Abstract
Background & Aims
Muscle wasting commonly occurs in COPD, negatively affecting outcome. The aim was to examine the net whole-body protein synthesis response to two milk protein meals with comparable absorption rates (hydrolyzed casein (hCAS) vs. hydrolyzed whey (hWHEY)) and the effects of co-ingesting leucine.
Methods
Twelve COPD patients (GOLD stage II-IV) with nutritional depletion, were studied following intake of a 15g hCAS or hWHEY protein meal with or without leucine-co-ingestion, according to a double-blind randomized cross-over design. The isotopic tracers L-[ring-2H5]-Phenylalanine, L-[ring-2H2]-Tyrosine, L-[2H3]-3-Methylhistidine (given via continuous intravenous infusion), and L-[15N]-Phenylalanine (added to the protein meals) were used to measure endogenous whole-body protein breakdown (WbPB), whole-body protein synthesis (WbPS), net protein synthesis (NetPS), splanchnic extraction and myofibrillar protein breakdown (MPB). Analyses were done in arterialized-venous plasma by LC/MS/MS.
Results
WbPS was greater after intake of the hCAS protein meal (P<0.05) whereas the hWHEY protein meal reduced WbPB more (P<0.01). NetPS was stimulated comparably, with a protein conversion rate greater than 70%. Addition of leucine did not modify the insulin, WbPB, WbPS or MPB response.
Conclusions
Hydrolyzed casein and whey protein meals comparably and efficiently stimulate whole-body protein anabolism in COPD patients with nutritional depletion without an additional effect of leucine co-ingestion.
Keywords: Chronic Obstructive Pulmonary Disease, whole-body protein kinetics, hydrolyzed casein protein, hydrolyzed whey protein, leucine, nutritionally depleted
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is considered a systemic disease in which muscle wasting, as independent co-morbidity, negatively affects mortality (1). The exact mechanisms underlying muscle wasting in COPD are complex and multifactorial. We have observed disturbances in protein turnover and intermediary amino acid metabolism on whole-body and skeletal muscle level (2-5). This suggests that dietary proteins, through enhancing protein anabolism, could help preserve and increase muscle mass in COPD patients. In particular the milk proteins casein and whey are of interest as both are high-quality proteins (6) because of their high essential amino acid (EAA) content. Previous studies indicate that primarily the EAA stimulate muscle protein synthesis and improve anabolism in healthy older adults (7, 8), with a specific role for the amino acid leucine (9).
Although casein and whey are both high-quality proteins, studies comparing their anabolic capacity in healthy individuals show variable results. In young men, several studies report a better whole-body leucine balance after casein protein intake, despite the higher leucine content of whey protein (10, 11). In elderly, researchers find a greater stimulation of protein anabolism in response to whey protein intake (12, 13). A complicating factor in several recent studies that examine the muscle anabolic response to milk proteins is the lack of information on muscle protein breakdown. With only information on muscle protein synthesis, the calculation of net muscle protein gain is not possible (14). In the present study we chose a protocol that is able to measure net whole-body protein synthesis, can delineate the underlying mechanisms (i.e. synthesis increase or breakdown decrease) and allowed us to have the same patient come back four times.
Several factors can influence the anabolic capacity of a protein meal besides the EAA or leucine content, including the relative proportion of the individual branched-chain amino acids (BCAA), the mode of administration (sip versus bolus meal) (10), and the presence of carbohydrates (15) that affects insulin kinetics (16). To exclusively compare protein meals for differences in amino acid profile, it is of key importance to use comparable amounts of protein and carbohydrates and to correct for differences in digestion and absorption pattern.
The purpose of the present study was to compare the anabolic properties of a hydrolyzed casein and whey protein bolus meal with and without co-ingestion of leucine in COPD patients characterized by nutritional depletion. A bolus meal was chosen as it is a more physiological approach compared to continuous feeding and thereby the preferred therapeutic modality to provide supplemental proteins in COPD. All meals contained a similar amount of proteins and carbohydrates. Hydrolyzed proteins were used to eliminate possible differences in digestion and absorption rates. Furthermore, we added leucine to the protein meals to 40% of its EAA content to examine whether leucine addition would further enhance the anabolic capacity of the meals.
Subjects and Methods
Subjects
The study population consisted of 12 patients (7 male, 5 female) ranging from 43 to 77 yrs., with moderate to severe airflow obstruction. Eligible patients were recruited from March 2010 to June 2011 from Little Rock, AR and surrounding areas and had COPD stage II-IV according to the established Gold Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (17). All patients were in clinically stable condition and not suffering from a respiratory tract infection or exacerbation of their disease at least 4 weeks prior to the study. Exclusion criteria were malignancy, cardiac failure, recent surgery and severe endocrine, hepatic or renal disorders. Also, patients who were using systemic corticosteroids within 1 month prior to the study were excluded. The number of present smokers was 5. For non-smokers the average time since smoking cessation was 10.4 ± 2.7 yrs. The maintenance treatment of all but one patient consisted of inhaled β2-agonists, inhaled anti-cholinergics, inhaled corticosteroids, or a combination and one-third was on long-term oxygen therapy. We selected patients that were nutritionally depleted based on the following criteria: body mass index (BMI) ≤ 24 kg/m2 (18) and/or muscle wasting (fat-free mass index (FFMI) ≤ 16 kg/m2 (male) and ≤ 15 kg/m2 (female)) (1) and/or recent involuntarily weight loss (5% in three months or 10% in six months). Written informed consent was obtained from all patients and the study was approved by the Institutional Review Board of the University of Arkansas for Medical Sciences.
Pulmonary function tests
All COPD patients underwent spirometry for determination of FEV1, as a marker of disease severity, with the highest value from ≥ 3 technically acceptable maneuvers being used. All values obtained were related to a predicted value (19) and expressed as percentages of the predicted value. In addition, the most recent pulmonary function test results of each patient were obtained (when available) from the treating physician.
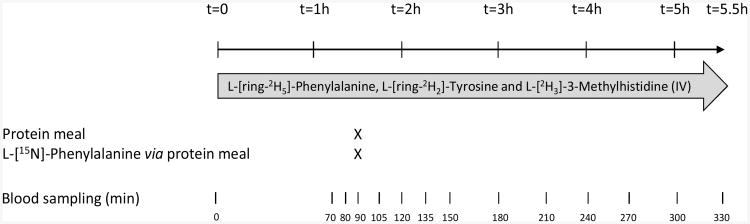
Study protocol (Figure 1)
Figure 1.
Overview of study design. Patients received four different protein meals (hydrolyzed casein versus hydrolyzed whey protein meal with or without added leucine), one on each experimental test day, according to a double-blind randomized cross-over design.
On four experimental test days within a timeframe of 2 weeks, patients were studied at the outpatient clinical research center of the University of Arkansas for Medical Sciences. The protocol started in the early morning, after an overnight fast from 10:00 pm onwards. All subjects were in supine position for 5.5 hours. Body weight and vital signs were measured at the start. After insertion of a catheter into an anticubital vein, the first blood sample was taken for baseline measurement. A primed-constant continuous infusion of stable isotopes (70 mL/h) was started with the use of a calibrated pump (CareFusion Corporation, San Diego, CA). The stable isotopes L-[ring-2H5]-Phenylalanine and L-[ring-2H2]-Tyrosine were used to determine net whole-body protein synthesis as the primary outcome measure. L-[2H3]-3-Methylhistidine was infused to measure whole-body myofibrillar protein breakdown. The following priming doses and infusion rates were used: L-[ring-2H5]-Phenylalanine: prime = 3.6 μmol/kg body weight (BW), infusion = 3.6 μmol/kg BW/h), L-[ring-2H2]-Tyrosine: prime = 1.14 μmol/kg BW, infusion = 1.14 μmol/kg BW/h). Moreover, a bolus dose of L-[ring-2H4]-tyrosine was given to prime the phenylalanine-derived plasma tyrosine pool (prime = 0.31 μmol/kg BW). L-[15N]-Phenylalanine was given orally (135.7 mg) together with each protein meal to measure splanchnic extraction. The stable isotopes were purchased from Cambridge Isotopic Laboratories (Woburn, MA, USA). A second catheter for arterialized venous blood sampling was placed in a superficial dorsal vein of the hand or lower arm of the contralateral arm. The hand was placed in a thermostatically controlled heated box, a technique to mimic direct arterial sampling (20). Triple arterialized-venous blood samples were taken between 70 and 90 min after the start of infusion. Arterialized-venous blood was sampled throughout the study for analysis of the enrichment and concentration of amino acids and of insulin. Total blood drawn on each test day was approx. 70 mL.
Anthropometric data and body composition
Body weight was measured by a digital beam scale and height by a stadiometer. BMI was calculated by dividing body weight by squared height. Fat-free mass (FFM) was obtained by Dual-Energy X-ray Absorptiometry (DXA) (Hologic QDR 4500/Version 11.2 (Bedford, MA)). FFM was standardized for height.
Protein meals
Patients received four different protein meals, one on each test day, according to a double-blind randomized cross-over design. Randomization was performed using http://randomizer.org and the allocation sequence was kept at the UAMS metabolic kitchen where the protein meals were prepared. The meals contained either 15 g casein protein hydrolysates (hCAS, CE90STL, 2.2 g N, 20% w/w leucine of EAA content, DMV International Veghel, The Netherlands), 15 g whey protein hydrolysates (hWHEY, WE90F, 2.4 g N, 28% w/w leucine of EAA content, DMV International Veghel, The Netherlands), 15 g casein protein hydrolysates and 1.5 g leucine (40% w/w leucine of EAA content, hCAS+LEU) or 15 g whey protein hydrolysates and 2.1 g leucine (40% w/w leucine of EAA content, hWHEY+LEU). Furthermore, all protein meals contained 15 g of maltodextrin (Ross Nutrition, Sturgis, MI). The protein hydrolysates and maltodextrin were dissolved in 250 ml water at 60 °C a day before each test day and kept at 4 °C until use. For amino acid composition of the protein meals, see Table 1.
Table 1. Amino acid composition of the hydrolyzed casein (hCAS), hydrolyzed whey (hWHEY), hydrolyzed casein with added leucine (hCAS+LEU) and hydrolyzed whey with added leucine (hWHEY+LEU) protein meals.
| hCAS | hCAS+LEUa | hWHEY | hWHEY+LEUa | |
|---|---|---|---|---|
| HIS (g) | 0.38 | 0.29 | ||
| ILE (g) | 0.78 | 0.90 | ||
| LEU (g) | 1.28 | 3.38 | 1.97 | 3.47 |
| LYS (g) | 1.23 | 1.32 | ||
| MET (g) | 0.36 | 0.32 | ||
| PHE (g) | 0.60 | 0.57 | ||
| THR (g) | 0.65 | 0.59 | ||
| TRP (g) | 0.11 | 0.24 | ||
| VAL (g) | 0.96 | 0.90 | ||
| ALA (g) | 0.47 | 0.89 | ||
| ARG (g) | 0.53 | 0.62 | ||
| ASP (g) | 1.05 | 1.65 | ||
| CYS (g) | 0.05 | 0.18 | ||
| GLU (g) | 3.45 | 2.58 | ||
| GLY (g) | 0.26 | 0.36 | ||
| PRO (g) | 1.38 | 0.59 | ||
| SER (g) | 0.83 | 0.24 | ||
| TYR (g) | 0.68 | 0.54 | ||
| Total AA (g) | 15.00 | 17.10 | 14.72 | 16.22 |
| Sum BCAA (g) | 3.02 | 5.12 | 3.77 | 5.27 |
| Sum EAA (g) | 6.33 | 8.43 | 7.08 | 8.58 |
ALA: alanine, ARG: arginine, ASP: asparagine, CYS: cysteine, GLU: glutamine, GLY: glycine, HIS: histidine, ILE: isoleucine, LEU: leucine, LYS: lysine, MET: methionine, PHE: phenylalanine, PRO: proline, SER: serine, THR: threonine, TRP: tryptophan, TYR: tyrosine, VAL: valine. BCAA: branched-chain amino acids, sum of LEU, VAL, and ILE. EAA: essential amino acids. AA; amino acids. The amino acid composition of the protein meals is based on the manufacturer factsheet.
Only differences are shown from the equivalent protein meal without the addition of leucine.
Sample processing and biochemical analysis
Arterialized-venous blood samples were put in Li-heparinized or EDTA tubes (Becton Dickinson Vacutainer system, Franklin Lakes, NJ) and immediately put on ice to minimize enzymatic reactions. The blood was centrifuged (4°C, 3000 × g for 5 min) and a portion of the plasma was put in 50% sulfosalicyl acid matrices for deproteinization. The plasma was instantly frozen and stored at −80°C until further analyses. All samples obtained were analyzed in a batch. Enrichments and concentrations were simultaneously determined on a LC/ESI/MS/MS system (QTrap 5500 MS (AB Sciex, Foster City, CA) with ExpressHT Ultra LC (Eksigent Div., AB Sciex, Foster City, CA) after derivatization with 9-fluorenylmethoxycarbonyl (Fmoc). Fmoc amino acid derivatives were fragmented to obtain specific and high sensitivity fragments. Enrichments were determined as tracer (labeled substance) / tracee (unlabeled substance). Details of the isotope enrichment analysis for amino acids and system precision were described by van Eijk et al (21). The plasma insulin concentrations were analyzed with a commercially available enzyme-linked immunosorbent assay (ELx808 Absorbance Microplate Reader; BioTek, Winooski, VT).
Calculations for protein metabolism
EAA represents the sum of the measurable α-amino acids threonine, histidine, valine, methionine, isoleucine, phenylalanine (PHE), tryptophan, leucine and lysine. BCAA represents the sum of the three BCAA valine, leucine and isoleucine. The tracer-tracee ratio of PHE and Tyrosine (TYR) reached an isotopic steady state within 1.5 hours of infusion in the postabsorptive state.
To assess whole-body protein kinetics in the postabsorptive state, the following calculations were used:
Whole-body rate of appearance (WbRa) PHE and TYR and 3-Methylhistidine = F/tracer-tracee ratio (TTR) in plasma
Whole-body protein breakdown (WbPB) = WbRa PHE
Whole-body protein synthesis (WbPS) is calculated by subtracting hydroxylation of plasma PHE to TYR from whole-body rate of disappearance (WbRd) (= WbRa PHE under steady-state) (3)
Hydroxylation of PHE into TYR (OHPHE>TYR) = WbRa L-[ring-2H2]-TYR × (TTR L-[ring-2H4]-TYR/TTR L-[ring-2H5]-PHE)
NetPS = WbPS − WbPB
F represents the intravenous tracer infusion rate (μmol · kg ffm−1 ·h−1). Because 3-Methylhistidine is released from myofibrillar protein breakdown, the WbRa of 3-Methylhistidine gives an indirect reflection of muscle protein breakdown (22).
From the start of intake, exogenous WbRa PHE (WbRa PHE coming from the protein meal) and PHE appearance not coming from the protein meal (endogenous WbRa = WbPB) are calculated using the one-pool non-steady state equations of Steele (23), further modified by Proietto et al. (24) and described by Boirie et al. (25). Enrichment is expressed as mole percent excess (MPE).
6. WbRa PHE = [F − pV · ((C1+C2) / 2) · ((MPEiv2 − MPEiv1) / (t2 − t1))] / (MPEiv1+MPEiv2) / 2
7. Exogenous WbRa PHE = WbRa · ((MPEoral1+MPEoral2) / 2) + [pV · ((C1+C2) / 2) · ((MPEoral2 −MPEoral1) / (t2 − t1))] / diet PHE MPE
8. Endogenous WbRa PHE = WbRa PHE − exogenous WbRa PHE − F
F is the intravenous tracer infusion rate (μmol · kg ffm−1 ·h−1), pV (0.125) is the distribution volume of PHE (25), (MPE2 − MPE1) / (t2 − t1) and (C2 − C1) / (t2 − t1) represent the time-dependent variations of plasma PHE enrichment (derived from the intravenous and oral tracer) and concentrations, and (MPE1+MPE2) / 2 and (C1+C2) / 2 are the mean plasma PHE enrichment (from the intravenous and oral tracer) and concentrations between 2 consecutive time points. The enrichment of the oral tracer is measured for each protein meal.
Splanchnic extraction represents the fraction (in %) of ingested PHE taken up by the gut and liver during its first pass and metabolized via oxidation or protein synthesis. Splanchnic extraction is calculated as follows (4):
9. Splanchnic extraction = [Exogenous WbRa PHE/(PHE in protein meal + PHE oral tracer)] * 100%
According to Steele's equations, WbRd PHE is calculated as follows:
10. WbRd PHE = WbRa PHE − (pV · ((C2−C1) / (t2 − t1))
where (C2−C1) / (t2 − t1) is the time difference in plasma PHE concentration between two points and WbRa PHE is as in formula [6]. The rate of total (endogenous + exogenous) PHE utilized for protein synthesis is then calculated as
11. WbPS = WbRd PHE − hydroxylation PHE to TYR in the prandial state for which we used the calculation as represented in formula [4].
WbPS, WbPB and NetPS are expressed as μmol · kg ffm−1 · h−1 or μmol · kg ffm−1 · 4h−1. Protein conversion (i.e. efficiency) was calculated as follows:
12. NetPS/PHE in protein meal *100
The amount of PHE is expressed in μmol · kg ffm−1 and NetPS is expressed in μmol · kg ffm−1 · 4h−1.
Statistical analysis
The power calculation to determine sample size was based on net whole-body protein synthesis measurements done in non-depleted COPD patients (4). We used a pooled standard deviation estimate of approximately 2.4 μmol/kg ffm/h. For nutritionally depleted COPD patients, to detect a change in the metabolic response to a hydrolyzed casein or whey protein meal of 1.8 μmol/kg ffm/h or larger, with an approximate power of 80% and a 5% α-level a sample size of 24 depleted COPD patients was calculated. Due to the high sensitivity of our new analytical equipment, we anticipated that fewer patients were necessary and that 12 subjects would be sufficient. Results are expressed as means ± standard error (SEM). The mean value of the measures of protein kinetics at the time points 70, 80 and 90 min was used as the postabsorptive state and at 105, 120, 135, 150, 180, 210, 240, 270, 300 and 330 min, expressed as area under the curve (AUC) over 4 h, as the postprandial state. If data failed the normality or equal variance test, they were log-transformed where appropriate.
Three-way repeated-measures analysis of variance (three-way RM ANOVA) with time, protein, and leucine addition as factors was used to compare differences between protein meals, leucine additive, and the two different phases of the experiment (postabsorptive versus postprandial) as well as all interactions. Bonferroni post hoc test was applied to evaluate within-time differences between protein meals. Expected mean squares were initially computed for all error terms, but non-significant error terms were pooled into residual error, retaining only significant error terms for testing main effects and interactions. To get insight whether there was a time and/or protein meal effect as well as a time × protein meal interaction for plasma enrichments, amino acid and insulin concentrations and WbRa, we used two-way repeated-measures analysis of variance (two-way RM ANOVO) with time and protein meal as factors, and Bonferroni post hoc test was used when applicable. For comparison of NetPS and PHE intake data were analyzed with 2-tailed tests of significance by using Pearson's correlation coefficients and linear regression. The level of significance was set at p<0.05. The statistical package Statistical Program for the Social Sciences (SPSS version 21) was used for the three-way RM ANOVA data analysis and the statistical package within Graphpad Prism (Version 5.04) was used for additional data analysis. Multiple imputation (MI) with 10 imputed data sets (26) was employed to estimate missing data for the two patients that did not complete all four study days. Each data set was analyzed separately and the ten estimates for each effect and its standard error were averaged according to MI estimation procedures. T-tests for each effect were computed using the pooled estimates.
Results
Twelve COPD patients whom were nutritionally depleted participated in the study and were analyzed for the primary outcome. Two out of 12 patients dropped out early (after one and two study days respectively). Patients (Table 2) had moderate to severe airflow obstruction (FEV1 predicted: 45±4%). Six patients had one or more exacerbations of their disease in the past year of which four required hospitalization.
Table 2. Characteristics of the study populationa.
| Range | |||
|---|---|---|---|
| Gender | (m/f) | 7 / 5 | |
| Age | (y) | 60.9 ± 2.9 | |
| Height | (m) | 1.71 ± 0.03 | 1.55 - 1.94 |
| Weight | (kg) | 64.7 ± 3.9 | 46.9 - 98.8 |
| BMI | (kg/m2) | 22.0 ± 0.7 | 18.1 - 26.3 |
| FFMI males | (kg/m2) | 16.7 ± 0.8 | 14.2 - 19.6 |
| FFMI females | (kg/m2) | 13.3 ± 0.5 | 12.4 - 15.1 |
| FEV1 | (% of predicted) | 45 ± 4 | 20 - 66 |
| Pack years | (y) | 33 ± 3 | 20 - 52 |
Values are means ± SEM. FFMI: fat-free mass index (fat-free mass/height2), FEV1: forced expiratory volume in one second.
Changes in plasma cTTR and amino acid concentrations
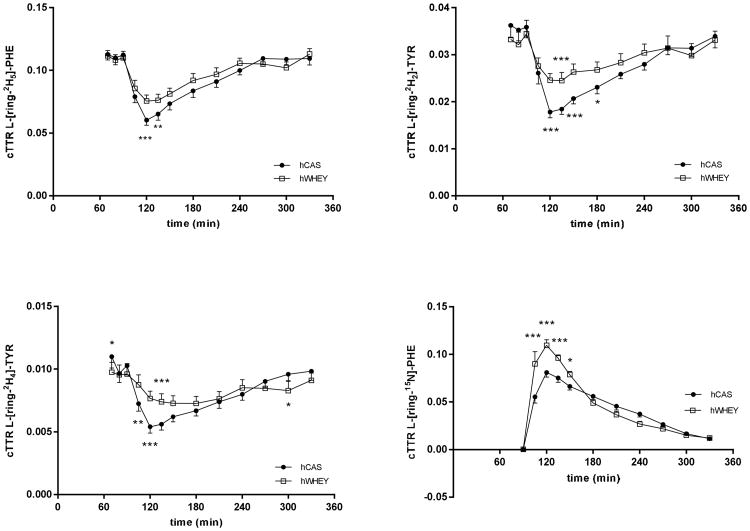
In response to the protein meals there was a time effect for the enrichment of the i.v. tracers L-[ring-2H5]-PHE (Figure 2a), L-[ring-2H2]-TYR (Figure 2b), L-[ring-2H4]-TYR (Figure 2c) and the oral tracer L-[15N]-PHE (added to the protein meal) (Figure 2d) (P<0.0001). A time by protein meal interaction was also found for all of the above. Data of the two protein meals to which leucine was added is not shown as we did not find an effect of leucine addition. A protein meal effect was found only for the enrichment of the oral tracer L-[15N]-PHE (P =0.0003).
Figure 2.
Mean (±SEM) plasma L-[ring-2H5]-Phenylalanine (L-[ring-2H5]-PHE, panel a), L-[ring-2H2]-Tyrosine (L-[ring-2H2]-TYR, panel b), L-[ring-2H4]-Tyrosine (L-[ring-2H4]-TYR, panel c), and L-[15N]-Phenylalanine (L-[15N]-PHE (panel c) enrichments expressed as tracer-tracee ratio corrected for background enrichment (cTTR) before and after intake of a hydrolyzed casein (hCAS) and hydrolyzed whey (hWHEY) protein meal (t=90 min). Plasma L-[ring-2H5]-PHE enrichment: time effect, P<0.0001; time × protein meal interaction, P<0.0001. Plasma L-[ring-2H2]-TYR: time effect, P<0.0001; time × protein meal interaction, P<0.0001. Plasma L-[ring-2H4]-TYR: time effect, P<0.0001; time × protein meal interaction, P<0.0001. Plasma L-[15N]-PHE enrichment: time effect, P<0.0001; protein meal effect, P=0.0003; time × protein meal interaction, P<0.0001. Significance of difference between hCAS and hWHEY: *: P<0.05; **: P<0.01; ***: P<0.001.
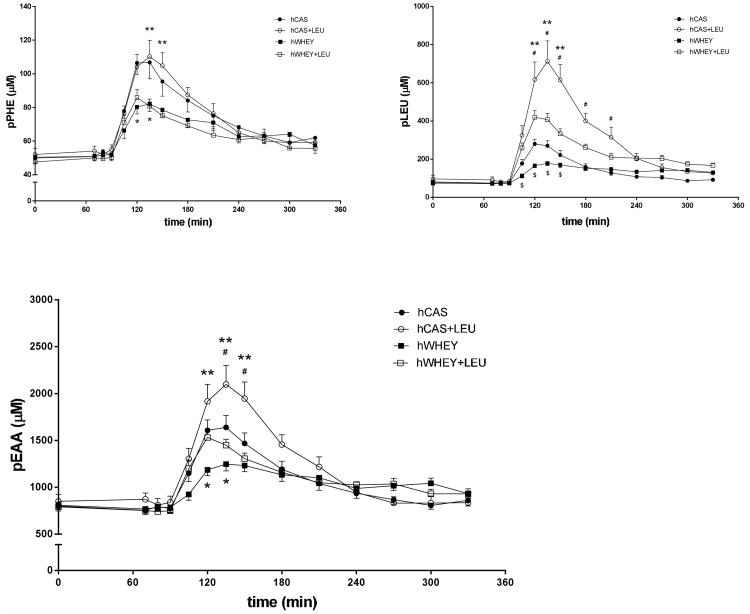
In response to the protein meals, there was a time effect for plasma PHE, leucine and EAA concentrations (Figure 3) (P<0.0001), indicating increases in plasma concentration of these amino acids after intake of the protein meals. There was also a protein meal effect for all above mentioned concentrations, indicating the highest plasma PHE concentrations after intake of the hCAS and hCAS+LEU protein meal (Figure 3a) and the highest plasma leucine and EAA concentration after intake of the hCAS+LEU meal (Figure 3b and c). A time by protein meal interaction was found for the concentration of PHE (P=0.0216), leucine (P<0.0001) and EAA (P<0.0001). We observed a small (not significant) reduction of the meal-related increase in isoleucine and valine concentration when leucine was added to the protein meals (data not shown).
Figure 3.
Mean (±SEM) plasma phenylalanine (pPHE, panel a), leucine (pLEU, panel b) and essential amino acid (pEAA, panel c) concentrations before and after intake of a hydrolyzed casein (hCAS), hydrolyzed casein with leucine addition (hCAS+LEU), hydrolyzed whey (hWHEY), and hydrolyzed whey with leucine addition (hWHEY+LEU) protein meal. pPHE concentration: time effect, P<0.0001; protein meal effect, P<0.0001; time × protein meal interaction, P=0.0216. pLEU concentration: time effect, P<0.0001; protein meal effect, P<0.0001; time × protein meal interaction, P<0.0001. pEAA concentration: time effect, P<0.0001; protein meal effect, P<0.0001; time × protein meal interaction, P<0.0001.*hCAS significantly different from hWHEY, P<0.05. **hCAS+LEU significantly different from hWHEY+LEU, P<0.05. #hCAS significantly different from hCAS+LEU, P<0.05. $hWHEY significantly different from hWHEY+LEU, P<0.05.
Tracer-tracee
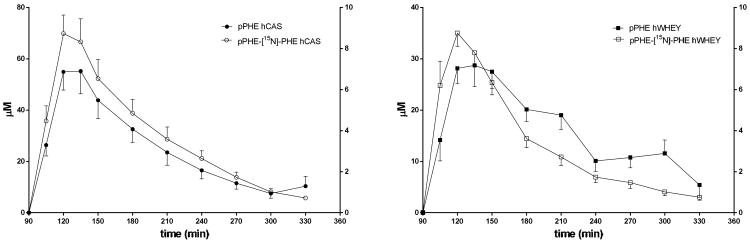
Figure 4 shows the mean plasma L-[15N]-Phenylalanine (tracer) and phenylalanine (tracee) concentration after intake of the hCAS and hWHEY protein meal (corrected for postabsorptive mean concentrations) (panel a and b respectively). Following intake of both protein meals tracer and tracee concentrations rapidly increased. The figure indicates that the tracer and tracee follow a comparable pattern for both meals.
Figure 4.
Mean (±SEM) plasma L-[15N]-Phenylalanine concentration (pL-[15N]-PHE) and phenylalanine concentration (pPHE) after intake of a hydrolyzed casein (hCAS) (panel a) and hydrolyzed whey (hWHEY) (panel b) protein meal.
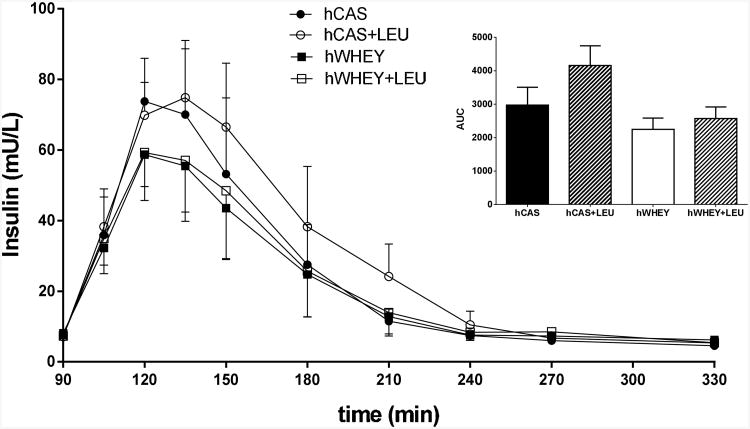
Plasma insulin
Plasma insulin concentrations (Figure 5) rapidly increased after intake of all four protein meals. A protein effect (P=0.0038), but no leucine effect or protein by leucine interaction was found for the postprandial (4h) area under the curve (AUC). This indicates a greater insulin response after intake of the hCAS compared to the hWHEY protein meals.
Figure 5.
Plasma insulin concentration after intake of a hydrolyzed casein protein (hCAS), hydrolyzed casein with leucine addition (hCAS+LEU), hydrolyzed whey (hWHEY), and hydrolyzed whey with leucine addition (hWHEY+LEU) protein meal. Data are also expressed as the 4h postprandial peak area under the curve (AUC). AUC insulin: protein meal effect, P=0.0038.
Splanchnic extraction
The splanchnic extraction of PHE for the hCAS, hWHEY, hCAS+LEU and hWHEY+LEU protein meals were (mean ± SEM): 34±4%, 36±3%, 36±4% and 38±3%, respectively. No significant protein or leucine effects were observed (data not shown).
Whole-body protein kinetics
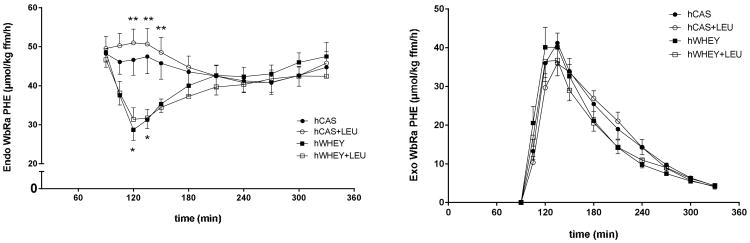
In the postabsorptive state, we found no difference for WbPS, WbPB, NetPS, and MPB (Table 3) between the protein meals. We found a difference in hydroxylation only between the hCAS+LEU and hWHEY+LEU protein meal (P<0.05). WbPB exceeded WbPS in the postabsorptive state, indicating net protein breakdown. There were time effects for endogenous WbRa (=WbPB) (Figure 6a) (P<0.01) and exogenous WbRa (Figure 6b) (P<0.0001) after intake of the protein meals, indicating a decrease in endogenous WbRa and an increase in exogenous WbRa. There was also a protein meal effect for endogenous WbRa (P<0.0001), indicating lower values after intake of the hWHEY compared to the hCAS protein meal. There was a time by protein meal interaction for endogenous WbRa (P<0.0001), showing that at t=120 and 135 min endogenous WbRa was significantly lower in response to the hWHEY vs. the hCAS protein meal and significantly lower in the response to the hWHEY+LEU vs. the hCAS+LEU protein meal from t=120-150 min. A time effect, but no protein meal effect or time by protein meal interaction was found for exo WbRa. Exo WbRa was significantly different from postabsorptive values at t=105-210 min for all protein meals apart from the hCAS+LEU protein meal at t=105 min. At t=240 min only for the hCAS and hCAS+LEU protein meal exo WbRa was still above postabsorptive values.
Table 3. Measures of protein metabolism before and after intake of the hydrolyzed casein (hCAS), hydrolyzed whey (hWHEY), hydrolyzed casein with added leucine (hCAS+LEU) and hydrolyzed whey with added leucine (hWHEY+LEU) protein mealsa.
| hCAS | hCAS+LEU | hWHEY | hWHEY+LEU | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Postabs | Prandial | Postabs | Prandial | Postabs | Prandial | Postabs | Prandial | |
| WbPSb | 44.3 ± 3.3 | 60.0 ± 4.6 | 45.2 ± 3.3 | 60.7 ± 4.4 | 45.1 ± 2.7 | 56.5 ± 3.6 | 43.2 ± 2.9 | 55.2 ± 3.2 |
| WbPBb | 47.8 ± 3.3 | 43.5 ± 3.2 | 49.1 ± 3.3 | 44.5 ± 3.0 | 48.7 ± 2.5 | 39.4 ± 1.9 | 47.2 ± 3.0 | 39.3 ± 2.1 |
| Hydroxylationb | 4.6 ± 1.1 | 7.4 ± 2.2 | 5.4 ± 1.2 | 7.4 ± 1.9 | 4.9 ± 1.6 | 6.5 ± 2.2 | 4.2 ± 1.1 | 6.0 ± 1.5 |
| NetPS | −3.7 ± 0.3 | 16.6 ± 1.8 | −4.6 ± 0.4 | 16.2 ± 1.9 | −4.1 ± 0.4 | 16.2 ± 1.8 | −4.4 ± 0.4 | 15.9 ± 1.6 |
| MPB | 1.60 ± 0.20 | 1.14 ± 0.12 | 1.60 ± 0.15 | 1.08 ± 0.10 | 1.53 ± 0.10 | 1.01 ± 0.06 | 1.57 ± 0.13 | 1.09 ± 0.08 |
Values are means ± SEM and expressed in μmol · kg ffm−1 · h−1. Data show postabsorptive values (postabs) and values for the 4 h prandial state (averaged per hour) following intake of the hCAS, hCAS+LEU, hWHEY or hWHEY+LEU protein meals. FFM: fat-free mass, MPB: myofibrillar protein breakdown, NetPS: net protein synthesis, WbPB: whole-body protein breakdown [reflects the rate of appearance of endogenous phenylalanine], WbPS: whole-body protein synthesis.
A significant protein meal effect was observed in the prandial state, P<0.01.
Figure 6.
Mean (±SEM) endogenous (endo) (panel a) and exogenous (exo) (panel b) whole-body rate of appearance of phenylalanine (WbRa PHE) after intake of a hydrolyzed casein protein (hCAS), hydrolyzed casein with leucine addition (hCAS+LEU), hydrolyzed whey (hWHEY), and hydrolyzed whey with leucine addition (hWHEY+LEU) protein meal. Endo WbRa: time effect, P=0.0013; protein meal effect, P<0.0001; time × protein meal interaction, P<0.0001. Exo WbRa: time effect, P<0.0001. *hCAS significantly different from hWHEY, P<0.01. **hCAS+LEU significantly different from hWHEY+LEU, P<0.05.
While lower values for WbPB were found after intake of the hWHEY protein meal compared to the hCAS protein meal and adversely higher values for WbPS after intake of the hCAS protein meal, the net anabolic response was comparable (Table 3).
Relation net whole-body protein synthesis and PHE intake
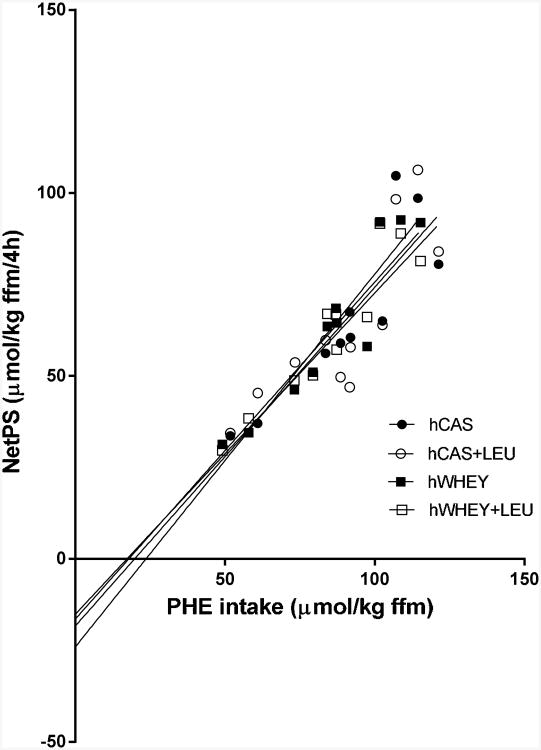
There was a significant and distinct relationship between netPS and the amount of PHE per kg FFM provided to the patients (Figure 7). On average (as no significant differences were found between the meals) postprandial PHE (i.e. protein) utilization was (mean ± SEM) 72 ± 2% (data not shown). There was no relationship between age and netPS/amount of PHE per kg FFM (data not shown).
Figure 7.
Correlation between net protein synthesis (NetPS) expressed as the total amount over four hours and phenylalanine intake (PHE intake) of a hydrolyzed casein (hCAS) (r = 0.88; NetPS = 0.93 · PHE intake − 18.3), hydrolyzed casein with leucine addition (hCAS+LEU) (r=0.83; net NetPS = 0.88 · PHE intake − 15.1), hydrolyzed whey (hWHEY) (r=0.92; NetPS = 0.84 · PHE intake − 16.4), and hydrolyzed whey with leucine addition (hWHEY+LEU) (r=0.94; NetPS = 1.02 · PHE intake − 24.0) protein meal. All correlations were statistically significant (P<0.01) and linear regression showed no significant difference between the slopes (P=0.9374).
Discussion
In the present study, we found that a bolus meal of casein vs. whey protein, containing a similar amount of carbohydrates and with comparable absorption characteristics, stimulated net whole-body protein synthesis comparably and efficiently in COPD patients characterized by nutritional depletion. Co-ingestion of leucine did not stimulate the net whole-body protein synthesis response further.
Use of hydrolyzed proteins
Casein and whey are both complete high-quality milk proteins with high amounts of EAA, but display very different digestion and absorption characteristics (10, 11). Casein is considered a “slow” and whey a “fast” protein. This difference in absorption rate is known to affect postprandial plasma amino acid availability and protein kinetics (10, 11) and can therefore mask the direct effects of differences in the amino acid composition between the proteins. We have previously studied the anabolic properties of casein and whey protein in normal-weight COPD patients (27). In that study, we provided the proteins in a “continuous” way (through frequent small (sip) feeds) to eliminate absorption differences between the proteins. The observed higher anabolic response with casein protein intake was therefore related to differences in the amino acid profile between the proteins. In the present study, we preferred to examine the anabolic response to a bolus meal of casein and whey protein as it better represents daily life food intake. Hydrolyzed proteins were used as hydrolysis converts the proteins into smaller tri- and dipeptides and free amino acids which increases the absorption rate (13). Furthermore, it made it possible to add free amino acids to the protein meals (leucine and the oral tracer L-[15N]-PHE), thereby simultaneously eliminating absorption differences between the proteins, free amino acids and tracer.
Protein kinetic response
The comparable exogenous WbRa of PHE between the hCAS and hWHEY protein meal indicated that both meals (i.e. proteins) were digested equally fast. This confirms that using hydrolyzed proteins is a good approach to compare the anabolic properties between milk proteins based solely on differences in amino acid composition. We observed a different protein kinetic response to the hCAS and hWHEY protein meal. Whole-body protein synthesis was stimulated more by the hCAS protein meal, likely related to a greater increase in plasma amino acid availability, and whole-body protein breakdown was reduced more by the hWHEY protein meal. Although the hWHEY protein meal contained more EAA, the lower postprandial plasma EAA concentration after intake of the hWHEY vs. the hCAS protein meal is likely associated with the higher postprandial reduction in protein breakdown after intake of the hWHEY protein meal. It would be helpful in development of nutritional interventions in COPD to have a better understanding of the mechanisms underlying the different kinetic response to the hCAS and hWHEY protein meal. The greater reduction in whole-body protein breakdown after the hWHEY protein meal was not similarly reflected by a reduced 3-methylhistidine production. We hypothesize that myofibrillar protein breakdown is more slowly affected after a protein meal in comparison to the more labile protein pools in muscle and that we were not able to find any changes using only a four hour observation period. In general, when carbohydrates are added to a pure protein meal it reduces the increase in postprandial plasma amino acid concentrations and enhances the retention of dietary amino acids in the splanchnic bed (15). It also reduces urea production and amino acid uptake by the liver, suggesting a decrease in liver protein breakdown (15). More research is needed to study the effects of carbohydrate addition on protein kinetics when added to hydrolyzed milk protein meals.
Anabolic capacity of milk proteins
The present study showed that COPD patients defined as nutritionally depleted are still capable of producing a high anabolic response to a high-quality milk protein meal. A comparable anabolic response to casein and whey protein is in contrast with our previous study in normal-weight COPD patients, which showed higher anabolism during “continuous” (sip) feeding of casein protein (27). Differences in the nutritional status of COPD patients, and the mode of administering protein meals (bolus versus sip feeding), resulting in different peak levels for plasma amino acid and insulin concentrations might be potential factors explaining the differences between the studies. Further research studying the dose-response curve between hydrolyzed casein and whey protein meals in relation to net whole-body protein synthesis is therefore warranted. Furthermore, we found a higher insulin response after casein protein compared to whey protein. It is unclear whether the higher plasma amino acid availability after casein protein intake is responsible for the greater insulin response and in this way contributed to a greater stimulation of whole-body protein synthesis, or if other intrinsic factors may play a role.
No anabolic effect of leucine co-ingestion
Adding leucine to the protein meals did not improve the whole-body net protein synthesis response. Therefore, we hypothesize that the use of hydrolyzed high-quality milk proteins and the addition of carbohydrates to the protein meals makes additional leucine to be of no benefit. A recent review discusses that a saturating dose of whey protein, containing 2-3 g leucine is sufficient to stimulate muscle protein synthesis in healthy young adults and that greater amounts of leucine do not result in a greater synthetic response (28). Our data, on whole-body level, suggest that 1.28 g of leucine is already sufficient to optimally stimulate protein anabolism in older adults with COPD when it is part of a hydrolyzed high quality protein meal with carbohydrates. That we did not observe a leucine effect on the insulin response is most likely associated with the already highly stimulated insulin response both by the rapid postprandial increase in plasma amino acids and the addition of carbohydrates. Some studies that found an anabolic effect of leucine did not add carbohydrates to the protein/amino acid meals (9, 13, 29). Recent research show that the effects of leucine are almost comparable to the effects of adding carbohydrates (29, 30), while it is unknown whether adding both leucine and carbohydrates is of additional benefit. We conclude based on our study that this is not the case and that protein anabolism is optimally stimulated when you use a high quality hydrolyzed milk protein meal with added carbohydrates. However, it remains to be determined whether adding carbohydrates alone is as effective as adding leucine. Some of the studies describing a protein anabolic effect of leucine co-ingestion in older adults also have the limitation that muscle protein breakdown is not measured (9, 13), making calculation of net muscle protein balance impossible (14) and thus the effect on protein anabolism of leucine cannot be established.
Previously, we observed an increase in WbPS in normal-weight COPD patients after adding BCAA to a soy protein meal (31). This positive effect on protein synthesis without affecting protein breakdown might be related to the fact that by adding the BCAA, the overall amount of EAA became higher and probably more balanced. Previous studies show that selective leucine administration reduces plasma valine and isoleucine concentrations (32, 33), related to an enhanced splanchnic bed uptake (32) and that therefore all three BCAA are needed to obtain additional protein anabolism. Therefore, it is unclear whether the addition of leucine alone in our study prohibited additional anabolism, due to the mild observed BCAA antagonism.
Relation between protein intake and net whole-body protein synthesis
The amount of muscle mass was variable in the studied patients, but the amount of protein that was provided to each patient was equal. Therefore, more dietary amino acids were available to the periphery of those with a low FFM. It appears that there is a strong correlation between the amount of PHE per kg FFM given to the patients in this study and the net whole-body protein synthesis response. The higher the PHE intake the higher the response is. PHE is one of the EAA of which the amount is present in a fixed proportion to the other EAA in casein and whey protein. Therefore the amount of EAA in high-quality milk proteins directly relates to net whole-body protein synthesis. With these results we must conclude that the amount of EAA that is available in the protein meal determines the protein anabolic effect of a high-quality protein meal in COPD patients with nutritional depletion. This is in line with the results of arecent study we performed in children with Cystic Fibrosis (34). The efficiency of the studied milk protein meals was very high in this study (conversion rate of PHE was >70%) and much higher than observed after continuous (sip) feeding of a low-quality soy protein meal in a group of normal weight COPD patients (conversion rate of PHE is approx. 40%) (31). The lower and less balanced EAA and BCAA content of soy protein is likely associated with this lower conversion rate. The mode of administration might be a contributing factor as well, since a rapid increase in extracellular plasma amino acids as seen after a bolus meal is an important stimulus for protein synthesis (11).
Limitations of the study
The current study focused on whole-body protein turnover in relation to muscle wasting in COPD. Although determining muscle protein synthesis and breakdown rate is the preferred method to investigate muscle loss in COPD, it was not feasible to obtain muscle biopsies on the four test days for the subjects. Therefore, we studied whole-body myofibrillar protein breakdown using the WbRa of 3-methylhistidine, which represents muscle myofibrillar protein breakdown for ≈ 90% (22). The inclusion of a healthy control group would have improved our study design, because we could have compared the effectiveness of the protein meals between COPD patients with nutritional depletion and healthy older adults. This would have helped us to better determine the clinical relevance of the protein meals tested in this study. However, even if the response in COPD patients was less compared to healthy controls, the protein meals induced an increase in net protein synthesis and we therefore consider them effective. Other protein meals, with e.g. other proteins could still be more anabolic, have a lower splanchnic extraction and have an even higher net conversion rate.
In conclusion, the results of the present study show that after elimination of absorption differences, a bolus meal of casein and whey protein with added carbohydrates comparably and efficiently stimulate whole-body protein anabolism in COPD patients with nutritional depletion and that co-ingestion of leucine does not stimulate the whole-body anabolic response further. Therefore, we suggest that hydrolyzed milk proteins are powerful anabolic proteins that can be used in rehabilitation programs to achieve muscle protein accretion over time and can improve overall outcome and physical performance in COPD patients.
Acknowledgments
RJ was involved in the conduct of the research, data analysis and writing of the manuscript. NEPD and MPKJE designed the research and were involved in the conduct of the research, data analysis and writing of the manuscript. MLE and PJA were involved in the recruitment of study participants. None of the authors had any financial or personal interest in any company or organization sponsoring the research, including advisory board affiliations.
The project described was sponsored by award number R-01HL095903 from the National Heart, Lung and Blood Institute, award number NIH S10RR027047 from the National Center For Research Resources and award number 1UL1RR029884 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, National Heart, Lung and Blood Institute or the National Institutes of Health.
This trial was registered at clinicaltrials.gov as NCT01154400.
List of abbreviations
- BCAA
branched-chain amino acids
- COPD
Chronic Obstructive Pulmonary Disease
- EAA
essential amino acid
- FEV1
forced expiratory volume in one second
- FFM
fat-free mass
- FFMI
fat-free mass index
- hCAS
hydrolyzed casein protein
- hCAS+LEU
hydrolyzed casein protein with added leucine
- hWHEY
hydrolyzed whey protein
- hWHEY+LEU
hydrolyzed whey protein with added leucine
- MPE
mole percent exces
- NetPS
net protein synthesis
- PHE
phenylalanine
- SEM
standard error of the mean
- TTR
tracer-tracee ratio
- TYR
tyrosine
- WbPB
whole-body protein breakdown
- WbPS
whole-body protein synthesis
- WbRa
whole-body rate of appearance
- WbRd
whole-body rate of disappearance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005 Jul;82(1):53–9. doi: 10.1093/ajcn.82.1.53. Epub 2005/07/09. eng. [DOI] [PubMed] [Google Scholar]
- 2.Engelen MP, Schols AM. Altered amino acid metabolism in chronic obstructive pulmonary disease: new therapeutic perspective? Curr Opin Clin Nutr Metab Care. 2003 Jan;6(1):73–8. doi: 10.1097/00075197-200301000-00011. eng. [DOI] [PubMed] [Google Scholar]
- 3.Engelen MP, Deutz NE, Wouters EF, Schols AM. Enhanced levels of whole-body protein turnover in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000 Oct;162(4 Pt 1):1488–92. doi: 10.1164/ajrccm.162.4.2002045. [DOI] [PubMed] [Google Scholar]
- 4.Engelen MP, Rutten EP, De Castro CL, Wouters EF, Schols AM, Deutz NE. Altered interorgan response to feeding in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2005 Aug;82(2):366–72. doi: 10.1093/ajcn.82.2.366. [DOI] [PubMed] [Google Scholar]
- 5.Rutten EP, Franssen FM, Engelen MP, Wouters EF, Deutz NE, Schols AM. Greater whole-body myofibrillar protein breakdown in cachectic patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2006 Apr;83(4):829–34. doi: 10.1093/ajcn/83.4.829. [DOI] [PubMed] [Google Scholar]
- 6.Phillips SM, Tang JE, Moore DR. The role of milk- and soy-based protein in support of muscle protein synthesis and muscle protein accretion in young and elderly persons. J Am Coll Nutr. 2009 Aug;28(4):343–54. doi: 10.1080/07315724.2009.10718096. Epub 2010/04/07. eng. [DOI] [PubMed] [Google Scholar]
- 7.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003 Aug;78(2):250–8. doi: 10.1093/ajcn/78.2.250. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Experimental gerontology. 2006 Feb;41(2):215–9. doi: 10.1016/j.exger.2005.10.006. eng. [DOI] [PubMed] [Google Scholar]
- 9.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. American journal of physiology. 2006 Aug;291(2):E381–7. doi: 10.1152/ajpendo.00488.2005. eng. [DOI] [PubMed] [Google Scholar]
- 10.Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P, et al. The digestion rate of protein is an independent regulating factor of postprandial protein retention. American journal of physiology. 2001 Feb;280(2):E340–8. doi: 10.1152/ajpendo.2001.280.2.E340. eng. [DOI] [PubMed] [Google Scholar]
- 11.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proceedings of the National Academy of Sciences of the United States of America. 1997 Dec 23;94(26):14930–5. doi: 10.1073/pnas.94.26.14930. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dangin M, Guillet C, Garcia-Rodenas C, Gachon P, Bouteloup-Demange C, Reiffers-Magnani K, et al. The rate of protein digestion affects protein gain differently during aging in humans. The Journal of physiology. 2003 Jun 1;549(Pt 2):635–44. doi: 10.1113/jphysiol.2002.036897. Epub 2003/04/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pennings B, Boirie Y, Senden JM, Gijsen AP, Kuipers H, van Loon LJ. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr. 2011 Mar 2; doi: 10.3945/ajcn.110.008102. Epub 2011/03/04. Eng. [DOI] [PubMed] [Google Scholar]
- 14.Deutz Ne, Wolfe RR. Is there a maximal anabolic response to protein intake with a meal? Clinical Nutrition. (0) doi: 10.1016/j.clnu.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deutz NE, Ten Have GA, Soeters PB, Moughan PJ. Increased intestinal amino-acid retention from the addition of carbohydrates to a meal. Clin Nutr. 1995 Dec;14(6):354–64. doi: 10.1016/s0261-5614(95)80053-0. Epub 1995/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 16.Fuller MF, Weekes TE, Cadenhead A, Bruce JB. The protein-sparing effect of carbohydrate. 2. The role of insulin. Br J Nutr. 1977 Nov;38(3):489–96. doi: 10.1079/bjn19770114. Epub 1977/11/01. eng. [DOI] [PubMed] [Google Scholar]
- 17.Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011 Available from: http://www.goldcopd.org/
- 18.Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998 Jun;157(6 Pt 1):1791–7. doi: 10.1164/ajrccm.157.6.9705017. eng. [DOI] [PubMed] [Google Scholar]
- 19.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999 Jan;159(1):179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 20.Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism: clinical and experimental. 1981 Sep;30(9):936–40. doi: 10.1016/0026-0495(81)90074-3. eng. [DOI] [PubMed] [Google Scholar]
- 21.van Eijk HM, Suylen DP, Dejong CH, Luiking YC, Deutz NE. Measurement of amino acid isotope enrichment by liquid chromatography mass spectroscopy after derivatization with 9-fluorenylmethylchloroformate. J Chromatogr B Analyt Technol Biomed Life Sci. 2007 Sep 1;856(1-2):48–56. doi: 10.1016/j.jchromb.2007.05.020. Epub 2007/06/15. eng. [DOI] [PubMed] [Google Scholar]
- 22.Ballard FJ, Tomas FM. 3-Methylhistidine as a measure of skeletal muscle protein breakdown in human subjects: the case for its continued use. Clin Sci (Lond) 1983 Sep;65(3):209–15. doi: 10.1042/cs0650209. eng. [DOI] [PubMed] [Google Scholar]
- 23.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Annals of the New York Academy of Sciences. 1959 Sep 25;82:420–30. doi: 10.1111/j.1749-6632.1959.tb44923.x. eng. [DOI] [PubMed] [Google Scholar]
- 24.Proietto J, Rohner-Jeanrenaud F, Ionescu E, Terrettaz J, Sauter JF, Jeanrenaud B. Non-steady-state measurement of glucose turnover in rats by using a one-compartment model. Am J Physiol. 1987 Jan;252(1 Pt 1):E77–84. doi: 10.1152/ajpendo.1987.252.1.E77. Epub 1987/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 25.Boirie Y, Gachon P, Corny S, Fauquant J, Maubois JL, Beaufrere B. Acute postprandial changes in leucine metabolism as assessed with an intrinsically labeled milk protein. Am J Physiol. 1996 Dec;271(6 Pt 1):E1083–91. doi: 10.1152/ajpendo.1996.271.6.E1083. Epub 1996/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 26.Schafer JL. Multiple imputation: a primer. Statistical methods in medical research. 1999 Mar;8(1):3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 27.Engelen MP, Rutten EP, De Castro CL, Wouters EF, Schols AM, Deutz NE. Casein protein results in higher prandial and exercise induced whole body protein anabolism than whey protein in Chronic Obstructive Pulmonary Disease. Metabolism: clinical and experimental. 2012 Apr 16; doi: 10.1016/j.metabol.2012.03.001. Epub 2012/04/20. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Churchward-Venne TA, Burd NA, Phillips SM. Nutritional regulation of muscle protein synthesis with resistance exercise: strategies to enhance anabolism. Nutrition & metabolism. 2012;9(1):40. doi: 10.1186/1743-7075-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wall BT, Hamer HM, de Lange A, Kiskini A, Groen BB, Senden JM, et al. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin Nutr. 2012 Sep 20; doi: 10.1016/j.clnu.2012.09.002. Epub 2012/10/10. Eng. [DOI] [PubMed] [Google Scholar]
- 30.Hamer HM, Wall BT, Kiskini A, de Lange A, Groen BB, Bakker JA, et al. Carbohydrate co-ingestion with protein does not further augment post-prandial muscle protein accretion in older men. Nutrition & metabolism. 2013 Jan 25;10(1):15. doi: 10.1186/1743-7075-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engelen MP, Rutten EP, De Castro CL, Wouters EF, Schols AM, Deutz NE. Supplementation of soy protein with branched-chain amino acids alters protein metabolism in healthy elderly and even more in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2007 Feb;85(2):431–9. doi: 10.1093/ajcn/85.2.431. eng. [DOI] [PubMed] [Google Scholar]
- 32.Hagenfeldt L, Eriksson S, Wahren J. Influence of leucine on arterial concentrations and regional exchange of amino acids in healthy subjects. Clin Sci (Lond) 1980 Sep;59(3):173–81. doi: 10.1042/cs0590173. eng. [DOI] [PubMed] [Google Scholar]
- 33.Eriksson S, Hagenfeldt L, Wahren J. A comparison of the effects of intravenous infusion of individual branched-chain amino acids on blood amino acid levels in man. Clin Sci (Lond) 1981 Jan;60(1):95–100. doi: 10.1042/cs0600095. eng. [DOI] [PubMed] [Google Scholar]
- 34.Engelen MP, Com G, Wolfe RR, Deutz NE. Dietary essential amino acids are highly anabolic in pediatric patients with cystic fibrosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2013 Jan 25; doi: 10.1016/j.jcf.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]