Abstract
Antenatal glucocorticoids are used to mature lung function in fetuses at risk for preterm delivery, but they also suppress cortisol synthesis in both pregnant women and their fetuses through blockade of ACTH and so adrenocortical P450c17 expression. We recently discovered in pregnant rabbits that even though exogenous betamethazone is not a mineralocorticoid, it also suppress production of aldosterone, a mineralocorticoid that does not require P450c17 for its biosynthesis. Lower aldosterone levels were linked to reduced P450 side chain cleavage (P450scc) mRNA levels in the rabbit maternal and fetal adrenal cortex. To establish if this occurs in humans, we assayed aldosterone levels in women and newborns treated with antenatal betamethasone for preterm labor. In mothers treated with betamethasone, maternal cortisol depression after 48 hours was accompanied by aldosterone depression. Both pregnant women and their newborns treated with betamethasone showed depressed aldosterone levels in a 1-3 day period after the first betamethasone dose. We conclude that suppression of aldosterone biosynthesis is a side effect of antenatal steroids that has been largely overlooked, but may be clinically relevant at a time when the newborn is learning to control plasma electrolytes and blood volume.
Introduction
One of the most important developments in recent years to enable survival of an extremely preterm infant is the realization that when signs of preterm labor present the mother can be treated with betamethasone (BMZ) to accelerate maturation of fetal lung function. Today, almost 80% of women who have preterm labor at 24 to 34 wks gestation receive synthetic steroids before delivery (1) as recommended by the 1994 NIH Consensus Panel, in order to promote lung maturation (2). In the US in 2004 an estimated 60,000 fetuses were exposed to antenatal steroids (3), some as early as 23 wks gestation (4). Antenatal steroids decrease neonatal death and reduce morbidity, including intraventricular hemorrhage (5-6). Treatment with antenatal steroids is not without its consequences, and a widely recognized side effect of antenatal BMZ is transient suppression of maternal and fetal cortisol biosynthesis for 4 -7 days after treatment (7-8). The practice of repeat steroid dosing decreases fetal growth and the risk: benefit ratio remains controversial (9-13), highlighting the particular importance of ongoing investigations into dosing strategies and side effects of antenatal steroids. Also, the data in premature infants at less than 32 wks gestation is limited (14-15).
A recent study shows the human fetal adrenal cortex synthesizes cortisol as early as 8-9 weeks post conception (16). Nonetheless it is only in the third trimester that, from 23 weeks on, the human fetal adrenal neocortex undergoes more recognizable zonation and develops the zonal ability to synthesize cortisol and aldosterone (17). In part this development of zonal structure and function is normally associated with a progressive rise in ACTH. Studies in humans, primates and H295R human adrenocortical cells have clearly identified the effects of ACTH on modulating levels of several enzymes that regulate adrenocortical steroid biosynthesis (Supplemental Figure 1). Some of these enzymes are responsible for both cortisol and aldosterone biosynthesis (reviewed in 18). Since exogenous glucocorticoids are known to block this ACTH drive of adrenal maturation, and many of the enzymes sensitive to ACTH are involved in aldosterone production in the developing zona glomerulosa, the question must arise whether clinical use of BMZ suppresses fetal aldosterone production. The possible influence of exogenous steroids on development of a functional zona glomerulosa has indeed been suggested by studies in rabbits (19). Nonetheless, while glucocorticoids are now widely used to treat preterm labor, neither basic nor clinical research studies have yet elucidated the effect of exogenous glucocorticoids on aldosterone biosynthetic capacity in the developing human zona glomerulosa, an event that is otherwise necessary to prepare for a role post term under the influence of circulating angiotensin II and potassium.
In this study we investigate whether mothers on standard BMZ treatment regimens for threatened preterm labor exhibit aldosterone suppression and further if it also occurs in the antenatal infant. Given this critical information, it will then be possible to decide if this is a concern and plan future strategies to compensate.
Methods
Rabbit animal model
Rabbit treatments, animal sacrifice, and tissue collection were completed as previously described and approved (19). The treatment regime and general procedure of RNA analysis have been reiterated here for convenience. One maternal and one fetal adrenal gland were removed from each animal and frozen in liquid nitrogen. Maternal and pooled fetal adrenal glands per litter were homogenized and total RNA was extracted as described (19). Following determination of recovery and purity, total RNA was used for slot blot hybridization. Hybridization was performed with 32P labeled, asymmetric PCR probes (19). For this study, expression of ACTH-receptor; steroidogenic acute regulatory protein (StAR); cytochrome P450 enzymes: and side chain cleavage (P450scc) were performed in addition to the prior analysis of 17 α-hydroxylase/17, 20 lyase (P450 c17), 21-hydroxylase (P450 c21); and 3β-hydroxysteroid dehydrogenase/delta5-delta4 isomerase (3βHSDII). GAPDH expression was used for normalization. Data were analyzed using one-way ANOVA and results were considered significant if P < 0.05.
Human subjects
Pregnant women at less than 34 wks gestation who were admitted in labor and had antenatal steroids prescribed by their physician were invited to participate. Twenty-seven women were enrolled from July 2001 to July 2003. Five women were excluded because either the pre-BMZ or 48 h post-BMZ venous blood was not done. Two women were excluded because they delivered prior to completing the 48 hr course of BMZ. The University of Wisconsin and Meriter Hospital Institutional Review Boards approved this study, and all participants gave informed, written consent. Antenatal steroids consisted of BMZ 12 mg IM q 24 hs for 2 doses (one course). One woman received dexamethasone 6 mg IM q 6 hs secondary to a temporary shortage of BMZ and was excluded from analysis.
In a separate study, further analysis was performed on samples routinely collected, with consent, as described previously (15). As in our own study, subjects were treated with a single BMZ course (15). Blood samples were collected from mothers and infants at the time of birth, with mean gestational ages of 30.5 +/− 2.1 (control) vs. 30.5 +/− 2.7 wks. Previously, the suppression of cortisol by BMZ treatment of < 7 days was reported (15). In this study, further analysis of individual cortisol values plus new analysis of aldosterone levels were reported in subjects treated with BMZ and considered relative to time since last dose.
Serum steroid levels
All blood samples were collected in red top tubes, and serum was isolated and frozen at-20°C. Steroid levels (aldosterone, cortisol) were subsequently evaluated by radioimmunoassay in batches as described previously (19) by investigators blinded to identification with the following modifications. Specifically, the cortisol assay was done using an antibody-coated tube radioimmunoassay kit (DiaSorin kit, formerly Incstar, # CA-1549) with coefficients of variation for inter-assay at 14% and intra-assay at 6.6%. The cross-reactivity of the cortisol assay with other steroids is <1% with the exception of 17-hydroxyprogesterol (1.2%). The aldosterone assay was also done using an antibody-coated tube radioimmunoassay kit (Diagnostic Systems Laboratory now with Beckman-Coulter, # DSL-8600) with coefficients of variation for inter-assay at 10.41% and intra-assay at 8.1%. The cross-reactivity of the aldosterone assay with other steroids is <1%. Corticosterone samples underwent chromatography prior to assay with coefficients of variation for inter-assay at 24.7% and intra-assay at 4.1%.
Venous blood was collected from mother within 2 hs of first BMZ dose, at 48 hs after first BMZ dose and at or within 2 hs after delivery. Paired maternal blood was successfully collected prior to and at 48 hs after BMZ in 19 women. One subject was excluded from analysis because the 48 h cortisol level was not depressed, as it was in all other samples. The time from completion of a single course of BMZ to delivery ranged from less than 24 hs to 56 days. Cord blood was drawn at the time of delivery. Cord blood was obtained from 17 newborns, including two sets of twins. One cord blood was excluded in parallel with exclusion of the mother’s sample (above). Five newborns delivered 0-3 days and 11 delivered 14-56 days after the first BMZ dose.
Urine electrolyte levels
Urine was collected from 19 babies. Clean catch urine samples were obtained on days of life 1, 3, 5 and 7. Urine Na+ and K+ levels were analyzed by ion selective electrode (General Medical Laboratories, Madison, WI).
Statistical analysis
Rabbit mRNA data were analyzed by one-way ANOVA. Maternal aldosterone levels in Table 1 were analyzed by a Wilcoxon signed-rank (Sigma Stat version 1). Cord and maternal aldosterone levels in Figure 2 were analyzed by a Student-Newman-Keuls regression analysis. Combined cord blood aldosterone levels were also analyzed by a Mann-Whitney U test. Regression was performed at the lowest order fit that described the data set and expressed with 95% confidence intervals (Sigmaplot 2000). Significance between groups was accepted at P<0.05.
Table 1.
Effect of 48h treatment BMZ on maternal steroid levels. Blood collection was undertaken by mothers prior to treatment with BMZ, and repeated 48hs later regardless of delivery. Mean gestational age (GA) is given along with values for cortisol and aldosterone. The gestational ages in wks at 48 hrs after betamethasone were 23(1), 24 (4), 26(1), 27(1), 28(1), 29(1), 30 (3), 31 (3), 32(2) and 33 (1). 18 subjects were involved in this study and values given are Mean +− SE or median +− SD. Significance (P<) is as shown for each data set.
| Group | n= | GA weeks |
Days Post BMZ |
Maternal Cortisol (ug/dl) |
Maternal Aldosterone (pg/ml) |
|
|---|---|---|---|---|---|---|
| Pretreatment | 18 | Mean | 28.4 | 0 | 21.90 | 240.1 |
| Median | 29.5 | 17.35 | 176.11 | |||
| SD | 3.28 | 12.87 | 202.4 | |||
| SE | 0.77 | 3.03 | 47.7 | |||
| 48h Post Treatment |
18 | Mean | 2 | 2.99 | 132.3 | |
| Median | 3.2 | 108.93 | ||||
| SD | 1.17 | 122.7 | ||||
| SE | 0.28 | 28.9 | ||||
| P< | <0.0001 | <0.0198 |
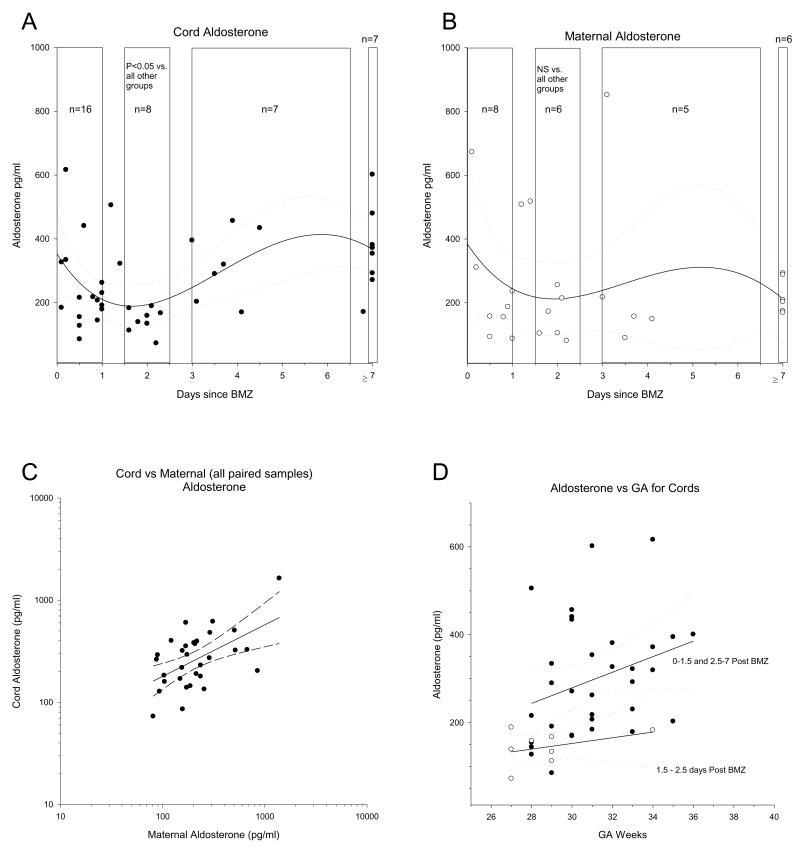
Figure 2.
Aldosterone values in maternal and fetal blood collected from deliveries according to Parker et al (15). The aldosterone levels in cord (upper left) and maternal (upper right) blood are shown. In both cases 3rd order regressions are shown with 95% confidence intervals to establish the initial minima. Note that while aldosterone does not drop immediately (consistent with acute aldosterone production being controlled by factors other than ACTH), there is a clustering of data points at a minima of 1.5-2.5 days, consistent with the known minima for cortisol and the data in Table 1. Data sets were subsequently compared as shown by the boxes. Only the cord blood set denoted in the period 1.5-2.5 days post BMZ was significantly different from all other groups (P<0.01). The relationship between cord aldosterone and maternal aldosterone are indicated bottom left; note linear regression with 95% confidence intervals are also shown. The relationship between aldosterone and gestational age in suppressed (open symbols 1.5-2.5 days post treatment) vs unsuppressed (solid symbols, 0-1.5 days or 2.5-7 or more days) are shown with linear regressions and 95% confidence intervals as indicated.
Results
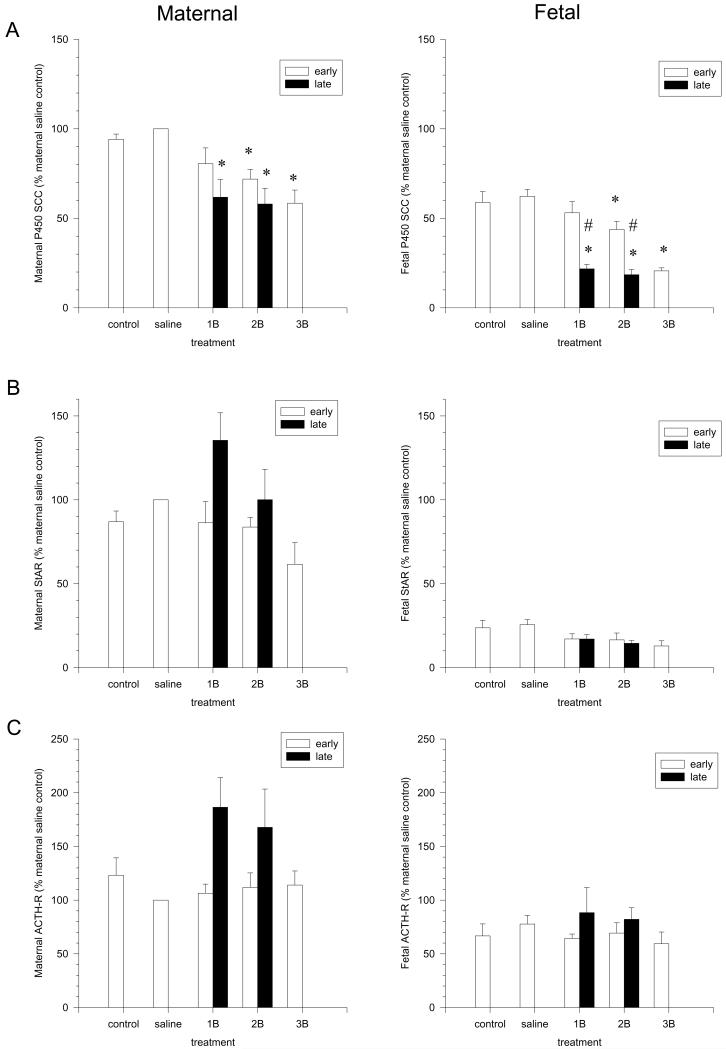
We previously showed that repeated BMZ courses in a widely used rabbit model of early and late steroid administration causes rapid and reversible cortisol suppression in the mother and fetus due to reduced expression of the rate-limiting enzyme for cortisol biosynthesis, P450c17 (19). Surprisingly, maternal aldosterone levels were decreased in parallel with cortisol, even though aldosterone biosynthesis is independent of P450c17 (19). Given the long-term effect of ACTH to maintain expression of biosynthetic enzymes including P450scc (18), we herein further examined in our rabbit model expression of the ACTH- sensitive enzymes common to both cortisol and aldosterone biosynthesis, along with the ACTH receptor itself. ACTH is known to chronically maintain expression of P450s necessary for both cortisol and aldosterone biosynthesis at the level of gene transcription (18). Analysis of both fetal and maternal adrenal mRNA showed decreased P450scc levels (Figure 1A) in addition to the known decline in P450c17 levels, with even greater suppression in fetal versus maternal adrenals. No comparable decreases were detected in the levels of StAR (Fig 1B), ACTH-R (Fig 1C), P450c21 (not shown), consistent with our previous report of no change in 3βHSDII message (19). Given suppression of both maternal and fetal P450scc mRNA, we hypothesized that exogenous glucocorticoids would decrease fetal aldosterone levels, as observed in the mother. Rabbit pups do not make detectable levels of aldosterone until they breathe, so it was not possible to examine rabbit fetal aldosterone suppression. This is not the case, however, in the subjects of clinical interest, humans.
Figure 1.
Effects of BMZ on adrenocortical mRNA levels for P450scc (A), StAR (B) and ACTH Receptor (C) in maternal (left) and pooled fetal (right) adrenals. Results are from n= 6-7 replicates in each group of rabbits. Significant differences between groups are indicated by * compared to saline control and # indicates the difference between early and late treatment (18).
To establish if antenatal glucocorticoids suppress aldosterone synthesis in pregnant women and their newborns, we initially collected serum from women who received BMZ for threatened preterm delivery at 23-34 wks gestation. Maternal blood was drawn prior to or within 2 hs of receiving the first BMZ dose. Maternal blood was also drawn at 48 hs after the first dose to confirm the suppressive effects of BMZ on cortisol and observe any parallel changes in aldosterone. Finally, a sample was collected from mother along with fetal cord blood at delivery. Total serum cortisol and aldosterone levels were batch analyzed by radioimmunoassay. In paired samples from 18 women, the expected cortisol suppression occurred 48 hs after the first BMZ dose and was clearly accompanied by a significant parallel drop in aldosterone (Table 1). Cord blood aldosterone levels were lower in babies delivered 0-3 days (n=5, mean 242.25 +/− 161.19 SD and median 180.8 +/− 72.09 SEM) after the first BMZ dose versus those delivered 14-56 days (n=11, mean 498.5 +/− 325.92 SD and median 416.36 +/− 98.27 SEM). Because of the smaller, unpaired sample size and inclusion of 2 babies that delivered only 24 hs after BMZ in the 1-3 day subgroup this difference was not significant (not shown). However, when our cord blood data are combined with a larger data set that we will discuss below and are subgrouped as cord bloods delivered 1.5-2.5 days after BMZ, the suppression of cord blood aldosterone is significant, as it is in maternal blood.
In order to examine the effect of antenatal BMZ on neonatal aldosterone levels in a larger data set, we further analyzed 42 cord and 29 maternal bloods collected at birth in a previous study (15). Prior analysis of these samples showed cortisol suppression for at least 3 days (15). Based on both the known time course of BMZ suppression of adrenocortical P450c17 expression (19) and the time course of ACTH re-induction of mRNA (18) we subgrouped the data shown in Figure 2. When our data and these cord bloods are combined, cord blood aldosterone levels are significantly lower in the 1.5-2.5 subgroup (n=9, median 152.96 +/− 12.1 SEM) versus the < 1.5 day subgroup (n=18, median 211.4 +/− 29.63 SEM) or the >2.5 day subgroup (n=30, median 376.5 +/−58.6 SEM, p<.05 by Mann-Whitney U tests). Moreover, regression analysis suggested suppressed maternal and fetal aldosterone levels at 1.5-2.5 days with full recovery to initial levels by 3-7 days (Figures 2 A, B). This is consistent with the expected time course for loss of steroidogenic enzxymes in the absence of ACTH (due to Betamethazone suppression of the pituitary) and subsequent re-expression of steroid biosynthetic enzymes as ACTH levels return to normal. More specifically it is well known that expression of both P450scc and P450c17 in human adrenocortical cells is controlled by ACTH through the second messenger cAMP, and corresponding induction of transcription is the determinant of mRNA and subsequently protein levels in each case (18). Increases in mRNA are detectable in hours and plateau at around 24 hs while stable expression of protein occurs within 36 hs. It is then no coincidence that in previous studies maximal suppression of cortisol and P450c17 expression has been reported at 48hs in response to BMZ blockade of ACTH. Statistical analysis revealed that this smaller maternal group (n=6) at 1.5-2.5 days post BMZ (ie a time when BMZ suppression of enzyme expression would be predicted to be maximal and before a rise in ACTH occurred due to BMZ excretion) failed to achieve significance when compared to women who delivered prior to or after this time window (pre and post-BMZ blood draws were not done in the Parker study as in our original data set). However, in this larger set of cord bloods at 1.5-2.5 days (when suppression is maximal and ACTH has not yet returned to normal), depression of aldosterone in cord bloods (n=8) was clearly significant and consistent with the greater depression of P450scc observed in rabbit fetal vs maternal adrenals (Figure 1A) when analyzed by regression analysis, consistent with our previous significant depression detected by Mann-Whitney U tests. Of clinical relevance, we also found that maternal levels still closely predicted cord blood levels (Figure 2C). Aldosterone suppression at 1.5-2.5 days post BMZ in cord blood occurred at all gestational ages in the time period studied (Figure 2D).
Discussion
Taken together, our most striking finding is that antenatal BMZ treatment depresses human maternal and fetal aldosterone levels in addition to the known suppression of cortisol levels. Furthermore, the degree of aldosterone suppression in the fetus is closely indicated by the fall in maternal levels. This new finding was previously unrecognized as a side effect of exogenous betamethasone administration for the threat of preterm labor.
Clearly reduced aldosterone levels could be considered to be due to a loss of circulating stimulus or a loss of biosynthetic capacity of the maternal or developing fetal zona glomerulosa. However, it should be noted in Figure 2 that aldosterone levels in the first day in mothers are not lower than controls and in mothers and infants are not different from that on day 7 or beyond. This contrasts with the overnight cortisol depression seen in response to synthetic glucocorticoids in the same subjects (15). In the case of the overnight fall in cortisol, this is explained by a rapid fall in circulating ACTH due to immediate negative feedback on the pituitary, so the acute stimulus of the adrenal cortisol biosynthesis is also immediately lost. The delay in the reduction of aldosterone levels suggests that ACTH blockade does lead to progressive loss of expression of key steroid biosynthetic enzymes but the acute stimulation of the zona glomerulosa occurs by factors other than ACTH, ie Angiotensin II which is not subject to the negative feedback by glucocorticoids at the level of the pituitary otherwise observed for ACTH. Since BMZ is not a mineralocorticoid, our rabbit model implicated BMZ action on aldosterone output may be via suppression of ACTH otherwise necessary for maintaining P450scc expression, the same enzyme acutely activated in the zona glomerulosa by angiotensin II.
Given that BMZ likely suppresses P450scc, as it does P450c17 expression, through negative feedback on the pituitary with a consequential drop in ACTH, the effects of many synthetic glucocorticoids in adults and children would be predicted to be similar and may have broad clinical implications beyond antenatal BMZ administration. Variability in the time course and magnitude of response would depend on the steroid of choice, the dose, and the rate of clearance.
Our study suggests that ACTH blockade by BMZ leads to a progressive loss of the expression of key steroid biosynthetic enzymes. We further suggest that a drop in P450scc expression/activity causes decreased aldosterone production, just as a drop in P450c17 leads to a decline of cortisol. Nonetheless, other explanations are worthy of consideration. The first such question which must be addressed is whether the steroid output of the adrenal was not simply reduced but instead was diverted to alternate steroid products. We previously reported deoxycorticosterone in cord blood after intrauterine exposure to exogenous glucocorticoids and showed that levels are indeed suppressed rather than elevated (22). This is consistent with other studies that showed decreased deoxycorticosterone levels in amniotic fluid (23), and no significant change in cord blood (24) acutely following exogenous glucocorticoids. In the current study we also assayed corticosterone levels in the same samples reported in Table 1 and showed approximately 50% reduction in corticosterone (not shown) alongside the decline in cortisol. As such we must conclude that the decline in cortisol is indeed due to a reduced zona fasciculata output in these subjects rather than diversion of substrate to alternate steroid products and further that the decline in aldosterone is not secondary to elevation of deoxycorticosterone or corticosterone.
An alternate explanation that we must also consider is that the dose of BMZ is sufficiently high to have a direct action to suppress renin and so circulating Angiotensin II. Previous animal studies have shown that infusion of endogenous glucocorticoids (cortisol) directly into the maternal or fetal sheep circulation does indeed suppress renin, but also that this occurs almost immediately (25-28). Thus if BMZ action to suppress aldosterone were through suppression of renin, aldosterone suppression would also be expected to be observed almost as quickly as BMZ crosses the placenta. In humans, we know that BMZ takes only a short time to cross the placenta as cortisol levels decline within about 6 hrs of BMZ administration (14). Given that the time from BMZ administration to decline in aldosterone was more than a day rather than simply hours, with further recovery by 3 days (Figure 2A), it seems more likely the decline in aldosterone in response to BMZ is due to reduced levels of P450scc rather than BMZ-induced suppression of renin. While further studies are needed, our data are more consistent with the known time course for suppression of P450scc expression on the loss of circulating ACTH and the reinduction of P450scc as BMZ is cleared and circulating ACTH returns.
The most important clinical factor in suppression of aldosterone levels was not gestational age, but rather the timing of delivery after the last BMZ course (Figure 2D). Aldosterone depression occurred in infants as young as 23 wks, and maternal levels were clearly a readily accessible biomarker for fetal levels (Figure 2C). Although our study does not address mode of delivery, other studies suggest that aldosterone suppression may be further exacerbated by Caesarian section delivery, for which the degree of stimulation of pituitary ACTH release is less than for vaginal delivery (29). In light of our findings, it is not surprising that previous studies have not demonstrated aldosterone suppression in cord blood following antenatal glucocorticoids (24, 30). Studies did not sub-classify data from the time of BMZ administration and those that did often grouped their data sets in a 1-7 days and greater than 7 days manner. Both adequate sample size and the time sampled after BMZ dose are required to detect aldosterone suppression.
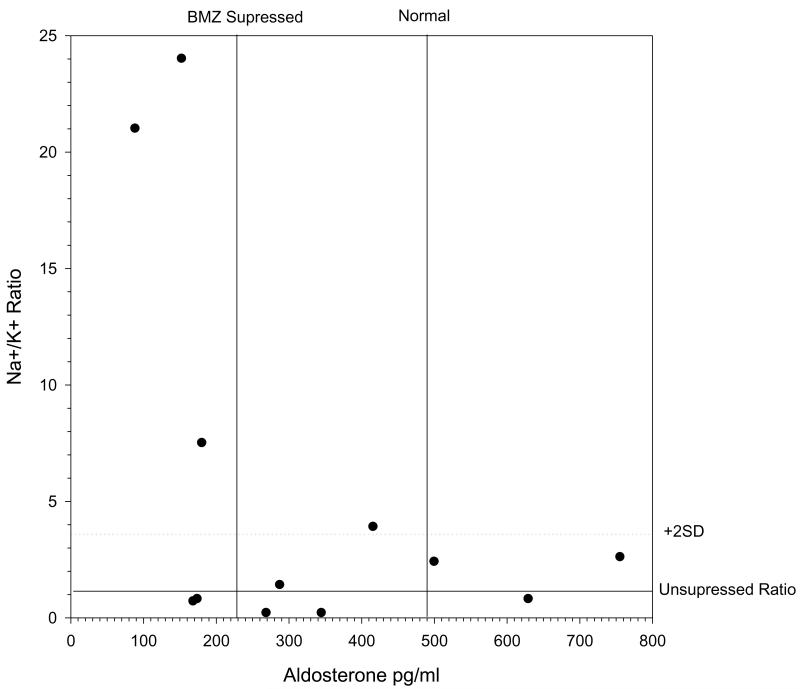
The physiologic effects of low aldosterone levels in preterm infants are not certain. Catarelli et al and others showed in fetal sheep that antenatal glucorticoids increase GFR and mature tubular function (25). In a study by Mortiz et al, the infusion of fetal sheep with aldosterone increases % change in GFR, increases K excretion and decreases the Na-to-K ratio (31), Antenatal dexamethasone blunted the change in GFR but did not alter K excretion or Na-to K ratio (31). It is unclear whether aldosterone has equivalent functions in the immature kidney of the preterm infant compared to the adult. Relatively few studies have examined normal aldosterone levels in preterm infants and none control for BMZ administration (32-33). To test possible clinical consequences in newborns born with suppressed aldosterone levels, we measured urine electrolytes in babies born in our study. On day one, 3 out of 5 newborns with aldosterone levels suppressed at or below 230 pg/ml had substantially elevated Na+/K+ ratios (Figure 3), suggestive of decreased renal epithelial cell K+ secretion or Na+ reabsorption.
Figure 3.
Effect of BMZ on urinary electrolytes. The first 24 hr urinary electrolytes were measured and compared to aldosterone levels at delivery in cord bloods. Note the tendency as aldosterone levels are observed in the suppressed range (at or below mean as determined in cortisol suppressed subjects), for the Na+:K+ ratio to rise dramatically above the ratio for subjects that were not cortisol suppressed (see horizontal reference lines for normal mean +2 SD).
The possible clinical consequences of low aldosterone in newborns following antenatal BMZ deserve recognition and further investigation. In adults, aldosterone acutely affects potassium and blood pressure homeostasis. In preterm infants, it is possible that under some circumstances low aldosterone levels affect the ability of the immature kidney to handle hyperkalemia, control blood volume, or regulate blood pressure. Non-oliguric hyperkalemia and corticosteroid-responsive hypotension are observed in preterm infants during the first week of life. Uga et al reported that antenatal BMZ improved hyperkalemia but did not control for time after BMZ (34). One proposed pathogenesis for non-oliguric hyperkalemia includes an intracellular to extracellular potassium shift combined with a decreased tubular potassium secretion secondary to immature activity of the Na+/K+ ATPase. Low aldosterone levels or aldosterone unresponsiveness could decrease the ability of tubules to compensate for increased serum potassium. If low aldosterone is identified in preterm babies with hyperkalemia (which could be done by cord blood analysis), then a single dose of ACTH, a drug already used in infants, might alleviate symptoms more quickly by promoting recovery of adrenal P450 expression.
The possible long-term implications of our findings are equally unclear. Intrauterine fetal programming is now a recognized phenomenon, and it is likely that such programming events continue in the first few days of life. Some animal studies suggest that alteration of the HPA axis may continue for a longer period of time than previously suspected (35), and a human study appears to support this observation (36). The newborn baby has to independently establish mechanisms controlling blood volume and blood pressure for the first time and learn “setpoints” that then affect long-term cardiovascular health. Although two long-term follow-up studies have not shown differences in blood pressure in young adults who received antenatal BMZ (37-38), one study detected an increase in insulin resistance. It is our sincere hope that perturbation of aldosterone secretion at this critical time does not have long-term consequences on these set points, but this is a question that clearly deserves closer attention in future studies.
In conclusion, the clinical implications of steroid-induced aldosterone suppression in human disease are not yet known. Clearly, antenatal glucocorticoids improve survival and morbidity of preterm infants and thereby represent a critical therapeutic strategy. However, our study highlights three areas for additional research. First, aldosterone suppression should be included as an outcome in trials that address the type of steroid administered and dosing regimen. Studies will need to be adequately powered to detect differences. Our findings suggest that the clinical effects will be best investigated by specifically sub-grouping those infants born 1-3 days following the last BMZ dose. Second, although the short-term effects of aldosterone deficiency on the blood pressure and electrolyte imbalance in the newborn are unknown, the clinician should be aware that infants born 1-3 days following antenatal BMZ are at risk for aldosterone deficiency. Finally, follow-up studies on newborns who received antenatal steroids should include longitudinal measurements of aldosterone levels and monitoring of long-term outcomes such as the metabolic syndrome (39).
Supplementary Material
Supplemental Figure 1: Pathways of steroid biosynthesis for zona glomerulosa, fasciculata and reticularis in humans, and the rate limiting steps for steroid biosynthesis (reviewed in 18). Note ACTH stimulates the expression of multiple steroid biosynthetic enzymes in the adrenal cortex including P450c17 and P450scc. Note that while P450c17 is necessary for cortisol biosynthesis it is not involved in aldosterone biosynthesis. Note also that P450scc is necessary for both cortisol and aldosterone biosynthesis.
Acknowledgments
We acknowledge Dr. David Abbott for steroid assays. GCRC nursing support Debbie Krumpos, Patricia Green, Tracy Gardner and Eva Dye in addition to the dedicated obstetrical nursing staff. Dr. Kessel was a recipient of a NIH K08 grant (K08 AI049238-04) during the period of study.
Abbreviations
- (BMZ)
betamethasone
- (GA)
gestational age
Footnotes
Competing Interests Statement: The authors declare that they have no competing financial interests.
This study was supported by funds from the Perinatal Foundation
References
- 1.Wilson-Costello D, Friedman H, Minich N, et al. Improved neurodevelopmental outcomes for extremely low birthweight infants in 2000-2002. Pediatrics. 2007;119(1):37–45. doi: 10.1542/peds.2006-1416. [DOI] [PubMed] [Google Scholar]
- 2.Meadow, Gyn Obstet. NIH Consensus Statement. 2003;12(2):1–24. 1994, Feb 28-March 2. [Google Scholar]
- 3.Hoyert DL, Mathews TJ, Menacker F, Strobino DM, Guyer B. Annual summary of vital statistics: 2004. Pediatrics. 2006;117(1):168–183. doi: 10.1542/peds.2005-2587. [DOI] [PubMed] [Google Scholar]
- 4.McElrath TF, Norwitz ER, Nour N, Robinson JN. Contemporary trends in management of delivery at 23 weeks’ gestation. Am. J. Perinatol. 2002;19(1):9–15. doi: 10.1055/s-2002-20176. [DOI] [PubMed] [Google Scholar]
- 5.Crowley PA. Antenatal corticosteroid therapy: A meta-analysis of the randomized trials, 1972 to 1994. Am. J. Obstet. Gynecol. 1995;173:322–35. doi: 10.1016/0002-9378(95)90222-8. [DOI] [PubMed] [Google Scholar]
- 6.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 1995;3 doi: 10.1002/14651858.CD004454.pub3. CD004454 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gennser G, Ohrlander S, Eneroth P. Cortisol in amniotic fluid and cord blood in relation to prenatal betamethasone load and delivery. Am. J. Obstet. Gynecol. 1976;124(1):43–50. doi: 10.1016/0002-9378(76)90009-0. [DOI] [PubMed] [Google Scholar]
- 8.Ballard PL, Granberg P, Ballard RA. Glucocorticoid levels in maternal and cord serum after prenatal betamethasone therapy to prevent respiratory distress syndrome. J. Clin. Invest. 1975;56:1548–1554. doi: 10.1172/JCI108236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institutes of Health Consensus Panel Antenatal corticosteroids revisited: repeat courses – National Institutes of Health Consensus Development Conference Statement. August 17-18, 2000. Obstet. Gynecol. 2001;98(1):144–150. doi: 10.1016/s0029-7844(01)01410-7. [DOI] [PubMed] [Google Scholar]
- 10.Crowther CA, Haslam RR, Hiller JE, Doyle LW, Robinson J. S. Australian Collaborative Trial of Repeat Doses of Steroids (ACTORDS) Study Group. Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: a randomised controlled trial. Lancet. 2006;367(9526):1913–1919. doi: 10.1016/S0140-6736(06)68846-6. [DOI] [PubMed] [Google Scholar]
- 11.Wapner RJ, Sorokin Y, Thom EA, et al. Single versus weekly courses of antenatal corticosteroids: evaluation of safety and efficacy. Am. J. Obstet. Gynecol. 2006;195(3):633–642. doi: 10.1016/j.ajog.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 12.Crowther CA, Doyle LW, Haslam RR, Hiller JE, Harding JE, Robinson JS. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. NEJM. 2007;357(12):1179–1189. doi: 10.1056/NEJMoa071152. [DOI] [PubMed] [Google Scholar]
- 13.Wapner RJ, Sorokin Y, Mele L, et al. Long-term outcomes after repeat doses of antenatal steroids. NEJM. 2007;357(12):1190–1198. doi: 10.1056/NEJMoa071453. [DOI] [PubMed] [Google Scholar]
- 14.Ballard PL, Gluckman PD, Liggins GC, Kaplan SL, Grumbach MM. Steroid and growth hormone levels in premature infants after prenatal betamethasone therapy to prevent respiratory distress syndrome. Pediatric Res. 1980;14(2):122–127. doi: 10.1203/00006450-198002000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Parker CR, Jr, Atkinson MW, Owen J, Andrews WW. Dynamics of the fetal adrenal, cholesterol, and apolipoprotein B responses to antenatal betamethasone therapy. Am. J. Obstet. Gynecol. 1996;174(2):562–565. doi: 10.1016/s0002-9378(96)70428-3. [DOI] [PubMed] [Google Scholar]
- 16.Goto M, Hanley KP, Marcos J, et al. In humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development. J Clin Invest. 2006;116:953–960. doi: 10.1172/JCI25091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr. Rev. 1997;18(3):378–403. doi: 10.1210/edrv.18.3.0304. [DOI] [PubMed] [Google Scholar]
- 18.Conley AJ, Bird IM. The role of cytochrome P450 17α-hydroxylase and 3β-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the Δ5 and Δ4 pathways of steroidogenesis in mammals. Biol. Reprod. 1997;56(4):789–799. doi: 10.1095/biolreprod56.4.789. [DOI] [PubMed] [Google Scholar]
- 19.Pratt L, Magness RR, Phernetton T, Hendricks S, Abbott DH, Bird I. Repeated use of betamethasone in rabbits: Effects of treatment variation on adrenal suppression, pulmonary maturation, and pregnancy outcome. Am. J. Obstet. Gynecol. 1999;180:995–1005. doi: 10.1016/s0002-9378(99)70672-1. [DOI] [PubMed] [Google Scholar]
- 20.Biglieri EG, Herron MA, Brust N. 17-hydroxylation deficiency in man. J Clin Invest. 1966;12:1946–1954. doi: 10.1172/JCI105499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biglieri EG. A prismatic case- 17α-hydroxylase deficiency: 1963-1966. J Clin Endocrinol Metab. 1997;82:48–50. doi: 10.1210/jcem.82.1.3653. [DOI] [PubMed] [Google Scholar]
- 22.Parker CR, Jr, Carr BR, Ragland SR, Morrison JC, Herbert WN, MacDonald PC. Ontogeny of human fetal plasma progesterone, deoxycortidosterone, and deoxycorticosterone sulfate. Am J Obstet Gynecol. 1983;147:955–959. doi: 10.1016/0002-9378(83)90253-3. [DOI] [PubMed] [Google Scholar]
- 23.Nolten WE, Holt LH, Rueckert PA. Desoxycorticosterone in normal pregnancy. III. Evidence of a fetal source of desoxycorticosterone. Am J Obstet Gynecol. 1981;139:477–82. doi: 10.1016/0002-9378(81)90328-8. [DOI] [PubMed] [Google Scholar]
- 24.Dorr HG, Versmold HT, Sippell WG, Bidlingmaier F, Knorr D. Antenatal betamethasone therapy: Effects on maternal, fetal and neonatal mineralocorticoids, glucocorticoids, and progestins. J. Pediatrics. 1986;108(6):990–993. doi: 10.1016/s0022-3476(86)80946-5. [DOI] [PubMed] [Google Scholar]
- 25.Cattarelli D, Chirico G, Simeoni U. Renal effects of antenatally or postnatally administered steroids. Pediatr Med Chir. 2002;24(2):157–62. Review. [PubMed] [Google Scholar]
- 26.Wood CE, Keil LC, Rudolph AM. Physiological inhibition of ovine fetal plasma renin activity by cortisol. Endocrinology. 1984;115:1792–6. doi: 10.1210/endo-115-5-1792. [DOI] [PubMed] [Google Scholar]
- 27.Wood CE. Sensitivity of cortisol-induced inhibition of ACTH and renin in fetal sheep. Am J Physiol. 1986;250:R795–802. doi: 10.1152/ajpregu.1986.250.5.R795. [DOI] [PubMed] [Google Scholar]
- 28.Keller-Wood M, Silbiger J, Wood CE. Progesterone-cortisol interaction in control of renin activity but not aldosterone. Am J Physiol. 1990;259:R350–6. doi: 10.1152/ajpregu.1990.259.2.R350. [DOI] [PubMed] [Google Scholar]
- 29.Vogl SE, Worda C, Egarter C, et al. Mode of delivery is associated with maternal and fetal endocrine stress response. BJOG. 2006;113:441–445. doi: 10.1111/j.1471-0528.2006.00865.x. [DOI] [PubMed] [Google Scholar]
- 30.Procianoy RS, de Oliveira-Filho EA. Aldosterone cord levels in preterm newborn infants. Acta. Pediatr. 1996;85:611–613. doi: 10.1111/j.1651-2227.1996.tb14097.x. [DOI] [PubMed] [Google Scholar]
- 31.Moritz KM, Jefferies A, Wintour EM, Dodic M. Fetal renal and blood pressure responses to steroid infusion after early prenatal treatment with dexamethasone. Am J Physiol Regul Integr Comp Physiol. 2005;288:R62–R66. doi: 10.1152/ajpregu.00282.2004. [DOI] [PubMed] [Google Scholar]
- 32.Bourchier D. Plasma aldosterone levels in the 1st week of life in infants of less than 30 weeks gestation. Eur. J. Pediatr. 2005;164:141–145. doi: 10.1007/s00431-004-1572-0. [DOI] [PubMed] [Google Scholar]
- 33.Doerr HG, Sippell WG, Versmold HT, Bidlingmaier F, Knorr D. Plasma mineralocorticoids, glucocorticoids, and progestins in premature infants: longitudinal study during the first week of life. Pediatric Research. 1988;23(5):525–529. doi: 10.1203/00006450-198805000-00018. [DOI] [PubMed] [Google Scholar]
- 34.Uga N, Nemoto Y, Ishii T, Kawase Y, Arai H, Jada H. Antenatal steroid treatment prevents severe hyperkalemia in very low-birthweight infants. Pediatr. Int. 2003;45(6):656–60. doi: 10.1111/j.1442-200x.2003.01807.x. [DOI] [PubMed] [Google Scholar]
- 35.Sloboda DM, Moss TJM, Li S, et al. Prenatal betamethasone exposure results in pituitary-adrenal hyporesponsiveness in adult sheep. Am. J. Physiol. Endocrinol. Metab. 2007;292:E61–E70. doi: 10.1152/ajpendo.00270.2006. [DOI] [PubMed] [Google Scholar]
- 36.Davis EP, Townsend EL, Gunnar MR, et al. Antenatal betamethasone treatment has a persisting influence on infant HPA axis regulation. J. Perinatol. 2006;26(3):147–153. doi: 10.1038/sj.jp.7211447. [DOI] [PubMed] [Google Scholar]
- 37.Keijzer-Veen MG, Finken MJ, Nauta J, et al. Is blood pressure increased 19 years after intrauterine growth restriction and preterm birth? A prospective follow-up study in the Netherlands. Pediatrics. 2005;116(3):725–731. doi: 10.1542/peds.2005-0309. [DOI] [PubMed] [Google Scholar]
- 38.Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomized controlled trial. Lancet. 2005;365:1856–62. doi: 10.1016/S0140-6736(05)66617-2. [DOI] [PubMed] [Google Scholar]
- 39.Bochud M, Nussberger J, Bovet P, et al. Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension. 2006;48:239–245. doi: 10.1161/01.HYP.0000231338.41548.fc. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Pathways of steroid biosynthesis for zona glomerulosa, fasciculata and reticularis in humans, and the rate limiting steps for steroid biosynthesis (reviewed in 18). Note ACTH stimulates the expression of multiple steroid biosynthetic enzymes in the adrenal cortex including P450c17 and P450scc. Note that while P450c17 is necessary for cortisol biosynthesis it is not involved in aldosterone biosynthesis. Note also that P450scc is necessary for both cortisol and aldosterone biosynthesis.