Summary
Critically ill children in the Pediatric Intensive Care Unit (PICU) are exposed to multiple physical, environmental and pharmacologic factors which increase the propensity for sleep disruption and loss and may, in turn, play a role in short-term recovery from critical illness and long-term neurocognitive outcomes. Mechanically ventilated children receive sedative and analgesic medications, often at high doses and for long durations, to improve comfort and synchrony with mechanical ventilation. Sedatives and analgesics can decrease slow wave sleep and rapid eye movement sleep. Paradoxically, sedative medications doses are often increased in critically ill children to improve the subjective assessment of sedation and sleep, leading to further agitation and deterioration of sleep quality. The heterogeneity in age and critical illness encountered in the PICU pose several challenges to research on sleep in this setting. The present article reviews the available evidence on sleep in critically ill children admitted to the PICU, with an emphasis on subjective and objective methods of sleep assessment used and special populations studied, including mechanically ventilated children and children with severe burns.
Keywords: sleep, pediatric, neonatal, intensive care, mechanical ventilation, circadian rhythm, delirium
INTRODUCTION
Approximately 250,000 children are admitted to the Pediatric Intensive Care Unit (PICU) each year in the United States.(1) Critical illnesses in children encompass a range of medical and surgical diagnoses, such as multi-system organ failure requiring extracorporeal support, complex congenital heart disease, and severe trauma. The modern PICU admits all critically ill infants and children ages 0-18 with the exception of critically ill neonates, who are admitted to the Neonatal Intensive Care Unit (NICU). As a result, the PICU provides care for a heterogeneous age range of patients at vastly different developmental stages and biological needs. Thus, during any given shift over a 24 hour period, physicians and nurses in the PICU may be concurrently responsible for the care of a one month old infant and a 12-year old adolescent. Admission to the PICU is a stressful experience for children who are going through active neurocognitive development, and sleep disturbances are often an unavoidable result of the critical illness and associated management.
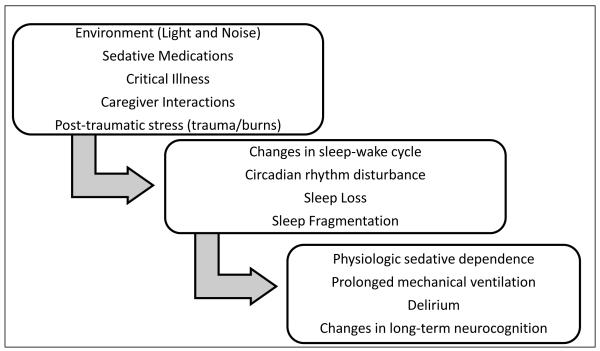
Although the importance of sleep in the intensive care unit setting has become a topic of significant research interest in recent years, there is a paucity of scientific evidence investigating sleep as a modulator of outcomes in critically ill children.(2) Studies that have used polysomnography in adults admitted to the ICU have demonstrated a decrease in sleep efficiency, an increase in arousal frequency, and a decrease or absence of slow wave sleep (SWS) and rapid eye movement (REM) sleep.(3-6). Furthermore, poor sleep quality and sleep onset and maintenance insomnia are the most frequent complaints noted by adult survivors of critical illness – impairments that persist even after discharge from the ICU.(7) When a child becomes critically ill, admission to the PICU brings with it a multitude of risk factors for disruption of the normal rhythm of the sleep-wake cycle, including a chaotic environment, administration of centrally acting medications, pain associated with the underlying illness, interruptions for nursing care, and invasive medical interventions (Figure 1). The resulting disruption in sleep continuity and reduction in sleep duration can interfere with a myriad of fundamental physiologic processes that, in turn, can lead to delirium, impaired immunity, catabolism and respiratory compromise - undesirable effects when a child is critically ill and recovery and healing are the goal.(2, 8-10) Despite obvious similarities between the pediatric and adult ICU environments, key differences exist in the exposures experienced by the critically ill child and adult. Moreover, across the age spectrum, there is substantial biological variability in normal sleep-wake behavior which may modify the detrimental impact of the ICU environment.
Figure 1.
Proposed causal pathway for changes in sleep behavior as a modulator of outcomes in critically ill children
It is well known that sleep needs are in a constant state of change as a child matures, reflecting neurologic maturation. Newborns typically sleep up to 18 hours per day on an irregular schedule, with periods of wakefulness that are limited to one to three hours. A newborn’s sleep cycle ranges from 50 to 60 minutes and is comprised of an equal proportion of REM and non-REM (NREM) sleep. Over the first twelve months of life, sleep becomes consolidated in infants, and by five years of age sleep patterns stabilize to one continuous period of sleep at night.(11-13) Healthy children between 3 to 12 years old sleep between nine to ten hours each night on average, and spend a greater proportion of the night in slow wave sleep than adults, from 20-38% of total sleep time.(12, 14, 15)
To understand key factors that disrupt the normal sleep-wake cycle in children admitted to the PICU, it is imperative to recognize that there are several features of critical care in children that are distinct from adults. Perhaps one of the most challenging components of pediatric critical care is sedation of mechanically ventilated children. Mechanically ventilated children universally receive sedative and analgesic drugs for pain and anxiety associated with invasive instrumentation, the most common being the endotracheal tube. The inability to communicate and the developmental limitations in understanding the need for ICU interventions compounds the challenges involved in adequately sedating children to maintain safety and prevent inadvertent extubation or decannulation of catheters.(16-18) Common medications used for sedation include opioids, benzodiazepines, ketamine, barbiturates and alpha-agonists such as dexmedetomidine and clonidine. Often these medications are used in combinations of two or more classes, the most common combination consisting of an opioid and a benzodiazepine after the initiation of mechanical ventilation. Opioids and benzodiazepines have been shown to decrease slow wave and REM sleep in adults.(19, 20) Paradoxically, sedative and hypnotic medications doses are often increased in critically ill children to improve the subjective assessment of sedation and sleep, leading to further agitation and deterioration of sleep quality. In a study of “difficult to sedate”, mechanically ventilated children in a tertiary care PICU (n=47), over 50% of these children received four or more distinct sedative or analgesic agents simultaneously highlighting the challenge of balancing patient comfort and medication side effects.(21) Indeed, excessive sedation is a risk in the critically ill child given the efforts that are usually made to help the patient “sleep” and maintain comfort and safety. Part of the difficulty in optimizing the use of centrally acting medications in critically ill children is the use of subjective measures to assess sedation such as the COMFORT scale, the State Behavioral Scale (SBS), and the Richmond Agitation and Sedation Scale (RASS). While helpful to some degree, these scoring systems are subjective and rely on the nurse’s assessment of patient comfort and sedation. Negative consequences of the escalating doses of sedative medications include lengthened time to extubation, withdrawal syndromes, and need for detoxification from sedatives due to pharmacological dependence. The addition of neuromuscular blockade as an adjunct to the sedation regimen adds an additional layer of complexity to the assessment of sedation and potentially compounds the problem of achieving adequate sleep quality in the ICU. Neuromuscular blockade diminishes the ability of care providers to use physical movement as an indicator of sleep state, and may lead to oversedation in an effort to ensure that the child is amnestic while receiving muscle relaxant. The role of impaired sleep quantity and quality in escalating sedative needs requires further evaluation in the PICU.
Previous research on sleep in the PICU has relied predominantly upon subjective assessments. As a result, assessment of sleep quality and quantity in the PICU remains an area for much needed research. An obvious challenge in characterizing sleep in the ICU results from the need to provide life-saving interventions and care for the critical illness that can interfere and confound the assessment of sleep. Interestingly, recommendations by the Society for Critical Care Medicine guidelines on sedation monitoring suggest that sleep assessment be a part of routine care.(4) Both subjective and objective sleep assessment tools are available for use in the ICU. Techniques for objectively characterizing sleep in the ICU include polysomnography, actigraphy, and bispectral index monitoring. There are also subjective methods that are based on observations of patient’s behavioral state.
Due to a lack of general awareness about the physical and psychological significance of sleep, hospital routines have been in place for decades that disrupt sleep continuity in the adult and pediatric ICU. Interestingly, many neonatal intensive care units nationally have adopted protocols to minimize sleep disruption, but neonates have distinct sleep physiology compared to children and adults, and many neonates do not require sedatives while mechanically ventilated. Lack of adequate sleep duration and quality has a known association with delirium, and the vast majority of PICUs internationally do not have protocols in place for sleep promotion and optimization. Given the limited information on sleep in critically ill children, the objective of the current study was to: (a) summarize the current evidence base; (b) highlight the challenges of sleep research in the PICU; and (c) demonstrate avenues for future research on sleep as a potential modulator of outcomes in critically ill children.
METHODS
Criteria for Selecting Studies
All prospective studies investigating sleep in children admitted to the pediatric intensive care unit (ages 1 month-18 years, inclusive) were included. Broad criteria for inclusion were employed to capture the entire breadth of sleep-related studies performed in the PICU environment, given the heterogeneous methodologies used and research questions posed. There were no exclusions for neurologic injuries. Studies in neonates were excluded because classification and evaluation of sleep in newborns is performed utilizing different criteria than in children, and the neonatal intensive care unit environment varies significantly from that of the PICU. Many newborns in the NICU are in incubators, and most of these patients do not require high doses of sedative medications to maintain invasive instrumentation such as the endotracheal tube and intravenous or intra-arterial catheters.
Literature Search Methodology
To identify relevant articles, the MEDLINE and EMBASE databases were searched. The search strategy focused on two main concepts: “sleep” and “pediatric intensive care unit”. There were no study period restrictions, and studies were limited to those performed in human subjects. The databases were last searched on August 20th, 2012. The search strategy for MEDLINE was as follows: ((“sleep”[MeSH Terms] OR “sleep”[All Fields] OR “sleeping”[All Fields]) AND (“Intensive Care Units”[Mesh] OR “Intensive Care Units, Pediatric”[MeSH] OR “Intensive Care Units, Neonatal”[MeSH] OR “intensive care units”[All Fields] OR “intensive care unit”[All Fields] OR “pediatric intensive care units”[All Fields] OR “pediatric intensive care unit”[All Fields] OR “neonatal intensive care units”[All Fields] OR “neonatal intensive care unit”[All Fields] OR “critical care”[tw] OR “Burn Units”[Mesh] OR “burn units”[All Fields] OR “burn unit”[All Fields]). For the EMBASE database the following search approach was utilized: ((‘sleep’/exp OR ‘sleeping’/exp OR sleep OR sleeping AND (‘intensive care units’/exp OR ‘intensive care unit’/exp OR ‘burn units’/exp OR ‘burn unit’/exp OR ‘pediatric intensive care units’ OR ‘pediatric intensive care unit’/exp OR ‘neonatal intensive care units’ OR ‘neonatal intensive care unit’ OR ‘critical care units’ OR ‘critical care unit’ OR ‘newborn intensive care’/exp OR ‘newborn intensive care’). A total of 3,153 articles were identified and title and abstract screening yielded 141 articles for full-text review. Furthermore, reference lists of the included studies, as well as review articles, were also examined to identify additional studies for inclusion.
RESULTS
Nine studies of sleep in children admitted to the PICU were identified and included in the current review (Table 1).(11, 16, 18, 22-27) Seven of the published studies utilized PSG for sleep measurement, although four resulted from the same randomized-controlled trial.(16, 18, 23-27) Two studies used the Patient Sleep Behavior Observation Tool (PSBOT) as the primary method of sleep measurement.(11, 22) Because of the significant heterogeneity in research objective, patient population (e.g., age, illness), and methodology among the nine studies identified, a quantitative synthesis of the published findings was not possible. Thus, in the following sections, available studies have been categorized by the method used for assessing sleep and summarized by specific patient subgroups. The subgroups focused on by the included studies were children with severe burns admitted to the PICU and mechanically ventilated children.
Table 1. Included Studies.
TST: Total sleep time; PSG: Polysomnography; PSBOT: Patient Sleep Behavior Observation Tool; REM: Rapid eye movement
| Authors (year) | Design | N | Age | Patient Sample | Sleep Assessment | Main findings |
|---|---|---|---|---|---|---|
| Al-Samsam and Cullen, 2005(16) |
Cross-sectional | 11 | 3-21 months | Intubated children on sedatives |
24 hour PSG | ↓ REM sleep No diurnal variation in TST or sleep stages |
| Armour, Gottschlich et al, 2008, 2009, 2011,2011* (24-27) |
Randomized Crossover Study |
40 | 3-18 years | PICU patients with severe burns randomized to zolpidem or haloperidol |
Nocturnal PSG (22:00- 07:00) for two 3-day periods in the 7-20 days postburn injury First night of PSG= control night (no treatment) Sleep assessment by observers q15 minutes over nighttime PSG periods |
↑ Wakefulness on control and treatment nights ↓ REM sleep Zolpidem: ↑ stage 3 and REM sleep Haloperidol: ↓ sleep latency and ↑ TST & N2 sleep Zolpidem + Haloperidol: ↑ sleep continuity Ketamine: ↓ REM sleep |
| Carno et al., 2004(18) |
Cross-sectional | 2 | 3 years | PICU patients on sedation and neuromuscular blockade after laryngotracheoplasty |
PSG for 96 hours beginning 2 hours after surgery |
↑ Stage 1 and 2 sleep and jslow wave sleep |
| Cureton-Lane and Fontaine, 1997(22) |
Cross-sectional | 9 | 15 months - 10.5 years |
Children in the PICU for at least 24 hours |
PSBOT for a 10 hour nighttime period |
Frequent awakenings Mean length of nighttime sleep less than at home ↑ Noise levels associated with wakefulness Abrupt changes in noise increased arousals ↑ Light levels and caregiver contact correlated with wakefulness |
| Corser et al., 1996(11) |
Cross-sectional | 12 | 13 months- 35 months |
PICU patients | PSBOT for 12 hour Nighttime Period Sleep Follow-up Interview Guide |
Arousals after sleep onset more than home baseline Benzodiazepines: ↑TST No correlation between time to return to pre-illness sleep pattern and PRISM score, ICU, or hospital length of stay |
| Gottschlich et al., 1994(23) |
Cross-sectional | 11 | 1.4-16 years | PICU patients with burns | Biweekly 24-hour PSG measurements through discharge |
Mean TST over 24-hour period of 10.5 hours Absence of stage 3/4 in 40% of PSG periods ↑ Stage 1 early in hospitalization Progressive ↓ Stage 2 and ↑ Stage 3 and REM sleep with recovery Normalization of sleep associated with clinical improvement |
Same Study
Polysomnography in Children Admitted to the PICU with Severe Burns
Studies have demonstrated that severe thermal injury has detrimental effects on sleep quality and quantity, and animal studies suggest that disruption of sleep continuity in the presence of severe burns may contribute to delayed wound healing and poor pain tolerance.(28-31). Data from subjective assessments and objective measurements with actigraphy have also shown poor sleep quality in adult burn patients in the hospital.(29, 31) Gottschlich and colleagues have previously summarized the entirety of PICU sleep literature in burned children.(23-27) The first study on sleep in critically ill children was published in 1994 and included children with burn injury that involved greater than 20% BSA admitted to the ICU with an endotracheal tube.(23) Using biweekly 24-hour eight-channel polysomnography in 11 children enrolled over 16 months, the observed total sleep time was, on average, 10.5 hours of sleep time/patient/24 hour period, which did not change significantly as days post-burn increased. Of the 43 sleep studies performed, 40% showed no evidence of slow wave sleep, and 19% demonstrated complete absence of REM sleep. Interestingly, slow wave and REM sleep percentages increased over the course of the hospital stay concomitant with a decrease in stage 1 and 2 sleep. Although the aforementioned findings confirm the disturbance of sleep in burn patients admitted to the PICU, the clinical implications for recovery after thermal injury and overall ICU stay remains to be determined.
Four of the identified manuscripts for the current review resulted from the same prospective, randomized controlled crossover study investigating the effects of two sleep-inducing medications in 40 pediatric acute burn patients admitted to the PICU.(24-27) The overarching objective was to evaluate the effects of zolpidem, a non-benzodiazepine short-acting hypnotic, and haloperidol, an antipsychotic agent frequently used to treat delirium in the adult ICU setting, on sleep in children ages 3-18 admitted within seven days with burns that covered greater than 20% body surface area. Children were randomized to zolpidem or haloperidol in the second week post-burn for two nights, and crossed over to the other drug for two nights in the third week post-burn. Each subject had a control night on the night prior to administration of each drug. Nocturnal polysomnography was performed for three nights for each of medications from 9:30 p.m. to 7 a.m., resulting in six nighttime PSG recordings per subject. All medications administered within 72 hours of the PSG periods were recorded. Sleep variables used for analysis were time in sleep stage, number of awakenings and longest periods of wake and sleep.(24) Thirteen of the forty patients enrolled were mechanically ventilated and the mean BSA burned was 50% (SD: 2.9%). Study subjects underwent anywhere from 2 to 21 surgical procedures during their hospitalization and 65% of subjects had inhalational injury. A majority of patients (82%) were receiving midazolam, while 42% and 44% were on methadone and morphine, respectively. Results from the control nights demonstrated a mean number of awakenings was 24.9 events/hr (SD: 10.3), with an average total sleep time of 4.2 hours (SD: 0.2 hours). At baseline, a five-fold increase in the proportion of time spent in stage 1 sleep was noted as compared to published normal values (13.1% vs. 2.8%). The greatest reduction was observed in the percentage of slow wave sleep, with an average 1.6% (SD: 2.5) on control nights in burned children compared to 21% in normal children. REM sleep amount was also reduced at 5.25% (SD: 5.0%). Zolpidem increased the number of awakenings compared to control nights (28.8 vs. 24.9 events/hr, p=.03), whereas haloperidol had no significant effect on awakenings (p=0.72). Total sleep time was not statistically significantly improved by zolpidem, with only a 12% increase, while children receiving haloperidol had an average of 23% higher total sleep than controls (p=0.02). Neither zolpidem or haloperidol had a significant effect on the average time spent in stage 1 sleep or REM sleep, but zolpidem was noted to increase the percentages of slow wave and REM sleep compared to controls (0.81 vs. 0.62 hrs., p=0.02). The overarching inference is that sleep duration and architecture are significantly affected among pediatric burn patients and that accelerated drug metabolism in burn patients may have had an effect on the outcome of the interventions, necessitating inclusion of pharmacokinetic parameters in future studies.
In a secondary analysis from the same randomized crossover trial, the correlation between the burn care provider’s subjective evaluation of sleep quality with simultaneous polysomnographic data was evaluated.(25) Trained field observers carried out sleep assessments with concurrent polysomnographic recordings, and recorded their observations every 15 minutes over each nine hour nighttime period. The observers classified the subjects as “awake”, “drowsy” or “asleep”, based on behavioral characteristics such as eye closure, vital sign changes, and body movement. Sedation scores were not monitored in order avoid waking thee patients. Sleep study epochs were analyzed in 2-minute and 10-minute intervals, and categorized as sleep, wakefulness or a combination of both. In order to be classified as sleep, all the epochs in the 2-minute and 10-minute intervals must be designated as either stage 1, 2, slow wave or REM sleep. The percentage of polysomnographic intervals rated as awake, asleep or mixed were calculated and averaged for all 40 patients. A secondary classification to facilitate statistical analysis was comprised of asleep (for all contiguous epochs) and not asleep (either mixed or awake). Correlation of visual observation and polysomnography showed a poor agreement, with a kappa statistic of 0.21. Children judged to be asleep by visual assessment were awake by polysomnography 56.3% of the time. The sensitivity and specificity of visual assessment of sleep state were 97% and 28%, respectively. Administration of zolpidem or haloperidol did not improve the intraclass correlation between subjective and objective measurements. Patients on ventilators were noted to have a larger amount of wake time compared to non-ventilated patients (p=0.001), and mechanical ventilation altered the level agreement between subjective and objective measures (p<0.05), although the direction of the association was not reported.
A third subanalysis of data collected from the randomized crossover study investigated the effect of ketamine administration on the quantity and quality of sleep in the pediatric burn patient.(26) Patients were grouped retrospectively into those who received ketamine on their first control day/night (a period of 30 hours beginning at midnight the day of the control PSG) and those who did not. Twenty-three of 40 patients received ketamine during the study period, and demographics including age, percentage of body surface area burned, sex, length of stay and mortality were similar between the ketamine and non-ketamine groups. Ketamine administration was associated with reduced REM sleep when compared to the non-ketamine group (4.54% vs. 1.66%, p=0.04). Ketamine had no effect on nocturnal total sleep time, frequency of awakenings, or the percentages of stages 1, 2, or slow wave sleep.
Finally, Gottschlich et al. evaluated the association between hormonal abnormalities and sleep stage distribution post-burn, as well as the effects of zolpidem and haloperidol on measures of endocrine function in these subjects.(27) At 6:00 a.m. on each study day, epinephrine, norepinephrine, and serotonin levels were measured, along with dehydroepiandrosterone (DHEA), growth hormone (GH), melatonin and cortisol. DHEA was the only hormone significantly affected by haloperidol or zolpidem. Serum levels of DHEA were higher than in the control group (p<0.03) but still below normal, and increases in DHEA were not associated with increases in slow wave or REM sleep. There was a significant inverse correlation between the amount of REM sleep and epinephrine levels (r=-0.34, p=0.004). Those subjects who had at least 2.5% REM sleep demonstrated reduced epinephrine levels, and groups with negligible REM had elevated concentrations of epinephrine. The same inverse association also existed between REM sleep percentage and norepinephrine, with subjects who experienced greater than 10% REM having normal norepinephrine levels compared to increased levels in those with <10% REM. Serotonin had a positive correlation with slow wave (r=0.24, p=0.01) and REM sleep (r=0.48, p=0.01). No significant associations were noted between GH, melatonin, or cortisol and slow wave sleep.
Polysomnography in Mechanical Ventilated Children
While the studies by Gottschlich and colleagues included a proportion of mechanically ventilated patients, two separate studies have focused on sleep in mechanically ventilated children using polysomnography.(16, 18) In a 2004 feasibility study of polysomnography in children receiving neuromuscular blockade, recordings were obtained for four days in two children following laryngotracheoplasty.(18) These post-operative patients were chosen due to their uniform need for immobility after repair and the difficulty in subjective assessment of sleep in children receiving neuromuscular blockade. Both subjects’ had a greater proportion of sleep during the daytime, and their sleep became more consolidated by day 4. There was a marked decrease of SWS and a pronounced shift to stages 1 and 2. REM sleep could not be documented due to neuromuscular blockade and resulting inability to record any eye movement and muscle activity data. There was significant variability in sedative and neuromuscular blockade dosing which may explain the lack of correlations between dose of drug of administered and sleep.
Al-Samsam and colleagues conducted 24-hour polysomnography, noise monitoring, and logging of staff interventions in infants from 3-21 months of age.(16) No interventions were made on medical management including sedative medications, and staff interventions were logged by the interveners on a bedside log, categorized as mildly, moderately or severely intrusive. Data collected by polysomnography were manually scored in 30-second epochs as quiet sleep, active sleep, wake or indeterminate using criteria for infants.(32, 33) Sixty percent of subjects were post-operative cardiac surgery patients, and all were mechanically ventilated and sedated with morphine and midazolam infusions. Chloral hydrate and trimeprazine were also used in all patients, and four patients were receiving dopamine. Active sleep was reduced to a mean of 3.0% (SD: 4.0%), although the 24-hour total sleep time was longer than expected for age, at 19 hours (SD: 2.6 hours). The average frequency of wake episodes per night was 40 (SD: 20), and the average of the longest sustained sleep period was 194 minutes (SD: 79 minutes). No statistically significant differences in percentages of total sleep time, wake, quiet or active sleep were present between daytime and nighttime, and the mean ratio of day/night total sleep time was 1.56 (SD: 2.3). Noise levels were ≥75 dB (A) for 19% of the nights, with minimum 24-hour Leq level of 48 dB (A). With regard to staff interventions, medical staff was in contact with subjects for a mean duration of 240±90 minutes in a 24-hour period. Nighttime severely intrusive interventions were associated with wake states 88±13% of the time.
Studies on Subjective Assessments of Sleep in the PICU
Two studies utilized observation of sleep state as the primary method of sleep measurement in the PICU.(11, 22) Both of these measured sleep with the Patient Sleep Behavior Observation Tool (PSBOT), which was developed in 1968 by Echols as part of a master’s thesis (Catholic University of America, Washington, DC). The PSBOT is an instrument which describes four tiers of cortical vigilance, identifying patient behaviors in each category. These tiers include awake, drowsy, paradoxical (REM) and orthodox (NREM) sleep. Using polysomnography, validity and reliability has been demonstrated for sleep latency, midsleep awakenings and waking after sleep onset when PSBOT is used for evaluation every five minutes.(11, 22, 34) Corser and colleagues examined the sleep of twelve 1-2 year old children during and after PICU admission, as well as environmental stimuli with sound and light measurement.(11) The study recruited subjects from a limited age group to minimize the potential confounding effects of psychosocial, cognitive and neurological development. In addition to the PSBOT which was performed for one 12-hour nighttime period every five minutes with light and sound monitoring, the Caregiver Activity Rating Scale (CARS) was developed to measure caregiver actions during the study period. The Sleep History Interview Guide and Sleep Follow-up Interview Guide were used to elicit sleep patterns before admission and after hospital discharge, respectively, and follow-up interviews were conducted weekly with parents for a six week period. Mean sleep time for children in the PICU was 436 minutes (SD: 167 minutes), and children awoke a mean of 9 times per 12-hour night (SD: 4.4). Sleep periods averaged 52 minutes and benzodiazepine use was associated with increased total sleep time (p=0.045). A negative correlation was noted between individual sleep state observations and noise, light, caregiver activity and pain (all p<0.05). Return to pre-illness total sleep time occurred at an average of 3.5 weeks (SD: 2.2), and the number of awakenings after discharge returned to baseline at 3.6 weeks (SD: 1.8). There was no association between time to return to baseline sleep patterns and extent of sleep change, disease severity or length of PICU stay or hospital stay.
One year after publication of the above study, Cureton-Lane and colleagues observed nine children from 15 months to 10 years of age admitted to the PICU for at least 24 hours.(22) The PSBOT was used for one 10-hour period, with simultaneous light and sound monitoring. The technician/staff member performing the PSBOT also recorded interactions between healthcare workers and the subject, as well as information about sources of noise and parental activities likely to influence variables. Mean total sleep time in that study was 4.7 hours (SD: 0.5 hours) over the 10 hour period observed, with a mean length of sleep episode of 27.6 minutes (SD: 25.9 minutes). Average number of awakenings was 9.8 per night (SD: 2.5). Noise levels averaged 55.1 dB (A) (SD: 6.8), and descriptions of noise exposures included frequent sharp elevations due to ventilators, cardiac monitors, oximeters, IV pumps, and staff conversations. Light levels, measured in foot candles, did vary distinctly according to the time of night, and remained low until 6 a.m. Caregivers were in direct contact with subjects 13.4 % (SD: 34.2) of all 5-minute observation points, and 22.5 % (SD: 41.2) of the time between observations. PRISM score, a measure of severity of illness and risk of mortality for PICU patients, was low or moderate for all subjects, due to exclusion of severely ill PICU patients in the convenience sample. Associations between sleep and metrics of noise, light, caregiver contact, parental presence and PRISM score indicated that noise (p<0.001), light (p<0.02), and caregiver contact (p<0.001) were predictive of sleep state.
DISCUSSION
In the characterization of sleep in critically ill children admitted to the PICU, it is important to note the complexity of sleep assessment in an environment with a multitude of confounding variables that include the age, nature of the critical illness, invasive instrumentation such as the endotracheal tube, mechanical ventilation, noise, light, staff interventions and medications. Each child admitted to the PICU has a unique sleep environment considering all the factors that are associated with the child’s illness. In the current study, nine publications were reviewed on sleep-related measurement in the PICU setting, resulting from six unique studies. The included studies assessed sleep in a wide range of ages, 3 months to 18 years, which is a major source of heterogeneity given the changes in sleep architecture that occur in development from infancy to adolescence.(14) All of the included studies excluded children with neurologic disorders such as traumatic brain injury or baseline severe neurologic deficits. The effects of traumatic brain injury represent a fertile area for additional research given evidence that children and adults with traumatic brain injury demonstrate sleep disturbances after discharge from the hospital.(35, 36) Specific sites of neurologic injury may impact the sleep-wake cycle differentially, with resultant consequences for short and long-term prognosis. In addition, all but one study excluded patients who required neuromuscular blockade. Although exclusion criteria were consistently imposed on all of the included studies, six varied considerably with regards to inclusion criteria with only two focusing solely on children undergoing mechanical ventilation, while the remainder included both spontaneously breathing and mechanically ventilated patients. There was also significant heterogeneity with regards to inclusion of children who had recently undergone surgery, which may play an important role when pain from the surgical intervention is factored into analgesic requirements.
Five of the included publications resulted from two studies focusing on sleep in children with severe burns admitted to the PICU.(23-27) In addition to the physical, environmental and pharmacologic factors that contribute to sleep disturbances in critically ill children admitted to the PICU, the child with severe burns has experienced a major traumatic event that can have significant implications for the child’s stress response in the acute and chronic phase of the injury.(37) These children have been shown to meet criteria for post-traumatic stress disorder soon after the injury that can extend months to years later.(22, 38) Much of the psychological distress is due to pain associated with the injury, but fear and anxiety may be difficult for medical providers to distinguish from pain, particularly in younger children.(39) As a result, children with severe burns are potentially at the highest risk for sleep disturbances in the PICU.
Perhaps one of the most interesting factors common to all studies that included polysomnography was the use of historical controls, normative age-based sleep stage and duration data for analysis of the sleep parameters observed in the PICU. Overall, these studies demonstrated that children in the PICU experience changes in sleep-wake behavior.
Mechanical Ventilation and Sedation
Children requiring mechanical ventilation in the PICU are the most critically ill patients and have the longest hospital length of stay, morbidity and mortality. Therefore, when considering sleep in the PICU, a special focus must be made on this vulnerable population. Many adult studies have focused on the effects of mechanical ventilation on sleep using polysomnography, demonstrating marked differences between the mechanically ventilated and spontaneously breathing patients.(2, 40) As mechanically ventilated patients spend a disproportionate amount of time in stage 1 sleep compared to the spontaneously breathing patient it is important to note that mechanically ventilated patients have the influence of an endotracheal or tracheostomy tube in addition to the other interventions (e.g., sedative medications, pulmonary toilet) from care providers.(7, 8) As a result, it is difficult to assess the true interplay between mechanical ventilation and sleep in the critically ill.
Mechanical ventilation modes may also have an effect on sleep. The irregularities in respiration and heart rate and the accompanying paralysis of respiratory muscles excepting the diaphragm during REM sleep may further influence mechanical ventilation and patient-ventilator synchrony.(2) Studies in adults have demonstrated that sleep fragmentation is more frequent with pressure support ventilation, and sleep efficiency is higher with assist control ventilation.(5, 40) Mechanically ventilated patients demonstrate more disruption of the natural diurnal fluctuation of 6-sulfatoxymelatonin and a decrease in excretion of the metabolite when compared to the spontaneously breathing patient.(41) Circadian fluctuation of melatonin can be completely abolished, but may not correspond to the level of sedation measured by a BIS monitor or sedation agitation scale, suggesting that sedative medications may play a greater role in circadian rhythmicity.(42)
Although the effects of sedative and analgesic medications on sedation depth have been given much attention, little is known of their effects on sleep in the critically ill population, particularly in children.(43) For example, benzodiazepines are known to increase sleep efficiency and duration while decreasing sleep latency and awakenings. Yet, benzodiazepines have been observed to decrease and suppress REM and slow wave sleep, the most restorative components of the sleep cycle. In addition, benzodiazepines decrease EEG frequency and amplitude at high doses and increase cortical EEG frequency at low doses.(44) The definition of high and low doses of these medications in children is complex given the plasticity of the developing brain and the wide range of ages and doses used in these critically ill children. Continuously changing dosing regimens add another confounding factor to the influence of mechanical ventilation, sedation and environmental stimuli on sleep architecture in the critically ill child.
Before strategies to improve sleep quality can begin in critically ill children, pediatric intensivists must begin to understand the physiology of normal sleep and the effect of sedative management on sleep. The majority of children in the ICU setting who require sedation are intubated; therefore a clear distinction between sleep and sedation becomes important. Sedation can be a form of augmented or artificial sleep, but it can also be used to define a patient’s level of unresponsiveness, or level of sedation, a complex interplay.(45)
Challenges in PICU Sleep Research
Given the data summarized and the growing evidence for the role of sleep as a potential modulator of outcomes in critically ill adults, there is a great need for increased sleep research in the PICU, where each patient is undergoing active neurocognitive development. The call for increased research is not without significant challenges. First, there is the challenge of measuring sleep in a complex and uncontrolled environment. In addition to varying ages and developmental levels, the potential confounding of each child’s individual illness, severity, medication exposures and external environment need to be adequately considered. Though some of these factors (e.g., noise, light, and medications) can be measured and accounted for, it is the effect of the child’s critical illness on the sleep process that is unknown. Added to the complex and heterogeneous milieu of the critically ill child are the effects of various medications (e.g., sedatives) on the neurobiology of the sleep process. Objective measurement of sleep in the PICU setting is ideal, utilizing the gold standard of polysomnography, but this approach has several operational limitations. Equipment needed for polysomnography is expensive and cumbersome and may be viewed by care providers as interfering with care of critically ill children. Parents may be reluctant to consent to non-therapeutic assessments which involve application of additional diagnostic sensors. In addition, to understand the sleep process of a child in the PICU, more than 24 hours of continuous recordings are likely required given that sleep may occur any time during the day and total sleep time during the night may not necessarily reflect a child’s experience. Studies that require extended monitoring periods are challenging particularly because critical illness increases the risk of sensor loss leading to missing data. Actigraphy, an alternative and simpler option given its ease of implementation and non-invasive nature, has not been validated in the ICU population, and results can be affected by movement not initiated by the child, such as routine nursing cares. Furthermore, actigraphy offers no information about sleep quality in a sedated child, which is a compelling question in this population.
Aside from implementation of the sleep assessment tools, specifically polysomnography, there are significant challenges in the analysis of the data obtained. The electroencephalogram of a critically ill child may be confounded by the derangements of their critical illness, in addition to the neurologic effects of medications administered. Neuromuscular blockade limits polysomnography given the inability to record muscle activity. Children add the additional layer of variability in normative sleep depending on age. Given these factors, traditional sleep staging may not be appropriate in the critically ill population.
Future Directions
This review has synthesized the available body of evidence in sleep in critically ill children admitted to the PICU and highlighted many of the confounders and challenges associated with research in this patient population. The role of sleep as a possible modulator of outcomes in these children needs to be investigated in a systematic fashion in order to understand the complex neurobiology at play. Given that there are a limited number of objective assessment tools available for use in the PICU setting, it is imperative that these are implemented in a rigorous fashion to obtain the most valid information possible. Although polysomnography and specifically the sleep EEG may be affected by independent factors such as critical illness, the variables that may prove to be the most dependent are dose and duration of sedative and analgesic medications. If a critically ill child’s sleep is adversely affected by centrally acting medications and these medications are being perhaps utilized to induce sleep, there is a clear need for developing methods for sleep optimization in the PICU. The potential effects of decreased REM sleep on autonomic function, inflammation and immunity present multiple avenues of future investigation.(46) Modalities that may help improve the sleep-wake cycle in the PICU may include non-invasive, non-pharmacologic approaches such as earplugs, noise reduction protocols, lighting optimization, and pharmacologic approaches including melatonin. Although these preventative and treatment approaches may be viewed as simple and inexpensive to implement, there is a major culture change that must occur in the pediatric critical care community to maintain these approaches. Scientific and clinical evidence is imperative to demonstrate that optimizing sleep in critically ill children can reduce morbidity through decreases in sedative medications, neuroinflammation, and hospital length of stay. Characterizing sleep and understanding the biology of circadian rhythmicity in critically ill children are possible avenues for research, using EEG, actigraphy, and biomarkers, in addition to long-term follow-up of neurodevelopmental outcomes. Finding the optimal balance between analgesia, anxiolysis and sleep is integral to the care of critically ill children in the PICU.
Practice Points.
Children in the Pediatric Intensive Care Unit (PICU) are exposed to many risk factors for sleep loss and disruption, including sedative medications, which are often increased to improve the subjective assessment of sleep;
Observational studies of children in the PICU demonstrate that critically ill children experience decreases in slow wave sleep and REM sleep, and subjective assessments do not correlate with objective measures such as polysomnography;
Noise is a major component of the environmental factors that may contribute to sleep loss and disruption
Research Agenda.
Determine the effects of the PICU environment on sleep across the age spectrum and test the efficacy of non-invasive behavioral sleep protocols (sleep hygiene, earplugs, light therapy) on ICU outcomes;
Investigate the efficacy of sedation management with pharmacologic agents least likely to affect sleep detrimentally, such as dexmedetomidine;
Characterize the role of sleep as a modulator of neurocognitive outcomes after recovery from critical illness through the use of objective measures such as EEG and actigraphy
Acknowledgments
The authors would like to thank Dr. Myron Yaster for his expertise and vision for pain and sedation management in critically ill children and ongoing support of our work to understand the role of sleep as a modulator of outcomes in the Pediatric Intensive Care Unit. We would also like to thank Ms. Blair Anton for guidance with the literature search strategy and Ms. Natasha Sukerkar for her assistance with data extraction for this study. This work was supported by the Johns Hopkins CTSA Award Number 5KL2RR025006 from the National Center for Advancing Translational Sciences of the National Institutes of Health. Dr. Punjabi has received research support from the National Institutes of Health (HL075078). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- db (A)
A-weighted decibels
- GH
growth hormone
- NICU
Neonatal Intensive Care Unit
- NREM
non-REM
- PICU
Pediatric Intensive Care Unit
- PRISM
Pediatric Risk of Mortality
- PSBOT
Patient Sleep Behavior Observation Tool
- REM
rapid eye movement
- SWS
slow wave sleep
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Randolph AG, Gonzales CA, Cortellini L, Yeh TS. Growth of pediatric intensive care units in the United States from 1995 to 2001. J Pediatr. 2004;144:792–8. doi: 10.1016/j.jpeds.2004.03.019. [DOI] [PubMed] [Google Scholar]
- *2.Kudchadkar S, Sterni L, Yaster M, Easley RB. Sleep in the Intensive Care Unit. Contemp Crit Care. 2009;7:1–12. [Google Scholar]
- 3.Friese RS. Sleep and recovery from critical illness and injury: A review of theory, current practice, and future directions. Crit Care Med. 2008;36:2963. doi: 10.1097/CCM.0b013e318187268d. [DOI] [PubMed] [Google Scholar]
- 4.Bourne RS, Minelli C, Mills GH, Kandler R. Clinical review: Sleep measurement in critical care patients: research and clinical implications. Crit Care. 2007;11:226. doi: 10.1186/cc5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parthasarathy S, Tobin MJ. Effect of ventilator mode on sleep quality in critically ill patients. American journal of respiratory and critical care medicine. 2002;166:1423–9. doi: 10.1164/rccm.200209-999OC. [DOI] [PubMed] [Google Scholar]
- 6.Parthasarathy S, Tobin MJ. Sleep in the intensive care unit. Intensive Care Med. 2004;30:197–206. doi: 10.1007/s00134-003-2030-6. [DOI] [PubMed] [Google Scholar]
- 7.Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163:451–7. doi: 10.1164/ajrccm.163.2.9912128. [DOI] [PubMed] [Google Scholar]
- 8.Gabor JY, Cooper AB, Crombach SA, Lee B, Kadikar N, Bettger HE, et al. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003;167:708–15. doi: 10.1164/rccm.2201090. [DOI] [PubMed] [Google Scholar]
- 9.Schieveld JN, Leroy PL, van Os J, Nicolai J, Vos GD, Leentjens AF. Pediatric delirium in critical illness: phenomenology, clinical correlates and treatment response in 40 cases in the pediatric intensive care unit. Intensive Care Med. 2007;33:1033–40. doi: 10.1007/s00134-007-0637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith HA, Fuchs DC, Pandharipande PP, Barr FE, Ely EW. Delirium: an emerging frontier in the management of critically ill children. Anesthesiology clinics. 2011;29:729–50. doi: 10.1016/j.anclin.2011.09.011. [DOI] [PubMed] [Google Scholar]
- *11.Corser NC. Sleep of 1- and 2-year-old children in intensive care. Issues in comprehensive pediatric nursing. 1996;19:17–31. doi: 10.3109/01460869609026852. [DOI] [PubMed] [Google Scholar]
- 12.Williams RL, Karacan I, Hursch CJ. Electroencephalography (EEG) of human sleep: clinical applications. xiv. Wiley; New York: 1974. p. 169. [Google Scholar]
- 13.Ross JJ, Agnew HW, Jr., Williams RL, Webb WB. Sleep patterns in pre-adolescent children: an EEG-EOG study. Pediatrics. 1968;42:324–35. [PubMed] [Google Scholar]
- 14.Coble PA, Kupfer DJ, Taska LS, Kane J. EEG sleep of normal healthy children. Part I: Findings using standard measurement methods. Sleep. 1984;7:289–303. doi: 10.1093/sleep/7.4.289. [DOI] [PubMed] [Google Scholar]
- 15.Palm L, Persson E, Elmqvist D, Blennow G. Sleep and wakefulness in normal preadolescent children. Sleep. 1989;12:299–308. doi: 10.1093/sleep/12.4.299. [DOI] [PubMed] [Google Scholar]
- *16.Al-Samsam RH, Cullen P. Sleep and adverse environmental factors in sedated mechanically ventilated pediatric intensive care patients. Pediatr Crit Care Med. 2005;6:562–7. doi: 10.1097/01.pcc.0000165561.40986.a6. [DOI] [PubMed] [Google Scholar]
- 17.Carno MA, Connolly HV. Sleep and sedation in the pediatric intensive care unit. Crit Care Nurs Clin North Am. 2005;17:239–44. doi: 10.1016/j.ccell.2005.04.005. [DOI] [PubMed] [Google Scholar]
- *18.Carno MA, Hoffman LA, Henker R, Carcillo J, Sanders MH. Sleep monitoring in children during neuromuscular blockade in the pediatric intensive care unit: a pilot study. Pediatr Crit Care Med. 2004;5:224–9. doi: 10.1097/01.pcc.0000124024.92280.f9. [DOI] [PubMed] [Google Scholar]
- 19.Johnson LC, Spinweber CL, Seidel WF, Dement WC. Sleep spindle and delta changes during chronic use of a short-acting and a long-acting benzodiazepine hypnotic. Electroencephal Clin Neurophysiol. 1983;55:662–7. doi: 10.1016/0013-4694(83)90276-6. [DOI] [PubMed] [Google Scholar]
- 20.Knill RL, Moote CA, Skinner MI, Rose EA. Anesthesia with abdominal surgery leads to intense REM sleep during the first postoperative week. Anesthesiology. 1990;73:52–61. doi: 10.1097/00000542-199007000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Rapan K, Veltri M, Easley RB. Use of dexmedetomidine in a Pediatric Intensive Care Unit. Crit Care. 2007;35:A876. [Google Scholar]
- *22.Cureton-Lane RA, Fontaine DK. Sleep in the pediatric ICU: an empirical investigation. Am J Crit Care. 1997;6:56–63. [PubMed] [Google Scholar]
- *23.Gottschlich MM, Jenkins ME, Mayes T, Khoury J, Kramer M, Warden GD, et al. The 1994 Clinical Research Award. A prospective clinical study of the polysomnographic stages of sleep after burn injury. J Burn Care Rehab. 1994;15:486–92. [PubMed] [Google Scholar]
- *24.Armour A, Gottschlich MM, Khoury J, Warden GD, Kagan RJ. A randomized, controlled prospective trial of zolpidem and haloperidol for use as sleeping agents in pediatric burn patients. J Burn Care Res. 2008;29:238–47. doi: 10.1097/BCR.0b013e31815f384e. [DOI] [PubMed] [Google Scholar]
- *25.Armour AD, Khoury JC, Kagan RJ, Gottschlich MM. Clinical assessment of sleep among pediatric burn patients does not correlate with polysomnography. J Burn Care Res. 2011;32:529–34. doi: 10.1097/BCR.0b013e31822ac844. [DOI] [PubMed] [Google Scholar]
- *26.Gottschlich MM, Mayes T, Khoury J, McCall J, Simakajornboon N, Kagan RJ. The effect of ketamine administration on nocturnal sleep architecture. J Burn Care Res. 2011;32:535–40. doi: 10.1097/BCR.0b013e31822ac7d1. [DOI] [PubMed] [Google Scholar]
- *27.Gottschlich MM, Khoury J, Warden GD, Kagan RJ. An evaluation of the neuroendocrine response to sleep in pediatric burn patients. J Parenter Enteral Nutr. 2009;33:317–26. doi: 10.1177/0148607108325180. [DOI] [PubMed] [Google Scholar]
- 28.Raymond I, Nielsen TA, Lavigne G, Manzini C, Choiniere M. Quality of sleep and its daily relationship to pain intensity in hospitalized adult burn patients. Pain. 2001;92:381–8. doi: 10.1016/S0304-3959(01)00282-2. [DOI] [PubMed] [Google Scholar]
- 29.Raymond I, Ancoli-Israel S, Choiniere M. Sleep disturbances, pain and analgesia in adults hospitalized for burn injuries. Sleep Med. 2004;5:551–9. doi: 10.1016/j.sleep.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Gumustekin K, Seven B, Karabulut N, Aktas O, Gursan N, Aslan S, et al. Effects of sleep deprivation, nicotine, and selenium on wound healing in rats. Int J Neurosci. 2004;114:1433–42. doi: 10.1080/00207450490509168. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence JW, Fauerbach J, Eudell E, Ware L, Munster A. The 1998 Clinical Research Award. Sleep disturbance after burn injury: a frequent yet understudied complication. The J Burn Care Rehab. 1998;19:480–6. doi: 10.1097/00004630-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Stern E, Parmelee AH, Akiyama Y, Schultz MA, Wenner WH. Sleep cycle characteristics in infants. Pediatrics. 1969;43:65–70. [PubMed] [Google Scholar]
- 33.Hoppenbrouwers T, Hodgman J, Arakawa K, Geidel SA, Sterman MB. Sleep and waking states in infancy: normative studies. Sleep. 1988;11:387–401. doi: 10.1093/sleep/11.4.387. [DOI] [PubMed] [Google Scholar]
- 34.Fontaine DK. Measurement of nocturnal sleep patterns in trauma patients. Heart and Lung: J Critical Care. 1989;18:402–10. [PubMed] [Google Scholar]
- 35.Baumann CR, Werth E, Stocker R, Ludwig S, Bassetti CL. Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain. 2007;130:1873–83. doi: 10.1093/brain/awm109. [DOI] [PubMed] [Google Scholar]
- 36.Li YC, Jin HQ, Owens JA, Hu CL. The association between sleep and injury among school-aged children in rural China: A case-control study. Sleep Medicine. 2008;9:142–8. doi: 10.1016/j.sleep.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 37.Stoddard FJ, Ronfeldt H, Kagan J, Drake JE, Snidman N, Murphy JM, et al. Young burned children: the course of acute stress and physiological and behavioral responses. Am J Pychiatry. 2006;163:1084–90. doi: 10.1176/ajp.2006.163.6.1084. [DOI] [PubMed] [Google Scholar]
- 38.Saxe GN, Stoddard F, Hall E, Chawla N, Lopez C, Sheridan R, et al. Pathways to PTSD, part I: Children with burns. Am J Pychiatry. 2005;162:1299–304. doi: 10.1176/appi.ajp.162.7.1299. [DOI] [PubMed] [Google Scholar]
- 39.Arceneaux LL, Meyer WJ., 3rd Treatments for common psychiatric conditions among children and adolescents during acute rehabilitation and reintegration phases of burn injury. Int Rev Psychiatry. 2009;21:549–58. doi: 10.3109/09540260903343984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parthasarathy S. Sleep during mechanical ventilation. Curr Opin Pulm Med. 2004;10:489–94. doi: 10.1097/01.mcp.0000143691.94442.fa. [DOI] [PubMed] [Google Scholar]
- 41.Frisk U, Olsson J, Nylen P, Hahn RG. Low melatonin excretion during mechanical ventilation in the intensive care unit. Clin Sci. 2004;107:47–53. doi: 10.1042/CS20030374. [DOI] [PubMed] [Google Scholar]
- 42.Olofsson K, Alling C, Lundberg D, Malmros C. Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol Scand. 2004;48:679–84. doi: 10.1111/j.0001-5172.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- 43.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–44. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 44.Wauquier A. EEG and neuropharmacology. In: Niedermeyer E, Da Silva F, editors. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Williams and Wilkins; Baltimore: 1993. pp. 619–29. [Google Scholar]
- 45.Bourne RS, Mills GH. Sleep disruption in critically ill patients--pharmacological considerations. Anaesthesia. 2004;59:374–84. doi: 10.1111/j.1365-2044.2004.03664.x. [DOI] [PubMed] [Google Scholar]
- 46.Gomez-Gonzalez B, Dominguez-Salazar E, Hurtado-Alvarado G, Esqueda-Leon E, Santana-Miranda R, Rojas-Zamorano JA, et al. Role of sleep in the regulation of the immune system and the pituitary hormones. Annals NY Acad Sci. 2012;1261:97–106. doi: 10.1111/j.1749-6632.2012.06616.x. [DOI] [PubMed] [Google Scholar]