Abstract
Small nerve fiber loss and damage (SNFLD) is a frequent complication of sarcoidosis that is associated with autonomic dysfunction and sensory abnormalities, including pain syndromes that severely degrade the quality of life. SNFLD is hypothesized to arise from the effects of immune dysregulation, an essential feature of sarcoidosis, on the peripheral and central nervous systems. Current therapy of sarcoidosis-associated SNFLD consists primarily of immune suppression and symptomatic treatment; however, this treatment is typically unsatisfactory. ARA 290 is a small peptide engineered to activate the innate repair receptor that antagonizes inflammatory processes and stimulates tissue repair. Here we show in a blinded, placebo-controlled trial that 28 d of daily subcutaneous administration of ARA 290 in a group of patients with documented SNFLD significantly improves neuropathic symptoms. In addition to improved patient-reported symptom-based outcomes, ARA 290 administration was also associated with a significant increase in corneal small nerve fiber density, changes in cutaneous temperature sensitivity, and an increased exercise capacity as assessed by the 6-minute walk test. On the basis of these results and of prior studies, ARA 290 is a potential disease-modifying agent for treatment of sarcoidosis-associated SNFLD.
INTRODUCTION
Sarcoidosis is an immune-mediated, inflammatory orphan disease of unclear etiology that can affect virtually any organ of the body (1). In most individuals diagnosed with sarcoidosis, the disease is mild, with pulmonary and hilar lymph node involvement that resolves within several years whether treated by immune suppression or not. However, in about one-third of patients, sarcoidosis evolves into a chronic, progressive disease (2). In these refractory cases, therapy has generally consisted of immune suppression, which has been associated with a variable response rate (3).
Recently, it has become apparent that a significant proportion of patients with chronic sarcoidosis report symptoms that suggest abnormal function of the small nerve fibers of the sensory and autonomic nervous systems (4–8). Clinical evaluation by skin biopsy, typically of the distal leg, has shown that many of these patients have a demonstrable reduction in intraepidermal nerve fibers (6,9). The affected nerves consist of unmyelinated C and lightly myelinated Aδ fibers that comprise the sensory and autonomic peripheral nervous systems. Patients having reduced nerve fiber densities typically complain of pain, numbness and/or dysesthesia, as well as autonomic symptoms that can be extremely variable depending on the organ affected (5). The neuropathic symptoms in these patients are frequently severe and therefore are major contributors to the poor quality of life of those afflicted (10).
The etiology of the loss of small nerve fibers in sarcoidosis has not been definitively identified, but one prevalent hypothesis is that nerve fiber dropout is the end result of systemic and/or local inflammation (11). Neuropathy arising from inflammation can affect both the peripheral nerve endings and the neuronal somata within the dorsal root ganglia of the spinal cord. At the present time, glucocorticoids and other immune suppressants are the principal therapeutic approach to small-fiber neuropathy but are often ineffective (8). In addition to immune modulators as potential disease modifiers, treatment is generally symptomatic, consisting of the analgesics, antiepileptics and antidepressants used for other painful neuropathies (12). Thus, there is a clear need for new therapeutics in sarcoidosis-associated small-fiber neuropathy.
ARA 290 is an 11–amino acid peptide derived from the structure of erythropoietin that possesses potent tissue- protective and tissue-repair activities without stimulating erythropoesis (13). The actions of ARA 290 are mediated through a receptor consisting of a complex formed by the erythropoietin receptor and β common receptor subunits (14), termed the innate repair receptor (IRR). In preclinical models of neuropathic pain, ARA 290 demonstrated beneficial effects that include IRR-dependent prevention of the development of allodynia in a peripheral nerve transection model (15) or in an inflammatory neuritis model (16), as well as attenuation of spinal cord inflammation (15). Also, erythropoietin, and its nonerythropoietic derivatives (for example, ARA 290) have been shown to support the regrowth of intraepidermal nerve fibers in preclinical models of neuropathy arising from toxins (17) or diabetes (18).
An initial open-label study of the effects of three intravenous doses of ARA 290 administered over 1 wk on neuropathic pain of patients with sarcoidosis or diabetes showed a 50% improvement without any safety concerns (19). The results of a follow-up trial of ARA 290 (20) administered intravenously three times weekly for 4 wks to sarcoidosis patients with symptoms of small-fiber neuropathy also appeared to be safe and was associated with a significant improvement in the patient-reported outcomes of the small-fiber neuropathy screening list (SFNSL) (21) and the pain and well-being components of the RAND-36.
On the basis of these observations, we have conducted the present study to assess the effects of ARA 290 on neuropathic symptoms when given as a daily subcutaneous (SC) injection for 28 d. Because of the association of small nerve fiber loss with neuropathic symptoms and the potential for ARA 290 to cause nerve fiber regrowth, we hypothesized that ARA 290 administration will improve symptoms and stimulate the re-growth of small nerve fibers. To evaluate this, the nerve fiber densities in the cornea, proximal thigh and distal leg were assessed. Additionally, cutaneous sensory testing of the face, hand and foot were determined by using quantitative sensory testing (QST), and quality of life was assessed with appropriate patient questionnaires. Finally, functional capacity, which is often reduced in chronic sarcoidosis (22), was assessed by using the 6-minute walk test (6MWT).
MATERIALS AND METHODS
Rationale for Dose Selection
Results of a previous study performed in sarcoidosis patients with painful small-fiber neuropathy showed that 2 mg ARA 290 administered intravenously (IV) three times weekly improved neuropathic symptoms. In the current trial, we sought to assess the potential of SC dosing, as the IV dosing is not practicable in the outpatient setting. Therefore, a crossover pharmacokinetic study was performed using 10 normal volunteers to compare a 2-mg IV dose that was used in the previous study, to 2, 4 or 6 mg ARA 290 administered subcutaneously (19). Results of preclinical and in vitro studies have shown that activation of the IRR requires concentrations of ARA 290 greater than or equal to ~1 nmol/L (~1.3 ng/mL) (14). Therefore, the area under the curve (AUC) of the pharmacokinetic data was calculated by using the trapezoidal rule for the period of time in which the plasma concentrations were >1.3 ng/mL. The results of this crossover study showed the following median AUCs: 2 mg IV = 65 ng/mL × min, 2 mg SC = 23 ng/mL × min, 4 mg SC = 59 ng/mL × min and 6 mg = 249 ng/mL × min, with only the 6-mg dose differing significantly from the others (p < 0.05; Kruskal-Wallis test). Based on these data, the 4-mg SC group was selected for the daily dosing regimen of this trial.
Study Design
The trial, entitled “Effects of ARA 290 on the regrowth of epidermal nerve fibers in patients with sarcoidosis,” was an investigator-initiated, single-site, double-blind, placebo-controlled trial carried out at the Leiden University Medical Center after receiving ethics committee approval. The trial was registered with the International Clinical Trials Registry (NTR3575) and was assigned EudraCT number 2012-001492-37. All study personnel and patients remained blinded to the treatments until the end of the follow-up period (16 wks from the beginning of dosing).
The primary outcomes were as follows: (a) change in epidermal or corneal nerve fiber density at d 28 versus baseline; (b) change in cutaneous sensitivity of d 28 versus baseline by using QST; and (c) change in visual acuity or retinal edema at d 28 versus baseline. Secondary outcomes assessed were as follows: (a) change in the SFNSL score at d 35 versus baseline; (b) change in the Brief Pain Inventory (BPI) at d 35 versus baseline; and (c) change in distance walked in the 6MWT at d 28 versus baseline.
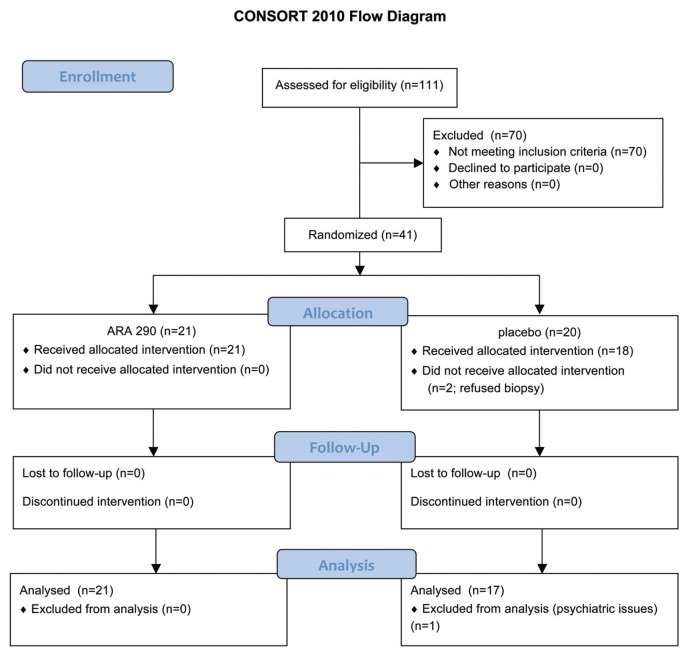
Patients who satisfied the international consensus statement for diagnosis of sarcoidosis (23) and had symptoms suggestive of neuropathy were recruited after referral by sarcoidosis specialists. The Consolidated Standards of Reporting Trial (CONSORT) flow chart corresponding to this trial is illustrated in Figure 1. After obtaining informed consent, a total of 38 patients (18 females, 20 males) of a mean age of 49.5 years (range 28–65) satisfying inclusion criteria were enrolled. These patients had a mean duration since sarcoidosis diagnosis of 8.4 years. The baseline characteristics of these patients with respect to the treatment groups are summarized in Table 1. Although all patients were diagnosed as having sarcoido sis, two patients also had Type 2 diabetes mellitus, a condition known to also be associated with small nerve fiber loss and damage (SNFLD) (12).
Figure 1.
CONSORT flow diagram.
Table 1.
Baseline patient characteristics.
| ARA 290 | Placebo | |
|---|---|---|
| n | 21 | 17 |
| Years since diagnosis of sarcoidosis (mean ± SEM) | 7.1 ± 1.2 | 9.9 ± 2.4 |
| Concomitant medical treatment [n (%)] | ||
| NSAIDS | 5 (23.8) | 8 (47.1) |
| Neurological/psychological drugs | 5 (23.8) | 6 (35.3) |
| Oral corticosteroids | 6 (28.6) | 7 (41.2) |
| Opioids | 6 (28.6) | 2 (11.8) |
| Systemic immune suppressants (methotrexate or azathioprine) | 7 (33.3) | 3 (17.7) |
| Prior TNFα antagonist treatment (n = yes) | 2 (9.5) | 0 |
| SFNSL | ||
| Total score | 43.9 ± 2.9 | 42.8 ± 3.2 |
| Autonomic component | 20.6 ± 2.0 | 20.8 ± 1.5 |
| Pain component | 23.3 ± 1.2 | 22.9 ± 1.2 |
| BPI | ||
| Mean score (pain now; range 0–10) | 5.0 ± 0.4 | 5.3 ± 0.5 |
| Pain interference (maximum 70) | 32.1 ± 1.9 | 36.5 ± 2.9 |
| 6-Min walk | ||
| Test, actual (m) | 468 ± 18 | 479 ± 26 |
| Test, predicted (m)a | 700 ± 12 | 683 ± 15 |
| Nerve fiber density | ||
| Corneal nerve fiber area (μm2) | 1,576 ± 94 | 1,304 ± 104 |
| Normal corneal nerve fiber areab | 3,134 ± 119 | |
| Ankle IENFD (n/mm) | 5.3 ± 0.5 | 4.6 ± 0.4 |
| Normal sex- and age-adjusted ankle IENFDc | 9.9 ± 0.3 | 9.8 ± 0.3 |
| Proximal thigh IENFD (n/mm) | 10.8 ± 0.7 | 11.1 ± 0.9 |
| Normal proximal thigh IENFDd | 21.1 ± 0.2 | 21.0 ± 0.1 |
| Laboratory markers | ||
| High sensitivity C-reactive protein (mg/L) | 1.5 ± 0.2 | 2.9 ± 1.1 |
| Angiotensin-converting enzymee | 47.4 ± 6.1 | 53.6 ± 8.0 |
| Number with elevated angiotensin-converting enzyme n(%) | 5 (23.8) | 6 (35.3) |
Study inclusion criteria required meeting three thresholds: (1) spontaneous pain level (“pain now” of the BPI) >5 (scale 0–10); (2) SFNSL score >22 (out of 84 possible), or pain <5 and SFNSL score >37; and (3) pain defined as distal extremity pain plus one of the following: dysesthesia, burning/painful feet worsening at night or intolerance of sheets/clothes touching the legs or feet. Additional inclusion criteria were as follows: age between 18 and 65 years (inclusive), a body mass index (BMI) between 18 and 30 kg/m2 (inclusive) and the ability to read and understand the written consent form, complete study-related procedures and communicate with the study staff. Exclusion criteria were as follows: abnormal blood pressure, history of alcoholism or illicit drug use, positive pregnancy test, refusal to use acceptable contraception throughout the study period (unless surgically sterilized or postmenopausal), vaccination or surgery within the prior 3 months, or use of anti–tumor necrosis factor (anti-TNF) therapy in the prior 6 months.
Safety was assessed by questioning the patient weekly during ARA 290 administration and throughout the 12-wk follow-up for the occurrence of adverse events. Additionally, the patients were examined at three occasions during the active treatment phase of the study: baseline, 2 wks and 4 wks at the end of dosing. Additionally, blood was drawn for routine hematology and chemistry at these times points. Finally, serum was obtained for determination of possible anti-ARA 290 antibodies.
Patient Questionnaires
Questionnaires were administered at the screening visit and then weekly during the dosing and follow-up period of 3 months (total 16 wks). Questionnaire data were also obtained approximately 6 months after the end of the follow-up period (that is, 9 months from the end of dosing) to assess durability of any effects. The BPI Short Form, consisting of pain intensity and pain interference sections, was administered in the validated Dutch language format. SFNSL is a questionnaire developed specifically for Dutch patients with sarcoidosis to assess pain and autonomic dysfunction consistent with small nerve fiber loss and damage (21). In addition to the total score, the questionnaire was divided into an autonomic component (questions 2–5, 9, 11–16) and a pain component (questions 1, 6–8, 17–21) to assess those dimensions of the patients’ neuropathic symptoms.
QST
Small nerve fiber and large fiber cutaneous sensory function was assessed by using QST of the face, hand and foot using a Medoc Advanced Medical Systems TSA-II device (Ramat Yishai, Israel), following the published protocol of the German Research Network on Neuropathic Pain (24). Normative data were obtained from Rolke et al. (24). Baseline data for each patient group are summarized in Table 2. To arrive at the percentages shown in Table 2, the three regions tested were pooled (that is, each patient’s data were considered abnormal if the results were >2 standard deviations from the normative population mean in at least one of the locations evaluated).
Table 2.
Results of baseline QST.
| Variable | Nerve fibers involved | ARA 290 (n = 21) | Placebo (n = 17) | ||
|---|---|---|---|---|---|
|
|
|
||||
| Change | Number of patients (%) | Change | Number of patients (%) | ||
| Cold detection threshold | Aδ and C | Decrease | 19 (91) | Decrease | 11 (65) |
| Warm detection threshold | Aδ and C | Decrease | 17 (81) | Decrease | 13 (77) |
| Increase | 1 (5) | ||||
| Thermal sensory limen | Aδ and C | Decrease | 4 (19) | Decrease | 4 (24) |
| Increase | 2 (10) | ||||
| Paradoxical heat sensation | Aδ | Decrease | 8 (38) | Decrease | 7 (41) |
| Cold pain threshold | Aδ and C | Increase | 3 (14) | — | 0 |
| Heat pain threshold | C | Decrease | 3 (14) | Decrease | 1 (6) |
| Increase | 5 (24) | ||||
| Mechanical detection threshold | Aβ | Decrease | 11 (52) | Decrease | 10 (59) |
| Mechanical pain threshold | Aβ | Decrease | 11 (52) | Decrease | 4 (24) |
| Increase | 4 (19) | Increase | 2 (12) | ||
| Mechanical pain sensitivity | Aβ + C | Decrease | 2 (10) | Decrease | 1 (6) |
| Increase | 5 (24) | ||||
| Dynamic mechanical allodynia | Aβ | Increase | 11 (52) | Increase | 3 (18) |
| Windup ratio | Aδ and C | Increase | 4 (19) | Increase | 2 (12) |
| Vibration detection threshold | Aβ | Decrease | 20 (95) | Decrease | 15 (88) |
| Pressure pain threshold | Aδ and C | Decrease | 3 (14) | Decrease | 1 (6) |
| Increase | 10 (48) | Increase | 7 (41) | ||
Patients in the ARA 290 and placebo groups showed functional impairment of both small nerve fibers (Aδ and C) as well as larger sensory nerve fibers (Aβ). Data are expressed as number of patients deviating beyond the 95% confidence interval of a sex- and age-matched normal population. Test sites of face, hand and foot are pooled for calculation of percentages. “Decrease” indicates a loss of function; “Increase” indicates a gain in function compared with a normal population. For example, a decreased CDT means that a patient required a lower temperature stimulus than normal to determine that an object was cold (that is, a decrease in sensitivity).
Skin Biopsy
Skin biopsies were obtained at baseline and after 28 d from the proximal thigh (20 cm below the anterior superior iliac spine) and the distal leg (10 cm above the lateral malleolus) by using a disposable punch biopsy (3 mm) and processed following established guidelines (25). After fixation of the biopsy specimens, free floating 50-μm-thick sections were cut and stained by using rabbit anti-protein gene product 9.5 antibody (Dako Netherlands BV, Eindhoven, the Netherlands) and visualized using a goat anti-rabbit Alexa fluor 488 antibody (Invitrogen,/Life Technologies, Carlsbad, CA, USA). A minimum of three sections selected from each end and the middle of each biopsy specimen were evaluated by using a Leica M5500 fluorescence microscope (Leica Microsystems, Rijswijk, the Netherlands) at a magnification of 1,000×. The nerve fibers were counted manually. Images of the sections were recorded by using the Leica Application Suite, magnification 400×, and the length of the epidermaldermal junction measured using ImageJ (National Institutes of Health, Bethesda, MD, USA). Sex- and age-dependent normative data of nerve fiber density used for the distal leg were those of Lauria et al. (26) and, for the thigh, Umapathi et al. (27). All measurements and counting was performed by the same individual, who was blinded to treatment modality. Technical problems during tissue preparation resulted in the loss of two placebo biopsies of the lower leg, one ARA 290 biopsy of the thigh and three placebo biopsies of the thigh.
Corneal Confocal Microscopy
Corneal nerve fiber density was determined by corneal confocal microscopy carried out by using the Rostock Cornea Module with the Heidelberg Retina Tomograph III using established methodology (28). Briefly, after the application of a topical anesthetic, the sterile objective of the confocal microscope was placed on the apex of the cornea, as determined by the characteristic orientation of the nerve fibers in a superior–inferior direction. By using the automatic scan feature of the device, confocal images of graduated depth in the plane of the cornea were acquired. The field of view of each image was 0.4 × 0.4 mm. Images containing sensory nerve fibers within the subbasal layer between the Bowman layer and the basal epithelium were further analyzed. Collected images were subjected to automated analysis using a custom macro written for Fiji, a public-domain image analysis program, version 1.47e (29). This macro maps all neurites in the image on the basis of their brightness and tubeness. The area covered by the mapping is then expressed as a percentage of total image area. For each patient, the 10 images with the highest nerve fiber density were averaged to generate a representative sample for that patient for that eye. Because the variation between eyes of different patients was similar to the variation between eyes of individual patients (standard deviation of the mean neurite area between patients = 562; standard deviation of the difference between eyes of individual patients = 501), each eye was treated as an independent sample. The automated analysis was validated by comparison of 78 randomly selected images in which total neurite length in each image was determined by manually outlining individual neurites. Linear regression analysis showed an excellent goodness of fit (95% confidence interval of the slope: 0.99–1.19; R2 = 0.76; p < 0.0001) between the computer-generated nerve fiber area and the manually measured total nerve fiber length for each image. Both the automated analyses and the manual measurements were performed by a researcher blinded to the treatment modality. The Shapiro-Wilk test showed that, at baseline, the corneal nerve fiber area data were not distributed normally; therefore, nonparametric statistical analysis was performed to determine if a significant treatment effect was observed.
Normative data were calculated from corneal confocal data that were previously reported (30). These data were obtained from 22 healthy volunteers (M/F, 9/13; age 49 ± 2.7 years) by determining the mathematical relationship between corneal nerve fiber area and corneal nerve fiber length. The results showed that a normal corneal nerve fiber area is 3,134 ± 119 μm2.
6-Minute Walk Test
The 6MWT, the distance in meters walked in 6 min, was conducted following American Thoracic Society guidelines (31). Normal 6MWT values were calculated by Troosters et al. (32) by using the regression equation developed from data obtained from a healthy, older normal Dutch population.
Ophthalmologic Tests
To assess for possible retinal edema, optical coherence tomography was carried out to quantitate retinal thickness by using the Zeiss CIRRUS1 system that includes normative values.
Visual acuity was carried out under standard uniform lighting conditions for patients wearing corrective lenses, if any, by using a SLOAN ETDRS chart and scoring system.
Statistical Analysis
Statistical analysis was performed by using JMP version 11.0 (SAS, Cary, NC, USA). Parametric and nonparametric tests, linear modeling and analysis of covariance were carried out where appropriate. p values <0.05 (two-tailed) were considered significant.
RESULTS
Safety
No medically significant deviations were noted in the general blood chemistry or hematology assessments. There was no pain or local irritation surrounding the site of the injection into the upper leg or lower abdomen. No serious adverse events were encountered during the dosing period or within the 12 wks of follow-up. Three adverse events judged to be moderate were noted in the placebo group that resolved spontaneously (diarrhea, irritability, light-headedness). One patient receiving ARA 290 suffered a moderate adverse event consisting of a long-term weight loss of 14 kg over several months that stabilized thereafter. Verification of the patient’s medical history showed that the weight loss began before entering the study. The etiology of the weight loss was undetermined and persisted after administration of ARA 290 ceased. Multiple, mild adverse events were recorded, all of which spontaneously resolved, and none were judged by the investigators as likely to be associated with administration of the study drug. All doses of ARA 290 were administered daily for the full 28-d period. One placebo patient suffering from diarrhea discontinued dosing for the last week of the study. No anti-ARA 290 antibodies were detected in any of the postexposure serum samples.
Primary Endpoints
Nerve fiber density
Corneal nerve fibers
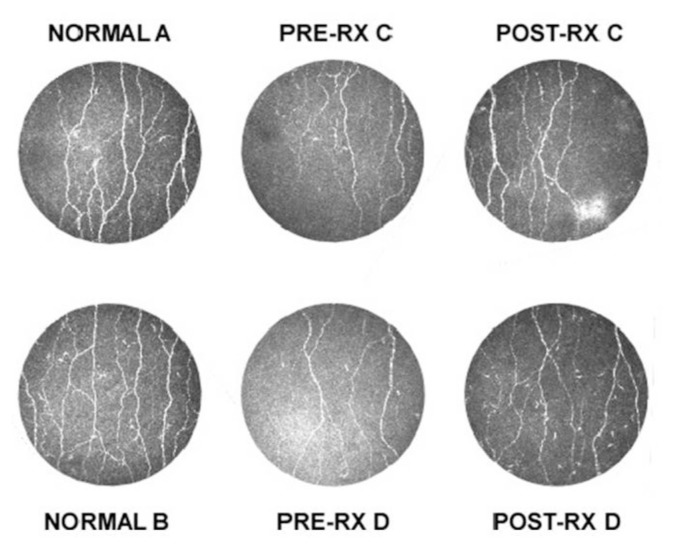
The baseline corneal nerve fiber area showed that the patient population exhibited about a 50% reduction compared with normal controls (Figure 2; Table 1). After 28 d of dosing, the ARA 290 group exhibited a significant increase in the median nerve fiber area over baseline of 14.5%, corresponding to an absolute median increase of 185 μm2 (p = 0.022; Wilcoxon signed rank test). In contrast, the placebo group had a nonsignificant decrease in median nerve fiber area over baseline of −5.3% and an absolute median decrease of 64 μm2 (p = 0.462). Figure 2C illustrates the corneal nerve density of two normal individuals compared with two ARA 290 patients who showed the best responses.
Figure 2.
ARA 290 administration is associated with an increase in corneal nerve fiber area. Examples of the distribution and density of corneal nerve fibers obtained via corneal confocal microscopy performed on two normal individuals (A and B) are shown (left panels). Examples of corneal nerve density obtained from two sarcoidosis patients (C and D) show a decreased density at baseline (middle panel: Pre-RX) and an increase when reimaged after 28 d of ARA 290 administration (right panel: Post-RX).
Intraepidermal nerve fibers
Similar to the corneal nerve fiber area, at baseline, the mean intraepidermal nerve fiber densities (IENFDs) of the proximal and distal leg were significantly reduced by approximately 50% in both treatment groups, compared with the median of age- and sex-matched normal controls (p < 0.0001; Table 1). The mean ratio of IENFD of the proximal thigh to the distal leg was 3.9 ± 1.5 standard error of the mean (SEM), with no patient having a ratio <0.9. The patients in this study, therefore, suffered from a peripheral neuropathy characterized by a length-dependent loss of epidermal nerve fibers. IENFD of the proximal leg was not significantly correlated to that of the distal leg (Pearson correlation coefficient = 0.20; p = 0.22).
After 28 d of dosing, the ARA 290 group exhibited a mean increase in IENFD in the distal leg of 0.38 ± 0.48 fibers/mm (7.2% of baseline; p = ns) compared with the placebo group, which had a mean reduction of nerve fiber density of 0.06 ± 0.42 fibers/mm (1.3% of baseline; p = ns). The thigh IENFD at 28 d showed a mean decrease of 0.49 ± 0.53 fibers/mm for the ARA 290 group (−2.3% of baseline; p = ns), and the placebo group had a mean decrease of 1.24 ± 0.88 fibers/mm (−5.7% of baseline; p = ns).
Cutaneous Sensitivity
Baseline QST data showed that, as a group, the patients with sarcoidosis and painful neuropathy exhibited findings consistent with both small fiber (Aδ and C) and large fiber (Aβ) dysfunction (Table 2). Most patients exhibited a reduced ability to determine cold temperatures (cold detection threshold, 79% of the study group) or warm temperatures (warm detection threshold, 79%) and to detect vibratory stimuli (vibratory stimuli threshold, 92%). A total of 55% of patients also experienced a reduced ability to detect graded mechanical stimuli elicited by von Frey fibers (mechanical detection threshold [MDT]) or pain caused by graded pin prick (mechanical pain threshold [MPT]) or to pressure (pressure pain threshold [PPT]). A minority of patients in each treatment group also exhibited abnormalities in a variety of the other sensory modalities tested, as summarized in Table 2.
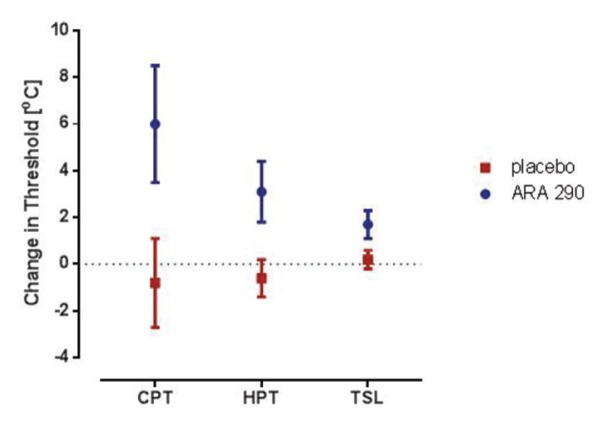
After 28 d of daily dosing, the cold pain threshold (CPT), heat pain threshold (HPT) and thermal sensory limen (TSL) significantly increased in the ARA 290 group, as illustrated in Figure 3, which summarizes data obtained from the hand testing location. In contrast, there were no changes noted in the placebo group. Although a decreased sensitivity was noted for these sensory modalities, the population means at baseline and after ARA 290 dosing remained within the normal range. Similar but smaller changes were noted in the face test location, whereas a nonsignificant trend was noted at the foot testing site (data not shown). Additionally, the cold detection and warm detection thresholds also decreased (that is, decreased sensitivity) at the hand and face sites, but the changes were not quite large enough to be statistically significant (data not shown). No changes were observed in any other sensory modality within the QST battery.
Figure 3.
ARA 290 administration increases the threshold for thermal pain and decreases thermal sensitivity in the hand. The CPT, HPT and TSL of most patients were within normal limits at baseline (Table 1). After ARA 290 administration, the mean threshold for determining a painful cold (p = 0.027; paired t test compared with baseline) or hot (p = 0.032) stimulus increased, whereas the placebo group remained unchanged (p = ns). Similarly, the thermal sensory limen (the temperature threshold at which they can discriminate a hot or cold stimulus) increased in the ARA 290 after exposure (p = 0.008). This decreased thermal sensitivity could correspond to reduced symptoms of temperature-induced allodynia. After ARA 290 treatment, the CPT, HPT and TSL remained within the normal range. The normative means for CPT, HPT and TSL were 9.7 ± 0.5, 44.8 ± 0.2 and 3.0 ± 0.1°C, respectively. Similar smaller changes were noted for the face, as well as a nonsignificant trend for the foot (data not shown).
Retinal Thickness and Visual Acuity
Baseline average thickness of the macula and central macula, and retinal nerve fibers of both eyes, were normal in all patients and did not significantly change over the 28-d observation period (data not shown). Visual acuity at baseline obtained with corrective lenses was normal except for one patient in the ARA 290 group (data not shown). The visual acuity of this patient, and that of all other patients, did not change after ARA 290 exposure.
Secondary Endpoints
SFNSL
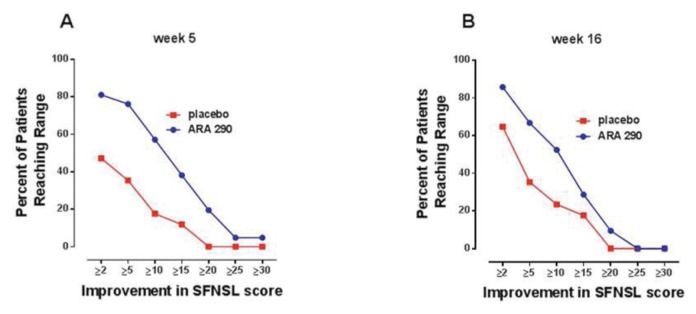
Baseline scores of the SFNSL developed specifically for sarcoidosis patients showed that the treatment groups were very symptomatic and well matched, with mean baseline values of 43.9 and 42.8 for the ARA 290 and placebo groups, respectively (not significantly different; t test). When evaluated at wk 5 (that is, 1 wk after the end of dosing), the ARA 290 group showed a mean reduction in the SFNSL score of 12.2 ± 1.9 (median 13.0; ~28% reduction from baseline) compared with 3.8 ± 2.1 (median 1.0; ~9% reduction from baseline) for placebo (difference between groups: p = 0.005; t test). Construction of proportional responders curves (Figure 4A) showed that the percentage of patients receiving ARA 290 having symptomatic improvement in the SFNSL score was greater than the placebo group at each response level. For example, 81% of the ARA 290 patients exhibited at least a two-point improvement in the SFNSL score, compared with only 47% of patients within the placebo group. This response profile was substantially maintained during the 12-wk follow-up period, at which time the mean score reduction from baseline for the ARA 290 group was 9.7 ± 1.8 (median 11.0) and for placebo was 4.1 ± 1.9 (median 3.0; difference between groups: p = 0.037; t test). The proportional responder curves at 16 wks (12 wks after the end of dosing) were similar to that observed immediately after treatment (Figure 4B). Follow-up at 6 months after the study observation period (that is, 9 months after the termination of dosing) was possible for 19 of 21 of the ARA 290 patients and all of the placebo patients and was notable for a mean SFNSL score of 38.1 ± 3.2 SEM versus 43.7 ± 3.2, respectively. This result represented a significant improvement over baseline for the ARA 290 group (5.2 ± 1.9) compared with the placebo group (−0.9 ± 2.0; p = 0.036).
Figure 4.
Evaluation of efficacy using the SFNSL shows a sustained improvement in the ARA 290 treatment group compared with placebo. (A) One week after the end of dosing, a larger percentage of patients in the ARA 290 group attained a specified range of score improvement over a broad range of responses. (B) This difference was largely maintained at the end of the 16 wks of follow-up.
With respect to the autonomic component of the SFNSL, the ARA 290 group demonstrated a significant improvement in the autonomic score when compared with the placebo group with mean improvements of 6.0 ± 1.1 and 1.2 ± 1.3, respectively (p = 0.009; t test). These improvements correspond to a 29% change from baseline for the ARA 290 group compared with a 6% improvement in the placebo group. A significant difference was observed in the pain component, with the ARA 290 group having a mean improvement of 6.2 ± 1.1 points (27% of baseline) compared with the placebo group, with a mean improvement of 2.6 ± 1.3 points (12% of baseline; p = 0.032; t test).
BPI
Pain intensity
One week after the last injection (that is, on d 35), the average BPI pain intensity score was reduced ~9% from baseline in both treatment groups, with a mean decrease of −3.4 (out of a maximum of 40). This result represented a significant improvement for both the active and placebo arms with respect to baseline (p = 0.01; t test), but with no significant difference between the treatment groups. The individual pain intensity scores were notable for a similar reduction in “most pain” (−1.2; p = 0.003), “average pain” (−1.1; p = 0.004) and “pain now” (−1.0; p = 0.03), whereas “least pain” did not change from baseline (−0.2; p = 0.54).
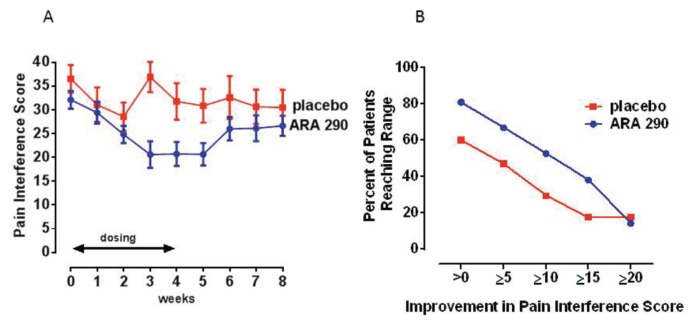
Pain interference
In contrast to the mean pain intensity scores that were significantly improved in both groups by wk 5, the mean change in the BPI pain interference score differed significantly between the treatment groups by the third week of dosing (p < 0.02; Figure 5A). Specifically, while the baseline values of the two groups were not different, the ARA 290 group dropped from a mean score of 32.1 ± 2.3 at baseline to 20.6 ± 2.7 by the week after dosing (a 36% reduction from baseline). This result compares to a change in the placebo group from a baseline of 36.5 ± 2.5 to 30.8 ± 3.1 (a 16% change from baseline). Proportional responder analysis (Figure 5B) illustrates that the ARA 290 group exhibited about a 20% greater proportion of responders across the response spectrum up to an improvement total of 20 points. At the time of evaluation 9 months after dosing, two patients in the ARA 290 group were lost to follow-up. For the remaining patients, the pain interference score did not significantly differ from the baseline values. Specifically, the score in the ARA 290 group mean was 30.0 ± 2.2 and in the placebo group was 33.9 ± 2.9.
Figure 5.
ARA 290 treatment improves the BPI pain interference score. (A) Weekly pain interference scores significantly decline over the 4 wks of daily dosing for the ARA 290 compared with the placebo group. (B) A proportional responder display illustrates that the ARA 290 group responded to a larger extent at all levels of improvement.
6MWT
The 6MWT is a measure of functional exercise capacity. Both groups had approximately the same baseline 6MWT distance (Table 1), which was significantly less than normal. By using a prediction formula for a normal population with the same approximate age spread (32), the patients in this study at baseline exhibited a mean reduction of 219 meters (p < 0.0001; 95% confidence interval of −186 to −253 m) in the actual distance walked in 6 min from a predicted value of 693 m. After 28 d of daily dosing, the 6MWT showed that the ARA 290 group increased the distance walked by a mean of 18.7 m, whereas the performance of the placebo group fell by a mean of −15.1 m (difference between groups: p = 0.049; t test). A proportional responder analysis (Figure 6) illustrates that about half of the patients in both treatment groups had improved their 6-min walk distance by up to 12 m. However, for an increase from >25 m, only 12% in the placebo group improved, compared with 52% of the ARA 290 group. Substantial percentages of the ARA 290 group exhibited even larger increases in the 6-min walk distance, whereas none of the placebo patients did.
Figure 6.
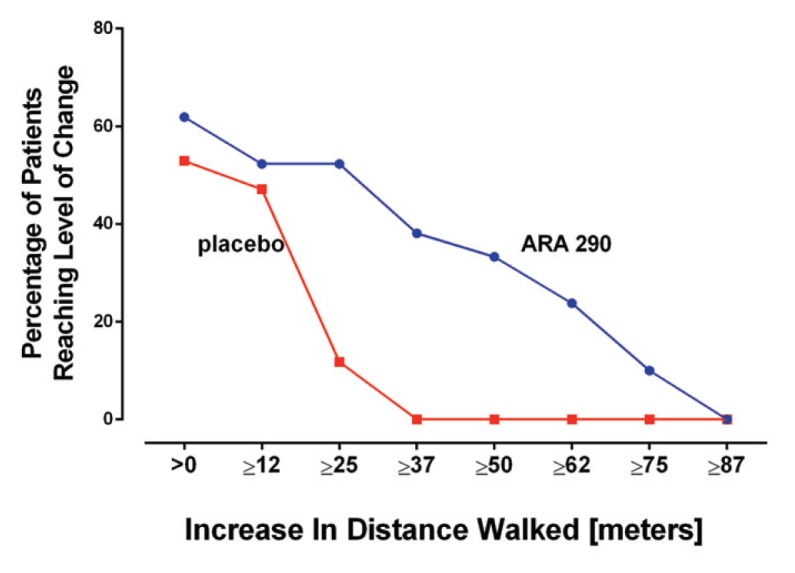
ARA 290 increases the distance patients can walk in 6 min. Similar to the results of symptom questionnaires, patients receiving ARA 290 performed better at all levels of response in the 6MWT.
A 6MWT was repeated at 9 months after dosing (two ARA 290 patients and one placebo patient were lost to follow-up). The mean change from baseline in the ARA 290 group was 8.3 ± 13.3 m and for placebo was −12.9 ± 13.9, neither of which constituted a significant change from baseline (p = ns; t test).
DISCUSSION
Sarcoidosis complicated by small-fiber neuropathy is a chronic disease characterized by the loss of small nerve fibers with associated pain, decreased temperature sensitivity, thermal allodynia and pronounced autonomic dysfunction that severely degrades quality of life. All patients included in this trial had painful neuropathic symptoms consistent with SFNSL that were unresponsive to the standard therapies for chronic sarcoidosis. Many patients continued on immune suppression and symptom-directed therapy throughout the trial.
The principal hypothesis to be tested in this study was whether exposure to ARA 290, a molecule demonstrating tissue-protective, antiinflammatory and reparative activities in numerous preclinical models, would stimulate nerve fiber regrowth with associated improvements in pain and other sensory symptoms, and autonomic function. To accomplish this, the trial was designed to focus on the assessment of objective endpoints such as small nerve fiber quantification by using both skin biopsy and corneal confocal microscopy and to relate these findings to semiobjective sensory testing by using QST, which directly assesses the effects of potential changes in cutaneous innervation. The 6MWT was also included as a simple semiobjective test that requires the integration of complex sensory stimuli of the lower limbs and good exertion by the patient. Finally, patient-reported outcomes were included for subjective assessments of pain and the degree to which pain interfered with activities of daily living, as well as symptoms of autonomic dysfunction that could also be potentially related to changes in nerve fiber density.
Baseline nerve fiber data from this study were analyzed (30), and these data show that corneal nerve quantification (density and length) correlates well with the IENFD of the distal, but not the proximal, lower limb when adjusted for the covariates of sex and age. Further, at baseline, the corneal nerve fiber density (and length) is inversely related to the BPI pain interference score and therefore has relevance for the symptoms that the patients report. Previous work performed in patients with diabetes has also shown a good correspondence between corneal nerve quantification and nerve fiber counts performed in the distal leg (33), thereby confirming the usefulness of corneal nerve assessment in patients with symptoms of small-fiber neuropathy.
The results of nerve fiber assessment after 28 d of dosing show that the corneal nerve fiber density improved significantly in the ARA 290 group when compared with the placebo group at the end of 28 d of dosing. In contrast, no change was observed in the IENFD obtained from the proximal thigh, although a trend was observed for the distal leg biopsy site. Notably, a recent study carried out in a diabetic population has reported positive effects of treatment on corneal nerve fiber density (although over a longer time scale, with no change in the skin biopsy nerve density of the distal extremity) (33). In this study, patients with Type 1 diabetes were followed after curative therapy by pancreas transplantation. Twelve months (but not 6 months) after normalization of blood glucose concentrations, a significant increase in corneal nerve fiber density was documented, whereas no changes were observed in the IENFD of the distal leg or in the results of QST. Additionally, Boyd et al. (34) were able to demonstrate a change in skin biopsy nerve densities after drug administration. These investigators studied Type 2 diabetic patients with small-fiber neuropathy after 12 wks of administration of the antiepileptic drug topiramate and documented an increase in cutaneous nerve fiber length at multiple biopsy sites and in nerve fiber density in the proximal leg. It would be of interest to know what assessment of the corneal nerve fibers would have shown.
Prior study (35) of re-innervation after experimental denervation by using capsaicin application to the skin of diabetics with neuropathy or normal individuals has shown that the natural rate of re-growth of sensory nerve fibers is slow in normal individuals and very slow in patients with diabetes. In contrast, re-growth of autonomic fibers is appreciably faster (40–50 d to return to baseline density) than sensory fibers (140–160 d for normalization) (36). Similar experiments have not been performed on corneal nerve fibers, but the results of a preclinical model shows that rapid regeneration (days to weeks) occurs after mechanical injury (37). It is possible that the cornea is an especially useful location to evaluate potential nerve regrowth. Corneal confocal microscopy has the benefit that it is a noninvasive technique that can be repeated many times in the same patient and thus is well suited for longitudinal interventional studies.
As a group, QST showed that the majority of patients in this study had significantly increased cold, warm and vibratory detection thresholds. For patients with sensitivity to cold or heat, this could translate into less pain during activities of daily living. Previous study of patients with diabetic neuropathy has reported similar findings in patients who specifically complained of pain (38). Because thermal sensory function depends on small fiber function, the admission criterion of neuropathic pain may have specifically selected patients that possess a high degree of fiber loss. This possibility was confirmed by the intraepidermal and corneal nerve fiber assessments that showed a marked reduction in the mean number of small fibers innervating cutaneous and corneal sites compared with a normal population.
It is currently unclear what sensory changes may be associated with the axon regeneration that occurs during the short time frame of this clinical trial, since the results of few relevant studies have been reported. Clinical studies performed by using nerve growth factor show that a single injection into normal individuals produces both mechanical and thermal hypersensitivity at the site of injection, which is rapid, reaching a maximum by 21 d and 3 d, respectively (39). Hypersensitivity has been observed at the injection site in longer-term clinical trials, with repeated injections carried out on patients with neuropathy, for example, diabetic polyneuropathy (40). As mentioned above, no injection site pain was noted after ARA 290 administration in the current study; yet changes in sensory thresholds occurred at distant test sites. This observation suggests that ARA 290 has effects within the central nervous system that underlie the altered sensory thresholds.
On the basis of preclinical work, it also appears that changes in responsiveness may occur within the time frame of the present clinical study. Tanelian and Monroe (37) studied a rabbit model in which they produced corneal nerve fiber injury by a small punch biopsy and subsequently used electrophysiological methods to directly determine the behavior of regenerating small nerve fibers to cold stimuli. Their findings document electro-physiological changes that returned to normal by 30 d after injury. If similar changes occur in patients during the early period of regrowth, we would expect to observe changes in thermal thresholds to the extent that axon sprouting has occurred. However, no assessments were carried out during the period of dosing that can provide relevant information. Additionally, the questionnaires administered do not provide information that is helpful in determining thermal sensory thresholds. It will be important to add these assessments in future trials. However, it is highly likely that any changes that might occur in the sensory system as a result of effects of 28 d of dosing with ARA 290 would not have reached a steady state.
The results of this study show that ARA 290 administration to patients with painful small-fiber neuropathy is associated with a significant improvement in patient-reported symptoms compared with patients receiving placebo, without any evident adverse events attributable to the drug. The changes in level of discomfort as assessed by the SFNSL after 4 mg ARA 290 administered subcutaneously daily was remarkably similar to what was observed in the previous blinded trial, in which 2 mg ARA 290 was administered three times weekly by the IV route (20). In the prior trial, approximately 80% of the patients in the active arm exhibited some improvement and ~40% showed improvement of ~50% over baseline. In contrast, whereas about 45% of the patients in the placebo arm showed some improvement, only ~12% showed a 50% improvement. Daily administration of 4 mg ARA 290 administered subcutaneously was well tolerated without any evident adverse effects. Also similar to the previous blinded trial, a large proportion of the change in SFNSL score was attributable to questions that are relevant to autonomic symptoms. Finally, it is remarkable how sustained the response to ARA 290 appears to be. This result may reflect the growth of small nerve fibers, as the corneal confocal nerve fiber data reveal.
Self-assessment of pain intensity by using the BPI showed that similar to the first blinded trial (20), both groups improved equally, indicating a significant placebo effect on this dimension. In contrast, assessment of to what extent the level of pain interfered with activities of daily living, mood and enjoyment of life showed that patients who received ARA 290 had an immediate reduction in mean score, reaching a nadir that was significantly different from placebo by the end of the dosing period. This result suggests that ARA 290 has a complex activity that extends beyond the sensation of pain to include effects on activities of daily living.
The 6MWT was originally developed to assess functional exercise capacity (that is, a measure of the ability to engage in physically demanding activities) in patients with chronic cardiopulmonary diseases. Since its introduction, the 6MWT has been used to evaluate functional capacity in a wide range of diseases and in healthy normal individuals (32) and has been used as a means to assess the effects of therapeutic interventions. Studies evaluating patients with chronic sarcoidosis have observed that ~50% of these patients have a markedly impaired baseline 6MWT (22,41). In the current study, we found that all of the patients had a reduction in expected walk distance (some very severe). The reason for the higher prevalence in this patient population is not clear, but could arise from the fact that the patients were selected for the presence of neuropathic symptoms that involved the feet, which could contribute to a poor performance on a walk test due to sensory deficits and pain.
At the end of dosing, the ARA 290 group had improved a mean of ~19 m, while the placebo group had declined by ~15 m, about a 4% improvement and 3% decrease of baseline, respectively. Although only about half of the patients improved in both groups (Figure 6), the improvement in distance walked in the 6MWT was limited in the placebo group to <37 m, whereas almost one-quarter of the ARA 290 patients improved the distance walked by up to 75 m. A minimally clinically significant difference has not been established for sarcoidosis patients with painful neuropathy, but for patients with cardiopulmonary disease, the minimally clinically significant difference was determined to be a low as 25 m (42).
The most prevalent form of small-fiber neuropathy occurs in patients with prediabetes or diabetes, and in this group, retinal edema and visual acuity changes are very common. Additionally, another major clinical manifestation of chronic sarcoidosis is ocular inflammation, especially uveitis, which often affects the retina (2). Alternatively, a recent study has shown that patients with neurosarcoidosis frequently have macular edema, even in the absence of ocular symptoms (43). It was of interest, therefore, to evaluate retinal thickness and visual acuity before and after dosing. At baseline, there were no significant abnormalities observed in the optical coherence tomographic evaluation of either retinal or optic nerve head thickness. Similarly, almost all patients had good visual acuity at baseline. Therefore, retinal abnormalities and visual acuity impairment do not appear to be a common feature of sarcoidosis complicated by small-fiber neuropathy.
The patients included in this trial all had longstanding sarcoidosis, with a mean time since diagnosis of 8.4 years. They all had failed existing therapy for neuropathy, including the use of antiinflammatory agents (nonsteroidal antiinflammatory drug [NSAIDs], glucocorticoids and methotrexate principally), as well as antiepileptics and antidepressants. About 30% of the patients were using a variety of these drugs during the conduct of this trial. Because of the small numbers of patient studied, it is not possible to evaluate synergistic effects with any of these agents. It will be interesting to assess for this possibility in future trials with ARA 290.
The principal limitations of this study are that only patients with pain were studied, and these patients did not have known active sarcoid involvement of any other organ. Circulating markers of inflammation were not significantly elevated and presumptive markers of active sarcoidosis (for example, angiotensin-converting enzyme levels) were only mildly increased in a minority of patients. Small nerve fiber loss is also well known to occur without associated painful symptoms, for example, in the prediabetic state (44). It will be of interest to determine whether corneal nerve fiber density is also abnormal in this patient group.
CONCLUSION
ARA 290 is the first drug that exhibits the ability to induce small nerve fiber regeneration in the cornea without serious side effects, showing a potential of true disease modification, not just symptom improvement. In addition, this trial design using the combination of objective and subjective endpoints offers insight into correlations with patient-reported outcomes and may provide a blueprint for superior trial design for future studies. Most importantly, the results of this study can provide some hope for sarcoidosis patients suffering from small nerve fiber loss and damage, since ARA 290 could substantially improve their quality of life.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the Dutch government to the Netherlands Institute for Regenerative Medicine (NIRM, grant FES0908). Joop van Heerikhuize, Department of Image Analysis of the Netherlands Institute for Neuroscience, provided crucial support by developing the macro used for automated quantification of the CCM images. The authors thank the patients and their families for agreeing to participate in this trial. We also wish to recognize M Drent and E Hoitsma for their pioneering work identifying the existence of small-fiber neuropathy and its major negative impact on quality of life in patients with sarcoidosis and for their invaluable input. We also thank L Aarts, R Baughman, F Breedveld, D Culver, G Lauria, R Kirk, N Lois, R Malik, A Rabelink and M Yamin for their support, assistance and helpful advice that made this study possible.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
A Dunne, A Cerami and M Brines are employees of Araim Pharmaceuticals and have stock or stock options in the company.
REFERENCES
- 1.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–65. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 2.Chen ES, Moller DR. Sarcoidosis: scientific progress and clinical challenges. Nat Rev Rheumatol. 2011;7:457–67. doi: 10.1038/nrrheum.2011.93. [DOI] [PubMed] [Google Scholar]
- 3.Baughman RP, et al. Defining the clinical outcome status (COS) in sarcoidosis: results of WASOG Task Force. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28:56–64. [PubMed] [Google Scholar]
- 4.Bakkers M, et al. Pain and autonomic dysfunction in patients with sarcoidosis and small fibre neuropathy. J Neurol. 2010;257:2086–90. doi: 10.1007/s00415-010-5664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heij L, Dahan A, Hoitsma E. Sarcoidosis and pain caused by small-fiber neuropathy. Pain Res Treat. 2012;2012;256024 doi: 10.1155/2012/256024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoitsma E, et al. Small fibre neuropathy in sarcoidosis. Lancet. 2002;359:2085–6. doi: 10.1016/s0140-6736(02)08912-2. [DOI] [PubMed] [Google Scholar]
- 7.Judson MA. Small fiber neuropathy in sarcoidosis: something beneath the surface. Respir Med. 2011;105:1–2. doi: 10.1016/j.rmed.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Tavee J, Culver D. Sarcoidosis and small-fiber neuropathy. Curr Pain Headache Rep. 2011;15:201–6. doi: 10.1007/s11916-011-0180-8. [DOI] [PubMed] [Google Scholar]
- 9.Bakkers M, et al. Intraepidermal nerve fiber density and its application in sarcoidosis. Neurology. 2009;73:1142–8. doi: 10.1212/WNL.0b013e3181bacf05. [DOI] [PubMed] [Google Scholar]
- 10.Hoitsma E, et al. Impact of pain in a Dutch sarcoidosis patient population. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:33–9. [PubMed] [Google Scholar]
- 11.Uceyler N, et al. Elevated proinflammatory cytokine expression in affected skin in small fiber neuropathy. Neurology. 2010;74:1806–13. doi: 10.1212/WNL.0b013e3181e0f7b3. [DOI] [PubMed] [Google Scholar]
- 12.Tavee J, Zhou L. Small fiber neuropathy: a burning problem. Cleve Clin J Med. 2009;76:297–305. doi: 10.3949/ccjm.76a.08070. [DOI] [PubMed] [Google Scholar]
- 13.Brines M, et al. Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc Natl Acad Sci U S A. 2008;105:10925–30. doi: 10.1073/pnas.0805594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brines M, Cerami A. The receptor that tames the innate immune response. Mol Med. 2012;18:486–96. doi: 10.2119/molmed.2011.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swartjes M, et al. ARA290, a peptide derived from the tertiary structure of erythropoietin, produces long-term relief of neuropathic pain: an experimental study in rats and beta-common receptor knockout mice. Anesthesiology. 2011;115:1084–92. doi: 10.1097/ALN.0b013e31822fcefd. [DOI] [PubMed] [Google Scholar]
- 16.Pulman KG, et al. The erythropoietin-derived peptide ARA290 reverses mechanical allodynia in the neuritis model. Neuroscience. 2013;233:174–83. doi: 10.1016/j.neuroscience.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Bianchi R, et al. Protective effect of erythropoietin and its carbamylated derivative in experimental Cisplatin peripheral neurotoxicity. Clin Cancer Res. 2006;12:2607–12. doi: 10.1158/1078-0432.CCR-05-2177. [DOI] [PubMed] [Google Scholar]
- 18.Bianchi R, et al. Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proc Natl Acad Sci U S A. 2004;101:823–8. doi: 10.1073/pnas.0307823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niesters M, et al. The erythropoietin-analogue ARA 290 for treatment of sarcoidosis-induced chronic neuropathic pain. Exp Opin Orphan Drugs. 2013;1:77–87. [Google Scholar]
- 20.Heij L, et al. Safety and efficacy of ARA 290 in sarcoidosis patients with symptoms of small fiber neuropathy: a randomized, double-blind pilot study. Mol Med. 2012;18:1430–6. doi: 10.2119/molmed.2012.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoitsma E, De Vries J, Drent M. The small fiber neuropathy screening list: construction and cross-validation in sarcoidosis. Respir Med. 2011;105:95–100. doi: 10.1016/j.rmed.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Marcellis RG, et al. Exercise capacity, muscle strength and fatigue in sarcoidosis. Eur Respir J. 2011;38:628–34. doi: 10.1183/09031936.00117710. [DOI] [PubMed] [Google Scholar]
- 23.Costabel U, Hunninghake GW. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735–7. doi: 10.1034/j.1399-3003.1999.14d02.x. [DOI] [PubMed] [Google Scholar]
- 24.Rolke R, et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10:77–88. doi: 10.1016/j.ejpain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Lauria G, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2010;17:903–12. e44–9. doi: 10.1111/j.1468-1331.2010.03023.x. [DOI] [PubMed] [Google Scholar]
- 26.Lauria G, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst. 2010;15:202–7. doi: 10.1111/j.1529-8027.2010.00271.x. [DOI] [PubMed] [Google Scholar]
- 27.Umapathi T, et al. Determinants of epidermal nerve fiber density in normal individuals. Muscle Nerve. 2006;33:742–6. doi: 10.1002/mus.20528. [DOI] [PubMed] [Google Scholar]
- 28.Tavakoli M, Malik RA. Corneal confocal microscopy: a novel non-invasive technique to quantify small fibre pathology in peripheral neuropathies. J. Vis. Exp. 2011 Jan;3 doi: 10.3791/2194. pii: 2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider CA, Rasband WS, Eliceiri KW. Nat Methods. Vol. 9. NIH; 2012. Image to ImageJ: 25 years of image analysis; pp. 671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brines M, et al. Corneal nerve quantification predicts the severity of symptoms in sarcoidosis patients with painful neuropathy. Technology. 2013;1:1–7. [Google Scholar]
- 31.ATS statement. Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 32.Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. Eur Respir J. 1999;14:270–4. doi: 10.1034/j.1399-3003.1999.14b06.x. [DOI] [PubMed] [Google Scholar]
- 33.Quattrini C, et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56:2148–54. doi: 10.2337/db07-0285. [DOI] [PubMed] [Google Scholar]
- 34.Boyd AL, et al. Topiramate improves neurovascular function, epidermal nerve fiber morphology, and metabolism in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2010;3:431–7. doi: 10.2147/DMSOTT.S13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polydefkis M, et al. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain. 2004;127:1606–15. doi: 10.1093/brain/awh175. [DOI] [PubMed] [Google Scholar]
- 36.Gibbons CH, Wang N, Freeman R. Capsaicin induces degeneration of cutaneous autonomic nerve fibers. Ann Neurol. 2010;68:888–98. doi: 10.1002/ana.22126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanelian DL, Monroe S. Altered thermal responsiveness during regeneration of corneal cold fibers. J Neurophysiol. 1995;73:1568–73. doi: 10.1152/jn.1995.73.4.1568. [DOI] [PubMed] [Google Scholar]
- 38.Sorensen L, Molyneaux L, Yue DK. The level of small nerve fiber dysfunction does not predict pain in diabetic neuropathy: a study using quantitative sensory testing. Clin J Pain. 2006;22:261–5. doi: 10.1097/01.ajp.0000169670.47653.fb. [DOI] [PubMed] [Google Scholar]
- 39.Rukwied R, et al. Nerve growth factor-evoked nociceptor sensitization in pig skin in vivo. J Neurosci Res. 2010;88:2066–72. doi: 10.1002/jnr.22351. [DOI] [PubMed] [Google Scholar]
- 40.Apfel SC, et al. Efficacy and safety of recombinant human nerve growth factor in patients with diabetic polyneuropathy: a randomized controlled trial. rhNGF Clinical Investigator Group. JAMA. 2000;284:2215–21. doi: 10.1001/jama.284.17.2215. [DOI] [PubMed] [Google Scholar]
- 41.Baughman RP, Sparkman BK, Lower EE. Six-minute walk test and health status assessment in sarcoidosis. Chest. 2007;132:207–13. doi: 10.1378/chest.06-2822. [DOI] [PubMed] [Google Scholar]
- 42.Gremeaux V, et al. Determining the minimal clinically important difference for the six-minute walk test and the 200-meter fast-walk test during cardiac rehabilitation program in coronary artery disease patients after acute coronary syndrome. Arch Phys Med Rehabil. 2011;92:611–9. doi: 10.1016/j.apmr.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 43.Eckstein C, et al. Detection of clinical and subclinical retinal abnormalities in neurosarcoidosis with optical coherence tomography. J Neurol. 2012;259:1390–8. doi: 10.1007/s00415-011-6363-8. [DOI] [PubMed] [Google Scholar]
- 44.Papanas N, Vinik AI, Ziegler D. Neuropathy in prediabetes: does the clock start ticking early? Nat Rev Endocrinol. 2011;7:682–90. doi: 10.1038/nrendo.2011.113. [DOI] [PubMed] [Google Scholar]