 = “Strongly agree” to 6 = “Strongly disagree,” with higher scores indicative of lower visceral sensitivity (Labus et al., 2004).
Reinterpretation of Pain Sensations
Cognitive coping with pain by reinterpreting painful sensations as sensory experiences was assessed via the reinterpreting pain sensations subscale of the CSQ (Rosenstiel & Keefe, 1983). This scale is comprised of items including “I don’t think of it as pain but rather as a dull or warm feeling,” and “I just think of it as another sensation such as numbness.” In the present sample, the reinterpretation of pain sensations subscale demonstrated high internal consistency (α = 0.88).
Psychological Distress
Psychological distress was measured by the Brief Symptom Inventory-18 (BSI-18) (Derogatis, 2000) The BSI-18 provides separate subscale scores for anxiety, depression, and somatization, as well a global symptom severity index. Here, the global severity index, which evidenced high internal consistency (α = 0.87), was used as an overall measure of psychological distress.
Statistical Analysis
First, we conducted paired t-tests to examine whether potential mediating variables changed from pre- to post-intervention within each treatment group. Next, we examined zero-order correlations between potential mediating and outcome variables, prior to estimating multivariate path models. Subsequently, multivariate path analysis within a structural equation modeling framework, which provides simultaneous estimation of multiple linear equations, was conducted with AMOS 17.0 to examine the direct and indirect relationships between treatment condition, potential psychological mediators, and therapeutic outcomes. Path analysis (a) tests the significance of the relationships between variables in a hypothetical model and (b) compares alternative models to determine which model best fits the observed data. The starting point for the specification of the measures and paths in our models was based on theory, as outlined in our original study proposal.
We screened all the potential variables that were candidate mediators of the effects of MT on IBS severity and IBS-related quality of life and dropped those which showed no difference between MT and SG and those which were not significantly correlated with IBS severity or quality of life. Although our theoretical model was centered on nonreactivity as a key therapeutic mechanism of mindfulness, we sought to determine if other FFMQ facets were integral mediating the effects of MT on cognitive risk factors (i.e., visceral sensitivity and catastrophizing) for IBS severity and impaired quality of life. As such, we examined correlations between FFMQ facets and the other study variables. In addition to nonreactivity, nonjudgment and acting with awareness were significantly associated with changes in visceral sensitivity and catastrophizing (r’s = .30 – .46; p’s < .05). Because there were no significant between-groups differences in nonjudgment (p = .18), this facet was rejected as a unique mediator of MT on IBS-related clinical outcomes. Further, neither the MT group nor the SG reported significant increases in acting with awareness, and thus this mindfulness facet could also not serve as a mediator the effects of MT on IBS-related clinical outcomes.
Consequently, our initial hypothetical model focused on nonreactivity and excluded the other 4 facets in the FFMQ. This simplified theoretical model is shown in Figure 1. The final model was generated using a data-driven approach to achieve the best model fit, where we examined correlation matrices for data suggesting that our a priori model had omitted important paths, and trimmed nonsignificant paths to improve model fit using the chi-square difference test to evaluate the comparative parsimony of nested models (Kline, 1998).
Subjects in both study arms (MT and SG) were included in path analyses. A dummy variable representing the MT versus the SG treatment conditions was created (where 1 = MT and 0 = SG) and was used as the independent (exogenous) variable in multivariate path analyses. Pre-post treatment change scores were used for all endogenous variables (Maris, 1998; Rogosa, 1988). Figure 1 depicts our main hypothetical model. Treatment condition was hypothesized to exert indirect effects on changes in IBS severity, as we hypothesized that MT would result in decreased abdominal pain through two main pathways: a) by increasing nonreactivity, resulting in decreased visceral sensitivity over the course of treatment; and b) by increasing reinterpretation of pain sensations, also resulting in decreased visceral sensitivity. Similarly, treatment condition was modeled to exert indirect effects on changes in IBS-related quality of life, potentially mediated by changes in visceral sensitivity, pain catastrophizing, and IBS severity.
We also tested a series of four alternative models. In model two we conducted a partial mediation model where treatment condition had both direct and indirect effects on treatment changes in IBS severity and IBS-related quality of life. This model proposed that treatment (i.e., mindfulness training) could directly reduce IBS severity and increase IBS-related quality of life in addition to influencing these outcomes indirectly via pathways through nonreactivity, reinterpretation of pain sensations, visceral sensitivity, and pain catastrophizing.
Next, because it is commonly assumed that psychological interventions ameliorate IBS symptoms by reducing psychological symptoms, we tested model three where changes in psychological distress could potentially mediate the treatment effect. This competing hypothesis was reasonable given the body of literature demonstrating therapeutic effects of mindfulness practice on psychological distress (e.g., Jones et al., 2011, Lackner et al., 2007), as well as our own findings of significantly improved psychological distress among IBS patients at follow-up (Gaylord et al., 2011).
Lastly, because our hypothetical model, like all causal models, is prone to specification error, two other alternative models were assessed to ensure that significant path coefficients identified were not artifactual. A priori, we reasoned that reductions in catastrophizing would be more directly linked with IBS-related quality of life than IBS severity, given that catastrophizing has been robustly associated with quality of life among IBS patients (Seres et al., 2008). To test this assumption, we ran model four, with a path between change in catastrophizing and change in IBS severity rather than IBS-related quality of life. Furthermore, we presumed a priori that because the mindfulness intervention offered explicit training in reinterpretation of pain sensations as a distinct technique from the cultivation of nonreactivity to thoughts and emotions, there would be a direct pathway from the treatment variable to change in reinterpretation of pain sensations. To confirm this assumption, we tested model five where the effect of treatment on change in reinterpretation of pain sensations was indirectly mediated by change in nonreactivity.
Because we found that sociodemographic (age, education, and race) variables were statistically unrelated to IBS severity and quality of life in the present sample, they were omitted from path analyses for the purposes of parsimony.
AMOS 17.0 was used to calculate model parameters, and missing data were handled using full-information maximum likelihood estimation. Model fit was determined comparing fit indices to widely accepted cut-offs (Kline, 1998), including nonsignificant χ2 (p > .05), comparative fit index (CFI) > 0.90, and the root-mean square error of approximation (RMSEA) < 0.08.
RESULTS
Pre-post intervention changes
Characteristics of the study sample are provided in Table 1. There were no significant differences between MT and SG participants on any of the demographic variables. In addition, descriptive statistics for MT and SG groups on changes in clinical outcomes and the hypothesized cognitive-affective mediators of the treatment effects are shown in Table 2. Participants in the MT group experienced significant improvements in IBS severity, IBS-related quality of life, nonreactivity, nonjudgment of experience, observing and attending to experience, visceral sensitivity, pain catastrophizing, psychiatric distress, and cognitive coping via reinterpretation of pain sensations from pre- to post-treatment. In contrast, no significant changes were observed among SG participants. Zero-order correlations between primary study variables are presented in Table 3.
Table 1.
Demographic characteristics
| Variable | Total
|
Mindfulness Group
|
Support Group
|
Drop outs (not treated)
|
||||
|---|---|---|---|---|---|---|---|---|
| Na | % | N | % | N | % | N | % | |
|
|
|
|
|
|
||||
| Race/ethnicity | ||||||||
| White | 54 | 76.1 | 29 | 82.9 | 25 | 69.4 | 15 | 68.2 |
| Non-white | 17 | 23.9 | 6 | 17.1 | 11 | 30.6 | 7 | 31.8 |
| Marital status | ||||||||
| Partnered | 40 | 54.1 | 15 | 41.7 | 25 | 65.8 | 11 | 50 |
| Not partnered | 34 | 45.9 | 21 | 58.3 | 13 | 34.2 | 11 | 50 |
| Income | ||||||||
| $40,000 or less | 24 | 36.4 | 9 | 27.3 | 19 | 51.4 | 15 | 71.4 |
| $41,000 – 80,000 | 27 | 40.9 | 14 | 42.4 | 13 | 35.1 | 3 | 14.3 |
| > $80,000 | 15 | 22.7 | 10 | 30.3 | 5 | 13.5 | 3 | 14.3 |
| Education | ||||||||
| High school or less | 3 | 4.1 | 0 | 0 | 3 | 7.9 | 3 | 13.6 |
| Some college | 23 | 31.5 | 9 | 25.7 | 14 | 36.8 | 8 | 36.4 |
| College graduate | 26 | 35.6 | 13 | 37.1 | 13 | 34.2 | 0 | 36.4 |
| Graduate degree | 21 | 28.8 | 13 | 37.1 | 8 | 21.1 | 3 | 13.6 |
| Age | ||||||||
| < 30 | 15 | 20.3 | 4 | 11.1 | 11 | 28.9 | 5 | 21.7 |
| 30 – 49 | 31 | 41.9 | 17 | 47.2 | 14 | 36.8 | 9 | 39.1 |
| 50 – 64 | 22 | 29.7 | 12 | 33.3 | 10 | 26.3 | 8 | 34.8 |
| 65 – 76 | 6 | 8.1 | 3 | 8.3 | 3 | 7.9 | 1 | 4.4 |
Several participants declined to give responses for demographic questions, resulting in a small proportion of missing data on several of the variables.
Table 2.
Pre-post treatment changes in clinical outcomes and hypothesized cognitive-affective mediators of the treatment effects.
| Variable | Change in score: Mindfulness Group | Change in score: Support Group | ||
|---|---|---|---|---|
|
| ||||
| Mean | S.D. | Mean | S.D. | |
|
|
|
|||
| IBS severity | −82.0*** | 97.1 | −22.5 | 88.7 |
| IBS-quality of life | 11.2*** | 15.9 | 4.4 | 16.7 |
| Visceral sensitivity | −6.3** | 11.7 | −1.5 | 14.2 |
| Pain catastrophizing | −3.5** | 6.2 | −2.9 | 8.1 |
| Reinterpreting pain sensations | 4.4*** | 5.5 | 0.8 | 4.7 |
| Nonreactivity | 2.7** | 4.6 | 0.1 | 4.2 |
| Nonjudging of experience | 3.7** | 7.7 | 1.4 | 5.8 |
| Acting with awareness | 0.7 | 5.2 | 1.2 | 4.7 |
| Observing/attending to experience | 2.9** | 4.2 | −0.2 | 3.3 |
| Describing/differentiating experiences | 1.0 | 3.9 | 0.5 | 4.9 |
| Psychological distress | −4.0** | 6.3 | −1.8 | 7.4 |
t-test p-value:
p < .05
p < .01
p < .001
Table 3.
Correlations between changes in clinical outcome variables and cognitive-affective mediators.
| 1. | 2. | 3. | 4. | 5. | 6. | |
|---|---|---|---|---|---|---|
| 1. IBS severity | 1 | |||||
| 2. IBS-related quality of life | −.52*** | 1 | ||||
| 3. Visceral sensitivity | .31* | −.56*** | 1 | |||
| 4. Pain catastrophizing | −.00 | −.40** | .29* | 1 | ||
| 5. Reinterpreting pain sensations | −.28* | .25 | −.01 | −.09 | 1 | |
| 6. Nonreactivity | −.20 | .38** | −.53*** | −.31* | −.01 | 1 |
p < .05;
p < .01;
p < .001
Test of the hypothesized model
Model one: full mediation model
Our hypothesized model (Figure 1) exhibited good fit: χ2/df = 1.26, p = .24; RMSEA = .05 (.00, .13), CFI = .96, AIC = 61.83. Results indicated that the therapeutic effect of MT on IBS severity was mediated by increasing reinterpretation of pain sensations and decreasing visceral sensitivity, the latter being mediated, in turn, by improvements in nonreactivity. Similarly, increases in nonreactivity mediated reductions in pain catastrophizing, which, along with reductions in visceral sensitivity and IBS severity, mediated improvements in IBS-related quality of life. However, the pathway between change in reinterpretation of pain sensations and visceral sensitivity was not significant. As such, we trimmed this pathway, and achieved a better model fit: χ2/df = 1.15, p = .31; RMSEA = .04 (.00, .12), CFI = .98, AIC = 59.83. Given that these models were nested (i.e., the second model without the path between change in reinterpretation of pain sensations and visceral sensitivity is a subset of the first model), a chi-square difference test was employed for model comparison, χD2 = χ2 (m2) - χ2 (m1), with dfD = df(model 2)-df(model 1), where a significant χD2 value indicates that the trimmed model is oversimplified (Kline, 1998). The non-significant chi-square value obtained (p = .86) indicated that trimmed model was most parsimonious. Given that this model (Figure 2) was more parsimonious and fit our a priori hypotheses, we henceforth report its parameter estimates in detail.
Mediation of the effect of MT on IBS severity
MT was significantly associated with increases in reinterpretation of pain sensations (β = .34, p < .01) and increases in nonreactivity (β = .29, p < .05). Additionally, increased nonreactivity was associated with decreased visceral sensitivity (β = .48, p < .001) and pain catastrophizing (β = .33, p < .01). In turn, decreased visceral sensitivity and (β = .26, p < .05) and increased reinterpretation of pain signals (β = .29, p < .05) were significantly associated with reduced IBS severity. The path model accounted for 16% of the variance in improvements in IBS severity.
Mediation of the effect of MT on IBS-related quality of life
There was a direct relationship between decreased IBS severity and improved IBS-related quality of life (β = .28, p = .002), which indicated that reductions in IBS severity over the course of the MT intervention predicted increases in quality of life. In addition, decreases in visceral sensitivity (β = .39, p < .001) and pain catastrophizing (β = .45, p < .001) over the course of treatment were associated with increased IBS-related quality of life. The path model accounted for 56% of the variability in improvements in quality of life.
Tests of alternative models
Model two: partial mediation model
We first tested an alternative, partial mediation model where intervention condition had both direct and indirect effects on IBS severity and IBS-related quality of life. This model also demonstrated good fit, χ2/df = 1.15, p = .32; RMSEA = .04 (.00, .12), CFI = .98, AIC = 61.48. Given that the model with only indirect effects of treatment was a subset of an alternate model with both direct and indirect effects of treatment, another chi-square difference test was employed for model comparison. The non-significant chi-square value obtained (p = .31) indicated that the additional direct effects of MT on IBS severity and IBS-related quality of life should not be retained in the final model, and that a full mediation model with only indirect effects of MT on IBS severity and IBS-related quality of life was more parsimonious.
Model three: psychological distress as a mediator
Although changes in reinterpretation of pain sensations, nonreactivity, visceral sensitivity, and catastrophizing were found to mediate the effects of MT, decreased IBS severity, and increased IBS-related quality of life, competing models explicating the pathways between these variables are possible. Given intervention effects on psychological distress observed in clinical outcomes research on MT for IBS (Gaylord et al., 2011) and in the studies of Lackner et al. (2007) and Jones et al. (2011) on cognitive-behavioral therapy for IBS, we next tested an alternative path model where changes in psychological distress could mediate the effect of MT on IBS-related quality of life. This model exhibited acceptable fit: χ2/df = 1.25, p = .23; RMSEA = .06 (.00, .13), CFI = .95, AIC = 77.47. However, in this model, after controlling for all of the aforementioned variables, pathways between MT and changes in psychological distress and IBS-related quality of life were statistically nonsignificant. Upon comparing fit indices of these two models, our original hypothetical model exhibited superior fit to the alternative model.
Models four and five
Neither of the other two alternative models fit the data as well as the final model presented in Figure 3 (model four: χ2/df = 2.69, p = .001; RMSEA = .13 (.08, .19), CFI = .74, AIC = 78.28; model five: χ2/df = 1.74, p = .05; RMSEA = .09 (.00, .15), CFI = .89, AIC = 66.83), nor did the parameter estimates derived contradict the basic conclusions made in the results and discussion sections of this paper. Hence, we do not present parameters of these alternative models. Given the presence of nonsignificant paths and decrements in model fit, our original (yet trimmed) hypothetical model was retained as the final model. The final model is presented in Figure 3 and Table 4.
Figure 3.
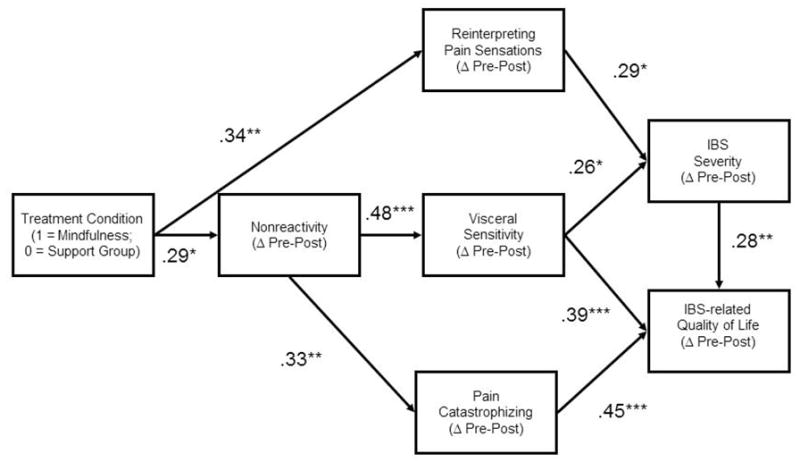
Path model (N = 75) testing mediational relationships between mindfulness training, reinterpretation of pain sensations, visceral sensitivity, nonreactivity, pain catastrophizing, IBS severity, and IBS-related quality of life.
Table 4.
Parameter estimates for the final model of the therapeutic effect of mindfulness training on IBS severity and quality of life.
| Path Coefficient | Unstandardized beta | Standard error | p | |
|---|---|---|---|---|
| Mindfulness training → Δ reinterpretation | .34 | 3.62 | 1.33 | .006 |
| Mindfulness training → Δ nonreactivity | .29 | 2.59 | 1.11 | .02 |
| Δ Nonreactivity → Δ visceral sensitivity | −.48 | 1.37 | .33 | <.001 |
| Δ Nonreactivity → Δ catastrophizing | .33 | .52 | .20 | .008 |
| Δ Reinterpretation → Δ IBS severity | .29 | 5.26 | 2.16 | .02 |
| Δ Visceral sensitivity → Δ IBS severity | −.26 | 1.93 | .87 | .03 |
| Δ Catastrophizing → Δ IBS-related quality of life | .45 | 1.03 | .20 | <.001 |
| Δ Visceral sensitivity → Δ IBS-related quality of life | −.39 | −.49 | .11 | <.001 |
| Δ IBS severity → Δ IBS-related quality of life | .28 | .05 | .02 | .002 |
Δ = change from pre- to post-treatment levels. Total R for change in IBS severity from pre- to post-treatment = .15; Total R2 for change in IBS-related quality of life from pretreatment to 3-month follow-up = .56
DISCUSSION
Our research suggests that MT substantially attenuates IBS symptoms and ameliorates the often severe impairments in quality of life that characterize this disorder (Gaylord et al., 2011). Results of the path analyses in the present investigation indicate that these therapeutic effects were mediated by a number of cognitive processes with known associations to the affective and sensory antecedents and consequences of pain. MT led to increased nonreactivity to cognitions, emotions, and physiological sensations which in turn was associated with decreased visceral sensitivity. Reductions in visceral sensitivity predicted decreased severity of IBS symptoms and improved IBS-related quality of life. In addition, increases in nonreactivity were associated with decreases in pain catastrophizing which also predicted improvements in quality of life. MT also led to increases in reinterpretation of pain sensations which predicted reductions in IBS severity. Overall, reduced IBS severity mediated improvements in IBS-related quality of life.
With regard to both IBS severity and the effect of IBS on quality of life, cultivating a nonreactive mindset toward potentially distressing thoughts, affective states, and somatic symptoms appears to be a key process. Based on our path analyses, it appears that as IBS patients learn through MT to let go of visceral sensations and their worried thoughts about such sensations and observe them with a dispassionate attentional stance, anxiety over sensations of gastrointestinal distention decreases, leading to reductions in abdominal pain and IBS-related impairments in quality of life. This causal pathway identified in our analysis may be subserved by the same brain mechanisms that underlie the neuromodulatory effects of anxiety on visceral hyperalgesia (Elsenbruch et al., 2010). Cultivating nonreactivity may also lead to less catastrophic primary appraisals of the threat value of painful sensations in the gut while increasing veracity of secondary appraisals of one’s ability to manage those sensations. Such mindfulness-related reductions in catastrophizing may thereby lessen impairments in psychosocial functioning secondary to IBS, as patients come to realize that they can successfully cope with unpleasant gastrointestinal symptoms without substantial disruption of their everyday lives.
Moreover, evidence from experimental and clinical studies demonstrates that negative mood induction increases pain perception via emotion-based tuning of the pain neuromatrix (Wiech & Tracey, 2009). Conversely, neuromodulatory activations of anterior cingulate cortex (ACC) and lateral prefrontal cortex (PFC) are associated with decreases in pain resulting from cognitive interventions that promote detachment from the affective components of pain (Kalisch et al., 2005). It is possible that the nonreactive awareness engendered by MT may influence the affective modulation of pain via increases in lateral PFC and ACC activity, brain structures which appear to subserve hypnosis-induced decreases in pain unpleasantness (Rainville et al., 1997). Although hypnosis is often used to facilitate dissociation from pain whereas MT encourages “being with” pain, both interventions share a component of attentional refocusing and detachment from affective reactions to noxious stimuli.
Concomitantly, reinterpretation of pain sensations as sensory signals, taught as part of the MT, appears to be another key mechanism underlying the therapeutic effect of the intervention on IBS symptoms. During the 8-week course of MT, IBS patients were instructed to attend to the sensory qualities of their visceral sensations rather than their thoughts about such sensations. Mindfully engaging attention toward gastrointestinal symptoms may objectify visceral sensations as innocuous sensory data rather than as an emotionally salient threat to physical wellbeing (Farb et al., 2010). By coping with abdominal pain in this manner, cognitive elaboration on the unpleasantness of pain is reduced while attention to interoceptive signals is increased. Such a shift from affective to sensory processing may result in interoceptive recovery from experiences of emotional distress stemming from abdominal pain. Indeed, a recent study of the effect of mindfulness on experimentally induced pain found that meditation-related decreases in pain intensity were associated with increased activation of the ACC and anterior insula (Zeidan et al., in press), brain regions that putatively may subserve the process of interoceptive recovery. Without brain imaging data, the foregoing, proposed linkages between therapeutic processes and neurocognitive mechanisms are merely informed speculations. Yet, current study findings robustly confirm a priori hypotheses about the ways in which MT operates to ameliorate somatic symptoms.
The present findings should be considered in light of several studies that investigated the therapeutic mechanisms of CBT for IBS. Lackner and colleagues (2007) identified a feedback loop between changes in psychological distress and IBS-related quality of life. Jones et al. (2009) identified indirect effects of CBT on IBS symptoms that were partially mediated by changes in anxiety and depression. In contrast, we found no significant relationship between changes in psychological distress and IBS-related quality of life after controlling for the influence of pain catastrophizing and visceral sensitivity. One explanation for this discrepancy may be that CBT and MT operate through distinct therapeutic mechanisms, where the former targets distressing cognitive-emotional content while the latter targets a maladaptive cognitive-emotional process (Hayes & Wilson, 2003), that is, conflating cognitive evaluations of sensory experience with the actual experience in and of itself. Indeed, two key therapeutic mechanisms identified in the present investigation, nonreactivity and reinterpretation of pain sensations, are both process-level factors. These factors relate to pre-verbal, nonconceptual modes of cognitive processing that appear to be opposed to the strong affective reactions to visceral stimuli characteristic of IBS patients. Yet, despite these possible differences, MT and CBT may target some similar processes. For example, RCTs have found that participation in CBT is associated with significant reductions in visceral sensitivity and catastrophizing (Craske, Wolitzky-Taylor, Labus, Wu, Frese, Mayer, & Naliboff, 2011; Hunt, Mosier, & Millanova, 2009).
This study has substantial strengths, including the simultaneous estimation of multiple causal pathways and outcomes within the same model and the use of an active and credible control condition. There are also a number of limitations. The sample size precluded the use of latent variables in our structural equation models, and thus, although the study utilized well-validated measures, the inclusion of manifest variables in the analyses may have biased statistical estimates due to measurement error. Future research should use questionnaire items as indicators to model the latent constructs of interest (e.g., nonreactivity, pain catastrophizing, etc.). Second, although our overall model fit was excellent, the specified pathways explained only a modest (yet statistically significant) amount of variance in IBS severity, which implies that variables were omitted from the present investigation that may have contributed significantly to the therapeutic effect of mindfulness on IBS. A third primary limitation of the study relates to its reliance on self-report data. Future investigations should incorporate behavioral, psychophysiological, and neuroimaging measures to counter social desirability biases and further parse the mechanisms underpinning the robust treatment effects observed. Finally, the present investigation cannot establish causal order of putative treatment mechanisms and changes in IBS severity. For causality to be established, changes in the mediator must precede changes in the dependent variable of interest (Baron & Kenny, 1986). Pre-post changes in reinterpretation of pain sensations, nonreactivity, visceral sensitivity, IBS severity, and IBS-related quality of life were measured concurrently and thus true causal order of the hypothesized pathways cannot be ascertained. Future research should employ an even greater number of measurement points to allow for greater precision in identifying mediational pathways. The results of path analysis are “data driven,” that is, dependent on the particular set of experimental observations to support the explanatory model. Because of this, study findings should be considered exploratory and require replication in additional samples of subjects.
Earlier, we (2007; Garland et al., 2009) asserted that disengagement from negative appraisals of aversive stimuli in favor of attending to the raw sensorium of present moment experience allows for the generation of new, more adaptive appraisals rather than reprocessing an existing cognitive set already tainted by negative emotional judgments. In the present data, we see traces of this hypothetical mechanism. MT appears to result in nonreactivity to gut-focused anxiety and catastrophic appraisals of the significance of abdominal sensations coupled with a refocusing of attention onto interoceptive data with less affective interference. By learning to mindfully disengage from negative cognitive appraisals of visceral sensations and re-orient attention to the sensory quality of interoceptive experience, IBS patients may come to appraise such sensations as innocuous and eminently manageable.
Acknowledgments
The authors gratefully acknowledge funding support for this trial from the National Institutes of Health, National Center for Complementary and Alternative Medicine Grant # R21AT003619 and Grant # T32AT003378 from the National Center for Complementary and Alternative Medicine, as well as the National Institutes of Health, National Institute of Diabetes, Digestive and Kidney Disease (NIDDK) grant #s R24 DK067674 and R01 DK031369.
References
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13(1):27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Carmody J, Baer RA. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. Journal of Behavioral Medicine. 2008;31(1):23–33. doi: 10.1007/s10865-007-9130-7. [DOI] [PubMed] [Google Scholar]
- Chambers R, Gullone E, Allen NB. Mindful emotion regulation: An integrative review. Clinical Psychology Review. 2009;29(6):560–572. doi: 10.1016/j.cpr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Craske MG, Wolitzky-Taylor KB, Labus J, Wu S, Frese M, Mayer EA, Naliboff BD. A cognitive-behavioral treatment for irritable bowel syndrome using interoceptive exposure to visceral sensations. Behavior Research and Therapy. 2011 doi: 10.1016/j.brat.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Empirical explorations of mindfulness: Conceptual and methodological conundrums. Emotion. 2010;10(1):8–11. doi: 10.1037/a0018480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean BB, Aguilar D, Barghout V, Kahler KH, Frech F, Groves D, et al. Impairment in work productivity and health-related quality of life in patients with IBS. Am J Manag Care. 2005;11(1 Suppl):S17–26. [PubMed] [Google Scholar]
- Derogatis LR. BSI-18: Administration, Scoring, and Procedures Manual. Minneapolis, MN: National Computer Systems; 2000. [Google Scholar]
- Drossman DA, Patrick DL, Whitehead WE, Toner BB, Diamant NE, Hu Y, et al. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterol. 2000;95(4):999–1007. doi: 10.1111/j.1572-0241.2000.01941.x. [DOI] [PubMed] [Google Scholar]
- Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, Gizewski ER. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: An fMRI study. Gut. 2010;59:489–495. doi: 10.1136/gut.2008.175000. [DOI] [PubMed] [Google Scholar]
- Engel GL. The need for a new medical model: A challenge for biomedicine. Science. 1977;196(4286):129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- Farb NA, Anderson AK, Mayberg H, Bean J, McKeon D, Segal ZV. Minding one’s emotions: mindfulness training alters the neural expression of sadness. Emotion. 2010;10(1):25–33. doi: 10.1037/a0017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis C, Morris J, Whorwell P. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Ailment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- Garland EL. The meaning of mindfulness: A second-order cybernetics of stress, metacognition, and coping. Complementary Health Practice Review. 2007;12(1):15–30. [Google Scholar]
- Garland EL, Gaylord SA, Park J. The role of mindfulness in positive reappraisal. Explore (NY) 2009;5(1):37–44. doi: 10.1016/j.explore.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Fredrickson BL, Kring AM, Johnson DP, Meyer PS, Penn DL. Upward spirals of positive emotions counter downward spirals of negativity: Insights from the broaden-and-build theory and affective neuroscience on the treatment of emotion dysfunctions and deficits in psychopathology. Clinical Psychology Review. 2010;30:849–864. doi: 10.1016/j.cpr.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaylord SA, Palsson O, Garland EL, Faurot K, Coble R, Frey W, et al. Mindfulness training reduces the severity of irritable bowel syndrome in women: Results of a randomized controlled trial. 2011. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaylord SA, Whitehead WE, Coble RS, Faurot KR, Palsson OS, Garland EL, et al. Mindfulness for irritable bowel syndrome: protocol development for a controlled clinical trial. BMC Complement Altern Med. 2009;9:24. doi: 10.1186/1472-6882-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalkorale WM, Houghton LA, Whorwell PJ. Hypnotherapy in irritable bowel syndrome: a large-scale audit of a clinical service with examination of factors influencing responsiveness. Am J Gastroenterol. 2002;97(4):954–961. doi: 10.1111/j.1572-0241.2002.05615.x. [DOI] [PubMed] [Google Scholar]
- Gonsalkorale WM, Toner BB, Whorwell PJ. Cognitive change in patients undergoing hypnotherapy for irritable bowel syndrome. J Psychosom Res. 2004;56(3):271–278. doi: 10.1016/S0022-3999(03)00076-X. [DOI] [PubMed] [Google Scholar]
- Gralnek IM, Hays RD, Kilbourne A, Naliboff B, Mayer EA. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119(3):654–660. doi: 10.1053/gast.2000.16484. [DOI] [PubMed] [Google Scholar]
- Grant JA, Rainville P. Pain sensitivity and analgesic effects of mindful states in Zen meditators: a cross-sectional study. Psychosom Med. 2009;71(1):106–114. doi: 10.1097/PSY.0b013e31818f52ee. [DOI] [PubMed] [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res. 2004;57(1):35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Wilson KG. Mindfulness: Method and process. Clinical Psychology: Science and Practice. 2003;10(2):161–165. [Google Scholar]
- Hunt MG, Moshier S, Milonova M. Brief cognitive-behavioral internet therapy for irritable bowel syndrome. Behaviour Research and Therapy. 2009;47:797–802. doi: 10.1016/j.brat.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Jones M, Koloski N, Boyce P, Talley NJ. Pathways connecting cognitive behavioral therapy and change in bowel symptoms of IBS. Journal of Psychosomatic Research. 2011;70(3):278–85. doi: 10.1016/j.jpsychores.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Critchley HD, Seymour B, O’Doherty JP, Oakley DA, et al. Anxiety reduction through detachment: subjective, physiological, and neural effects. J Cogn Neurosci. 2005;17(6):874–883. doi: 10.1162/0898929054021184. [DOI] [PubMed] [Google Scholar]
- Kaplan KH, Goldenberg DL, Galvin-Nadeau M. The impact of a meditation-based stress reduction program on fibromyalgia. Gen Hosp Psychiatry. 1993;15(5):284–289. doi: 10.1016/0163-8343(93)90020-o. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. NY: Guilford Press; 1998. [Google Scholar]
- Labus JS, Bolus R, Chang L, Wiklund I, Naesdal J, Mayer EA, et al. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004;20(1):89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- Lackner JM, Jaccard J, Krasner SS, Katz LA, Gudleski GD, Blanchard EB. How does cognitive behavior therapy for irritable bowel syndrome work? A mediational analysis of a randomized clinical trial. Gastroenterology. 2007;133(2):433–444. doi: 10.1053/j.gastro.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea R, Whorwell PJ. Psychological influences on the irritable bowel syndrome. Minerva Med. 2004;95(5):443–450. [PubMed] [Google Scholar]
- Ljotsson B, Falk L, Vesterlund A, Hedman E, Lindfors P, Ruck C, Hursti T, Andreewitch S, Jansson L, Lindefors N, Andersson G. Internet-delivered exposure and mindfulness based therapy for irritable bowel syndrome-a randomized controlled trial. Behavior Research and Therapy. 2010;48(6):531–539. doi: 10.1016/j.brat.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Maris E. Covariance adjustment versus gain scores--revisited. Psychological Methods. 1998;3:309–327. [Google Scholar]
- Mayer EA. Emerging disease model for functional gastrointestinal disorders. Am J Med. 1999;107(5A):12S–19S. doi: 10.1016/s0002-9343(99)00277-6. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med. 2010 doi: 10.1146/annurev-med-012309-103958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V, Whorwell P. Hypnotherapy for functional gastrointestinal disorders: a review. International Journal of Clinical and Experimental Hypnosis. 2009;57(3):279–92. doi: 10.1080/00207140902881098. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Waters AM, Labus JS, Kilpatrick L, Craske MG, Chang L, et al. Increased acoustic startle responses in IBS patients during abdominal and nonabdominal threat. Psychosom Med. 2008;70(8):920–927. doi: 10.1097/PSY.0b013e318186d858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsson OS, Turner MJ, Johnson DA, Burnett CK, Whitehead WE. Hypnosis treatment for severe irritable bowel syndrome: investigation of mechanism and effects on symptoms. Dig Dis Sci. 2002;47(11):2605–2614. doi: 10.1023/a:1020545017390. [DOI] [PubMed] [Google Scholar]
- Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43(2):400–411. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- Payne A, Blanchard EB. A controlled comparison of cognitive therapy and self-help support groups in the treatment of irritable bowel syndrome. J Consult Clin Psychol. 1995;63(5):779–786. doi: 10.1037//0022-006x.63.5.779. [DOI] [PubMed] [Google Scholar]
- Perlman DM, Salomons TV, Davidson RJ, Lutz A. Differential effects on pain intensity and unpleasantness of two meditation practices. Emotion. 2010;10(1):65–71. doi: 10.1037/a0018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277(5328):968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Robinson ME, Riley JL, Myers CD, Sadler IJ, Kvaal SA, Geisser ME, et al. The Coping Strategies Questionnaire: A large sample, item level factor analysis. The Clinical Journal of Pain. 1997;13(1):43–49. doi: 10.1097/00002508-199703000-00007. [DOI] [PubMed] [Google Scholar]
- Rogosa D. Myths about longitudinal research. In: Campbell RT, Meredith W, Rawlings SC, editors. Methodological issues in aging research. New York: Springer; 1988. pp. 171–210. [Google Scholar]
- Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: Relationship to patient characteristics and current adjustment. Pain. 1983;17:33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- Rosenzweig S, Greeson JM, Reibel DK, Green JS, Jasser SA, Beasley D. Mindfulness-based stress reduction for chronic pain conditions: variation in treatment outcomes and role of home meditation practice. J Psychosom Res. 2010;68(1):29–36. doi: 10.1016/j.jpsychores.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Seres G, Kovacs Z, Kovacs A, Kerekgyarto O, Sardi K, Demeter P, Meszaros E, Tury F. Different associations of health related quality of life with pain, psychological distress and coping strategies in patients with irritable bowel syndrome and inflammatory bowel disorder. Journal of Clinical Psychology in Medical Settings. 2008;15(4):287–295. doi: 10.1007/s10880-008-9132-9. [DOI] [PubMed] [Google Scholar]
- Strassman RJ, Galanter M. The Abhidharma: a cross-cultural model for the psychiatric application of meditation. Int J Soc Psychiatry. 1980;26(4):293–299. doi: 10.1177/002076408002600407. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. 2000;68(4):615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- Whorwell PJ, Prior A, Faragher EB. Controlled trial of hypnotherapy in the treatment of severe refractory irritable-bowel syndrome. Lancet. 1984;2(8414):1232–1234. doi: 10.1016/s0140-6736(84)92793-4. [DOI] [PubMed] [Google Scholar]
- Wiech K, Tracey I. The influence of negative emotions on pain: behavioral effects and neural mechanisms. Neuroimage. 2009;47(3):987–994. doi: 10.1016/j.neuroimage.2009.05.059. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Gordon NS, Merchant J, Goolkasian P. The effects of brief mindfulness meditation training on experimentally induced pain. J Pain. 2009;11(3):199–209. doi: 10.1016/j.jpain.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.5791-10.2011. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]