Abstract
Damage-associated molecular pattern (DAMP) molecules are essential for the initiation of innate inflammatory responses to infection and injury. The prototypic DAMP molecule, high-mobility group box 1 (HMGB1), is an abundant architectural chromosomal protein that has location-specific biological functions: within the nucleus as a DNA chaperone, within the cytosol to sustain autophagy and outside the cell as a DAMP molecule. Recent research indicates that aberrant activation of HMGB1 signaling can promote the onset of inflammatory and autoimmune diseases, raising interest in the development of therapeutic strategies to control their function. The importance of HMGB1 activation in various forms of liver disease in relation to liver damage, steatosis, inflammation, fibrosis, tumorigenesis and regeneration is discussed in this review.
INTRODUCTION
The high-mobility group (HMG) chromosomal proteins were discovered in mammalian cells in 1973 and named according to their high electrophoretic mobility in polyacrylamide gels (1). High-mobility group box 1 (HMGB1), as a member of the HMG family, has location-specific biological functions: within the nucleus as a DNA chaperone, within the cytosol to sustain autophagy and outside the cell as a damage-associated molecular pattern (DAMP) molecule. HMGB1 is essential for life because the absence of this protein is postnatally lethal; newborn knockout mice succumb to hypoglycemia (2). Although initial studies demonstrated HMGB1 as a late mediator of lethal systemic inflammation, recent findings indicate that HMGB1 has an important role in noninfectious inflammation, such as autoimmunity, cancer, trauma and ischemia/reperfusion (I/R) injury (3–5). During the past decade, studies in both patients and animal models have established that HMGB1 represents a potential biomarker and novel therapeutic target in certain diseases. There have been important advances recently in the understanding of the structure and function of HMGB1 (Figure 1). In this review, we will summarize the basics of HMGB1 and focus on the current understanding of connections between HMGB1 and liver disease and its potential as a therapeutic target.
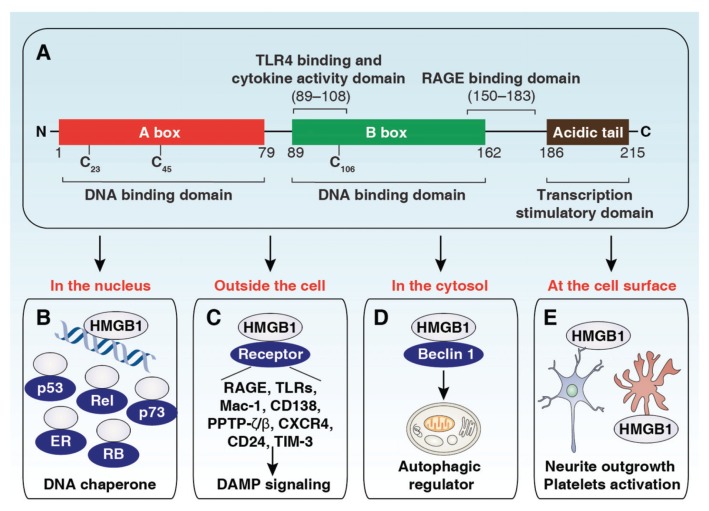
Figure 1.
Structure and function of the HMGB1 protein. (A) HMGB1 is composed of the A box, B box and C tail domains. (B) As a DNA chaperone, nuclear HMGB1 participates in DNA replication, recombination, transcription and repair. In particular, HMGB1 interacts with and enhances the activities of a number of transcription factors, including p53, p73, the retinoblastoma protein (RB), members of the Rel/NF-κB family and estrogen receptor (ER). (C) Once released, HMGB1 binds to various receptors to activate DAMP signaling involved in multiple cellular processes. (D) Cytoplasmic HMGB1 protein binds with beclin 1 to induce autophagy. (E) Membrane HMGB1 promotes neurite outgrowth and platelet activation.
HMGB1 STRUCTURE
HMGB1 is encoded on human chromosome 13q12–13 and consists of 215 amino acids. HMGB1 is a highly conserved protein containing two DNA-binding domains (HMGB A and B) and a natively charged C tail (for transcription stimulation) domain (Figure 1A). Both the HMGB1 A and B boxes are about 75–80 amino acids long and are formed by two short and one long a-helix that, upon folding, produce an L- or V-shaped three-dimensional domain structure (6–8). The B box has been identified as a function domain, which can be recognized by toll-like receptor (TLR)-4 and trigger the release of proinflammatory cytokines (9). In comparison, the purified recombinant A box has antiinflammatory properties in vivo and in vitro (10). Amino acids 150–183 of HMGB1 are responsible for the receptor for advanced glycation end products (RAGE) binding (11), whereas amino acids 89–108 of HMGB1 are responsible for binding to TLR4 (9). Structurally, cytokine-HMGB1 (the form with cytokine activity) has a disulfide bridge between cysteine residues 23 and 45 and a reduced cysteine residue 106, whereas chemotaxis-HMGB1 (the chemoattractant form) is completely reduced (12,13).
HMGB1 FUNCTION
In 1978, HMGB1 was identified as a nonhistone chromosomal protein involved in DNA binding and bending and participating in regulation of DNA structure (14). In the last decades, accumulated evidence has demonstrated that HMGB1 plays a critical role in controlling multiple DNA signaling pathways (for example, gene transcription, genetic recombination, chromatin remodeling and DNA damage repair) by protein–DNA or protein–protein interactions (Figure 1B). In addition, nuclear HMGB1 is also involved in the regulation of the nucleosome dynamics, telomere length and chromosomal stability. In 1999, HMGB1 was identified as a potent proinflammatory cytokine that can be released into the extracellular milieu and mediate endotoxin lethality in mice (15). Once outside the cell, HMGB1 interacts with several receptors and coordinates various cellular responses such as immune system activation, cell migration, cell growth, angiogenesis and tissue repair and regeneration (Figure 1C and Figure 2). The redox status of HMGB1 has been regarded as an important mechanism to positively or negatively regulate its extracellular activities in inflammation and death (12,13,16,17) (Figure 2). Extracellular HMGB1 may form heterocomplexes with other immune coactivators such as interleukin (IL)-1β, C-X-C motif chemokine 12 (CXCL12), deoxyribonucleic acid (DNA), nucleosomes or lipopolysaccharide (LPS) that generate synergistic responses in inflammation and immunity (18,19) (Figure 2). Autophagy is a highly conserved intracellular catabolic pathway that involves degradation and recycling of cytoplasmic components, such as long-lived proteins and organelles. In 2010, HMGB1 was identified as an autophagy regulator (20). Cytoplasmic HMGB1 promotes autophagy by binding beclin-1 (Figure 1D). Nuclear and extracellular HMGB1 have the ability to sustain autophagy by regulating the expression of heat shock protein β1 and binding RAGE, respectively (16,21). In addition, membrane HMGB1 promotes neurite outgrowth and platelet activation (Figure 1E). Thus, HMGB1 performs contrasting, location-dependent roles inside and outside the cell.
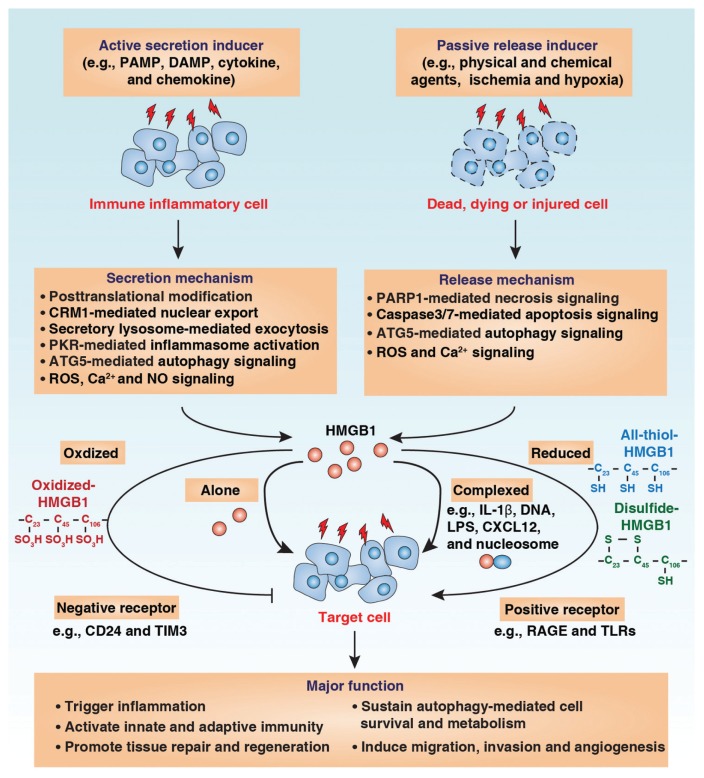
Figure 2.
HMGB1 release and extracellular function. HMGB1 can be actively secreted by immune inflammatory cells or passively released by dead, dying or injured cells into the extracellular milieu by several different mechanisms as indicated. Extracellular HMGB1 acts as a DAMP molecule and plays a vital role in several pathophysiological processes. The activity of extracellular HMGB1 depends on redox state, receptors and their interactive partners.
HMGB1 RELEASE
Extracellular HMGB1 is derived either by active secretion by innate immune cells (for example, macrophages, monocytes and dendritic cells [DCs]) or by passive release by dead, dying or injured cells (for example, cancer cells and hepatocytes) (Figure 2). Depending on the inducing stimulus, the mechanism of HMGB1 secretion and release can vary. In response to exogenous pathogen-associated molecular patterns (PAMPs, such as endotoxin or cytosine-phosphate-guanine [CpG]-DNA) or endogenous inflammatory stimuli (for example, tumor necrosis factor [TNF], interferon [IFN]-γ, hydrogen peroxide or DAMP molecules), HMGB1 is modified by different posttranscriptional modifications (for example, acetylation or phosphorylation) and actively released by innate immune cells (22,23). HMGB1 lacks a leader signal sequence and cannot be released via the classic endoplasmic reticulum–Golgi-dependent secretory pathway. Instead, HMGB1 can be secreted through noncanonical, secretory, lysosome-mediated exocytosis in active immune and inflammatory cells (24). In addition, inflammasome, a large caspase-1–activating multiprotein complex, was recently shown to regulate HMGB1 release in immune and inflammatory cells (25). Notably, double-strand RNA (dsRNA)-dependent protein kinase–mediated inflammasome activation is an essential regulator of HMGB1 secretion (26). HMGB1 release has been considered a feature of necrosis but not apoptosis (27). Recent studies suggest that HMGB1 can also be released by apoptotic cells at a late stage (a stage that can be called secondary necrosis) and induce immune tolerance (28). Autophagy can regulate both active secretion and passive release of HMGB1 in macrophages and cancer cells, respectively (20,29). The differences observed in HMGB1 release during death may reflect the experimental systems used, including the cell lines and death inducers.
HMGB1 RECEPTORS
The receptors used by HMGB1 are not entirely clear and may vary by context. These receptors include RAGE, the TLRs, Mac-1, syndecan-1 (CD138), phosphacan protein–tyrosine phosphatase (PPTP)-ξ/β, CD24, chemokine (C-X-C motif) receptor 4 (CXCR4), T-cell immunoglobulin mucin-3 (TIM-3) and possibly others (Figure 1C). RAGE, a member of the immunoglobulin superfamily of cell surface molecules expressed on a variety of cell types, has a high affinity for HMGB1. HMGB1 interaction with RAGE can mediate cell proliferation, growth and migration by activating intracellular signals such as nuclear factor (NF)-κB and the mitogen-activated protein kinase (MAPK) pathway (30,31). In addition to RAGE, the importance of TLRs has been demonstrated in HMGB1 signaling pathways. TLRs are highly conserved proteins and important pathogen-recognized patterns both in innate and adaptive immunity. Three of the TLRs have been reported to be involved in HMGB1 signaling: TLR2, TLR4 and TLR9. Signaling by activation of these TLRs culminates in NF-κB and MAPKs that regulate gene expression of various immune and inflammatory mediators (32). Recently, it was suggested that bacterial DNA or other contaminants in bacterially expressed HMGB1 may activate both TLR9 and RAGE (33). In addition, CD24 and TIM-3 act as negative receptors and inhibit immune activity of HMGB1 in macrophages and tumor-associated DCs, respectively (Figure 2) (34,35). HMGB1 also promotes recruitment of inflammatory cells to damaged tissue by forming a complex with the chemokine CXCL12 and signaling via CXCR4 independent of RAGE and TLR4 (36). Taken together, these studies support the possibility that tissue and cell-type specific roles for HMGB1 receptors exist. Interplay between HMGB1 receptors within the same tissue and cells remains largely unknown.
HMGB1 IN LIVER DISEASES
The liver plays a key role in integration of immune responses after severe systemic insult such as trauma or surgery. In the liver, TLRs and RAGE are expressed by parenchymal and nonparenchymal cell types, which is likely biologically important, since these receptors can initiate innate immune cascades through the recognition of HMGB1 in the pathogenesis of liver disease as discussed below.
HMGB1 AND LIVER I/R
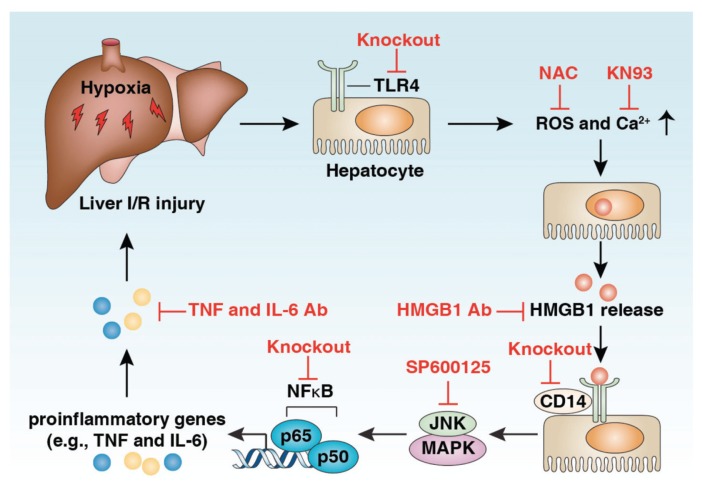
I/R injury refers to a set of deleterious phenomena that emanate from the temporary deprivation of circulation and subsequent restoration of oxygen supply. There are two different forms of hepatic I/R: warm I/R and cold I/R, which differ with respect to the hepatic blood flow stage. Warm I/R can occur in low-flow states, surgery, injury and trauma. In contrast, cold I/R occurs in the time during organ preservation before transplantation. Local cellular damage causes the release of DAMP molecules and subsequent systemic sterile inflammatory response, two characteristics in the pathophysiology of hepatic I/R injury (37). The involvement of HMGB1 in warm I/R injury was first described in 2005 (38,39). HMGB1 was rapidly released into circulation by hepatocytes in I/R. TLR4 plays a central role in liver I/R (Figure 3). TLR4 is required for HMGB1-mediated proinflammatory cytokine generation and organ damage in warm hepatic I/R injury by activation of the JNK MAPK–NF-κB pathway (38). Injection of recombinant HMGB1 exacerbates, whereas treatment with HMGB1-neutralizing antibody protects hepatic I/R damage in TLR4 wild-type mice, but not TLR4-deficient mice (38). In addition, TLR4 from parenchymal cells but not myeloid cells and DCs also regulates hepatic I/R–induced HMGB1 release by activation of reactive oxygen species and calcium/calmodulin-dependent protein kinase (CaMK) signaling pathway (38,40). Treatment with CaMK inhibitor (KN93) and antioxidant N-acetylcysteine inhibit HMGB1 release and protect against liver I/R injury in animal models. CD14 is known to participate in ligand recognition by the TLR4 receptor complex and is involved in both the injury and the inflammation induced by liver I/R (41). However, the release of HMGB1 in liver I/R did not require CD14, whereas the recognition of HMGB1 needs CD14 (41). TLR9 (42) and RAGE (43) inhibition also confer protection from liver I/R injury, suggesting that HMGB1 may interact with multiple receptors to mediate warm liver I/R injury. In addition, a range of mechanisms such as inflammasome activation (44), decreased histone deacetylase activity (45) and upregulation of nuclear interferon regulatory factor 1 (46) have been identified to contribute to HMGB1 release in warm liver I/R damage. Recent studies show that Bβ15–42 (the fibrin-derived peptide), PNU-282987 (a selective α7 nicotinic acetylcholine receptor agonist), EPC-K1 (the vitamin E derivative), melatonin, cisplatin and glycyrrhizin attenuate warm liver I/R damage partly through inhibition of HMGB1 release (47–52). Interestingly, pretreatment of mice with HMGB1 protein significantly decreased liver I/R injury through upregulation of IL-1R–associated kinase-M, a negative regulator of TLR4 signaling (53). Thus, extracellular HMGB1 may have a dual role in regulation of hepatic I/R injury, which depends on its posttranslational modifications and receptors.
Figure 3.
HMGB1-TLR4 signaling mediates liver I/R injury. HMGB1 is an early mediator of injury and inflammation in liver I/R, and TLR4 is the major receptor that is involved in the process by which the JNK MAPK/NF-κB pathway is activated. The release of HMGB1 in liver I/R requires TLR4 but not CD14. Different strategies of HMGB1-TLR4 pathway inhibition as indicated have been shown to reduce liver I/R injury.
HMGB1 AND LIVER TRANSPLANTATION
Liver transplantation remains the primary treatment for patients with end-stage liver disease, including cirrhosis. During liver transplantation, the stage of cold preservation after organ harvest often results in predominantly sinusoidal cell injury and a warm phase of organ reperfusion, in which the expected ischemic injury with disruption of multiple cellular metabolic processes is initiated (54,55). A second type of injury occurs on reperfusion and consists of a rapid inflammatory response (56). The extent of damage is associated with the length of ischemia time and mechanical stress. TLR4 has been implicated in cold I/R injury during liver transplantation. A recent clinical study indicates that HMGB1 is a useful biomarker of hepatocellular injury in human liver transplantation (57). They found the following: (a) reperfusion after cold ischemia in human liver transplantation was related to extensive HMGB1 efflux from the graft (57); and (b) the level of HMGB1 expression and release in hepatocytes correlated with peak postoperative alanine aminotransferase (ALT) levels (57). In addition to cold I/R injury, immunologic tolerance and rejection are major impediments to successful liver transplantation. However, whether HMGB1 is linked to the subsequent immunologic tolerance and graft rejection and its precise role and mechanism remain unknown. Redox state and cell death form have been reported to determine whether HMGB1 is tolerogenic or immunogenic in macrophages and DCs (28).
HMGB1 AND VIRAL HEPATITIS
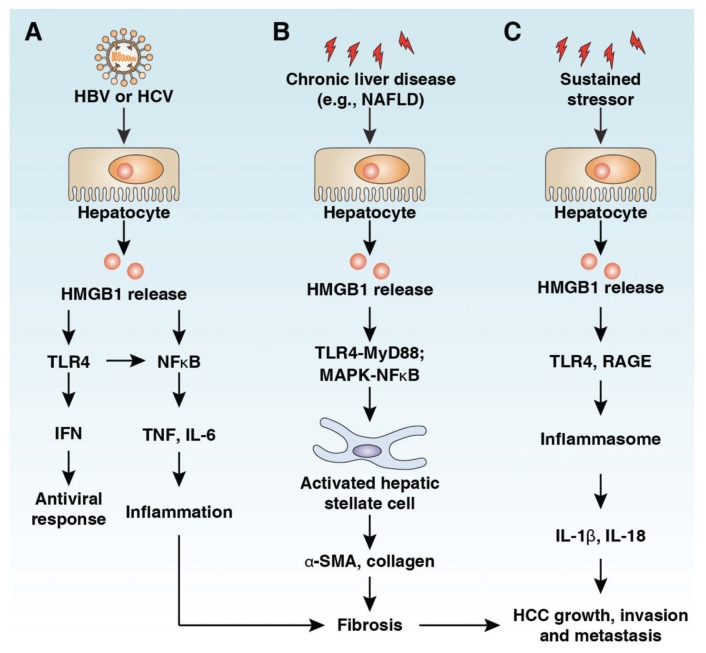
At least five different hepatitis viruses (hepatitis A to E viruses) cause liver disease in humans. All five cause acute hepatitis, whereas hepatitis B (HBV), C (HCV), and D (HDV) viruses can also lead to persistent infection and chronic hepatitis. HBV is the major cause of chronic hepatitis, cirrhosis and liver cancer worldwide. Most HBV infections are silent; thus, a significant number of persistently infected individuals remain unaware of infection, even for decades, which has become a major public health problem (58). Chronic infection with either HBV or HCV can result in inflammation and oxidative stress. A prolonged fibrotic response resulting in cirrhosis is also common in both infections, which is accompanied by the appearance of localized hypoxia, rearrangement of tissue architecture (epithelial–mesenchymal transition) and angiogenesis (59). Thus, viral hepatitis confers a risk of developing a chronic infection that can lead to liver fibrosis and eventually evolve into liver cirrhosis and hepatocellular carcinoma (60). Although multiple signaling networks are responsible for coordinating the inflammatory and immune response during virus hepatitis, HMGB1 is critical in initiating and mediating these effects. HMGB1 is translocated from the nucleus to the cytoplasm and subsequently is released into the extracellular milieu by HCV or HBV infection (61–63). Extracellular HMGB1 during HCV infection can trigger the interferon antiviral response through a TLR4-dependent pathway (61,63). HMGB1 serum levels are significantly higher in patients with chronic HBV than in normal healthy people and are closely related to the stage of inflammation and fibrosis in the liver (Figure 4A) (64). In addition, HMGB1 plays an important role in the pathogenesis of liver failure in chronic HBV patients by downregulation of Foxp3 expression and inhibiting the immune activity of regulatory T cells (65). These studies suggest that HMGB1 may have a different role in regulation of antiviral immunity and inflammation during HBV and HCV infection.
Figure 4.
Role of HMGB1 in hepatic pathology. (A) HBV and HCV infection confer a risk of developing a chronic inflammation that can lead to liver fibrosis and eventually evolve into liver cirrhosis and hepatocellular carcinoma, and HMGB1 participates in this process. HCV or HBV infection induces HMGB1 translocation and release. Extracellular HMGB1 causes chronic inflammation by activation of NF-κB pathways or results in TLR4-dependent interferon antiviral response. (B) Serum HMGB1 level is increased in several chronic liver diseases. HMGB1 induces HSC activation by TLR4-MyD88/MAPK-NF-κB pathways. HMGB1 promotes the expression of collagen and α-SMA in HSCs and results in accumulation of excess extracellular matrix and liver fibrosis. (C) Extracellular HMGB1 binds to its receptors (for example, TLR4, TLR9 and RAGE) to induce inflammasome activation and proinflammatory cytokine release, which is essential for tumor growth, invasion and metastasis.
HMGB1 AND NONALCOHOLIC FATTY LIVER DISEASE
Nonalcoholic fatty liver disease (NAFLD), one of the most common causes of chronic liver disease in developed countries, is caused by an accumulation of lipid deposits in the liver. NAFLD includes a wide variety of liver damage, ranging from steatosis (only fat accumulation, also called fatty liver), to nonalcoholic steatohepatitis (fatty infiltration associated with inflammation and liver injury), to advanced fibrosis and cirrhosis (permanent damage/injury to the liver). The initial theory for the pathogenesis of NAFLD was the “two-hit” hypothesis (66). The “first hit,” hepatic triglyceride accumulation (also called steatosis), sensititizes the liver to injury by “second hits,” such as those from cytokines, endotoxin, adipokines, mitochondrial dysfunction and oxidative stress, which result in de novo lipogenesis and increased release of free fatty acid and abnormal lipid deposition in the liver (67). Emerging evidence suggests that HMGB1-TLR4-MyD88 signaling is involved in the progression of NAFLD in the early stage (Figure 4B) (68). Knockout of TLR4 and its cytosolic adapter protein MyD88 in mice decreases serum HMGB1 level and suppresses high-fat diet–induced liver dysfunction. The accumulation of free HMGB1 in the plasma may further enhance inflammation and liver damage. Treatment with HMGB1-neutralizing antibody inhibits free fatty acid–induced proinflammatory cytokines (for example, TNF and IL-6) production (68). These studies indicate that HMGB1 serves as an important mediator to accelerate local liver damage and systemic inflammation during the early stage of NAFLD.
HMGB1 AND LIVER FIBROSIS
Liver fibrosis is the final common pathway for a number of chronic liver diseases. Regardless of the underlying etiology, hepatic fibrosis is a kind of liver injury and scar repair, characterized by the accumulation of excess extracellular matrix (ECM), a reduction of ECM-removing matrix metalloproteinase (MMP) and an upregulation of tissue inhibitors of MMP (69). Hepatic stellate cells (HSCs) are quiescent cells in the perisinusoidal space in the liver that facilitate hepatocyte interactions in the process of liver fibrosis via the release of soluble inflammatory factors and the production of ECM (69). HMGB1 is involved in the development and progression of liver fibrosis (Figure 4B) (60). Recombinant HMGB1 protein remarkably stimulates HSC growth, promotes α-smooth muscle actin (α-SMA) expression and inhibits MMP-2 activity (70). In a rat model of hepatic fibrosis, the level of HMGB1 is upregulated, and its expression is closely correlated with the deposition of collagen. Suppression of HMGB1 expression by small interfering RNA (siRNA) significantly inhibits synthesis of α-SMA and collagen (types I and III) in HSCs (71). In addition, HMGB1 released during the rejection phase of orthotropic liver transplantation has the ability to activate HSCs and exhibit profibrogenic effects on liver grafts (70). HMGB1 also has a synergistic effect with transforming growth factor β1 (TGF-β1) to stimulate expression of fibrogenic protein (for example, collagen α2 and α-SMA) in HSCs (72). HMGB1 promotes the proliferation/migration of HSCs and liver fibrosis partly via TLR4-MyD88 and the MAPK–NF-κB pathway (Figure 4B) (72,73). These studies have demonstrated that HMGB1 and its profibrotic function may be effective targets to treat liver fibrosis.
HMGB1 AND HEPATOCELLULAR CARCINOMA
Hepatocellular carcinoma (HCC) is one of the most common forms of cancer in the world, especially in Asia and Africa (58). Approximately 90% of HCCs are preceded by chronic liver disease, hepatic fibrosis and cirrhosis (Figure 4C). HCC is a typical inflammation-related carcinoma, commonly arising in a damaged organ, featuring extensive inflammation and fibrosis. Different players, including immune cells, hepatic stellate cells and macrophages, react to liver injury by producing cytokines and components of the ECM, which promote angiogenesis and survival of damaged hepatocytes or cancer stem cells. HMGB1 is dysfunctional in various tumors and plays a context-dependent role in the regulation of tumor development and therapy (74). Extracellular HMGB1-induced chronic inflammation within the tumor microenvironment favors tumor survival, proliferation, metastasis and angiogenesis. Expression of HMGB1 is closely correlated with pathological grade and distant metastases of liver cancer, and knockdown of HMGB1 inhibits liver cancer growth and metastasis (75,76). Serum HMGB1 levels were significantly higher in HCC and HBV patients and were associated with clinicopathological features and outcome in HCC patients (64,77). Ethyl pyruvate, a potent inhibitor of HMGB1 release, can suppress tumor growth in liver metastasis models of colon cancer (78). HMGB1 and its receptor RAGE were shown to modulate the proliferation and cell cycle of human HCC cell lines in vitro (79). Hypoxia-induced HMGB1 release in HCC cell lines can activate TLR4- and RAGE-signaling pathways to induce activation of the inflammasome and secretion of IL-1β and IL-18, which, in turn, promote HCC invasion and metastasis (Figure 4C) (80). A recent study indicated that activation of PAMP-TLR4 signaling contributes to injury/inflammation-driven tumor promotion and progression in late stages of hepatocarcinogenesis (81). Thus, TLR4 activation by PAMPs and DAMP molecules has a critical role in HCC pathogenesis.
HMGB1 AND DRUG-INDUCED LIVER INJURY
Drug-induced liver injury, also known as hepatotoxicity, refers to liver injury caused by drugs or other chemical agents and represents a special type of adverse drug reaction. Acetaminophen (APAP) overdoses are currently the most frequent cause of acute liver failure in the United States (82). Hepatocyte necrosis, apoptosis and innate immune activation have been defined as the dominant features of the toxicological response associated with APAP. HMGB1 has been reported as a circulating mechanistic indicator of cell death in animal and clinical studies on APAP-induced hepatotoxicity (83–85). HMGB1 also becomes a sensitive serum diagnosis and severity assessment biomarker of APAP-induced acute liver injury (83–85). APAP-induced HMGB1 release contributes to innate immune activation during liver injury (27,86). For example, the HMGB1–TLR4–IL-23–IL-17A signaling pathway mediates interplay between macrophages and γδ T cells, which contributes to APAP-induced liver inflammation (87). The change of cysteine redox isoforms of HMGB1 is an important mechanism to regulate HMGB1 activity during inflammation, immunity and cell death (88). Caspase-dependent oxidation of HMGB1 prevents inflammatory response in mice treated with APAP (85). It is also important to note that the release of the specific HMGB1 isoform such as acetylated HMGB1 is related to drug-induced liver injury prognosis in the patient and mouse model (89,90). Blocking HMGB1 release or activity by ethyl pyruvate and HMGB1-neutralizing antibody prevents APAP-induced hepatotoxicity and restores liver structure by inhibition of oxidative injury and inflammation (91,92). In addition, HMGB1 cytoplasmic translocation and release during tissue damage and cell death promotes pathological processes in several drug-induced acute liver failures (93,94).
HMGB1 AND LIVER REGENERATION
Compared with other organs, liver has the greatest capacity to regenerate in response to a variety of stimuli. This regeneration capacity is critical for survival after partial hepatectomy and acute and chronic liver damage. There are many different factors such as growth factors, cytokines, DNA synthesis and cell cycle progression that affect liver regeneration (95). Growing evidence indicates that the expression and release of HMGB1 is associated with liver regeneration after various liver injuries (63,92). Blocking HMGB1 activity by HMGB1-neutralizing antibody increases cyclin D1 expression and improves hepatocyte regeneration in APAP-injected mice (92). Inhibition of HMGB1 release by glycyrrhizin also improves hepatocyte regeneration after I/R in rats (48). These studies suggest that extracellular HMGB1 limits liver regeneration, although the potential mechanism of this association still remains largely unknown.
HMGB1-TARGETING THERAPEUTIC STRATEGIES
As discussed below, several strategies have been shown to protect against HMGB1 release and biological function in preclinical animal studies (Table 1) (96); however, these approaches have not yet been performed in clinical studies.
Table 1.
HMGB1-targeting therapeutic strategies.
| Therapeutic strategies | Mechanism of action | References |
|---|---|---|
| HMGB1-neutralizing antibody | ||
| Mouse anti-HMGB1 DPH1.1 antibody | Inhibition of HMGB1 activity | 99 |
| Mouse anti-HMGB1 2G7 antibody | Inhibition of HMGB1 activity | 100 |
| Anti-HMGB1 chicken IgY polyclonal antibody | Inhibition of HMGB1 activity | 101 |
| A box protein | Inhibition of HMGB1 activity | 10 |
| RNA interference | ||
| HMGB1 siRNA | Suppression of HMGB1 expression | 102 |
| HMGB1 shRNA | Suppression of HMGB1 expression | 103 |
| Adsorption | Inhibition of HMGB1 activity | 104 |
| Chemicals | ||
| Molecular hydrogen | Inhibition of HMGB1 release | 105 |
| Quercetin | Inhibition of HMGB1 release | 106 |
| Atorvastatin | Suppression of HMGB1 expression | 107 |
| Tanshinone II A | Suppression of HMGB1 expression | 108 |
| Dexamethasone | Inhibition of HMGB1 release | 109 |
| Gold sodium thiomalate | Inhibition of HMGB1 release | 109 |
| Chloroquine | Inhibition of HMGB1 release | 110 |
| Ethyl pyruvate | Inhibition of HMGB1 release | 111 |
| Glycyrrhizin | Inhibition of HMGB1 activity and release | 112 |
| Stearoyl lysophosphatidylcholine | Inhibition of HMGB1 release | 113 |
| Chinese medicinal herbs | ||
| Danggui (Angelica sinensis) | Inhibition of HMGB1 release | 114 |
| Danshen (Salvia miltiorrhiza) | Inhibition of HMGB1 release | 115 |
| Green tea (Camellia sinensis) | Inhibition of HMGB1 release and activity | 116,117 |
| Mung bean (Vigna radiata) | Inhibition of HMGB1 release | 118 |
| HMGB1 receptor and signaling pathway inhibition | ||
| sRAGE | Inhibition of HMGB1-RAGE pathway | 119 |
| Other | ||
| Anticoagulant agents (for example, thrombomodulin) | Inhibition of HMGB1 activity | 101 |
| Endogenous hormones (for example, insulin) | Inhibition of HMGB1 release | 120 |
| Vagus nerve stimulation (for example, nicotine) | Inhibition of HMGB1 release | 121 |
HMGB1 Neutralizing Antibody
Currently used neutralizing antibodies include the monoclonal mouse anti-HMGB1 DPH1.1 antibody (HMGBiotech, Milan, Italy), the monoclonal mouse anti-HMGB1 2G7 antibody (Kevin Tracey’s lab, The Feinstein Institute for Medical Research, Manhasset, NY, USA) and the anti-HMGB1 chicken IgY polyclonal antibody (IBL International, Gunma, Japan).
A Box Protein
A recombinant fusion protein of the A box with thrombomodulin or a truncated HMGB-1–derived A box protein can effectively inhibit HMGB1 cytokine activity.
RNA interference (RNAi)
Suppression of HMGB1 expression through shRNA or siRNA can directly reduce intracellular and extracellular levels of HMGB1. However, intracellular and extracellular HMGB1 may have different roles in cancer and inflammation.
Adsorption
Hemoperfusion therapy using a cellulofine sulfate bead column that adsorbs HMGB1 reduces serum HMGB1 to decrease reperfusion injury of the rat liver.
Chemicals
Several chemicals and bioactive substances affect HMGB1 expression and release, including molecular hydrogen, quercetin, statins (atorvastatin or tanshinone II A), dexamethasone, gold sodium thiomalate, chloroquine, ethyl pyruvate, glycyrrhizin and stearoyl lysophosphatidylcholine. Additionally, certain Chinese medicinal herbs, such as Danggui (Angelica sinensis), Danshen (Salvia miltiorrhiza), green tea (Camellia sinensis) and Mung bean (Vigna radiata) can inhibit HMGB1 release and activity.
HMGB1 Receptor and Signaling Pathway Inhibition
The inhibition of HMGB1-related receptors (for example, RAGE and TLRs) and downstream signaling molecules can inhibit HMGB1 activity. For example, pathological effects mediated via RAGE are physiologically inhibited by soluble RAGE (sRAGE).
CONCLUSIONS AND PERSPECTIVES
As a basic pathological process of various diseases, inflammation contributes to progressive liver disease ranging from liver damage to fibrosis, as well as tumorigenesis (97,98). HMGB1 released by damaged liver tissue can lead to prolonged inflammatory and immune responses and influence the progression of liver disease. Despite significant progress, the mechanisms underlying HMGB1 release, the surface receptors that interact with HMGB1 and the intra-cellular signal transduction pathways of HMGB1 remain relatively poorly defined. Large human patient populations with well-defined clinical disease characteristics and more animal liver disease models are needed to more thoroughly characterize HMGB1 function in liver disease. In addition, although HMGB1-specific antagonists have been proven effective in preclinical animal models of diverse conditions mentioned above, this approach has not yet been performed in patients in well-controlled clinical studies.
ACKNOWLEDGMENTS
We apologize to the researchers who were not referenced because of space limitations. We thank Christine Heiner (Department of Surgery, University of Pittsburgh) for her critical reading of the manuscript. This work was supported by the National Institutes of Health (grant R01CA160417 to D Tang) and the National Natural Sciences Foundation of China (grant 81272253 to X-G Fan).
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Goodwin GH, Sanders C, Johns EW. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem. 1973;38:14–9. doi: 10.1111/j.1432-1033.1973.tb03026.x. [DOI] [PubMed] [Google Scholar]
- 2.Calogero S, et al. The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat Genet. 1999;22:276–80. doi: 10.1038/10338. [DOI] [PubMed] [Google Scholar]
- 3.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–62. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 5.Kang R, Zhang Q, Zeh HJ, 3rd, Lotze MT, Tang D. HMGB1 in cancer: good, bad, or both? Clin Cancer Res. 2013;19:4046–57. doi: 10.1158/1078-0432.CCR-13-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weir HM, et al. Structure of the HMG box motif in the B-domain of HMG1. EMBO J. 1993;12:1311–9. doi: 10.1002/j.1460-2075.1993.tb05776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardman CH, et al. Structure of the A-domain of HMG1 and its interaction with DNA as studied by heteronuclear three- and four-dimensional NMR spectroscopy. Biochemistry. 1995;34:16596–607. doi: 10.1021/bi00051a007. [DOI] [PubMed] [Google Scholar]
- 8.Read CM, Cary PD, Crane-Robinson C, Driscoll PC, Norman DG. Solution structure of a DNA-binding domain from HMG1. Nucleic Acids Res. 1993;21:3427–36. doi: 10.1093/nar/21.15.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, et al. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol Med. 2003;9:37–45. [PMC free article] [PubMed] [Google Scholar]
- 10.Yang H, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huttunen HJ, Fages C, Kuja-Panula J, Ridley AJ, Rauvala H. Receptor for advanced glycation end products-binding COOH-terminal motif of amphoterin inhibits invasive migration and metastasis. Cancer Res. 2002;62:4805–11. [PubMed] [Google Scholar]
- 12.Yang HA, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A. 2010;107:11942–7. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venereau E, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209:1519–28. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Javaherian K, Liu JF, Wang JC. Nonhistone proteins HMG1 and HMG2 change the DNA helical structure. Science. 1978;199:1345–6. doi: 10.1126/science.628842. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 16.Tang D, et al. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene. 2010;29:5299–310. doi: 10.1038/onc.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang H, et al. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1) Mol Med. 2012;18:250–9. doi: 10.2119/molmed.2011.00389. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Hreggvidsdottir HS, et al. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J Leukoc Biol. 2009;86:655–62. doi: 10.1189/jlb.0908548. [DOI] [PubMed] [Google Scholar]
- 19.Wahamaa H, et al. High mobility group box protein 1 in complex with lipopolysaccharide or IL-1 promotes an increased inflammatory phenotype in synovial fibroblasts. Arthritis Res Ther. 2011;13:R136. doi: 10.1186/ar3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang D, et al. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010;190:881–92. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang D, et al. High-mobility group box 1 is essential for mitochondrial quality control. Cell Metab. 2011;13:701–11. doi: 10.1016/j.cmet.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonaldi T, et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–60. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Youn JH, Shin JS. Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J Immunol. 2006;177:7889–97. doi: 10.4049/jimmunol.177.11.7889. [DOI] [PubMed] [Google Scholar]
- 24.Gardella S, et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vande Walle L, Kanneganti TD, Lamkanfi M. HMGB1 release by inflammasomes. Virulence. 2011;2:162–5. doi: 10.4161/viru.2.2.15480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu B, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670–4. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 28.Kazama H, et al. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dupont N, et al. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1beta. EMBO J. 2011;30:4701–11. doi: 10.1038/emboj.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taguchi A, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–60. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 31.Palumbo R, et al. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol. 2004;164:441–9. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park JS, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–24. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 33.Tian J, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–96. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 34.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–5. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiba S, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. 2012;13:832–42. doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schiraldi M, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209:551–63. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Golen RF, Reiniers MJ, Olthof PB, van Gulik TM, Heger M. Sterile inflammation in hepatic ischemia/reperfusion injury: present concepts and potential therapeutics. J Gastroenterol Hepatol. 2013;28:394–400. doi: 10.1111/jgh.12072. [DOI] [PubMed] [Google Scholar]
- 38.Tsung A, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–43. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe T, et al. The role of HMGB-1 on the development of necrosis during hepatic ischemia and hepatic ischemia/reperfusion injury in mice. J Surg Res. 2005;124:59–66. doi: 10.1016/j.jss.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 40.Nace GW, et al. Cellular-specific role of toll-like receptor 4 in hepatic ischemia-reperfusion injury in mice. Hepatology. 2013;58:374–87. doi: 10.1002/hep.26346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai C, et al. CD14 contributes to warm hepatic ischemia-reperfusion injury in mice. Shock. 2013;40:115–21. doi: 10.1097/SHK.0b013e318299d1a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bamboat ZM, et al. Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury. Hepatology. 2010;51:621–32. doi: 10.1002/hep.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng S, et al. Receptor for advanced glycation end product (RAGE)-dependent modulation of early growth response-1 in hepatic ischemia/ reperfusion injury. J Hepatol. 2009;50:929–36. doi: 10.1016/j.jhep.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 44.Zhu P, et al. Gene silencing of NALP3 protects against liver ischemia-reperfusion injury in mice. Hum Gene Ther. 2011;22:853–64. doi: 10.1089/hum.2010.145. [DOI] [PubMed] [Google Scholar]
- 45.Evankovich J, et al. High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histone deacetylase activity. J Biol Chem. 2010;285:39888–97. doi: 10.1074/jbc.M110.128348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dhupar R, et al. Interferon regulatory factor 1 mediates acetylation and release of high mobility group box 1 from hepatocytes during murine liver ischemia-reperfusion injury. Shock. 2011;35:293–301. doi: 10.1097/SHK.0b013e3181f6aab0. [DOI] [PubMed] [Google Scholar]
- 47.Cardinal J, et al. Cisplatin prevents high mobility group box 1 release and is protective in a murine model of hepatic ischemia/reperfusion injury. Hepatology. 2009;50:565–74. doi: 10.1002/hep.23021. [DOI] [PubMed] [Google Scholar]
- 48.Ogiku M, Kono H, Hara M, Tsuchiya M, Fujii H. Glycyrrhizin prevents liver injury by inhibition of high-mobility group box 1 production by Kupffer cells after ischemia-reperfusion in rats. J Pharmacol Exp Ther. 2011;339:93–8. doi: 10.1124/jpet.111.182592. [DOI] [PubMed] [Google Scholar]
- 49.Li F, et al. The protective effect of PNU-282987, a selective alpha7 nicotinic acetylcholine receptor agonist, on the hepatic ischemia-reperfusion injury is associated with the inhibition of high-mobility group box 1 protein expression and nuclear factor kappaB activation in mice. Shock. 2013;39:197–203. doi: 10.1097/SHK.0b013e31827aa1f6. [DOI] [PubMed] [Google Scholar]
- 50.Liu A, et al. The fibrin-derived peptide Bss15–42 attenuates the liver damage in a rat model of liver ischemia reperfusion injury. Shock. 2013 2013 Feb 20; doi: 10.1097/SHK.0b013e31828c2b75. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 51.Oishi K, et al. The vitamin E derivative, EPC-K1, suppresses inflammation during hepatic ischemia-reperfusion injury and exerts hepatoprotective effects in rats. J Surg Res. 2012;176:164–70. doi: 10.1016/j.jss.2011.03.080. [DOI] [PubMed] [Google Scholar]
- 52.Kang JW, Koh EJ, Lee SM. Melatonin protects liver against ischemia and reperfusion injury through inhibition of toll-like receptor signaling pathway. J Pineal Res. 2011;50:403–11. doi: 10.1111/j.1600-079X.2011.00858.x. [DOI] [PubMed] [Google Scholar]
- 53.Izuishi K, et al. Cutting edge: high-mobility group box 1 preconditioning protects against liver ischemia-reperfusion injury. J Immunol. 2006;176:7154–8. doi: 10.4049/jimmunol.176.12.7154. [DOI] [PubMed] [Google Scholar]
- 54.Shibayama Y, Asaka S, Nishijima A. Mechanism of liver injury following ischemia. Exp Mol Pathol. 1991;55:251–60. doi: 10.1016/0014-4800(91)90005-i. [DOI] [PubMed] [Google Scholar]
- 55.Abu-Amara M, et al. Liver ischemia/ reperfusion injury: processes in inflammatory networks: a review. Liver Transpl. 2010;16:1016–32. doi: 10.1002/lt.22117. [DOI] [PubMed] [Google Scholar]
- 56.Montalvo-Jave EE, Escalante-Tattersfield T, Ortega-Salgado JA, Pina E, Geller DA. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J Surg Res. 2008;147:153–9. doi: 10.1016/j.jss.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ilmakunnas M, et al. High mobility group box 1 protein as a marker of hepatocellular injury in human liver transplantation. Liver Transpl. 2008;14:1517–25. doi: 10.1002/lt.21573. [DOI] [PubMed] [Google Scholar]
- 58.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 59.Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer. 2013;13:123–35. doi: 10.1038/nrc3449. [DOI] [PubMed] [Google Scholar]
- 60.Albayrak A, et al. Is HMGB1 a new indirect marker for revealing fibrosis in chronic hepatitis and a new therapeutic target in treatment? Viral Immunol. 2010;23:633–8. doi: 10.1089/vim.2010.0080. [DOI] [PubMed] [Google Scholar]
- 61.Jung JH, et al. Hepatitis C virus infection is blocked by HMGB1 released from virus-infected cells. J Virol. 2011;85:9359–368. doi: 10.1128/JVI.00682-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu HB, et al. [Serum level of HMGB1 in patients with hepatitis B and its clinical significance]. Zhonghua Gan Zang Bing Za Zhi. 2007;15:812–5. [PubMed] [Google Scholar]
- 63.Zhou RR, et al. HMGB1 cytoplasmic translocation in patients with acute liver failure. BMC Gastroenterol. 2011;11:21. doi: 10.1186/1471-230X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng BQ, et al. Serum high mobility group box chromosomal protein 1 is associated with clinicopathologic features in patients with hepatocellular carcinoma. Dig Liver Dis. 2008;40:446–52. doi: 10.1016/j.dld.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 65.Wang LW, Chen H, Gong ZJ. High mobility group box-1 protein inhibits regulatory T cell immune activity in liver failure in patients with chronic hepatitis B. Hepatobiliary Pancreat Dis Int. 2010;9:499–507. [PubMed] [Google Scholar]
- 66.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–5. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 67.Zuiderweg ER, et al. Allostery in the hsp70 chaperone proteins. Top Curr Chem. 2013;328:99–153. doi: 10.1007/128_2012_323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li L, et al. Nuclear factor high-mobility group box1 mediating the activation of Toll-like receptor 4 signaling in hepatocytes in the early stage of nonalcoholic fatty liver disease in mice. Hepatology. 2011;54:1620–30. doi: 10.1002/hep.24552. [DOI] [PubMed] [Google Scholar]
- 69.Guo J, Friedman SL. Hepatic fibrogenesis. Semin Liver Dis. 2007;27:413–26. doi: 10.1055/s-2007-991517. [DOI] [PubMed] [Google Scholar]
- 70.Kao YH, et al. High-mobility group box 1 protein activates hepatic stellate cells in vitro. Transplant Proc. 2008;40:2704–5. doi: 10.1016/j.transproceed.2008.07.055. [DOI] [PubMed] [Google Scholar]
- 71.Ge WS, Wu JX, Fan JG, Wang YJ, Chen YW. Inhibition of high-mobility group box 1 expression by siRNA in rat hepatic stellate cells. World J Gastroenterol. 2011;17:4090–8. doi: 10.3748/wjg.v17.i36.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Z, et al. High mobility group box 1 activates toll like receptor 4 signaling in hepatic stellate cells. Life Sci. 2012;91:207–12. doi: 10.1016/j.lfs.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 73.Wang FP, et al. High mobility group box-1 promotes the proliferation and migration of hepatic stellate cells via TLR4-dependent signal pathways of PI3K/Akt and JNK. PLoS One. 2013;8:e64373. doi: 10.1371/journal.pone.0064373. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.Kostova N, Zlateva S, Ugrinova I, Pasheva E. The expression of HMGB1 protein and its receptor RAGE in human malignant tumors. Mol Cell Biochem. 2010;337:251–8. doi: 10.1007/s11010-009-0305-0. [DOI] [PubMed] [Google Scholar]
- 75.Liu F, et al. High expression of high mobility group box 1 (hmgb1) predicts poor prognosis for hepatocellular carcinoma after curative hepatectomy. J Transl Med. 2012;10:135. doi: 10.1186/1479-5876-10-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dong YD, et al. Expression and clinical significance of HMGB1 in human liver cancer: knockdown inhibits tumor growth and metastasis in vitro and in vivo. Oncol Rep. 2013;29:87–94. doi: 10.3892/or.2012.2070. [DOI] [PubMed] [Google Scholar]
- 77.Jiang W, Wang Z, Li X, Fan X, Duan Y. High-mobility group box 1 is associated with clinicopathologic features in patients with hepatocellular carcinoma. Pathol Oncol Res. 2012;18:293–8. doi: 10.1007/s12253-011-9442-3. [DOI] [PubMed] [Google Scholar]
- 78.Liang X, et al. Ethyl pyruvate administration inhibits hepatic tumor growth. J Leukoc Biol. 2009;86:599–607. doi: 10.1189/jlb.0908578. [DOI] [PubMed] [Google Scholar]
- 79.Yaser AM, et al. The role of receptor for advanced glycation end products (RAGE) in the proliferation of hepatocellular carcinoma. Int J Mol Sci. 2012;13:5982–97. doi: 10.3390/ijms13055982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yan W, et al. High-mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases. Hepatology. 2012;55:1863–75. doi: 10.1002/hep.25572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dapito DH, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–16. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee WM. Acetaminophen and the U.S. Acute Liver Failure Study Group: lowering the risks of hepatic failure. Hepatology. 2004;40:6–9. doi: 10.1002/hep.20293. [DOI] [PubMed] [Google Scholar]
- 83.Antoine DJ, et al. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56:1070–9. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Antoine DJ, et al. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013;58:777–87. doi: 10.1002/hep.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Antoine DJ, Williams DP, Kipar A, Laverty H, Park BK. Diet restriction inhibits apoptosis and HMGB1 oxidation and promotes inflammatory cell recruitment during acetaminophen hepatotoxicity. Mol Med. 2010;16:479–90. doi: 10.2119/molmed.2010.00126. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Wang H, Li W, Goldstein R, Tracey KJ, Sama AE. HMGB1 as a potential therapeutic target. Novartis Found Symp. 2007;280:73–85. discussion 85–91, 160–164. [PubMed] [Google Scholar]
- 87.Wang XF, Sun R, Wei HM, Tian ZG. High-mobility group box 1 (HMGB1)-toll-like receptor (TLR)4-interleukin (IL)-23-IL-17A axis in drug-induced damage-associated lethal hepatitis: interaction of gamma delta T cells with macrophages. Hepatology. 2013;57:373–84. doi: 10.1002/hep.25982. [DOI] [PubMed] [Google Scholar]
- 88.Tang D, Billiar TR, Lotze MT. A Janus tale of two active high mobility group box 1 (HMGB1) redox states. Mol Med. 2012;18:1360–2. doi: 10.2119/molmed.2012.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Antoine DJ, et al. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56:1070–9. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90.Antoine DJ, et al. High-mobility group box-1 protein and keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo. Toxicol Sci. 2009;112:521–31. doi: 10.1093/toxsci/kfp235. [DOI] [PubMed] [Google Scholar]
- 91.Dragomir AC, Laskin JD, Laskin DL. Macrophage activation by factors released from acetaminophen-injured hepatocytes: potential role of HMGB1. Toxicol Appl Pharmacol. 2011;253:170–77. doi: 10.1016/j.taap.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang R, et al. High mobility group B1 impairs hepatocyte regeneration in acetaminophen hepatotoxicity. BMC Gastroenterol. 2012;12:45. doi: 10.1186/1471-230X-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gong QA, et al. High-mobility group box 1 exacerbates concanavalin A-induced hepatic injury in mice. J Mol Med. 2010;88:1289–8. doi: 10.1007/s00109-010-0681-7. [DOI] [PubMed] [Google Scholar]
- 94.Zhou RR, et al. HMGB1 cytoplasmic translocation in patients with acute liver failure. BMC Gastroenterol. 2011;11:21–31. doi: 10.1186/1471-230X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–47. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 96.Wang H, Zhu S, Zhou R, Li W, Sama AE. Therapeutic potential of HMGB1-targeting agents in sepsis. Expert Rev. Mol. Med. 2008;10:e32. doi: 10.1017/S1462399408000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Szabo G, Mandrekar P, Dolganiuc A. Innate immune response and hepatic inflammation. Semin Liver Dis. 2007;27:339–50. doi: 10.1055/s-2007-991511. [DOI] [PubMed] [Google Scholar]
- 98.Berasain C, et al. Inflammation and liver cancer: new molecular links. Ann N Y Acad Sci. 2009;1155:206–21. doi: 10.1111/j.1749-6632.2009.03704.x. [DOI] [PubMed] [Google Scholar]
- 99.Venereau E, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209:1519–28. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang H, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A. 2010;107:11942–7. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abeyama K, et al. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest. 2005;115:1267–74. doi: 10.1172/JCI22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim ID, et al. Intranasal delivery of HMGB1 siRNA confers target gene knockdown and robust neuroprotection in the postischemic brain. Mol Ther. 2012;20:829–39. doi: 10.1038/mt.2011.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Livesey K, et al. p53/HMGB1 complexes regulate autophagy and apoptosis. Cancer Res. 2012;72:1996–2005. doi: 10.1158/0008-5472.CAN-11-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamamoto T, et al. Hemoperfusion with a high-mobility group box 1 adsorption column can prevent the occurrence of hepatic ischemia-reperfusion injury in rats. Crit Care Med. 2010;38:879–85. doi: 10.1097/CCM.0b013e3181c58951. [DOI] [PubMed] [Google Scholar]
- 105.Xie K, et al. Protective effects of hydrogen gas on murine polymicrobial sepsis via reducing oxidative stress and HMGB1 release. Shock. 2010;34:90–7. doi: 10.1097/SHK.0b013e3181cdc4ae. [DOI] [PubMed] [Google Scholar]
- 106.Tang D, et al. Quercetin prevents LPS-induced high-mobility group box 1 release and proinflammatory function. Am J Respir Cell Mol Biol. 2009;41:651–60. doi: 10.1165/rcmb.2008-0119OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang L, et al. Atorvastatin protects rat brains against permanent focal ischemia and downregulates HMGB1, HMGB1 receptors (RAGE and TLR4), NF-kappaB expression. Neurosci Lett. 2010;471:152–6. doi: 10.1016/j.neulet.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 108.Wang L, et al. Tanshinone II A down-regulates HMGB1, RAGE, TLR4, NF-kappaB expression, ameliorates BBB permeability and endothelial cell function, and protects rat brains against focal ischemia. Brain Res. 2010;1321:143–51. doi: 10.1016/j.brainres.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 109.Schierbeck H, Wahamaa H, Andersson U, Harris HE. Immunomodulatory drugs regulate HMGB1 release from activated human monocytes. Mol Med. 2010;16:343–51. doi: 10.2119/molmed.2010.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang M, et al. Chloroquine inhibits HMGB1 inflammatory signaling and protects mice from lethal sepsis. Biochem Pharmacol. 2013;86:410–8. doi: 10.1016/j.bcp.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ulloa L, et al. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A. 2002;99:12351–6. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mollica L, et al. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol. 2007;14:431–41. doi: 10.1016/j.chembiol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 113.Chen G, et al. Suppression of HMGB1 release by stearoyl lysophosphatidylcholine: an additional mechanism for its therapeutic effects in experimental sepsis. J Lipid Res. 2005;46:623–7. doi: 10.1194/jlr.C400018-JLR200. [DOI] [PubMed] [Google Scholar]
- 114.Wang H, et al. The aqueous extract of a popular herbal nutrient supplement, Angelica sinensis, protects mice against lethal endotoxemia and sepsis. J Nutr. 2006;136:360–5. doi: 10.1093/jn/136.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li W, et al. A cardiovascular drug rescues mice from lethal sepsis by selectively attenuating a late-acting proinflammatory mediator, high mobility group box 1. J Immunol. 2007;178:3856–64. doi: 10.4049/jimmunol.178.6.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li W, et al. A major ingredient of green tea rescues mice from lethal sepsis partly by inhibiting HMGB1. PLoS One. 2007;2:e1153. doi: 10.1371/journal.pone.0001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li W, et al. EGCG stimulates autophagy and reduces cytoplasmic HMGB1 levels in endotoxin-stimulated macrophages. Biochem Pharmacol. 2011;81:1152–63. doi: 10.1016/j.bcp.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu S, et al. It is not just folklore: the aqueous extract of mung bean coat is protective against sepsis. Evid Based Complement Alternat Med. 2012;2012;498467 doi: 10.1155/2012/498467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liliensiek B, et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004;113:1641–50. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hagiwara S, Iwasaka H, Hasegawa A, Koga H, Noguchi T. Effects of hyperglycemia and insulin therapy on high mobility group box 1 in endotoxin-induced acute lung injury in a rat model. Crit Care Med. 2008;36:2407–13. doi: 10.1097/CCM.0b013e318180b3ba. [DOI] [PubMed] [Google Scholar]
- 121.Wang H, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–21. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]