Abstract
Like humans, rhesus macaques (Macaca mulatta) are large, long-lived diurnal primates, and show similar age-related changes in the secretion of many steroid hormones, including oestradiol, testosterone, cortisol, and dehydroepiandrosterone (DHEA). Consequently, they represent a pragmatic animal model in which to examine the mechanisms by which these steroidal changes contribute to perturbed sleep-wake cycles and cognitive decline in the elderly. Using remote serial blood sampling we have found the circulating levels of DHEA sulphate, as well as oestradiol and testosterone, decline markedly in old monkeys. Furthermore, using real-time PCR, we have shown that genes associated with the conversion of DHEA to oestradiol and testosterone (e.g., 3BHSD, 17BHSD, and aromatase) are highly expressed in brain areas associated with cognition and behavior, including the hippocampus, prefrontal cortex, and amygdala. Taken together, these findings suggest that administration of supplementary DHEA in the elderly may have therapeutic potential for cognitive and behavioral disorders, but with fewer negative side effects outside of the central nervous system. To test this we have developed a novel steroid supplementation paradigm for use in old animals; this involves oral administration of DHEA and testosterone at physiologically relevant times of the day to mimic the circadian hormone patterns observed in young adults. We are currently evaluating the efficacy of this steroid supplementation paradigm at reversing age-associated disorders, including perturbed sleep-wake cycles and cognitive decline as well as impaired immune response.
Keywords: dehydroepiandrosterone, oestradiol, testosterone, circadian rhythms
Introduction
Rhesus macaques (Macaca mulatta) have many attributes that make them suitable for translational neuroendocrine studies. Like humans, they are large, diurnal, long-lived primates, with similar brain morphology and organization of key neuroendocrine systems. Additionally, during aging they show many similar changes in their physiology, cognition, behavior, and immune function as elderly humans (1). Further, rhesus macaques can be readily maintained under highly controlled environmental conditions (e.g., photoperiod, ambient temperature, diet, and medication), and they can yield high-quality postmortem tissue from scheduled necropsies. Consequently, they represent a pragmatic animal model in which to investigate the neuroendocrine mechanisms that underlie normal and pathological human aging. In this review we highlight some of the key similarities between the neuroendocrine systems of rhesus macaques and humans, and focus on novel insights that have been gained from using this translational nonhuman primate model in aging research.
Neuroendocrine aging in humans and nonhuman primates
Communication between different organ systems is essential for normal physiological functions, and this relies on neuronal as well as endocrine signaling. From the perspective of primate aging, many steroid hormones from the gonads (oestradiol, progesterone, and testosterone) and adrenal gland (cortisol and dehydroepiandrosterone [DHEA]) are particularly important (2). Not only do they show marked age-related changes in their circulating levels but many of them show attenuation of their 24-hour pattern of release (3, 4), which may contribute to the etiology of perturbed circadian rhythms such as the sleep-wake cycle. In turn, lack of sleep has been linked to poor cognitive performance and deficits in attention and executive function (5), as well as to impaired immune response (6–9). Although the underlying causal mechanism is still poorly understood, human studies have recently shown that insufficient sleep can significantly affect the expression of genes associated with inflammatory, immune and stress responses, amongst other biological processes (10).
The hypothalamic-pituitary-gonadal (HPG) axis and the hypothalamic-pituitary-adrenal (HPA) axis of humans and rhesus macaques are remarkably similar. Within the HPG axis, gonadotropin-releasing hormone (GnRH) serves as the primary neuroendocrine link between the brain and the anterior pituitary gland, stimulating the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Interestingly, rhesus macaques and humans are one of the few mammalian species in which two distinct molecular forms of GnRH have been identified (GnRH-I and GnRH-II), suggesting that different subpopulations of GnRH neurons contribute differentially to the regulation of reproductive function in primates (11). The two pituitary gonadotropins in turn stimulate gametogenesis and sex-steroid hormone production within the ovary (oestradiol and progesterone) and testis (testosterone). The coordinated release of these reproductive hormones is essential for the onset of puberty and for the subsequent maintenance of fertility in adults, as well as for the development and maintenance of secondary sexual characteristics and other physiological functions. The similarity between the HPG axis of women and adult female rhesus macaques is emphasized by similar hormonal changes that occur across the menstrual cycle, and the subsequent precipitous loss of sex-steroid output after menopause (12, 13) (Fig. 1), which is thought to be triggered by loss of ovarian follicles (14, 15), as well as reduced responsiveness of the hypothalamus to GnRH and estrogen feedback (16–18). This decline in sex-steroid levels is associated with a decrease in the output of other ovarian hormones, such as anti-Müllerian hormone and Inhibin B, and a concomitant increase in circulating LH and FSH levels due to the loss of negative feedback to the hypothalamus and pituitary gland (13, 19). This age-related change within the primate HPG axis differs greatly from that observed in rodents, in which the first sign of reproductive senescence appears to be an attenuation of GnRH signaling (20, 21), resulting in a dampened and delayed preovulatory LH surge (22, 23), further supporting the use of the nonhuman primate as model for human aging over that of the rodent.
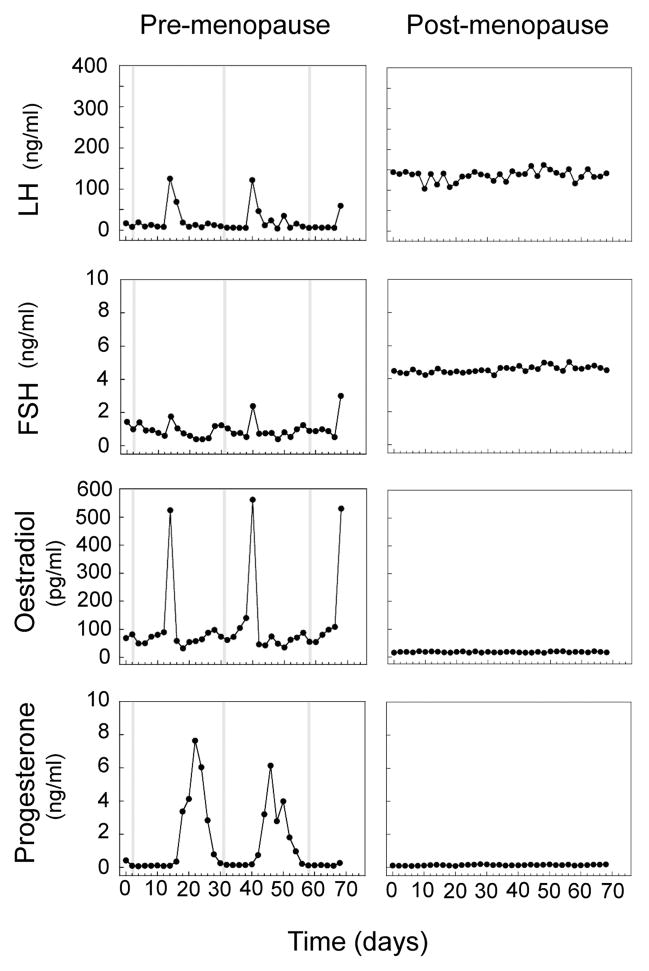
Fig. 1.
Reproductive serum hormone profiles of representative pre-menopausal and post-menopausal rhesus macaques. Plasma oestradiol, progesterone, LH, and FSH values were determined every two days for ~70 consecutive days. Shaded vertical bars represent days of menstruation. Note the marked attenuation of circulating oestradiol and progesterone levels after menopause, with a compensatory increase in LH and FSH levels. Figure adapted from Downs and Urbanski (13).
Although male primates show an age-related decline in circulating testosterone levels, this is generally very gradual and less extreme than the precipitous decrease of oestradiol observed in females around the time of menopause (4, 24, 25). What makes detection of age-related changes in testosterone output particularly difficult to study, however, is its episodic pattern of release, which is driven by pulsatile secretion of GnRH and LH every few hours. In addition, circulating testosterone levels are characterized by a distinctive 24-hour release pattern, which means that single measurements of testosterone in the circulation are unreliable indicators of overall testosterone output. To overcome this problem in rhesus macaques, we have used a remote blood sampling system to serially collect blood samples from young and old males across the 24-hour day (26), and have detected a significant age-related decline (Fig. 2A). This was reflected as a significant age-associated decrease in the overall mean, maximum, and minimum testosterone levels (4), similar to circadian sampling studies previously reported in humans (27, 28). It should be emphasized, however, that the attenuated testosterone levels of the old males were still considerably higher than those typically observed before puberty (29), and so the physiological impact of declining testosterone levels during normal aging is unclear.
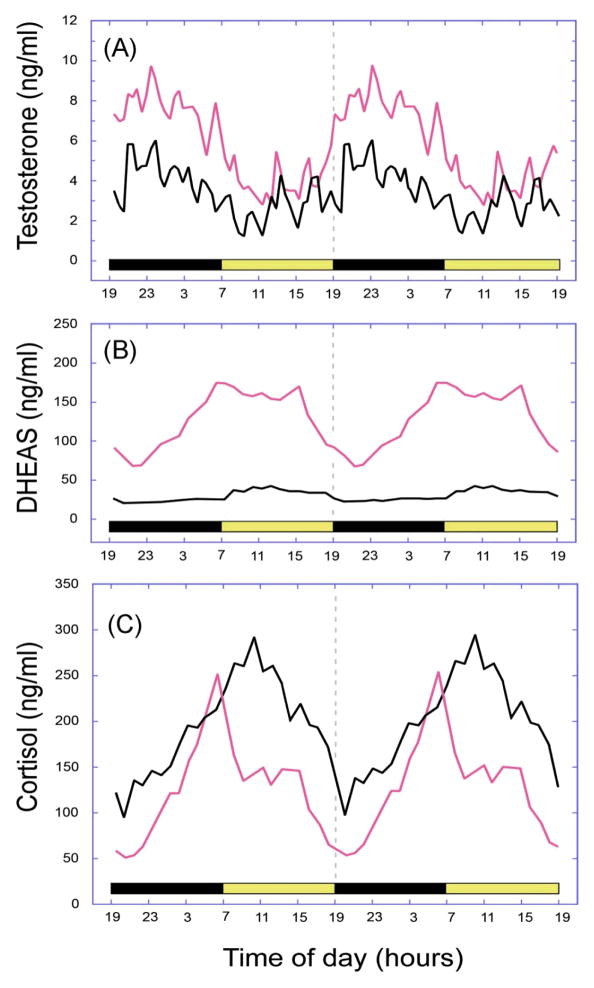
Fig. 2.
Age-related changes in the 24-h plasma concentrations of (A) testosterone, (B) dehydroepiandrosterone sulphate (DHEAS), and (C) cortisol in male rhesus macaques. The panels depict mean hormone profiles from 10 adult (~10 years, shown in red) and 10 aged (~26 years, shown in black) animals, and the horizontal light and dark bars correspond to the 12L:12D lighting schedule; note that the profiles have been double plotted to facilitate observation of day–night differences. Each hormone showed a distinct 24-h rhythm, with a peak occurring either during the night (testosterone) or early in the morning (cortisol and DHEAS). Both testosterone and DHEAS showed a significant age-related attenuation in the plasma levels whereas cortisol showed a significant increase. Figure adapted from Urbanski and Sorwell (9).
Within the HPA axis, corticotrophin-releasing hormone (CRH) from the paraventricular nucleus of the hypothalamus stimulates the release of adrenocorticotrophic hormone (ACTH) from the anterior pituitary gland. In turn, ACTH signals the adrenal cortex to release cortisol from the zona fasciculata and dehydroepiandrosterone (DHEA) from the zona reticularis. Under normal circumstances, cortisol exerts negative feedback on both the hypothalamus and pituitary to reduce secretion of CRH and ACTH, respectively. While acute increases in cortisol can be adaptive in times of stress, prolonged increases can result in hippocampal excitotoxicity (30–32) and oxidative damage (32, 33). In addition, high levels of cortisol can decrease hippocampal volume and interfere with the structural changes necessary for learning and memory (34–37). As the hippocampus, itself, responds to glucocorticoids by exerting additional negative feedback on the hypothalamus, these changes result in a disruption of HPA axis activity and further elevations in cortisol (35). DHEA, meanwhile, can act as a “functional antagonist” of cortisol (38, 39), in part by promoting neuronal and glial survival (40, 41). An increase in circulating cortisol with advanced age has been observed in both humans (42, 43) and nonhuman primates (44), with a concurrent marked decline in DHEAS (the sulphated form of DHEA) throughout adulthood (44–47) (Figs. 2B, 2C). This resulting increase in the cortisol:DHEA ratio may have drastic implications for many physiological process, including learning and memory (38, 39), a view that is supported by the finding that higher cortisol:DHEA ratios are associated with greater cognitive impairment (48, 49).
Impact of age-related hormonal changes on cognitive function
Close associations exist between age-related hormonal changes and cognitive decline, even in healthy individuals (50, 51). Consequently, extensive studies have examined not only the effect of steroid hormone deprivation on learning and memory, but also the therapeutic potential of hormone supplementation. Circulating testosterone levels show an age-related decline in men (52–54), as well as in male nonhuman primates (4, 24, 25), and aged men with higher levels of endogenous testosterone exhibit greater cognitive performance (55–57). Additionally, testosterone supplementation in men with low endogenous testosterone levels has been shown to improve certain aspects of cognitive function (58–60). Although the exact mechanism is unclear, it is possible that the beneficial effects of supplementation are mediated by conversion of testosterone to oestradiol. This rationale is based on the observation that men undergoing androgen deprivation therapy experience cognitive deficits that can be rescued by oestradiol supplementation (61), and that aromatization of testosterone to oestradiol appears to be necessary for some of the cognitive benefits of testosterone supplementation (62). In women, oestradiol deprivation is associated with cognitive impairments (63, 64), which can be overcome with oestrogen therapy (64, 65). As emphasized in Figure 2, the adrenal steroids cortisol and DHEAS both have significant age-related patterns of secretion, and the endogenous cortisol:DHEA ratio is associated with cognitive performance in aged humans (48, 49). Interestingly, however, there is little evidence from clinical studies that DHEA supplementation incurs cognitive benefits in the elderly (66–71), despite improvements in episodic memory performance in young men (72). High endogenous levels of cortisol alone have also been associated with cognitive impairment in both middle age (73) and old age (74–76).
Circadian perturbations associated with age
Rhesus macaques, like humans, are diurnal and generally confine their eating and activity to daylight hours. Although the underlying mechanism is still unclear, there is a wealth of evidence showing that the suprachiasmatic nucleus (SCN) of the hypothalamus plays a major role in controlling circadian rhythms in mammals (77, 78). Furthermore, there is now evidence from several species including the rhesus macaque that many peripheral organs, such as the pituitary and adrenal glands, also express circadian clock mechanisms (79–81). Consequently, the prevailing view is that human circadian physiology is regulated by a hierarchical clock mechanism, involving a master oscillator in the SCN and numerous subordinate peripheral oscillators. It is also clear that the SCN receives photoperiodic information from the retina, which it uses as a primary Zeitgeber to synchronize its circadian rhythm with that of the external environment. The mechanism responsible for synchronizing individual peripheral oscillators remains to be elucidated, but it is likely to involve both neural and hormonal cues. Indeed, circadian hormone rhythms have been implicated in the control of many human behaviors, including learning and memory (82–84), and aging-related changes in the peaks and/or phase relationships may interfere with cognitive processes (5, 82, 85–88). Given that many steroid hormones, show clear-cut 24-hour rhythms (e.g., Fig. 2), it is plausible that an age-related attenuation of these rhythms contributes to age-related perturbed sleep-wake cycles as well as other pathologies (8, 9); note that in humans and rhesus macaques circulating cortisol levels do not decline during aging but the age-related increase in basal cortisol levels means that there is less circadian information being relayed from the adrenal gland to other peripheral targets, such as the liver and muscle (78).
Intracrinology and the role of precursor steroids
Thus far we have discussed the impact of gonadal steroids on physiology; however, in primates the gonads are not the sole source of androgens and oestrogens. Young adult humans and rhesus macaques have characteristically very high circulating levels of the adrenal steroid hormone precursor DHEA, and many tissues are capable of locally converting DHEA into active steroids such as testosterone or oestradiol - a phenomenon termed “intracrinology” (89). Due to the possible local intracrine conversion of DHEA to testosterone and oestradiol (Fig. 3), circulating levels of these sex steroids may not accurately reflect the amount of hormone acting within individual tissues. This further introduces a mechanism by which individual tissues can titrate the amount of active hormone they are exposed to. In conditions of low oestradiol, for example, expression of the enzyme aromatase increases within the rhesus macaque hippocampus as compared to conditions of high oestradiol (46), potentially compensating for decreased oestradiol of ovarian origin. This mechanism also provides an additional potential target for hormone replacement therapy, as supplementation of DHEA may be useful to increase local oestrogen levels without impacting circulating oestrogen, thus reducing the risks of negative side effects associated with oestrogen replacement therapy.
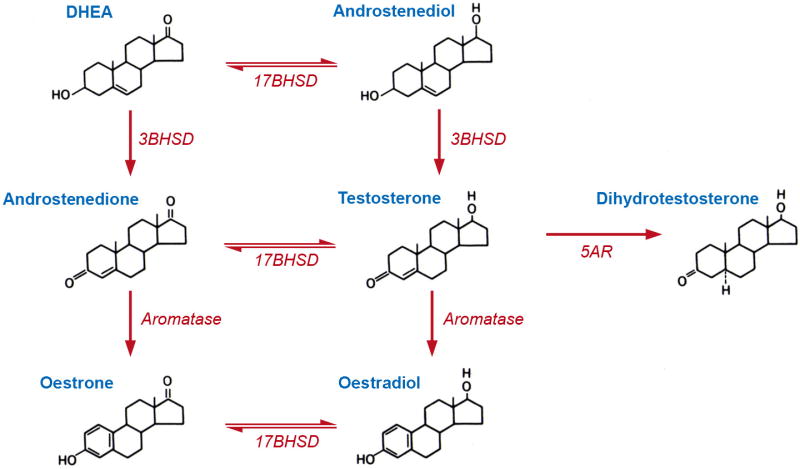
Fig. 3.
Schematic showing the biochemical conversion of dehydroepiandrosterone (DHEA) to oestradiol and dihydroxytestosterone (DHT). Genes coding for the key converting enzymes are depicted in italics. 3BHSD = 3β-hydroxysteroid dehydrogenase; 17BHSD = 17β-hydroxysteroid dehydrogenase, also known as 17-ketosteroid oxidoreductase. Importantly, tissues that express the key converting enzymes have the potential to activate oestrogen and androgen receptors, using DHEA as a sex-steroid hormone precursor.
Intracrine conversion of DHEA to oestradiol appears to be involved in mediating actions of exogenous DHEA within the rodent hippocampus, because administration of DHEA increases spine synapse density and the effect can be blocked with letrozole, an aromatase inhibitor (90). We have previously shown that the rhesus macaque hippocampus expresses all of the key enzymes (Fig. 3) necessary to convert DHEA to oestradiol (46), suggesting that a similar intracrine mechanism may be operative in primates. To test the hypothesis that DHEA improves cognition though conversion to oestradiol, we recently supplemented reproductively-intact peri-menopausal female rhesus macaques with daily oral DHEA. The animals were tested while on and off treatment, allowing for a within-subjects examination of DHEA’s effect. Despite promising evidence from rodent studies that DHEA improves cognitive performance (91–93), we saw no improvement of performance in a delayed match-to-sample test or a spatial delayed response task (94, 95). While the lack of an effect is discouraging, it does contribute to the validation of the aged macaque as a model for human aging, as the finding is consistent with the plethora of clinical studies that have failed to observe cognitive benefit of DHEA supplementation in aged men or women (56–71).
Given that DHEA can exert pro-cognitive effects in the rodent brain (via conversion to oestradiol), and cognitive areas of the primate brain express the enzymes required for the DHEA-to-oestradiol conversion, it is puzzling why there is no obvious benefit of DHEA supplementation on cognition in humans and nonhuman primates. One possibility is that there is a significant age-related dampening of the intracrine mechanism within these brain areas. Consequently, a decline in expression or action of any of the enzymes involved in the conversion of DHEA to oestradiol (3β-hydroxysteroid dehydrogenase [3BHSD], 17β-hydroxysteroid dehydrogenase [17BHSD], and aromatase) (Fig. 3) would result in decreased ability to perform such a conversion. Indeed, the discordance between our findings and previous reports of improved cognitive performance in aged female macaques treated with oestradiol (96) suggests that this intracrine conversion may be attenuated during aging. To examine this possibility we investigated hippocampal expression of the key enzymes across the life span of rhesus macaques, and observed a significant age-related decrease in expression of 3BHSD, an enzyme responsible for the conversion of DHEA to androstenedione and androstenediol to testosterone (Fig. 3). Importantly, this decrease in 3BHSD was observed in animals that were within the age range used in the DHEA supplementation study (90, 95). Thus we have identified three steroidal mechanisms by which aging may impact cognition: 1) an age-related decline of gonadal oestradiol, 2) a decline in adrenal DHEA production that serves as the intracrine precursor to oestradiol, and 3) a decline in the ability of the hippocampus to synthesize oestradiol from circulating DHEA.
Androgen supplementation in the aged rhesus macaque
Although an age-related decline in 3BHSD expression in cognitive brain areas could account for the disappointing findings from clinical DHEA supplementation studies, potential cognitive benefits may still be possible from other steroid hormone supplementations, at least in males. Our rhesus macaque study (90) found that hippocampal aromatase gene expression was maintained even into old age, suggesting that while DHEA may no longer be efficiently converted to oestradiol, testosterone may still serve as a beneficial intracrine supplement (Fig. 3). Indeed, in human studies, testosterone supplementation has been shown to increase spatial performance (97–99), and administration of oestradiol can rescue cognitive deficits induced by androgen deprivation in men (61); this suggests that the effects of endogenous testosterone on at least some aspects of cognition may depend on conversion to oestradiol. In fact, the benefits of testosterone on verbal memory have been shown to require aromatization to oestradiol (62), giving further support to the hypothesis that intracrine conversion of steroids within the brain helps to maintain cognitive performance during aging.
Conclusions
Oestrogen hormone therapy (HT) has been available for many years and has demonstrated efficacy in alleviating symptoms associate with post-menopausal disorders. On the other hand, increasing concern about possible side-effects of long-term HT has provided the impetus for developing safer HT paradigms, especially ones that do not include oestrogens. The rhesus macaque HPG and HPA neuroendocrine axes are remarkably similar to those of the human, showing the same age-associated changes. This makes the rhesus macaque a pragmatic animal model in which to investigate mechanisms that underlie normal and pathological human aging, and to develop more effective therapies, such as the administration of safer precursor hormones (89) or non-feminizing oestrogens (100–102). So far, data from old female rhesus macaques have failed to show significant beneficial effects of DHEA supplementation on cognitive function, which is in general agreement with human studies and in contrast with rodent studies. In part this is likely to be due to an aging associated dampening of the intracrine mechanism responsible for converting DHEA to sex steroids. It is also possible that existing hormone supplementation paradigms do not adequately mimic the endogenous circadian hormone profiles and so are less effective, or even detrimental. To explore this possibility, we have recently initiated a study involving old male rhesus macaques, in which we are assessing the efficacy of androgen supplementation on a wide range of physiological functions, including sleep-wake cycles, cognition and immune function (94). What makes this study especially pertinent is that the daily combined testosterone-DHEA supplementation paradigm not only raises mean circulating levels of DHEAS, testosterone, dihydrotestosterone (DHT), and oestradiol to juvenile levels, it also preserves the normal 24-hour pattern of these hormones in the circulation (Fig. 2). Thus testosterone, oestradiol, and DHT levels of androgen-supplemented old males continue to show a peak during the night, and DHEAS levels continue to show a peak in the morning (unpublished observations). Given that many primate behaviors and physiological functions have strong circadian components (8, 9), we anticipate that more physiological hormone supplementation paradigms may prove to be safer and more efficacious at treating disorders in the elderly.
Acknowledgments
This research was supported by the following grants from the National Institutes of Health: AG-023477, AG-029612, AG-036670, HD-007133 and OD-011092.
References
- 1.Messaoudi I, Urbanski HF, Kohama SG. Integrative models of aging in the nonhuman primate. In: Williams RM, editor. Monkeys: Biology, Behavior and Disorders. Chapter 1. Hauppauge: Nova Science Publishers; 2011. pp. 1–54. [Google Scholar]
- 2.Conrad CD, Bimonte-Nelson HA. Impact of the hypothalamic-pituitary-adrenal/gonadal axes on trajectory of age-related cognitive decline. Prog Brain Res. 2010;182:31–76. doi: 10.1016/S0079-6123(10)82002-3. [DOI] [PubMed] [Google Scholar]
- 3.Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–129. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- 4.Faraut B, Boudjeltia KZ, Vanhamme L, Kerkhofs M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med Rev. 2012;16:137–149. doi: 10.1016/j.smrv.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Reid KJ, McGee-Koch LL, Zee PC. Cognition in circadian rhythm sleep disorders. Prog Brain Res. 2011;190:3–20. doi: 10.1016/B978-0-444-53817-8.00001-3. [DOI] [PubMed] [Google Scholar]
- 6.Haley GE, Landauer N, Renner L, Weiss A, Hooper K, Urbanski HF, Kohama SG, Neuringer M, Raber J. Circadian activity associated with spatial learning and memory in aging rhesus monkeys. Exp Neurol. 2009;217:55–62. doi: 10.1016/j.expneurol.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haley GE, Urbanski HF, Kohama SG, Messaoudi I, Raber J. Spatial memory performance associated with measures of immune function in elderly female rhesus macaques. Eur Geriatr Med. 2011;2:117–121. doi: 10.1016/j.eurger.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urbanski HF. Role of circadian neuroendocrine rhythms in the control of behavior and physiology. Neuroendocrinology. 2011;93:211–222. doi: 10.1159/000327399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urbanski HF, Sorwell KG. Age-related changes in neuroendocrine rhythmic function in the rhesus macaque. Age (Dordr) 2012;34:1111–1121. doi: 10.1007/s11357-011-9352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Möller-Levet CS, Archer SN, Bucca G, Laing EE, Slak A, Kabiljo R, Lo JC, Santhi N, von Schantz M, Smith CP, Dijk DJ. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proc Soc Natl Acad Sci USA. 2013;110:E1132–1141. doi: 10.1073/pnas.1217154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urbanski HF. Differential roles of GnRH-I and GnRH-II neurons in the control of the primate reproductive axis. Front Endocrinol (Lausanne) 2012;3:20. doi: 10.3389/fenod.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall JE. Neuroendocrine changes with reproductive aging in women. Semin Reprod Med. 2007;25:344–351. doi: 10.1055/s-2007-984740. [DOI] [PubMed] [Google Scholar]
- 13.Downs JL, Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macaca mulatta) Biol Reprod. 2006;75:539–546. doi: 10.1095/biolreprod.106.051839. [DOI] [PubMed] [Google Scholar]
- 14.Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65:1231–1237. doi: 10.1210/jcem-65-6-1231. [DOI] [PubMed] [Google Scholar]
- 15.Richardson SJ, Nelson JF. Follicular depletion during the menopausal transition. Ann N Y Acad Sci. 1990;592:13–20. doi: 10.1111/j.1749-6632.1990.tb30312.x. [DOI] [PubMed] [Google Scholar]
- 16.Weiss G, Skurnick JG, Goldsmith LT, Santoro NG, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA. 2004;292(24):2991–2996. doi: 10.1001/jama.292.24.2991. [DOI] [PubMed] [Google Scholar]
- 17.Shaw ND, Srouji SS, Histed SN, McCurnin KE, Hall JE. Aging attenuates the pituitary response to gonadotropin-releasing hormone. J Clin Endocrinol Metab. 2009;94:3259–3264. doi: 10.1210/jc.2009-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang K, Zeitlian G, Adel G, Santoro NF, Pal L. Enhanced hypothalamic-pituitary sensitivity to estrogen in premenopausal women with diminished ovarian reserve compared with older perimenopausal controls. Menopause. 2011;18:880–885. doi: 10.1097/gme.0b013e31820cc564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramezani Tehrani F, Solaymani-Dodaran M, Tohidi M, Reza Gohari M, Azizi F. Modeling age at menopause using serum concentration of anti-Müllerian hormone. J Endocrinol Metab. 2013;98:729–735. doi: 10.1210/jc.2012-3176. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd JM, Hoffman GE, Wise PM. Decline in immediate early gene expression in gonadotropin-releasing hormone neurons during proestrus in regularly cycling, middle-aged rats. Endocrinology. 1994;134:1800–1805. doi: 10.1210/endo.134.4.8137745. [DOI] [PubMed] [Google Scholar]
- 21.Wise PM, Smith MJ, Dubal DB, Wilson ME, Rau SW, Cashion AB, Böttner M, Rosewell KL. Neuroendocrine modulation and repercussions of female reproductive aging. Recent Prog Horm Res. 2002;57:235–256. doi: 10.1210/rp.57.1.235. [DOI] [PubMed] [Google Scholar]
- 22.Wise PM. Alterations in proestrous LH, FSH, and prolactin surges in middle-aged rats. Proc Soc Exp Biol Med. 1982;169:348–354. doi: 10.3181/00379727-169-41356. [DOI] [PubMed] [Google Scholar]
- 23.Downs JL, Wise PM. The role of the brain in female reproductive aging. Mol Cell Endocrinol. 2009;299:32–38. doi: 10.1016/j.mce.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sitzmann BD, Brown DI, Garyfallou VT, Mattison JA, Ingram DK, Roth GS, Ottinger MA, Urbanski HF. Impact of moderate calorie restriction on testicular morphology and endocrine function in adult rhesus macaques (Macaca mulatta) Age (Dordr) 2013 doi: 10.1007/s11357-013-9563-6. (in press). doi 10.1007/s 11357-013-9563-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlatt S, Pohl CR, Ehmcke J, Ramaswamy S. Age-related changes in diurnal rhythms and levels of gonadotropins, testosterone, and inhibin B in male rhesus monkeys (Macaca mulatta) Biol Reprod. 2008;79:93–99. doi: 10.1095/biolreprod.107.066126. [DOI] [PubMed] [Google Scholar]
- 26.Urbanski HF. Circadian variation in the physiology and behavior of humans and nonhuman primates. In: Raber J, editor. Animal Models of Behavioral Analysis, Neuromethods 50. Chapter 9. NY: Humana Press; 2011. pp. 217–235. [Google Scholar]
- 27.Bremner WJ, Vitiello MB, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278–1281. doi: 10.1210/jcem-56-6-1278. [DOI] [PubMed] [Google Scholar]
- 28.Plymate SR, Tenover JS, Bremner WJ. Circadian variation in testosterone, sex hormone-binding globulin, and calculated non-sex hormone-binding globulin bound testosterone in healthy young and elderly men. J Andol. 1989;10:366–371. doi: 10.1002/j.1939-4640.1989.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 29.Urbanski HF, Pau KY. A biphasic developmental pattern of circulating leptin in the male rhesus macaque (Macaca mulatta) Endocrinology. 1998;139:2284–2286. doi: 10.1210/endo.139.5.5962. [DOI] [PubMed] [Google Scholar]
- 30.Moghaddam B, Boliano ML, Stein-Behrens B, Sapolsky R. Glucocorticoids mediate the stress-induced extracellular accumulation of glutamate. Brain Res. 1994;655:251–254. doi: 10.1016/0006-8993(94)91622-5. [DOI] [PubMed] [Google Scholar]
- 31.Goodman Y, Bruce AJ, Cheng B, Mattson MP. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid beta-peptide toxicity in hippocampal neurons. J Neurochem. 1996;66:1836–1844. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- 32.Schubert MI, Kalisch R, Sotiropoulos I, Cataia C, Sousa N, Almeida OF, Auer DP. Effects of altered corticosteroid milieu on rat hippocampal neurochemistry and structure—an in vivo magnetic resonance spectroscopy and imaging study. J Psychiatr Res. 2008;42:902–912. doi: 10.1016/j.jpsychires.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Sato H, Takahashi T, Sumitani K, Takatsu H, Urano S. Glucocorticoid generates ROS to induce oxidative injury in the hippocampus, leading to impairment of cognitive function of rats. J Clin Biochem Nutr. 2010;47:224–232. doi: 10.3164/jcbn.10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conrad CD. Chronic stress-induced hippocampal vulnerability: the glucocorticoids vulnerability hypothesis. Rev Neurosci. 2008;19:395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tata DA, Marciano VA, Anderson BJ. Synapse loss from chronically elevated glucocorticoids: relationship to neuropil volume and cell number in hippocampal area CA3. J Comp Neurol. 2006;498:363–374. doi: 10.1002/cne.21071. [DOI] [PubMed] [Google Scholar]
- 36.Schloesser RJ, Maji HK, Martinowich K. Suppression of adult neurogenesis leads to an increased hypothalamo-pituitary-adrenal axis response. Neuroreport. 2009;20:553–557. doi: 10.1097/WNR.0b013e3283293e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tata DA, Anderson BJ. The effects of chronic glucocorticoids exposure on dendritic length, synapse numbers and glial volume in animal models: implications for hippocampal volume reductions in depression. Physiol Behav. 2010;99:186–193. doi: 10.1016/j.physbeh.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23:921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 39.Ferrari E, Magri F. Role of neuroendocrine pathways in cognitive decline during aging. Ageing Res Rev. 2008;7:225–233. doi: 10.1016/j.arr.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Roberts E, Bologa L, Flood JF, Smith GE. Effects of dehydroepiandrosterone and its sulfate on brain tissue in culture and on memory in mice. Brain Res. 1987;406:357–362. doi: 10.1016/0006-8993(87)90807-9. [DOI] [PubMed] [Google Scholar]
- 41.Bologa L, Sharma J, Roberts E. Dehydroepiandrosterone and its sulfated derivative reduce neuronal death and enhance astrocytic differentiation in brain cell cultures. J Neurosci Res. 1987;17:225–234. doi: 10.1002/jnr.490170305. [DOI] [PubMed] [Google Scholar]
- 42.Dodt C, Theine J, Uthgenannt D, Born J, Fehm HL. Basal secretory activity of the hypothalamo-pituitary-adrenocortical axis is enhanced in healthy elderly. An assessment during undisturbed night-time sleep. Eur J Endocrinol. 1994;131:443–450. doi: 10.1530/eje.0.1310443. [DOI] [PubMed] [Google Scholar]
- 43.Deuschle M, Gotthardt U, Schweiger U, Weber B, Körner A, Schmider J, Standhardt H, Lammers CH, Heuser I. With aging in humans the activity of the hypothalamus-pituitary-adrenal system increases and its diurnal amplitude flattens. Life Sci. 1997;61:2239–2246. doi: 10.1016/s0024-3205(97)00926-0. [DOI] [PubMed] [Google Scholar]
- 44.Downs JL, Mattison JA, Ingram DK, Urbanski HF. Effect of age and caloric restriction on circadian adrenal steroid rhythms in rhesus macaques. Neurobiol Aging. 2008;29:1412–1422. doi: 10.1016/j.neurobiolaging.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Labrie F, Bélanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;82:2396–2402. doi: 10.1210/jcem.82.8.4160. [DOI] [PubMed] [Google Scholar]
- 46.Sorwell KG, Kohama SG, Urbanski HF. Perimenopausal regulation of steroidogenesis in the nonhuman primate. Neurobiol Aging. 2012;33:1487.e1–13. doi: 10.1016/j.neurobiolaging.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urbanski HF, Mattison JA, Roth GS, Ingram DK. Dehydroepiandrosterone sulfate (DHEAS) as an endocrine marker of aging in calorie restriction studies. Exp Gerontol. 2013 doi: 10.1016/j.exger.2013.01.001. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalmijn S, Launer LJ, Stolk RP, de Jong FH, Pols HAS, Hofman A, Breteler M, Lamberts SW. A prospective study on cortisol, dehydroepiandrosterone sulfate, and cognitive function in the elderly. J Clin Endocrinol Metab. 1998;83:3487–3492. doi: 10.1210/jcem.83.10.5164. [DOI] [PubMed] [Google Scholar]
- 49.Van Niekerk JK, Huppert FA, Herbert J. Salivary cortisol and DHEA: association with measures of cognition and well-being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrinology. 2001;26:591–612. doi: 10.1016/s0306-4530(01)00014-2. [DOI] [PubMed] [Google Scholar]
- 50.Holland J, Bandelow S, Hogervorst E. Testosterone levels and cognition in elderly men: a review. Maturitas. 2011;69:322–337. doi: 10.1016/j.maturitas.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 51.Zimmerman ME, Lipton RB, Santoro N, McConnell DS, Derby CA, Katz MJ, Baigi K, Saunders-Pullman R. Endogenous estradiol is associated with verbal memory in nondemented older men. Brain Cogn. 2011;76:158–165. doi: 10.1016/j.bandc.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plymate SR, Tenover JS, Bremner WJ. Circadian variation in testosterone, sex hormone-binding globulin, and calculated non-sex hormone binding globulin bound testosterone in healthy youn and elderly men. J Androl. 1989;10:366–371. doi: 10.1002/j.1939-4640.1989.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 53.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 54.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 55.Muller M, Aleman A, Grovvee DE, de Haan EH, van der Schouw YT. Endogenous sex hormone levels and cognitive function in aging men: is there an optimal level? Neurology. 2005;64:866–871. doi: 10.1212/01.WNL.0000153072.54068.E3. [DOI] [PubMed] [Google Scholar]
- 56.Barret-Connor E, Goodman-Gruen D, Patay B. Endogenous sex hormones and cognitive function in older men. J Clin Endocrinol Metab. 1999;84:3681–3685. doi: 10.1210/jcem.84.10.6086. [DOI] [PubMed] [Google Scholar]
- 57.Yaffe K, Lui LY, Zmuda J, Cauley J. Sex hormones and cognitive function in older men. J Am Geriatr Soc. 2002;50:707–712. doi: 10.1046/j.1532-5415.2002.50166.x. [DOI] [PubMed] [Google Scholar]
- 58.Kenny AM, Bellantonio S, Gruman CA, Acosta RD, Prestwood KM. Effects of transdermal testosterone on cognitive function and health perception in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2002;57:M321–M325. doi: 10.1093/gerona/57.5.m321. [DOI] [PubMed] [Google Scholar]
- 59.Janowsky JS, Chavez B, Orwoll E. Sex steroids modify working memory. J Cogn Neurosci. 2000;12:407–414. doi: 10.1162/089892900562228. [DOI] [PubMed] [Google Scholar]
- 60.Beer TM, Bland LB, Bussiere JUR, Neiss MB, Wersinger EM, Garotto M, Ryan CW, Janowsky JS. Testosterone loss and estradiol administration modify memory in men. J Urol. 2006;175:130–135. doi: 10.1016/S0022-5347(05)00049-2. [DOI] [PubMed] [Google Scholar]
- 61.Holland J, Bandelow S, Hogervorst E. Testosterone levels and cognition in elderly men: a review. Maturitas. 2011;69:322–337. doi: 10.1016/j.maturitas.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 62.Cherrier MM, Matsumoto AM, Amory JK, Ahmed S, Bremner W, Peskind ER, Raskind MA, Johnson M, Craft S. The role of aromatization in testosterone supplementation: effects on cognition in older men. Neurology. 2005;64:290–296. doi: 10.1212/01.WNL.0000149639.25136.CA. [DOI] [PubMed] [Google Scholar]
- 63.Farraq AK, Khedr EM, Abdel-Aleem H, Rageh TA. Effect of surgical menopause on cognitive functions. Dement Geriatr Cogn Disord. 2002;13:193–198. doi: 10.1159/000048652. [DOI] [PubMed] [Google Scholar]
- 64.Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically postmenopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- 65.Rocca WA, Groassardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res. 2011;1379:188–198. doi: 10.1016/j.brainres.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sorwell KG, Urbanski HF. Dehydroepiandrosterone and age-related cognitive decline. Age (Dordr) 2010;32:61–67. doi: 10.1007/s11357-009-9113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolf OT, Neumann O, Hellhammer DH, Geiben AC, Strasburger CJ, Dressendorfer RA, Pirke KM, Kirschbaum C. Effects of a two-week physiological dehydroepiandrosterone substitution on cognitive performance and well-being in healthy elderly women and men. J Clin Endocrinol Metab. 1997;82:2363–2367. doi: 10.1210/jcem.82.7.4056. [DOI] [PubMed] [Google Scholar]
- 68.Wolf OT, Kirschbaum C. Actions of dehydroepiandrosterone and its sulfate in the central nervous system: effects on cognition and emotion in animals and humans. Brain Res Rev. 1999;30:264–288. doi: 10.1016/s0165-0173(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 69.Arlt W, Callies F, Koehler I, van Vlijmen JC, Fassnacht M, Strasburger CJ, Seibel MJ, Huebler D, Ernst M, Oettel M, Reincke M, Schulte HM, Allolio B. Dehydroepiandrosterone supplementation in healthy men with an age-related decline of dehydroepiandrosterone secretion. J Clin Endocrinol Metab. 2001;86:4686–4692. doi: 10.1210/jcem.86.10.7974. [DOI] [PubMed] [Google Scholar]
- 70.Grimley Evans J, Malouf R, Huppert F, van Niekerk JK. Dehydroepiandrosterone (DHEA) supplementation for cognitive function in healthy elderly people. Cochrane Database Syst Rev. 2006;18:CD006221. doi: 10.1002/14651858.CD006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kritz-Silverstein D, von Muhlen D, Laughlin GA, Bettencourt R. Effects of dehydroepiandrosterone supplementation on cognitive function and quality of life: the DHEA and Well-Ness (DAWN) Trial. J Am Geriatr Soc. 2008;56:1292–1298. doi: 10.1111/j.1532-5415.2008.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alhaj HA, Massey AE, McAllister-Williams RH. Effects of DHEA administration on episodic memory cortisol and mood in healthy young men: a double-blind, placebo-controlled study. Psychopharmacology (Berl) 2006;188:541–551. doi: 10.1007/s00213-005-0136-y. [DOI] [PubMed] [Google Scholar]
- 73.Geoffroy MC, Hertzman C, Li L, Power C. Morning salivary cortisol and cognitive function in mid-life: evidence from a population-based birth cohort. Psychol Med. 2012;42:1763–1773. doi: 10.1017/S0033291711002704. [DOI] [PubMed] [Google Scholar]
- 74.Li G, Cherrier MM, Tsuang DW, Petrie EC, Colasurdo EA, Craft S, Scellenberg GD, Peskind ER, Raskind MA, Wilkinson CW. Salivary cortisol and memory function in human aging. Neurobiol Aging. 2006;27:1705–1714. doi: 10.1016/j.neurobiolaging.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 75.Lee BK, Glass TA, McAtee MJ, Wand GS, Bandeen-Roche K, Bolla KI, Schwartz BS. Associations of salivary cortisol with cognitive function in the Baltimore memory study. Arch Gen Psychiatry. 2007;64:810–818. doi: 10.1001/archpsyc.64.7.810. [DOI] [PubMed] [Google Scholar]
- 76.Comijs HC, Gerritsen L, Penninx BW, Bremmer MA, Deeq DJ, Geerlings MI. The association between serum cortisol and cognitive decline in older persons. Am J Geriatr Psychiatry. 2010;18:42–50. doi: 10.1097/JGP.0b013e3181b970ae. [DOI] [PubMed] [Google Scholar]
- 77.Hastings M, O’Neill JS, Maywood ES. Circadian clocks: regulators of endocrine and metabolic rhythms. J Endocrinol. 2007;195:187–198. doi: 10.1677/JOE-07-0378. [DOI] [PubMed] [Google Scholar]
- 78.Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 2012;74:246–260. doi: 10.1016/j.neuron.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 79.Lemos DR, Downs JL, Urbanski HF. Twenty-four-hour rhythmic gene expression in the rhesus macaque adrenal gland. Mol Endocrinol. 2006;20:1164–1176. doi: 10.1210/me.2005-0361. [DOI] [PubMed] [Google Scholar]
- 80.Sitzmann BD, Lemos DR, Ottinger MA, Urbanski HF. Effects of age on clock gene expression in the rhesus macaque pituitary gland. Neurobiol Aging. 2010;31:696–705. doi: 10.1016/j.neurobiolaging.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Urbanski HF, Noriega NC, Lemos DR, Kohama SG. Gene expression profiling in the rhesus macaque: experimental design considerations. Methods. 2009;49:26–31. doi: 10.1016/j.ymeth.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Winocur G, Hasher L. Age and time-of-day-effects on learning and memory in a non-matching-to-sample test. Neurobiol Aging. 2004;25:1107–1115. doi: 10.1016/j.neurobiolaging.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 83.Evans PD, Fredhoi C, Loveday C, Hucklebridge F, Aitchison E, Forte D, Clow A. The diurnal cortisol cycle and cognitive performance in the healthy old. Int J Psychophysiol. 2011;79:371–377. doi: 10.1016/j.ijpsycho.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 84.Evans P, Hucklebridge F, Loveday C, Clow A. The cortisol awakening response is related to executive function in older age. Int J Psychophysiol. 2012;84:201–204. doi: 10.1016/j.ijpsycho.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 85.Issa AM, Rowe W, Cauthier S, Meaney MJ. Hypothalamic-pituitary-adrenal activity in aged, cognitively impaired and cognitively unimpaired rats. J Neurosci. 1990;10:3247–3254. doi: 10.1523/JNEUROSCI.10-10-03247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cochrane A, Robertson IH, Coogan AN. Association between circadian rhythms, sleep and cognitive impairment in healthy older adults: an actigraphic study. J Neural Transm. 2012;119:1233–1239. doi: 10.1007/s00702-012-0802-2. [DOI] [PubMed] [Google Scholar]
- 87.Tranah GJ, Blackwell T, Stone KL, Ancoli-Israel S, Paudel ML, Ensrud KE, Cauley JA, Redline S, Hillier TA, Cummings SR, Yaffe K SOF Research Group. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70:722–732. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schmidt C, Peigneux P, Cajochen C. Age-related changes in sleep and circadian rhythms: impact on cognitive performance and underlying neuroanatomical networks. Front Neurol. 2012;3:118. doi: 10.3389/fneur.2012.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Labrie F. Intracrinology. Mol Cell Endocrinol. 1991;78:C113–C118. doi: 10.1016/0303-7207(91)90116-a. [DOI] [PubMed] [Google Scholar]
- 90.Hajsan T, MacLusky NJ, Leranth C. Dehydroepiandrosterone increases hippocampal spine synapse density in ovariectomized female rats. Endocrinology. 2004;145:1042–1045. doi: 10.1210/en.2003-1252. [DOI] [PubMed] [Google Scholar]
- 91.Flood JF, Roberts E. Dehydroepiandrosterone sulfate improves memory in aging mice. Brain Res. 1988;448:178–181. doi: 10.1016/0006-8993(88)91116-x. [DOI] [PubMed] [Google Scholar]
- 92.Flood JF, Smith GE, Roberts E. Dehydroepiandrosterone and its sulfate enhance memory retention in mice. Brain Res. 1988;447:269–278. doi: 10.1016/0006-8993(88)91129-8. [DOI] [PubMed] [Google Scholar]
- 93.Markowski M, Ungeheuer M, itran D, Locurto C. Memory-enhancing effects of DHEAS in aged mice on a win-shift water escape task. Physiol Behav. 2001;72:521–525. doi: 10.1016/s0031-9384(00)00446-7. [DOI] [PubMed] [Google Scholar]
- 94.Sorwell KG, Garten J, Renner L, Weiss A, Garyfallou VT, Kohama SG, Neuringer M, Urbanski HF. Hormone supplementation during aging: how much and when? Rejuvenation Res. 2012;15:128–131. doi: 10.1089/rej.2011.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sorwell KG, Neuringer M, Renner L, Weiss A, Garten J, Garyfallou VT, Kohama SG, Urbanski HF. Cognitive testing in nonhuman primate models of hormone replacement therapy. Soc Neurosci Abstract. 2012:835.08. [Google Scholar]
- 96.Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behav Neurosci. 1994;108:325–332. doi: 10.1037//0735-7044.108.2.325. [DOI] [PubMed] [Google Scholar]
- 98.Cherrier MM, Asthana S, Plymate S, Baker L, Matsumoto AM, Peskind E, Raskind MA, Brodkin K, Bremner W, Petrova A, LaTendresse S, Craft S. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology. 2001;57:80–88. doi: 10.1212/wnl.57.1.80. [DOI] [PubMed] [Google Scholar]
- 99.Gray PB, Singh AB, Woodhouse LJ, Storer TW, Casaburi R, Dzekov J, Dzekov C, Sinha-Hikim I, Bhasin S. Dose-dependent effects of testosterone on sexual function, mood and visuospatial cognition in older men. J Clin Endocrinol Metab. 2005;90:3838–3846. doi: 10.1210/jc.2005-0247. [DOI] [PubMed] [Google Scholar]
- 100.Honjo H, Iwasa K, Fushiki S, Hosoda T, Tatsumi H, Mihara M, Hirasugi Y, Oida M, Kariya K, Kikuchi N, Kawata M. Estrogen and non-feminizing estrogen for Alzheimer’s disease. Endocr J. 2003;50:361–367. doi: 10.1507/endocrj.50.361. [DOI] [PubMed] [Google Scholar]
- 101.Yi KD, Perez E, Yang S, Liu R, Covey DF, Simpkins JW. The assessment of non-feminizing estrogens for usin neuroprotection. Brain Res. 2011;1379:61–70. doi: 10.1016/j.brainres.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Simpkins JW, Richardson TE, Yi KD, Perez E, Covey DF. Neuroprotection with non-feminizing estrogen analogues: and overlooked possible therapeutic strategy. Horm Behav. 2013;63:278–283. doi: 10.1016/j.yhbeh.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]