Abstract
A series of block copolymers based on 2-methacryloyloxyethyl phosphorylcholine (MPC) were synthesized by reversible addition fragmentation chain transfer (RAFT) polymerization. Incorporation of dihydrolipoic acid (DHLA) into the hydrophobic block led to formation of block copolymer micelles in water. The micelles were between 15 and 30 nm in diameter, as characterized by dynamic light scattering (DLS), with some size control achieved by adjusting the hydrophobic/hydrophilic balance. Cross-linked micelles were prepared by disulfide formation, and observed to be stable in solution for weeks. The micelles proved amenable to disassembly when treated with a reducing agent, such as dithiothreitol (DTT), and represent a potential delivery platform for chemotherapeutic agents. As a proof-of-concept, camptothecin (CPT) was conjugated to the polymer scaffold through a disulfide linkage, and release of the drug from the micelle was monitored by fluorescence spectroscopy. These CPT-loaded prodrug micelles showed a reduction in release rate compared to physically encapsulated CPT. The use of disulfide conjugation facilitated drug release under reducing conditions, with a half-life (t1/2) of 5.5 hours in the presence of 3 mM DTT, compared to 28 hours in PBS. The toxicity of the micellar prodrugs was evaluated in cell culture against human breast (MCF7) and colorectal (COLO205) cancer cell lines.
Keywords: Polymer-drug conjugate, polymer pro-drug, poly(methacryloyloxyethyl phosphorylcholine), cancer drugs camptothecin, drug delivery, polymer micelle
Introduction
Recent advances in polymer therapeutics provide opportunities for improving pharmaceutical administration and delivery methods, including advances in experimental approaches to chemotherapy.1,2,3 Covalently conjugating a small molecule drug to a water-soluble polymer scaffold affords pro-drugs with massively improved aqueous solubility, longer in vivo circulation time (t1/2), and reduced side effects.1 The use of macromolecular scaffolds affords increased hydrodynamic size compared to the drug alone, resulting in slower renal clearance, and increased uptake in tumor tissue by the enhanced permeability and retention (EPR) effect.4 The EPR effect exploits preferential uptake of large molecules due to the porous vasculature of tumor tissue, and subsequent retention as a result of poor lymphatic drainage relative to healthy tissue.
Effective polymer pro-drugs employ water-soluble, biocompatible polymers that introduce potent cancer drugs (which are often hydrophobic compounds) effectively into the bloodstream. Examples of hydrophilic polymers suitable for cancer drug delivery include poly(ethylene glycol) (PEG),5 poly-N-(2-hydroxypropyl)methacrylamide (HPMA),6–9 cyclodextrin-based polymers,10,11 and more recently poly(methacryloyloxyethyl phosphorylcholine) (MPC).12,13 In addition to conventional linear polymer-drug conjugates, alternative architectures have been used with impressive results, including branched structures, such as a dendritic PEG-polyester doxorubicin (DOX) conjugate,14,15 as well as numerous reports of encapsulated drugs in micelle and liposomal systems.16–18 Polymer micelles are enabling materials in nanoscale therapeutics, generally prepared from amphiphilic block copolymers where, in water, the hydrophobic block sequesters drug within the core, and the hydrophilic block serves as an encapsulating corona, imparting both water solubility and stealth properties to the micelles.19–22 The ease with which drug-loaded micelles can be prepared (usually by dialysis or dilution) makes these nanostructures attractive for injectable therapeutics. However, their use can be problematic as they tend to suffer from “burst release” kinetics, where a large percentage of the payload is released very quickly.23 Dilution upon injection is also a concern: self-assembled polymer micelles are dynamic structures with equilibrium between free and associated polymer chains. Though polymeric micelles often have low critical micelle concentrations (CMC), extreme dilution arising from intravenous injection shifts the equilibrium toward free polymer, resulting in disassociation of the micelles and liberation of the payload.23 An effective method to overcome these challenges is to stabilize polymer micelles by covalent cross-linking.
Both shell and core cross-linked micelles have been prepared from a variety of different chemistries, including cross-linking with bi-functional additives,24,25 free radical polymerization,26,27 and photo-cross-linking.28,29 Reversing the cross-linking with an environmental trigger is an area of great interest, and examples of pH cleavable and redox reducible cross-links have been reported.20–33 Cross-linking with disulfides may be particularly important for drug delivery due to their triggered bond breakage under physiologically-relevant and intracellular-specific conditions, since the intracellular environment is up to 1000 times more reducing than extracellular fluids.34 Several recent reports utilized disulfide cross-linked micelles as drug delivery vehicles. Thiols have been introduced to polymers by post-polymerization modification, for example by thiol functionalization of PEGylated poly(lysine) with N-succinimidyl 3-(2-pyridyldithio)-propionate.35 Following micellization, oxidation led to core cross-linking, and treatment with dithiothreitol (DTT) resulted in micelle dissociation. In another example, a random copolymer of methacryloyloxyethyl phosphorylcholine (MPC), glycidyl methacrylate, and stearyl methacrylate formed micelles ~100 nm in diameter, with disulfide cross-linking achieved by reaction of the epoxide with cystamine.36 The micelles were responsive to DTT, and biocompatible. Disulfide cross-linked polymer micelles have been reported as carriers for chemotherapeutic agents including doxorubicin (DOX)37,38 camptothecin (CPT),39 and paclitaxel (PTX).40,41 A recent example employed a block copolymer of PEG and HPMA, where a percentage of the HPMA block was coupled to lipoic acid. Micelles from this polymer were loaded with DOX, and cross-linked using DTT.42 However, this post-polymerization modification lacked control over, and characterization of, the degree of substitution, and the block copolymers were water insoluble.
Here we report the synthesis of novel block copolymers based on polyMPC, where the second block is prepared from a lipoic acid-based methacrylate. This synthesis precludes the need for post-polymerization modification to introduce thiols, and ensures the presence of a functional hydrophobic block with known and easily tunable thiol content. These MPC-based block copolymers proved water soluble, even with high percentages of the lipoic acid-containing block, and self-assembled readily into nanoscale micelles. The micelles were characterized by dynamic light scattering (DLS) and transmission electron microscopy (TEM), and cross-linked by oxidation to disulfides. We describe these structures as carriers for CPT, in which a pyridyldithio-functionalized CPT39 was conjugated to the DHLA block, thus sequestering CPT to the micelle core. The disulfide linkages allow for controlled CPT release upon exposure to reducing conditions, as would be found upon cellular internalization. This environmental stimulus, coupled with the passive targeting of the EPR effect inherent to polymer-based drug delivery systems, is of interest for improving the outcome of polymer-based drug delivery.
Materials and Methods
4-(dimethylamino)pyridine (DMAP), N-(3-Dimethylaminopropyl)-N´-ethylcarbodiimide hydrochloride (EDC), lipoic acid, 3-mercaptopropionic acid, 2,2'-dithiodipyridine, 4-cyano-4-(phenylcarbonothioylthio)pentanoic acid, 4,4'-azobis(4-cyanovaleric acid) (ACVA), 2-methacryloyloxyethyl phosphorylcholine (MPC), 2-hydroxyethylmethacrylate (HEMA), methanol (anhydrous), and dimethyl sulfoxide (DMSO, anhydrous) were purchased from Sigma Aldrich. HEMA was purified by Kugelrohr distillation prior to use. Camptothecin (CPT) was purchased from 21CEC. Dichloromethane was distilled over calcium hydride. Dialysis cassettes (MWCO 3,500; total volume 0.5–3 mL) were purchased from Fisher Scientific and hydrated in water prior to use. Human colorectal (COLO205) and breast (MCF7) adenocarcinoma cells were purchased from American Type Culture Collection (ATCC). RPMI 1640 and MEM cell culture media were purchased from Life Technologies and Mediatech, respectively. Fetal bovine serum (FBS) was purchased from Atlanta Biologicals and bovine insulin from Aldrich. Cell viability was measured using CellTiter-Glo luminescent cell viability assay from Promega.
Instrumentation
Nuclear magnetic resonance (NMR) spectroscopy was performed on a Brüker Spectrospin DPX300. Aqueous GPC was performed in 0.1 M sodium nitrate and 0.02 weight percent sodium azide buffer against poly(ethylene oxide) calibration standards, operating at 1.0 mL/ min with three Waters Ultrahydrogel columns (7.8 × 300 mm) equipped with RI and UV/Vis detectors. GPC in 1,1,1-trifluoroethanol (TFE) (with 0.2 M sodium trifluoroacetate) was performed against poly(methyl methacrylate) (PMMA) standards, operating at 0.75 mL/min at 40 °C with three Agilent PL HFIPgel columns (300 × 7.5 mm) equipped with RI and UV/Vis detectors. UV/Visible spectroscopy was performed on a Perkin-Elmer Lambda 25 spectrometer. Fluorescence measurements were taken on a Perkin-Elmer LS 55 fluorimeter. Dynamic light scattering was performed on a Malvern Zetasizer Nano-ZS. Transmission electron microscopy (TEM) was performed using a TEM JEOL 2000FX with samples prepared on carbon-coated copper grids purchased from Electron Microscopy Sciences.
Synthesis of HEMA-LA (1)
Lipoic acid (4.00 g, 19.4 mmol) and 2-hydroxyethyl methacrylate (2.50 g, 19.4 mmol) were dissolved in 60 mL of anhydrous dichloromethane in a dry round bottom flask. The stirring solution was cooled to 0 °C, and EDC (7.40 g, 38.8 mmol) and DMAP (2.40 g, 19.4 mmol) were added as solids. The reaction mixture was warmed to room temperature, and stirred for 18 hours. The reaction was diluted with dichloromethane, and washed with 1 M HCl (aqueous), saturated NaHCO3 (aqueous), and brine solutions. The organic layers were dried over MgSO4, filtered, and concentrated by rotary evaporation, to give monomer 1 as a yellow oil (4.9 g, 80 % yield). 1H NMR (300 MHz, CDCl3): δ = 6.06 (s, 1H), 5.36 (s, 1H), 4.26 (s, 4H), 3.5 (m, 1 H), 3.11 (m, 2H), 2.40 (m, 1H), 2.3 (t, 2H), 1.87 (s, 3H), 1.35–1.70 (m, 8H). 13C NMR (75 MHz, CDCl3): δ = 18.31, 24.60, 28.70, 33.87, 34.58, 38.49, 40.21, 56.29, 61.99, 62.43, 126.10, 135.89, 167.08, 173.22.
Synthesis of poly(MPC-block-HEMA-DHLA) by RAFT (2, 3)
MPC (1.00 g, 3.37 mmol), 4-cyano-4-(phenylcarbonothioylthio)pentanoic acid (19 mg, 0.067 mmol), and 4,4'-azobis(4-cyanovaleric acid) (ACVA) (4.0 mg, 0.014 mmol) were added to a dry round bottom flask. Methanol (3 mL) and dimethylsulfoxide (DMSO) (2 mL) were added and the solution was degassed for 20 minutes by bubbling with dry nitrogen gas. The reaction mixture was placed in a preheated oil bath at 70 °C and stirred for 6 hours. In a separate vial, HEMA-LA 1 (212 mg, 0.67 mmol) was dissolved in DMSO (1 mL) and degassed for 30 minutes. The solution of 1 was added rapidly to the reaction flask by syringe, and stirring was continued for 12 hours. Propagation was terminated by placing the solution in liquid nitrogen, then allowing the mixture to warm while open to air. The solution was then passed through a short plug of silica gel, eluting with methanol, then precipitated into THF to afford polymer 2 as a pink solid. This solid was dissolved in 20 mL of degassed water, and stirred at 0 ° C. Sodium borohydride (102 mg, 2.68 mmol) was added under a stream of nitrogen. The reaction mixture was stirred at 0 °C for 1 hour, then at 25 °C for 1 hour. HClconc was added to adjust the pH to ~3, and the polymer was purified by dialysis (MWCO 1,000) against methanol and water at 4 °C. Lyophilization afforded the desired block copolymer 3 in 80% yield as white solids. 1H NMR (300 MHz, MeOD/CDCl3): δ = 4.32 (2H, br), 4.22 (2H, br), 4.07 (2H, br), 3.75 (2H, br), 2.95 (2H, br), 2.71 (2H, br), 2.45 (2H, br), 1.37–2.28 (H, br), 0.52, 1.23 (3H, br). 13C NMR (175 MHz, MeOD/CDCl3): δ = 16.91, 18.59, 21.81, 24.45, 26.31, 33.78, 38.43, 39.01, 42.70, 44.70, 45.10, 53.76, 59.09, 64.70, 66.09, 173.44, 176.79, 177.60, 177.87. GPC (TFE + 0.2 M Na trifluoroacetate, 1 eq DTT, PMMA standards): Mn, 26,900; PDI 1.24.
Synthesis of 3-(2-pyridyldithio)-propionic acid (5)
2,2'-Dithiodipyridine (500 mg, 2.27 mmol) was dissolved in ethyl acetate (2.5 mL) with stirring in a roundbottom flask. Separately, 3-mercaptopropionic acid (160 mg, 1.51 mmol) was dissolved in ethyl acetate (1.5 mL), and added dropwise to the stirring solution, which gradually became yellow. One drop of boron trifluoride diethyl etherate was added. After 7 hours, the reaction mixture was concentrated by rotary evaporation and purified by column chromatography on silica gel, eluting with methanol/dichloromethane mixtures to give the desired product as a yellow oil in 95 % yield (307 mg). 1H NMR (300 MHz, CDCl3): δ = 12.8 (s, 1H), 8.4 (br, 1H), 7.6 (br, 2H), 7.1 (br, 1H), 3.04 (tr, 2H), 2.8 (tr, 2H). 13C NMR (75 MHz, CDCl3): δ = 33.71, 34.01, 114.32, 121.19, 134.00, 138.06, 149.93, 176.16.
Synthesis of Camptothecin-pyridyl disulfide (6)
Compound 5 (232 mg, 1.07 mmol) was dissolved in anhydrous dichloromethane (30 mL) in a roundbottom flask. Camptothecin (250 mg, 0.718 mmol) was added to form a pale yellow suspension. EDC (276 mg, 1.44 mmol) and DMAP (175 mg, 1.44 mmol) were added. The mixture was stirred for 24 hours, then diluted with dichloromethane, and washed with 1M HCl (aq), brine, and water. The organic layer was dried over MgSO4, filtered, and concentrated by rotary evaporation. The residue was further purified by column chromatography on silica gel, eluting with methanol/dichloromethane to give the desired product as a yellow solid in 50 % yield (195 mg). 1H NMR (300 MHz, DMSO): δ = 8.71 (s, 1H), 8.48 (d, 1H), 8.14 (m, 2H), 7.62–7.83 (m, 3H), 7.36 (d, 1H), 7.11–7.2 (m, 2H), 5.51 (s, 2H), 5.31 (s, 2H), 3.34 (m, 2H), 2.96 (m, 2H), 2.15 (m, 2H), 0.90 (tr, 3H). 13C NMR (175 MHz, DMSO): δ = 171.01, 167.57, 157.61, 156.96, 153.82, 150.01, 148.33, 146.37, 145.73, 137.10, 132.00, 130.90, 130.25, 129.29, 129.00, 128.42, 128.14, 122.75, 120.40, 119.28, 95.66, 76.56, 66.72, 50.66, 34.25, 30.65, 29.36, 7.99. HRMS-FAB [M+H]: calculated: 546.115, found: 546.113 g/mole.
Determination of critical micelle concentration (CMC)
CMC was determined using a pyrene fluorescence probe. Briefly, a stock solution of pyrene in acetone (1.2 × 10−4 M) was prepared. Polymers 3A–C were dissolved in PBS, and diluted from 5 mg/ml to 1.25 µg/mL, with each solution having a total volume of 1 mL. 5 µL of pyrene solution was added to each for a final pyrene concentration of 6 × 10−7 M. The polymer solutions were kept at 18 °C for 18 hours. An excitation spectrum was recorded of each solution from 300–360 nm at a scan rate of 100 nm/s, with emission set to 394 nm. CMC was determined by plotting log(concentration) vs. the ratio of the intensities at 339 and 334 nm.
Preparation of poly(MPC-block-DHLA) micelles
Micelles were prepared by dissolution of polymer into phosphate buffered saline (PBS) at a concentration above the CMC (generally 1 mg/mL). To facilitate cross-linking through disulfide formation, the solutions were continuously bubbled with air for 48 hours, and loss of free thiol was monitored using Ellman's test.43
Preparation of CPT-loaded poly(MPC-block-DHLA) micelles
Polymer 3C (50 mg, 0.064 mmol DHLA) and camptothecin-pyridyl disulfide (16 mg, 0.029 mmol) were dissolved in methanol/DMSO (5 mL). The solution was stirred vigorously at 37 °C for 72 hours, then dialyzed against methanol to remove unconjugated camptothecin. The polymer solution was dialyzed against water to induce micelle formation, and bubbled with air to promote disulfide formation from residual thiols. Micelle solutions were passed through 0.45 µm filters to remove any free CPT, and lyophilized to produce off-white solids which were re-dissolved in water or methanol for characterization. CPT loading, as a weight percent, was determined using UV/Vis spectroscopy, comparing to a sample of known concentration.
CPT release from cross-linked polymer micelles
Release of CPT from the polymer micelles was monitored by dialysis. Briefly, lyophilized polymer micelles containing CPT were dissolved in PBS (1 mL). The solution was transferred to a dialysis cassette (MWCO 3500) by syringe. The cassette was suspended in a sealed container with 300 mL of PB, or PBS containing 3 mM DTT. Containers were kept in a water bath at 37 °C, and at select time points 1 mL aliquots were removed from the external media and replaced with fresh buffer. The fluorescence intensity at 440 nm (λex=370 nm) was monitored, and the experiments were carried out until a plateau was reached.
Size determination by dynamic light scattering (DLS)
DLS was performed on samples of polymer micelles to determine the size of the structures formed in PBS at a concentration of 1 mg/mL. Three measurements were performed, each consisting of 10 runs for 10 seconds. All experiments were performed at 25 ° C, and equilibrated for 3 minutes. Prior to analysis, samples were passed through a 0.45 µm filter.
Transmission electron microscope (TEM) imaging
Solutions of polymer micelles were diluted to 0.25 mg/ml in water, and drop cast, then allowed to dry at room temperature for 24 hours.
Cell culture
COLO205 cancer cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), while MCF7 cells were cultured in MEM medium supplemented with 10% FBS and 0.01 mg/mL bovine insulin. All cells were grown in 5% CO2 incubators at 37 °C. For in vitro cytotoxicity assays, cells were seeded in 96 well plates, and after reaching about 40% cell density were incubated for 72 hours with varying camptothecin equivalent concentrations of prodrug micelles, as well as control samples, including polymer only and polymer micelles (physically entrapped CPT). Cell viability post-treatment was measured using CellTiter-Glo luminescence cell viability assay (Promega) following the manufacturer instructions on a FLUOstar OPTIMA plate reader (BMG LABTECH). The CPT-mediated toxicity was calculated with respect to untreated cells, and graphed to give dose-response curves. IC50 values for each treatment were then calculated using GraphPad Prism4 statistical analysis software.
Results and Discussion
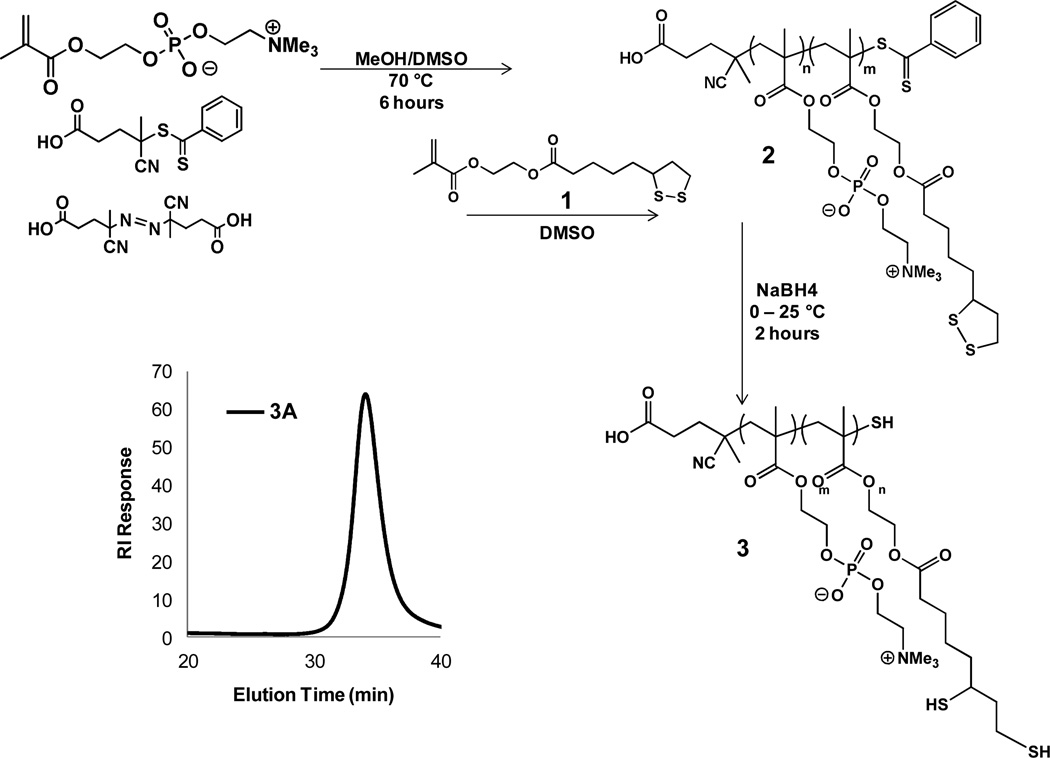
PolyMPC-DHLA block copolymers were synthesized by RAFT polymerization, employing sequential monomer addition to form the block copolymers shown as 3 in Figure 1. Monomer 1 was prepared in 80% yield by carbodiimide coupling of 2- hydroxyethyl methacrylate and lipoic acid.44 The monomer was isolated as a yellow oil, and stored as a CH2Cl2 solution at −80 °C to prevent disulfide exchange. Stored in this way, monomer 1 was stable for months.
Figure 1.
Synthesis of polyMPC-DHLA block copolymer 3. Lower left: GPC trace of purified block copolymer 3A, eluting in TFE.
PolyMPC-DHLA diblock copolymers were prepared by first polymerizing MPC using 4-cyano-4-(phenylcarbonothioylthio)pentanoic acid and 4,4'-azobis(4-cyanovaleric acid) (ACVA) as the chain transfer agent and initiator, respectively, at 70 °C in MeOH/DMSO solution. Following conversion of MPC to polymer, a DMSO solution of 1 was introduced under inert atmosphere, and the mixture was stirred at 70 °C for 12 hours. The polymerization was terminated by immersing the flask in liquid nitrogen, then allowing the mixture to warm while open to air. The polymerizations were generally taken to >90 % conversion, as judged by 1H NMR spectroscopy, comparing vinyl protons of the monomer (5.5 and 6.0 ppm) to methyl protons on the polymer backbone (1.0 ppm). Polymer 2 was isolated as a pink solid following purification by passage through a short plug of silica gel, and precipitation into THF.
Block copolymer 2 was reduced to the free thiol form with NaBH4 (4 molar equivalents relative to lipoic acid). The reaction was complete in 2 hours, at which point concentrated HCl was added to adjust the solution pH to ~3. The polymer solution was dialyzed against methanol, then water, at 4 °C (MWCO 1,000). Lyophilization gave polyMPC-DHLA (3) as a white solid. Polymer 3 was characterized by NMR spectroscopy (Figures S1–S2), and GPC (eluting with 0.1 M aqueous sodium nitrate + 0.02% (wt) sodium azide or trifluoroethanol (TFE) (0.2 M sodium trifluoroacetate), against linear PEO or PMMA calibration standards, respectively) (Table 1). The extent of DHLA incorporation was determined by 1H NMR spectroscopy in 1:1 CDCl3:MeOD, comparing the DHLA methylene signal at 2.5 ppm with the methyl protons of the polymer backbone at 1.0 ppm. This showed the DHLA content to be well-controlled by adjusting monomer feed ratios. GPC in TFE revealed a well-defined (monomodal) polymer signal, with PDI ~ 1.2. In water, a high molecular weight signal was also seen, attributed to copolymer micellization in solution. Interestingly, aggregation of this sort was not observed in our characterization of random copolymers of similar composition and molecular weight (Figure S2). We hypothesize that this behavior arises from (1) the distinct amphiphilicity of these diblock copolymers that leads to rapid solution assembly, and (2) the dense concentration of thiols in the DHLA block that provides additional stability to the micelles through disulfide formation. We note that these diblock copolymers maintained excellent water solubility (to the eye), even at the highest DHLA incorporation of 41 mole percent (sample 3C).
Table 1.
Characterization of polyMPC block copolymers 3A–C. Target molecular weights were calculated from chain transfer agent:monomer ratio; Target % DHLA was calculated from relative monomer feed ratios; DHLA incorporation was determined experimentally by 1H NMR spectroscopy; Mn (number average molecular weight) and PDI were determined using GPC in TFE (0.2 M TFAc) against PMMA standards.
| Sample | Target Mol. Wt (g/mole) |
Target % DHLA |
TFE GPC | % DHLA | |
|---|---|---|---|---|---|
| Mn | PDI | ||||
| 3A | 16,000 | 10 % | 24,800 | 1.19 | 15 % |
| 3B | 18,000 | 20 % | 26,900 | 1.24 | 23 % |
| 3C | 21,000 | 40 % | 22,300 | 1.15 | 41 % |
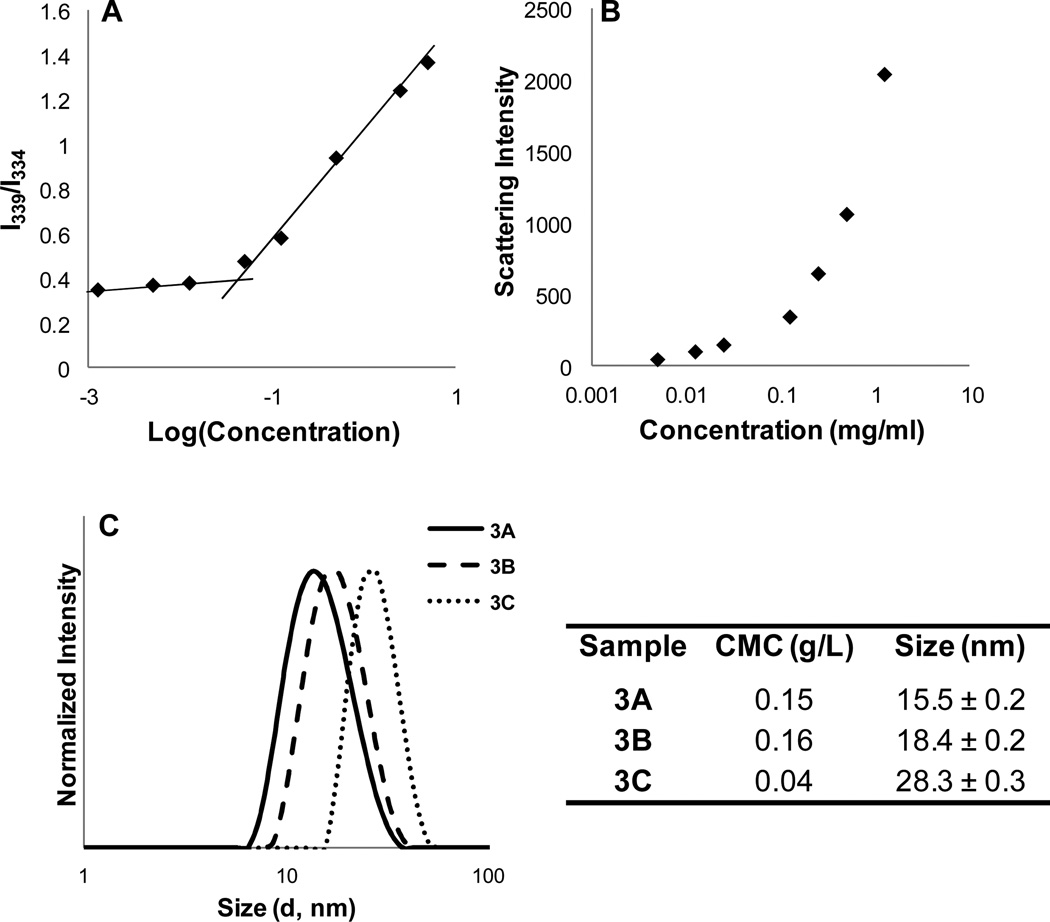
The critical micelle concentration (CMC) of block copolymers 3A–C was examined using a pyrene fluorescence probe. Briefly, serial dilutions of polymer were prepared in PBS, and 5 µL of pyrene solution in acetone was added to each, giving a pyrene concentration of 0.6 µM. The samples were equilibrated at room temperature for 18 hours. Pyrene exhibits a shift in peak fluorescence intensity as it transitions from a hydrophilic (334 nm) to hydrophobic environment (339 nm), and CMC is determined by plotting peak intensity against the log of the polymer concentration, as shown for polymer 3C in Figure 2A. The onset of the sharp change in slope of the line is taken as the CMC. Dynamic light scattering (DLS) analysis of the same series of samples/concentrations shows a non-linear relationship between the scattering intensity and concentration, confirming the presence of nanoscale micelles above CMC (Figure 2B). CMC varied among the polymer samples, with an observed dependence on hydrophobic content; as expected, the CMC for polymer 3C was lowest due its higher DHLA content (41 mole %). DLS measurements of block copolymers 3A, B and C showed an increase in size with hydrophobic block length, with hydrodynamic diameters of 15, 18 and 28 nm, respectively, measured at 1 mg/mL in PBS.
Figure 2.
Summary of micelle characterization: (A) CMC determination using pyrene fluorescence for block copolymer 3C; (B) scattering intensity vs. concentration from dynamic light scattering for block copolymer 3C; (C) size (diameter) of micelles from copolymers 3A–C.
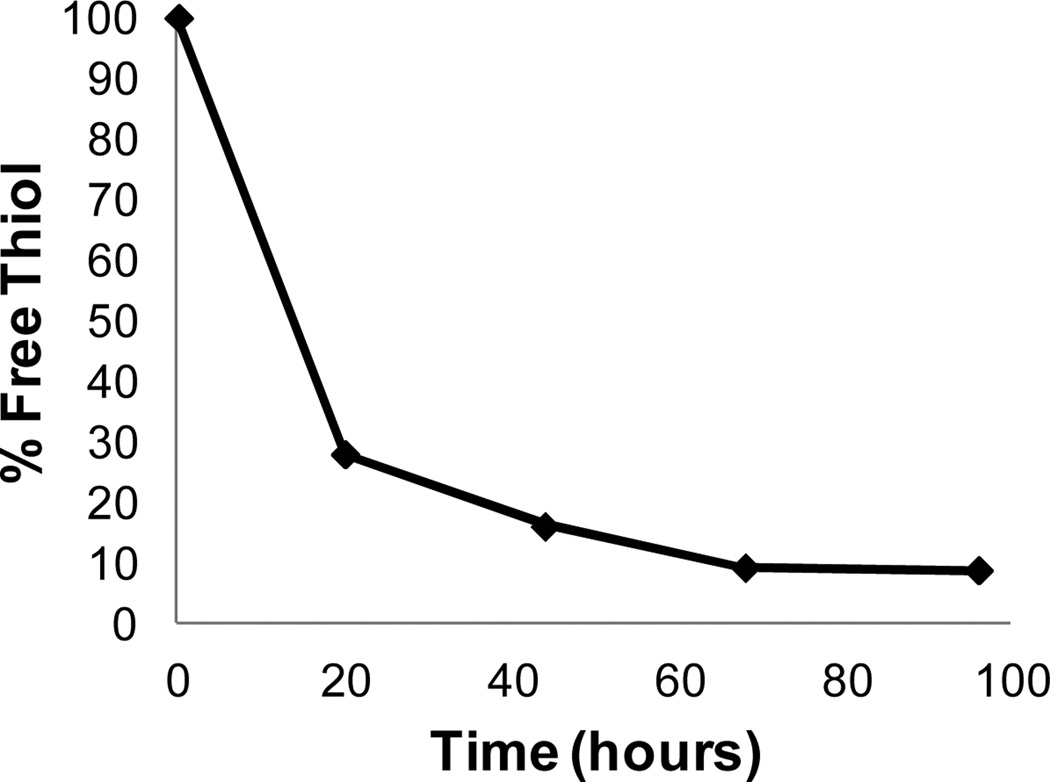
Thiol-containing micelles can self-cross-link by disulfide formation, while continually purging the system with air. The extent of disulfide formation was monitored using Ellman's test,43 performed on polymer dissolved in PBS above the CMC and agitated by bubbling a slow stream of air through the mixture. At various time points, 10 uL aliquots were removed and added to a buffered solution of Ellman's reagent, resulting in a decrease in intensity of the UV absorption at 412 nm as the free thiol converted to disulfide (Figure 3). This provides a spectroscopic handle to monitor cross-linking efficiency. Samples generally reached 85% conversion in 48 hours. Solutions simply left open to air, without bubbling, gave significantly lower conversion (~20% after two days).
Figure 3.
Percent decline of free thiol over time for micelles prepared from copolymer 3B.
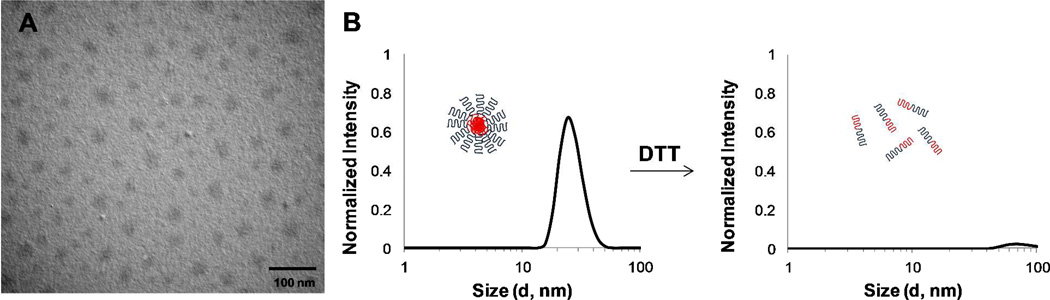
Cross-linked micelles were characterized by DLS and TEM (Figure 4). DLS of cross-linked micelles formed from polymer solutions at 1 mg/mL showed no difference from the uncross-linked samples, suggesting that cross-linking neither disrupts the structure of the micelles nor promotes inter-micelle cross-linking. 0.25 mg/mL aqueous solutions of cross-linked micelles were cast on copper grids and imaged by TEM. Micelles observed by TEM supported DLS data, with an average micelle diameter of 26 ± 4 nm (Figure 4A). The micelles imaged by TEM appeared as spherical structures and were dispersed cleanly on the substrate.
Figure 4.
(A) TEM of cross-linked micelles formed from polymer 3B; (B) DLS of cross-linked polymer micelles from copolymer 3A below the CMC (0.01 mg/mL) (left), and DLS of the same sample after treatment with DTT (right); DTT cleavage of disulfide linkages gives free polymer in solution (below CMC).
Cross-linked micelle solutions were stable, as characterized by DLS, well below the CMC (0.01 mg/mL). However when treated with 5 mM dithiothreitol (DTT) at 37 °C, then cooled to room temperature and analyzed again by DLS, no signal was detected (as for the uncross-linked polymer at the same concentration) indicating complete dissolution of the polymer micelle by disruption of the disulfide cross-links (Figure 4B).
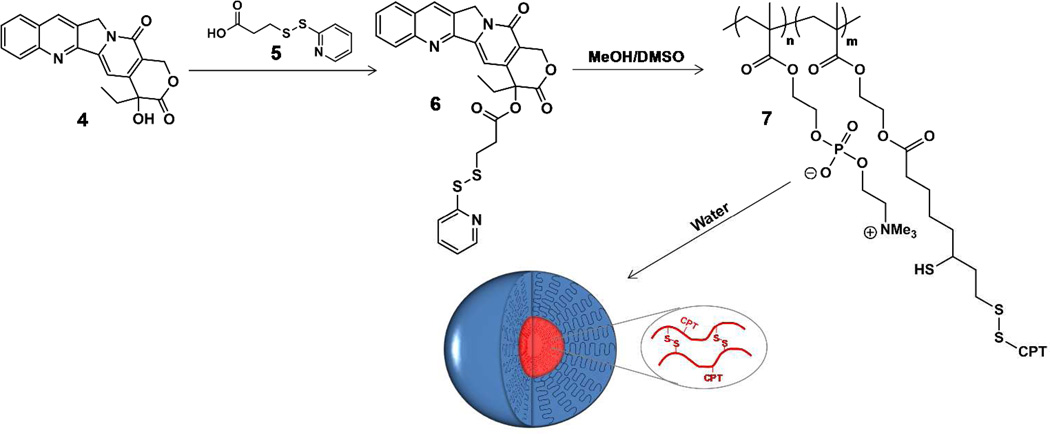
These redox-sensitive core cross-linked PC polymer micelles comprise a potentially suitable delivery platform for therapeutics, in which a triggered release can enable selective and targeted delivery of a drug, as the cytosol and nucleus are known to have a significantly higher reducing potential (mM) than the extracellular fluids (µM). We use CPT as the chemotherapeutic, specifically a pyridyl disulfide-functionalized CPT derivative, prepared similarly as reported in the literature,39 to facilitate conjugation to the polymer by disulfide formation. Briefly, 3-(2-pyridyldithio)-propionic acid (5) was prepared by reaction of 2,2'-dithiodipyridine with 3-mercaptopropionic acid in ethyl acetate, and purified by column chromatography on silica gel to yield the desired product in 95 % yield.45 Carbodiimide coupling of camptothecin (4) and linker 5 was achieved in anhydrous methylene chloride39 (Figure 6). Following purification by column chromatography on silica gel, eluting with methanol/methylene chloride mixtures, camptothecin derivative 6 was obtained as a yellow solid in ~50% yield. The structure was confirmed by NMR spectroscopy (Figure S3) and high resolution mass spectrometry (calculated, 546.115; found, 546.113).
Figure 6.
Synthesis of CPT-pyridyl disulfide (6), conjugation to poly(MPC-b-(HEMA-DHLA)), and cross-linked micelle formation.
CPT-pyridyl disulfide 6 was conjugated to polyMPC-DHLA by stirring in a 2:3 mixture of MeOH/DMSO for 72 hours at 37 °C. The solution was dialyzed against methanol (MWCO 1,000) to remove unreacted 6, then against water to induce micelle formation. After complete removal of the organic solvents, the aqueous solution was transferred to a vial and purged with air to form CPT-loaded core-cross-linked polymer micelles, as depicted in Figure 6. CPT loading was characterized by UV/Vis spectroscopy, comparing absorbance at 370 nm with a CPT solution of known concentration. Polymer-CPT prodrugs prepared in this way achieved from 5 to 10 wt % CPT-loading as determined by UV/Vis spectroscopy (Figures S5). CPT release from the polymer micelles was monitored by dialysis, where 1 mL of micelle solution was transferred to a cassette (MWCO 3,500), and dialyzed against PBS, or PBS + 3 mM DTT (300 mL), in a closed container at 37 °C. At select time points, aliquots were removed from the external medium, and replaced with fresh buffer, while monitoring fluorescence intensity at 440 nm over time (λex = 370 nm).
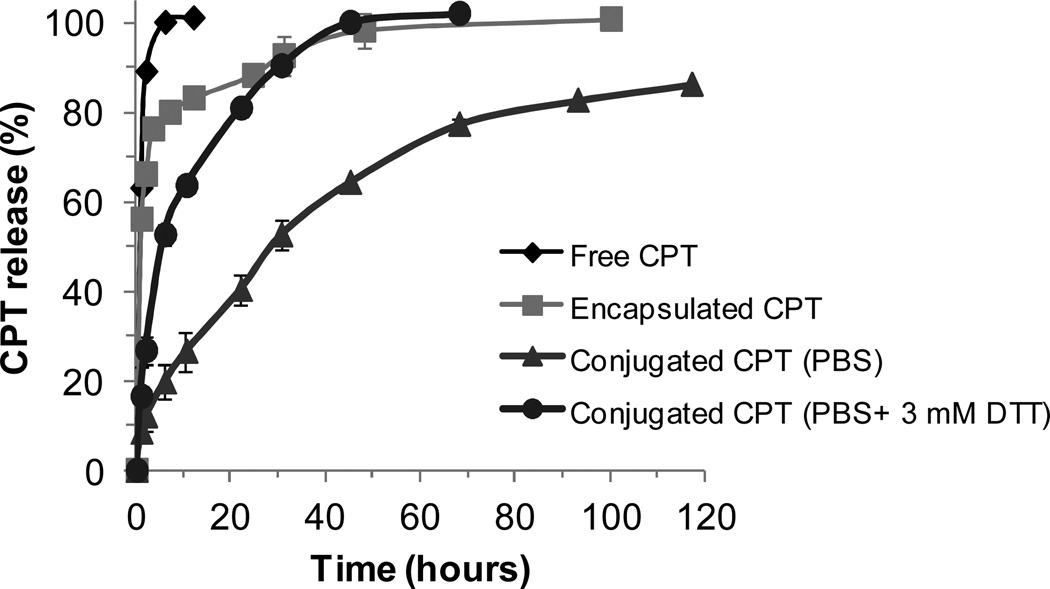
Free CPT was dialyzed against PBS to demonstrate that diffusion out of the dialysis cassette is not a limiting factor, and to establish a benchmark for assessing the performance of the micelle-based systems. CPT (without encapsulation) diffused through the cassette within 6 hours, with 90% released in the first 2 hours (Figure 7). For further comparison, polymer micelles were prepared containing CPT simply encapsulated in the core (i.e. having no disulfide linkage). Physically encapsulated systems showed little difference from CPT alone, with an initial burst release of 75% in 4 hours, followed by slow release of the remaining drug over two days. In contrast, the disulfide-conjugated CPT prodrug micelles showed much different release profiles. In PBS containing DTT (3 mM), CPT release was fast, with 50% release in 5.5 hours, and complete release in 2 days. In PBS at pH 7.4, CPT release was slow (85 % over 5 days), presumably due to slow hydrolysis of the ester linkage, with a half-life (t1/2) of 28 hours. These results suggest these polyMPC-CPT prodrug micelles as a potential drug delivery system that is relatively stable in a neutral environment, and can exploit the redox characteristics of the intracellular environment.
Figure 7.
Release profiles of CPT, encapsulated CPT, conjugated CPT in PBS, and conjugated CPT in PBS + 3 mM DTT.
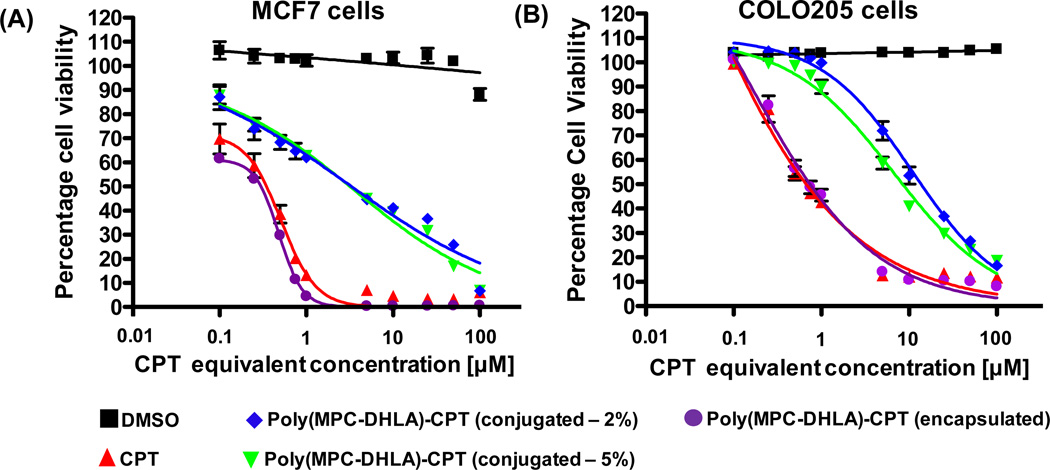
The cytotoxicity of poly(MPC-b-DHLA)-CPT conjugates was tested in vitro against human breast cancer (MCF7) and colorectal (COLO205) adenocarcinoma cells. This was done by incubating CPT-equivalent concentrations of poly(MPC-b-DHLA)-CPT conjugates with these cells for 72 hours, followed by cell viability measurements using a luminescence plate reader. Dose response curves (Figure 8) showed that micellar conjugates (with CPT loadings of 2 and 5 weight percent) were potent against the cancer cell lines tested. The observed cytotoxicity arises from released CPT (a result of ester bond cleavage), and the polymer itself exhibits no toxicity even at extremely high concentrations (Figure S6).
Figure 8.
In vitro cytotoxicity of poly(MPC-b-DHLA) micelles loaded with CPT in cell culture of (A) human breast (MCF7) and (B) colorectal (COLO205) adenocarcinoma cells. Error bars represent ± standard deviation.
The half-maximal inhibitory concentration (IC50) values of poly(MPC-b-DHLA)-CPT prodrug micelles were in the range of 3–9 µM, as shown in Table 2, where the comparable IC50 values for both poly(MPC-b-DHLA)-CPT conjugates originate from their similar release rates. The data shows poly(MPC-b-DHLA) micelles containing CPT conjugated by disulfide linkage induce toxicity at higher concentrations than CPT alone. This is expected for polymer prodrugs, and is a key feature that allows higher maximum tolerated dose (MTD) of prodrugs in vivo.46,47 Interestingly, CPT that was physically encapsulated within the micelles (i.e. no covalent linkage) showed nearly identical toxicity to native CPT. This is likely due to the fast release observed for these structures, as compared to the gradual release of CPT from the prodrugs. The combination of redox triggered release and the cell culture data presented here will be beneficial for controlling drug release in vivo, while exploiting the very high water solubility arising from the phosphorylcholine-substituted polymer.
Table 2.
IC50 values (µM) of poly(MPC-b-DHLA)-CPT micelles in MCF7 and COLO205 cancer cell lines.
| IC50[µM] | MCF7 | COLO205 |
|---|---|---|
| CPT | 0.51 ± 0.05 | 0.43 ± 0.1 |
| Poly(MPC-DHLA)-CPT (encapsulated) | 0.48 ± 0.01 | 0.44 ± 0.1 |
| Poly(MPC-DHLA)-CPT (2 wt %) | 3.0 ± 1.8 | 8.3 ±0.8 |
| Poly(MPC-DHLA)-CPT (5 wt %) | 3.6 ± 0.8 | 4.7 ± 0.3 |
Conclusion
In summary we have demonstrated the synthesis of novel diblock copolymers and micelles based on phosphorylcholine and dihydrolipoic acid-containing methacrylates, and shown their potential utility as a drug delivery platform. Use of DHLA as the hydrophobic block allows for post-polymerization conjugation and cross-linking reactions by disulfide formation. Camptothecin was successfully conjugated to the DHLA block, then released in a controlled manner in buffer, with the benefit of a trigger in a reducing environment. The CPT-loaded micelles demonstrated cytotoxicity at higher CPT concentrations than with the drug alone, as expected for polymer prodrugs due to the covalent connection of CPT to the backbone. The combination of robust, highly water soluble micelles and stimuli-responsive drug release yields a system that is promising for overcoming challenges faced by micellar delivery vehicles, including in vivo stability and fast, non-specific release of their contents.
Supplementary Material
Acknowledgements
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant No. S121000000211 (S.M.P). The authors acknowledge facilities support from the NSF Materials Research Science and Engineering Center (MRSEC) on Polymers at UMass (NSF-DMR-0820506), the NSF MRSEC Research Experience for Undergraduate (REU) Program at UMass (M.M.), and the National Institutes of Health (award R21CA167674).
Footnotes
Supporting Information. 1H NMR spectra and GPC traces of polyMPC-DHLA block copolymer 3, 1H NMR spectrum of CPT-pyridyl disulfide 6, and NMR spectra, UV/Vis spectroscopy of CPT-loaded polymer micelles, and cytotoxicity of poly(MPC-b-DHLA) polymers. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Duncan R. Polymer conjugates as anticancer nanomedicines. Nature Reviews Cancer. 2006;6:688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 2.Pasut G, Veronese FM. PEG conjugates in clinical development or use as anticancer agents: An overview. Adv. Drug Deliv. Rev. 2009;61:1177–1188. doi: 10.1016/j.addr.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Joralemon MJ, McRae S, Emrick T. PEGylated polymers for medicine: from conjugation to self-assembled systems. Chem. Commun. 2010;46:1377–1393. doi: 10.1039/b920570p. [DOI] [PubMed] [Google Scholar]
- 4.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 5.Greenwald RB, Choe YH, McGuire J, Conover CD. Effective drug delivery by PEGylated drug conjugates. Adv. Drug Deliv. Rev. 2003;55:217–250. doi: 10.1016/s0169-409x(02)00180-1. [DOI] [PubMed] [Google Scholar]
- 6.Thomson AH, Vasey PA, Murray LS, Cassidy J, Fraier D, Frigerio E, Twelves C. Population pharmacokinetics in phase I drug development: a phase I study of PK1 in patients with solid tumours. Br. J. Cancer. 1999;81:99–107. doi: 10.1038/sj.bjc.6690657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seymour LW, Ferry DR, Kerr DJ, Rea D, Whitlock M, Poyner R, Boivin C, Hesslewood S, Twelves C, Blackie R, Schatzlein A, Jodrell D, Bissett D, Calvert H, Lind M, Robbins A, Burtles S, Duncan R, Cassidy J. Phase II studies of polymer-doxorubicin (PK1, FCE 28068) in the treatment of breast, lung, and colorectal cancer. Int. J. Oncol. 2009;34:1629–1636. doi: 10.3892/ijo_00000293. [DOI] [PubMed] [Google Scholar]
- 8.Bissett D, Cassidy J, Bono JS, Muirhead F, Main M, Robson L, Fraier D, Magne ML, Pellizzoni C, Porro MG, Spinelli R, Speed W, Twelves C. Phase I and pharmacokinetic (PK) study of MAG-CPT (PNU 166148): a polymeric derivative of camptothecin (CPT) Br. J. Cancer. 2004;91:50–55. doi: 10.1038/sj.bjc.6601922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopecek J, Kopeckova P, Minko T, Lu Z. HPMA copolymer-anticancer drug conjugates: design, activity, and mechanism of action. Eur. J. Pharm. Biopharm. 2006;50:61–81. doi: 10.1016/s0939-6411(00)00075-8. [DOI] [PubMed] [Google Scholar]
- 10.Schluep T, Hwang J, Cheng JJ, Heidel JD, Bartlett DW, Hollister B, Davis ME. Preclinical efficacy of the camptothecin-polymer conjugate IT-101 in multiple cancer models. Clin. Cancer Res. 2006;12:1606–1614. doi: 10.1158/1078-0432.CCR-05-1566. [DOI] [PubMed] [Google Scholar]
- 11.Numbenjapon T, Wang JY, Colcher D, Schluep T, Davis ME, Duringer J, Kretzner L, Yen Y, Forman SJ, Raubitschek A. Preclinical results of camptothecin-polymer conjugate (IT-101) in multiple human lymphoma xenograft models. Clin. Cancer Res. 2009;15:4365–4373. doi: 10.1158/1078-0432.CCR-08-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, McRae S, Parelkar S, Emrick T. Polymeric Phosphorylcholine-Camptothecin Conjugates Prepared by Controlled Free Radical Polymerization and Click Chemistry. Bioconjugate Chem. 2009;20:2331–2341. doi: 10.1021/bc900339x. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Parelkar S, Henchey E, Schneider S, Emrick T. PolyMPC-Doxorubicin Prodrugs. Bioconjugate Chem. 2012;23:1753–1763. doi: 10.1021/bc200667s. [DOI] [PubMed] [Google Scholar]
- 14.Lee CC, Gillies ER, Fox ME, Guillaudeu SJ, Fréchet JMJ, Dy EE, Szoka FC. A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc. Natl. Acad. Sci. 2006;103:16649–16654. doi: 10.1073/pnas.0607705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guillaudeu S, Fox M, Haidar Y, Dy E, Szoka F, Fréchet JMJ. PEGylated dendrimers with core functionality for biological applications. Bioconjugate Chem. 2008;19:461–469. doi: 10.1021/bc700264g. [DOI] [PubMed] [Google Scholar]
- 16.Alakhov V, Klinski E, Li S, Pietrzynski G, Venne A, Batrakova EV, Bronitch T, Kabanov AV. Block copolymer-based formulation of doxorubicin. From cell screen to clinical trials. Colloids Surf. B. Biointerfaces. 1999;16:113–134. [Google Scholar]
- 17.Nakanishi T, Fukushima S, Okamoto K, Suzuki M, Matsumura Y, Yokoyama M, Okano T, Sakurai Y, Kataoka K. Development of the polymer micelle carrier system for doxorubicin. J. Contol. Release. 2001;74:295–302. doi: 10.1016/s0168-3659(01)00341-8. [DOI] [PubMed] [Google Scholar]
- 18.Christian DA, Cai S, Garbuzenko OB, Harada T, Zajac AL, Minko T, Discher DE. Flexible Filaments for in Vivo Imaging and Delivery: Persistent Circulation of Filomicelles Opens the Dosage Window for Sustained Tumor Shrinkage. Mol. Pharmaceutics. 2009;6:1343–1352. doi: 10.1021/mp900022m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Nostrum CF. Covalently cross-linked amphiphilic block copolymer micelles. Soft Matter. 2011;7:3246–3259. [Google Scholar]
- 20.Gohy J-F. Block Copolymer Micelles. Adv. Polym. Sci. 2005;190:65–136. [Google Scholar]
- 21.Jones M-C, Leroux J-C. Polymeric micelles- a new generation of colloidal drug carriers. Eur. J. Pharm. Biopharm. 1999;48:101–111. doi: 10.1016/s0939-6411(99)00039-9. [DOI] [PubMed] [Google Scholar]
- 22.O'Reilly RK, Hawker CJ, Wooley KL. Cross-linked block copolymer micelles: functional nanostructures of great potential and versatility. Chem. Soc. Rev. 2006;35:1068–1083. doi: 10.1039/b514858h. [DOI] [PubMed] [Google Scholar]
- 23.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 24.Li YT, Lokitz BS, McCormick CL. RAFT synthesis of a thermally responsive ABC triblock copolymer incorporating N-acryloxysuccinimide for facile in situ formation of shell cross-linked micelles in aqueous media. Macromolecules. 2006;39:81–89. [Google Scholar]
- 25.Duong HTT, Nguyen TLU, Stenzel MH. Micelles with surface conjugated RGD peptide and crosslinked polyurea core via RAFT polymerization. Polym. Chem. 2010;1:171–182. [Google Scholar]
- 26.Iijima M, Nagasaki Y, Okada T, Kato M, Kataoka K. Core-polymerized reactive micelles from heterotelechelic amphiphilic block copolymers. Macromolecules. 1999;32:1140–1146. [Google Scholar]
- 27.Lee W-C, Li Y-C, Chu I-M. Amphiphilic poly(D,L-lactic acid)/poly(ethylene glycol)/poly(D,L-lactic acid) nanogels for controlled release of hydrophobic drugs. Macromol. Biosci. 2006;6:846–854. doi: 10.1002/mabi.200600101. [DOI] [PubMed] [Google Scholar]
- 28.Henselwood F, Liu G. Water-soluble nanospheres of poly(2-cinnamoylethyl methacrylate)-block-poly(acrylic acid) Macromolecules. 1997;30:488–493. [Google Scholar]
- 29.Saito K, Ingalls LR, Lee J, Warner JC. Core-bound polymeric micellar system based on photocrosslinking of thymine. Chem. Commun. 2007;24:2503–2505. doi: 10.1039/b700686a. [DOI] [PubMed] [Google Scholar]
- 30.Meng F, Hennink WE, Zhong Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials. 2009;30:2180–2198. doi: 10.1016/j.biomaterials.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 31.Cheng R, Feng F, Meng F, Deng C, Feijen J, Zhong Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J. Control. Rel. 2011;152:2–12. doi: 10.1016/j.jconrel.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 32.Hu X, Li H, Luo S, Liu T, Jiang Y, Liu S. Thiol and pH dual-responsive dynamic covalent shell cross-linked micelles for triggered release of chemotherapeutic drugs. Polym. Chem. 2013;4:695–706. [Google Scholar]
- 33.Convertine AJ, Diab C, Prieve M, Paschal A, Hoffman AS, Johnson PH, Stayton PS. pH-Responsive polymeric micelle carriers for siRNA drugs. Biomacromolecules. 2010;11:2904–2911. doi: 10.1021/bm100652w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. 2011;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 35.Kakizawa Y, Harada A, Kataoka K. Environment-sensitive stabilization of core-chell structured polyion complex micelle by reverible cross-linking of the core through disulfide bond. J. Am. Chem. Soc. 1999;121:11247–11248. [Google Scholar]
- 36.Zhang J, Gong M, Yang S, Gong Y-K. Crosslinked biomimetic random copolymer micelles as potential anti-cancer drug delivery vehicle. J. Control. Rel. 2011;152:e1–e132. doi: 10.1016/j.jconrel.2011.08.099. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, Meng F, Cheng R, Zhong Z. Reduction-sensitive reversible crosslinked biodgradable micelles for triggered release of doxorubicin. Macromol. Biosci. 2009;9:1254–1261. doi: 10.1002/mabi.200900233. [DOI] [PubMed] [Google Scholar]
- 38.Vetvicka D, Hruby M, Hovorka O, Etrych T, Vetrik M, Kovar L, Kovar M, Ulbrich K, Rihova B. Biological evaluation of polymeric micelles with covalently bound doxorubicin. Bioconjugate Chem. 2009;20:2090–2097. doi: 10.1021/bc900212k. [DOI] [PubMed] [Google Scholar]
- 39.Cabral H, Nakanishi M, Kumagai M, Jang W-D, Nishiyama N, Kataoka K. A photo- activated targeting chemotherapy using glutathione sensitive camptothecin-loaded polymer micelles. Pharma. Res. 2009;26:82–92. doi: 10.1007/s11095-008-9712-2. [DOI] [PubMed] [Google Scholar]
- 40.Konna T, Watanabe J, Ishihara K. Enhanced solubility of paclitaxel using water-soluble and biocompatible 2-methacryloyloxyethyl phosphorylcholine polymers. J. Biomed. Mater. Res. A. 2002;65:209–214. doi: 10.1002/jbm.a.10481. [DOI] [PubMed] [Google Scholar]
- 41.Chu H, Liu N, Wang X, Jiao Z, Chen Z. Morphology and in vitro release kinetics of drug-loaded micelles based on well-defined PMPC-b-PBMA copolymer. Int. J. Pharm. 2009;371:190–196. doi: 10.1016/j.ijpharm.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 42.Wei R, Cheng L, Zheng M, Cheng R, Meng F, Deng C, Zhong Z. Reduction-responsive disassemblable core-cross-linked micelles based on poly(ethylene glycol)-b-poly(N-2-hydroxypropyl methacrylamide)-lipoic acid conjugates for triggered intracellular anticancer drug release. Biomacromolecules. 2012;13:2429–2438. doi: 10.1021/bm3006819. [DOI] [PubMed] [Google Scholar]
- 43.Ellman GL. Tissue Sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 44.Chen X, Lawrence J, Parelkar S, Emrick T. Novel Zwitterionic Copolymers with Dihydrolipoic Acid: Synthesis and Preparation of Nonfouling Nanorods. Macromolecules. 2012;46:119–127. [Google Scholar]
- 45.Digilio G, Menchise V, Gianolo E, Catanzaro V, Carrera C, Napolitano R, Fedeli F, Aime S. Exofacial protein thiols as a route for the internalization of Gd(III)-based complexes for magnetic resonance imaging cell labeling. J. Med. Chem. 2010;53:4877–4890. doi: 10.1021/jm901876r. [DOI] [PubMed] [Google Scholar]
- 46.Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 47.Duncan R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.