Abstract
Objective
To investigate in vitro adipocyte differentiation in baboon fetuses in response to reduced maternal nutrition.
Design
Cross-sectional comparison of adipocyte differentiation in normally grown fetuses and fetuses of pregnant baboons fed 70% control global diet from 30 days of pregnancy to term.
Subjects
Control (CTR) fetuses of ad libitum fed mothers (5 females and 5 males) and fetuses of mothers fed the 70% global diet eaten by CTR (MNR, 5 females and 5 males). The expression of genes/proteins involved in adipogenesis (PPARγ, FABP4 and adiponectin) and brown adipose tissue development (UCP1, TBX15 and COXIV) were determined in in vitro differentiated stromal-vascular cultures from subcutaneous abdominal, subcutaneous femoral, and omental adipose tissue depots. Adipocyte number per area (mm2) was determined histologically to assist in evaluating adipocyte size.
Results
Maternal suboptimal nutrition suppressed growth of male but not female fetuses and led to adipocyte hypertrophy accompanied by increased markers of white and particularly brown-type adipogenesis in male but not female fetuses.
Conclusion
Adipose tissue responses to fetal nonhuman primate under nutrition are sexually dimorphic. While female fetuses adapt adequately, males enhance pathways involved in white and brown adipose tissue development but are unable to compensate for a delayed development of adipose tissue associated with intrauterine growth restriction. These differences need to be considered when assessing developmental programming of adiposity in response to sub-optimal maternal nutrition.
Keywords: Adipocyte, adipogenesis, preadipocyte, fetal programming, nonhuman primate fetus, sexual dimorphism
Introduction
Enlargement of upper-body adipose tissue depots, either visceral or truncal subcutaneous depots, in obese individuals is a risk factor for metabolic dysfunction in diabetes, obesity and atherosclerosis whereas lower-body adiposity appears protective1–5. Although men tend to store lipid more in the visceral depot and women in the lower-body, both men and women demonstrate marked variability regarding regional fat distribution. Epidemiological studies indicate that a suboptimal intrauterine environment – including poor maternal nutrition, predisposes to diabetes, visceral obesity and metabolic syndrome6 suggesting that adiposity levels and fat distribution phenotype could be programmed prenatally. Current research efforts are focused on elucidating the alterations in adipose tissue development, hormone levels, and epigenome (reviewed in 7) that may lead to adult obesity. However, there are limited data on the influence of poor maternal nutrition on the variation in maturation of fetal adipose tissue from different depots that may contribute to the development of specific distinct body fat distribution phenotypes later in life.
Adipose tissue mass is a function of adipocyte size (hypertrophy) and number (hyperplasia). The contribution of each may vary among fat depots. Fat cell number depends on the abundance of adipocyte precursor cells and ability of adipocyte progenitor cells (preadipocytes) to form new fat cells through proliferation and adipocyte differentiation (adipogenesis). Studies in mice and pigs show that the formation of the pool of adipocyte precursors through commitment of mesenchymal stem cells to adipocyte lineage and the preadipocyte proliferation starts prenatally within the mural compartment of the adipose tissue vasculature (a progenitor niche), which further governs adipocyte differentiation8–10. Adipogenesis in perirenal adipose tissue during fetal development and early postnatal life in sheep undergoes profound modifications11. Adipogenesis starts with a phase of intense proliferative activity of primordial adipose tissue followed by adipocyte differentiation characterized by dominant features of brown adipose tissue (pronounced expression of the specific marker UCP1) in late gestation to meet the increased need for heat production associated with the changes in the temperature from ~40°C in the womb to the lower temperature of the extrauterine environment. After birth, there is a gradual transformation of brown to white phenotype of adipogenesis characterized by a complete loss of UCP1 expression to adapt to the new diet containing higher amounts of lipids11. Thus, the fetal and early postnatal periods appear to be a critical time for the enrichment of the adipocyte precursor pool and to accomplish functional adjustments.
To improve understanding of effects of maternal suboptimal nutrition on adipose tissue function, we examined the impact of moderate maternal nutrient reduction (MNR) on in vitro adipocyte differentiation in adipose-derived stromal-vascular cells (ASCs) from omental, subcutaneous abdominal and femoral adipose tissue depots in control normally grown baboon fetuses (CTR) of well-nourished mothers and fetuses of mothers fed 70% global diet of CTR from 30 d pregnancy to term (MNR), a nutrient challenge that leads to intrauterine growth restriction (IUGR) 12 and a pre-diabetic phenotype by puberty13. We hypothesized that decreased fetal nutrient availability would accelerate the brown-to-white alteration in differentiation of adipose tissue in a fetal sex dependent manner.
Materials and methods
Animal management and sample collection
Baboon (Papio species) singleton pregnancies were studied at the Southwest National Primate Research Center at the Texas Biomedical Research Institute (TBRI). Healthy female baboons of similar body weights (10–15 kg) were randomly assigned to outdoor group cages and maintained in social groups of 10–16 with a vasectomized male. At the end of the acclimation period (30 days), a fertile male was placed in each breeding cage. Pregnancy was dated initially by timing of ovulation and changes in sex skin color and confirmed at 30 days of gestation (0.16G; term, ~184 days) by ultrasonography. Details of animal housing and environmental enrichment have been published elsewhere14. The model of 30% global maternal nutrient reduction from 30 days of pregnancy to term has been described in detail15. The pregnant baboons were fed Purina Monkey Diet 5038 containing protein 15.7%, fat 6% by acid hydrolysis, and glucose 0.29% (the full composition of Monkey Diet 5038 can be found at http://labdiet.com/pdf/5037-5038.pdf). Cesarean sections were performed under general anesthesia using standard techniques as previously described16. Fetuses were euthanized by exsanguination while still under general anesthesia. Mothers were allowed to recover from surgery and returned to their group housing. Paired fetal adipose tissue samples from omental (OM), subcutaneous abdominal (scA), and subcutaneous femoral (scF) regions were collected from twenty near-term (165 days gestation (dG)) baboon fetuses. We studied CTR fetuses of ad libitum fed mothers (5 female and 5 males) and fetuses of mothers fed the 70% global diet eaten by CTR (MNR, 5 female and 5 males). All procedures were approved by the TBRI and University of Texas Health Science Center, San Antonio Institutional Animal Care and Use Committees and studies were conducted in AAALAC accredited facilities. Tissues were removed from the fetus under aseptic conditions, placed in Hank's buffered salt solution and shipped at room temperature to the Pennington Biomedical Research Center.
Adipocyte differentiation
Within ~24 h from collection, adipose tissue was digested enzymatically and adipose derived stromal-vascular cells (ASCs) isolated as previously described17. Cultures were expanded in 10% fetal bovine serum (FBS) and third passages were frozen until samples from all fetuses were available for batch processing in a single assay.
Frozen adipose tissue culture samples were thawed and further expanded in culture plates coated with a soluble extract of Engelbreth-Holm-Swarm tumors (E-C-L; Millipore, cat No. 08-110), 5µg/cm2. Upon reaching confluence, cells were switched to a differentiation cocktail comprised of DMEM-F12 (1:1) medium supplemented with 3% FBS, 10 mg/mL transferin, 33 µM biotin, 17 µM calcium pantothenate, 0.5 µM insulin, 0.1 µM dexamethasone, 0.2 nM tri-iodo-thyronine, 60 µM indomethacin, 1 µM roziglitazone, and 0.5 M IBMX (the last three components for the first 3 days only) for 9 days. Cells were harvested for RNA and protein isolation.
Quantitative real-time PCR
RNA was isolated and cleaned using RNeasy kit (QIAGEN, Valencia, CA). The yield and purity RNA were determined using Nanodrop® ND1000. qRT-PCR was used to determine the relative gene expression levels of fatty acid binding protein 4 (FABP4, Hs01086177_m1), adiponectin (Hs00605917_m1), peroxisome proliferator-activated receptor gamma (PPARγ, Hs01115513_m1), uncoupling protein 1 (UCP1, Hs00222453_m1), and T-box 15 (TBX15, Hs00537087_m1) after ASC differentiation. TBP (Hs99999910_m1) was used as an internal control. Of note, all the primers were from Applied Biosystems (Life Technologies, US). Fifty ng of cDNA template per sample was amplified on the ABI prism 7900HT by qPCR. The gene expression was determined in relation to the mean value for the subcutaneous abdominal ACS using the ΔΔCt method.
Immunoblotting
At the end of the differentiation protocol, ASC cells were harvested in a non-denaturing buffer containing 150 mM NaCl, 10 mM Tris pH 7.4, 1mM EGTA, 1mM EDTA, 1% Triton-X 100, 0.5% Igepal CA-630, 1 µM phenylmethylsulfonyl fluoride (PMSF), 1 µM pepstatin, 50 trypsin inhibitory milliunits of aprotinin, 10 µM leupeptin, and 2 mM sodium vanadate and frozen. Next, the samples were thawed, needled, and centrifuged at 14,000 × g at 4°C for 10 minutes. Supernatants containing whole cell extracts were analyzed for protein content using bicinchoninic acid assay. Three males and three females from each CTR and MNR, which has the largest, smallest and the median values of the respective mRNA levels were selected for the Western blotting. Samples from the three depots of the individual fetuses were pooled and 50 µg of the samples were loaded on to the 10% polyacrylamide gel. Protein from brown adipose tissue of mice (30 µg) was also loaded as a positive control for UCP1. Proteins were then transferred to a PVDF membrane and were probed with antibodies that recognize UCP1 [kindly donated by Dr. Gettys, Pennington Biomedical Research Center, Baton Rouge, LA; see more details in 18, PPARγ (Santa Cruz #Sc-22022, dilution 1:200; run on a separate blot), FABP4 (R&D-AF804, 1:2000), peroxisome proliferator-activated receptor gamma, co-activator 1 alpha (PGC1α; Abcam-ab77210, 1:1000), cytochrome c oxidase subunit 4 (COXIV; Cell Signaling-4844S, 1:1000), and tubulin (loading control; Cell Signaling-2148S), followed by secondary antibody conjugated with horseradish peroxidase. Signals were detected by enhanced chemiluminescence, and quantitated using AlphaEaseFC analyzer software and normalized to tubulin in the corresponding blot.
Histological analysis of adipose tissue
At necropsy white adipose tissues from OM, scA and scF depots of male and female baboon fetuses were fixed in 4% paraformaldehyde overnight and paraffin embedded. Sections (5 µm) were stained with hematoxylin and eosin. Images from 5 fetuses from each sex in both treatment groups were collected using a Hamamatsu NanoZoomer Digital Slide Scanning System (Hamamatsu City, Japan). Adipocyte lobules were manually outlined and adipocytes within these clusters counted using 20X magnification and Image J (NIH) software by an investigator blinded to the tissue source. Adipocyte number was expressed per unit area (mm2). By this method, a larger adipocyte number per unit area signifies smaller adipocyte size.
Statistical analysis
Expression of adipogenesis-related genes was analyzed by two-way ANOVA, in which we used 1) maternal diet, depot and maternal diet × depot interaction and 2) maternal diet, sex and maternal diet × sex interaction as fixed effects and the baboon fetus ID as a random effect, followed by Tukey adjustment for the pair-wise comparisons. The effect of maternal diet on protein expression was analyzed for each sex using Student’s t-test. The effect of maternal diet and adipose tissue depot and their interaction on adipocyte number per unit area (an indirect inverse measure of adipocyte size) were analyzed for each sex and then combined by two-way ANOVA. SAS (Version 9.1; SAS Institute, Cary, NC) was used for analysis. Data are expressed as Mean ± SEM and significance set at P < 0.05.
Results
Fetal Morphometry
Male MNR fetuses had lower weight and abdominal circumference than CTR males (762 ± 24 g vs. 884 ± 49 g, p = 0.03 and 13.5 ± 0.3 cm vs. 15.5 ± 0.6 cm, p = 0.01, respectively). Body weight and abdominal circumference were similar between MNR and CTR female fetuses (752 ± 43 g vs. 794 ± 40 g, p = 0.2 and 13.9 ± 0.6 cm, vs. 14.7 ± 0.5 cm, p = 0.2, respectively). The crown-rump length was similar in CTR and MNR fetuses of both sexes (males: CTR, 27.4 ± 0.9 cm vs. MNR, 26.4 ± 0.8 cm, p = 0.5 and females: CTR, 25.3 ± 1.9 cm vs. MNR, 26.4 ± 1.6 cm, p = 0.7).
Expression of white and brown adipogenesis-related genes
Although the maternal diet and depot each had no effect on the gene expression of terminal adipogenic genes, we found a significant interaction between diet and the fetal sex (Figure 1); i.e. maternal suboptimal nutrition increased the expression of adipogenesis-related genes in male but not female fetuses. Specifically, while expression of adipogenic genes in CTR female and male fetuses was similar, expression of the classical adipogenic transcription factor PPARγ and its targets FABP4, adiponectin, and to a remarkable degree UCP1 was higher in male MNR compared to female fetuses (Figure 1). Likewise, we found a significant interaction between diet and the fetal sex regarding TBX15 gene expression (Figure 1). Specifically, the expression of TBX15 was higher in CTR female vs. male fetuses (p = 0.03). MNR females showed lower TBX15 expression than female CTR (p = 0.03) as opposed to males, in whom MNR was associated with a trend to increased expression (p = 0.08). Thus, the expression of TBX15 tended (p = 0.09) to be lower in female vs. male fetuses in the MNR group.
Figure 1.
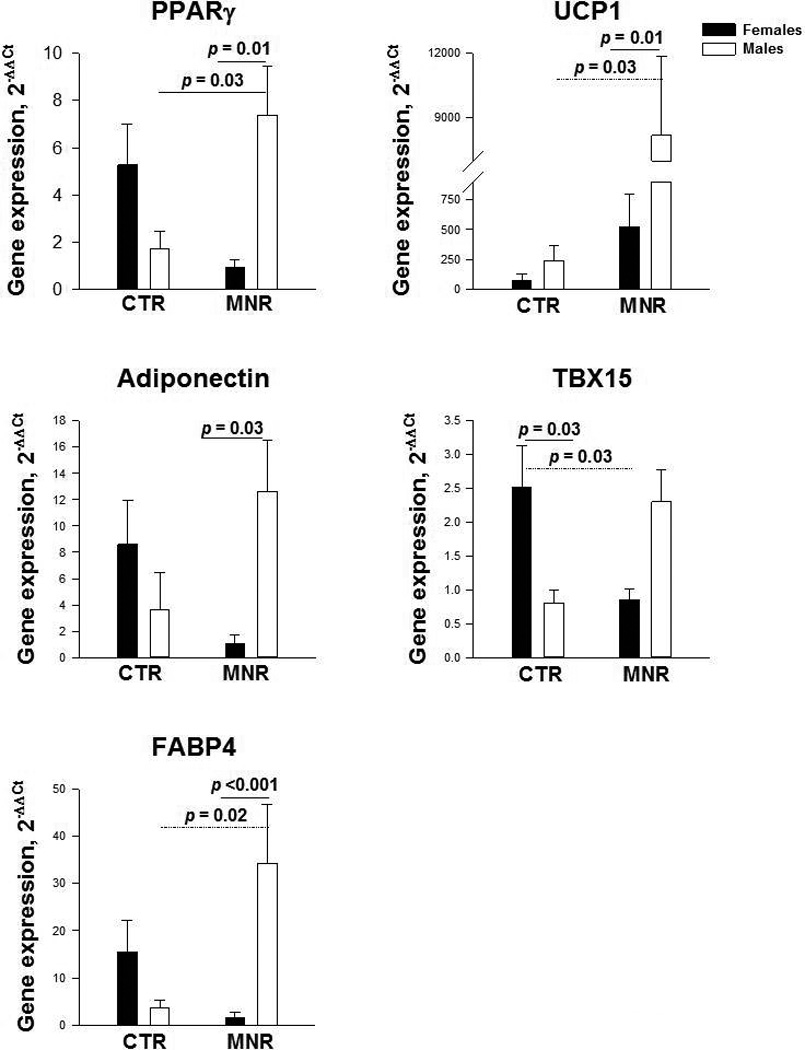
Sex-dependent expression of white (PPARγ, adiponectin, FABP4) and brown (UCP1, TBX15) adipocyte differentiation-related genes in 9-day differentiated adipose-derived stromal-vascular cell cultures from omental, subcutaneous abdominal and subcutaneous femoral adipose tissue combined in CTR and MNR baboon fetuses (5 males and 5 females in each treatment group).
Immunoblots
In males (Figure 2A), all proteins related to white (PPARγ and FABP4 ) and brown (UCP1 and PGC-1α) adipogenesis as well as mitochondrogenesis (COXIV) in in-vitro differentiated ASCs from pooled OM, scA and scF adipose tissue samples were higher in MNR than CTR fetuses. In contrast, some of these proteins (PPARγ, UCP1 and COXIV) were unchanged and others (FABP4 and PGC-1α) were lower in MNR than CTR female fetuses (Figure 2B).
Figure 2.
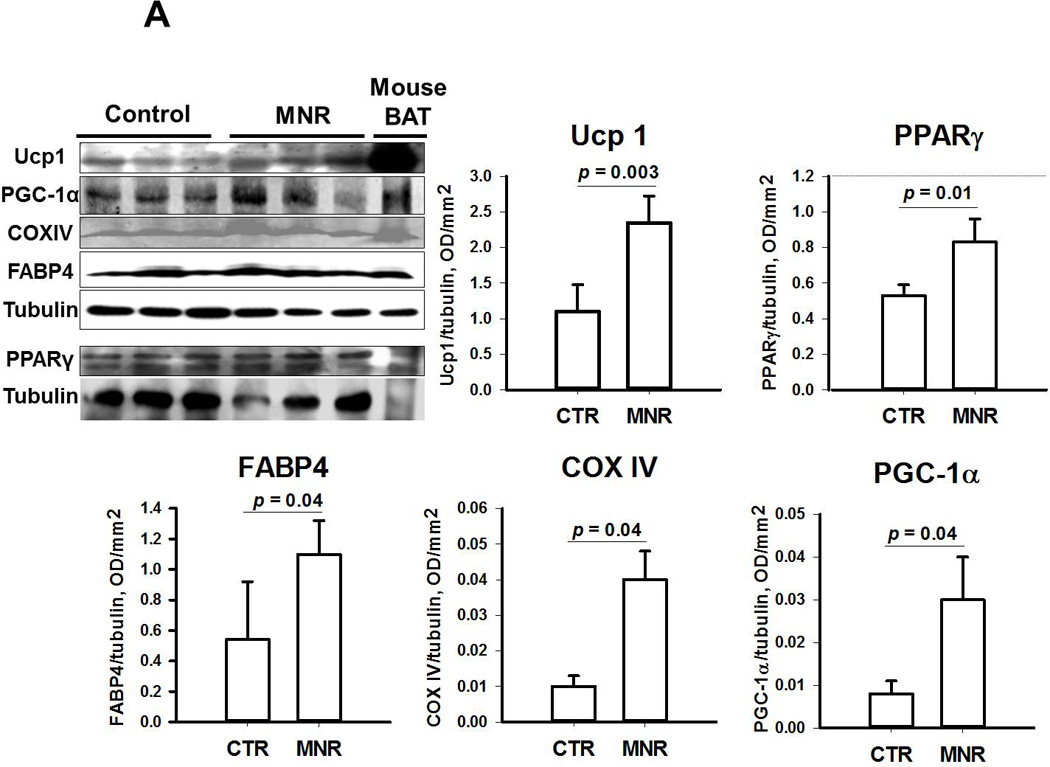
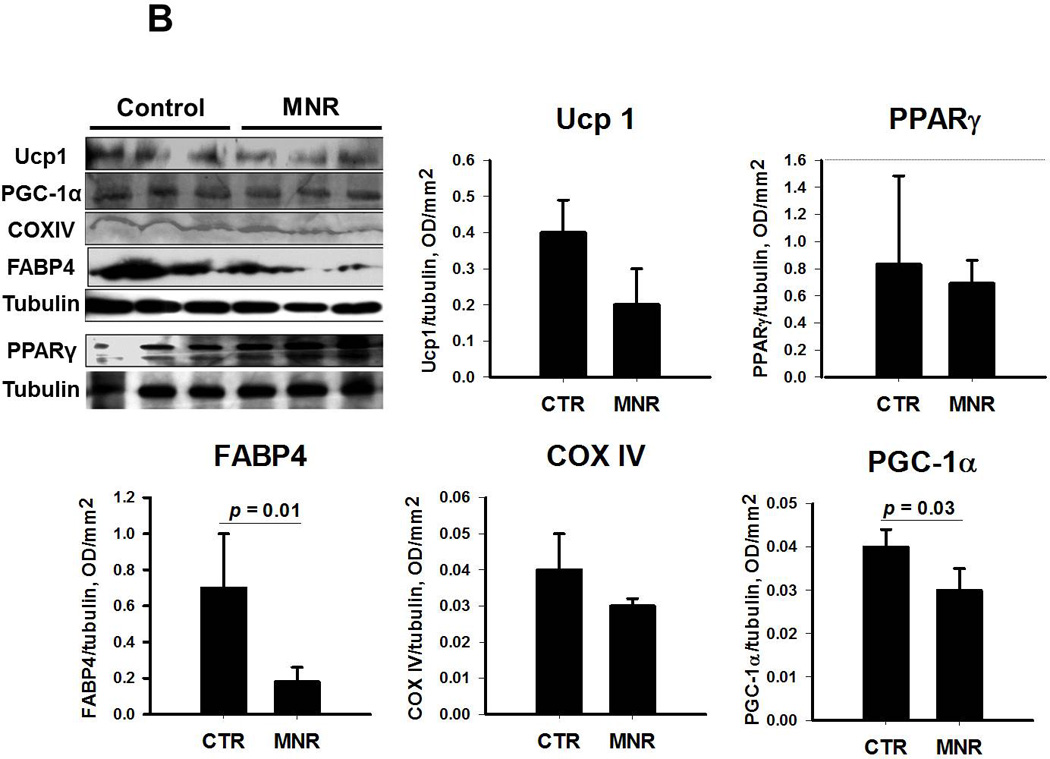
Immunoblotting of proteins related to white (PPARγ and FABP4 ) and brown (UCP1 and PGC-1α) type of adipocyte differentiation and mitochondrial biogenesis (COXIV) in pooled samples from differentiated omental, subcutaneous abdominal and subcutaneous femoral adipose tissue samples from male (panel A) and female (panel B) baboon fetuses; Mean ± SEM; n=3 for both fetuses of CTR and MNR mothers. Of note, PPARγ protein expression was assessed in a separate blot and protein from brown adipose tissue of mice was used as a positive control for UCP1.
Histology
The adipocytes per unit area are less abundant in MNR in both sexes (p = 0.02) combined indicating that MNR promotes adipocyte hypertrophy (Table 1). This effect of the maternal diet was primarily evident in males in whom the difference in adipocyte number per field reached borderline significance (p = 0.08) but not in females. However, the female fetuses tended to have a higher number of adipocytes in the omental compared to both subcutaneous depots (p = 0.09) indicating that subcutaneous adipocyte size tend to be larger than the size of omental adipocytes. We found no interaction between maternal diet and depot effects on the adipocyte number per field.
Table 1.
Adipocyte number per area (mm2) in ten CTR and ten MNR fetuses (5 males and 5 females in each treatment group) by maternal diet and depot
| Fetuses | CTR | MNR | p value | OM | scA | scF | p value |
|---|---|---|---|---|---|---|---|
| Female | 543 ± 60 | 361 ± 42 | 0.3 | 652 ± 89 | 409 ± 55 | 354 ± 37 | 0.095 |
| Male | 638 ± 99 | 431 ± 32 | 0.08 | 651 ± 109 | 434 ± 62 | 498 ± 91 | 0.7 |
| Both sexes | 589 ± 57 | 397 ± 26 | 0.02 | 651 ± 70 | 420 ± 40 | 425 ± 51 | 0.4 |
Values are mean ± SE.
Discussion
This study investigated the impact of decreased fetal nutrient availability on differentiation and functional properties of adipose tissue depots in male and female baboon fetuses. Our nonhuman primate model of development in a precocial species provides many advantages and strengths when translating to human development. MNR male fetuses weighed significantly less (approximately 14%) than CTR indicating development of IUGR, while the decrease in fetal weight in females was only 5% in absolute terms and was not statistically significant. The greater slowing of growth in male than female fetuses has been extensively reported and is usually attributed to the faster growth rate in normal male vs. female fetuses19. Thus, male baboon fetuses may have experienced a greater relative degree of nutritional deprivation. The lower abdominal circumference in MNR vs. CTR male fetuses but comparable crown-rump lengths in both groups suggest that the MNR male fetuses may have preferential reduction in total adipose tissue rather than lean mass similar to findings from previously published “ultrasound’ studies on human fetal body composition20, 21. Furthermore, rodent studies on effects of poor maternal nutrition show that body weight changes in the offspring are largely accounted for by variation in body fat22, 23.
In addition, nutrient deprivation exerted differential effects on adipocyte differentiation between male and female fetuses. Specifically it stimulated mostly brown adipogenesis in male fetuses as judged by the 26-fold increase in UCP1 gene expression and the modest 1.5-fold increase in the expression of its transcriptional regulator and a marker of white adipogenesis PPARγ, and its target genes FABP4 and adiponectin. These results are similar to the findings of substantial upregulation of UCP-1 and a concomitant modest increase in several transcriptional regulators [PGC-1α, PPARα, and type 2 deiodinase (DIO2)] in response to adrenergic stimulation of adipose tissue in male mice24. Although PPARγ binding sites are present in both the UCP-1 and FABP4 promoters, the PPARγ co-activator PGC-1α activates only UCP-125. Additional findings of higher protein expression of UCP-1 together with that of PGC-1α and of the marker of mitochondrial content COXIV26 (brown adipocytes have a high mitochondrial content) in differentiated ASCs of male MNR vs. CTR fetuses provides further support of the overall predominant increase in brown over white adipogenesis. The expression of UCP1 and PGC-1α is in part dependent on adrenergic stimulation by norepinephrine released by sympathetic efferents in white adipose tissue27 through the stimulatory β1-, β2-, and β3-adrenergic receptors and the inhibitory α2-adrenoreceptors (reviewed in 28). We have previously shown in this MNR model that there is a decrease in β1- and no change in β2-receptor levels in the fetal liver at term29 but changes in adipose tissue in this model have not been analyzed. However, in vitro studies show that sex hormones differentially affect adrenergic receptor expression in 3T3-L1 preadipocytes and adipocytes30 and maternal under-nutrition during early phases of gestation in male sheep decreases plasma testosterone levels31. These data suggest a potential role of sex hormones in regulating brown adipogenesis through modulation of the adrenergic systems. The reduced adipose tissue mass in the face of comparable adipocyte size in IUGR male fetuses compared to females lends support to the idea of smaller pool of adipocytes and their precursor cells. Given that development of adequate pool of brown adipocytes is a major requirement to provide sufficient thermogenesis for neonatal survival, the overall stimulation of adipogenesis and establishment of more brown than white adipocyte phenotype appear to be adaptive mechanisms to attain the critical number of brown adipocytes.
In contrast to findings in males, MNR did not affect fetal growth and did not stimulate adipogenesis in female fetuses. Indeed there was a decrease in protein expression of FABP4 and PGC-1α. Glucocorticoids are potential mediators that could explain this phenomenon since they are a major factor regulating terminal differentiation in a wide range of fetal tissues32. We have shown increased activity of the fetal hypothalamo-pituitary adrenal axis and elevated fetal circulating cortisol in this MNR model33. Although there are no fetal sex differences in circulating cortisol, we have demonstrated increased local production of cortisol at term that is fetal sex specific, being increased in female adipose tissue but not male and in male liver but not female liver34. Glucocorticoids enhance recruitment of stem cells towards the adipocyte lineage35 and cause adipocyte hypertrophy in primary in vitro differentiated porcine adipocytes36. A recent study reports that increased expression and activity of 11 beta-hydroxysteroid dehydrogenase type 1 (11β-HSD1), an enzyme that converts inactive cortisone to the active glucocorticoid cortisol, leads to suppression of genes characteristic of brown adipose tissue. We recently demonstrated increased activity of 11β-HSD1 in female but not male adipose tissue in this MNR model at term34. We hypothesize that increased local production of cortisol stimulates prenatal differentiation by switching adipocyte differentiation from the fetal brown fat-like to white fat type. If this occurs too late – in the MNR males, or too early in the MNR females it may be maladaptive, predisposing to obesity and insulin resistance. However, further studies are required to identify the molecular mechanisms underlying these interesting sex differences.
An alternative mechanism may involve genes encoding transcription factors regulating embryonic and fetal development and pattern specification based on data from transcriptional profiling studies in rodents and humans showing depot- and sex-dependent differences in their expression37–40. The expression of several developmental genes, TBX15, GLYP4, and HOXA5, correlates with levels of obesity (body mass index) and fat distribution (waist-to-hip ratio)39. We focused on TBX15 as it is expressed predominantly in brown adipose tissue and in those white adipose depots that are capable of giving rise to brown-in-white adipocytes41. Also, siRNA-mediated silencing of TBX15 expression in primary preadipocyte cultures from epididymal white and interscapular brown adipose tissue from 129/Sv mouse pups down-regulates the adipogenic genes (PPARγ and FABP4) and the brown phenotypic marker genes (PRDM16, PGC-1α, COXIV, UCP1) in brown adipocytes. Our findings of decreased expression of TBX15 gene in females and a trend for increased expression in male fetuses from MNR mothers could explain the trend for decreased adipogenesis in females and, in part, the enhanced white and particularly brown adipogenesis in male fetuses. It is noteworthy that the CTR females show increased TBX15 expression compared to CTR males. This corresponds to the higher expression of UCP1 in adult women compared to men42 suggesting a possible contribution of TBX15 to development of brown phenotype of white adipose tissue in adulthood. Recent evidence shows a relationship of UCP1 mRNA abundance with a member of the homeobox group of developmental genes, HOXA1 in the perinatal period11. Furthermore, HOXA2 gene [located adjacent to HOXA1 gene on chromosome 7 and thus theoretically its expression will overlap with that of HOXA1 both spatially (same adipose tissue sites) and temporally (same fetal period)] is expressed more in men compared to women43. Together, these data suggest that both HOXA1 and HOXA2 may be additional candidates for the sex-dependent regulation of fetal brown adipogenesis. Future studies of sex-differences in ontogeny of expression of an extended panel of embryonic patterning and developmental genes are warranted to gain a better understanding about their role as mediators of sex-hormone related intrinsic identity of preadipocytes and subsequent sex-specific adipose tissue programming events.
It remains to be shown how these responses of adipocyte differentiation affect adipose tissue function and metabolic health in adulthood. Studies investigating the dynamics of adipocyte cellularity with weight gain, using a cross-sectional design44 or obtained longitudinally by serial biopsies of inguinal adipose tissue depots45 suggest an oscillatory pattern of adipose tissue remodeling, involving simultaneous and repetitive cycles of hyperplasia, hypertrophy, and hypoplasia (decreased adipocyte number), presumably reflecting proliferation and differentiation of adipocyte precursor cells, development of mature adipocytes, and apoptosis, respectively. Interestingly, the rate of enlargement of adipocytes (hypertrophy) is proportional to the difference between the lipid load and the storage capacity of adipocytes45, which likely depends on both adipocyte number and metabolic properties. The high rate of adipocyte hypertrophy and low contribution of hyperplasia in white adipose tissue predispose some individuals to increased susceptibility to apoptosis, increased initiation of a local inflammatory response (infiltration of adipose tissue with immune cells and increased secretion of pro-inflammatory molecules by immune cells and adipocyte precursor cells)46. Given that maternal under nutrition appears to reduce the preadipocyte pool in males but to maintain or increase the abundance of preadipocyte in females suggest that males may be preconditioned to develop adipocyte hypertrophy, and hence local inflammation, more readily than females. This predisposition may be further enhanced by a potential catch up postnatal growth observed in IUGR47. In support, a study of developmental ontology in ovine fetuses and early newborns shows that the intrauterine nutritional environment elicits a lower inflammatory response prenatally with higher local inflammation in adipose tissue in offspring suggesting a possible role of inflammation in mediating, the long-term unfavorable metabolic consequences of poor maternal nutrition48.
An important question that arises is whether the increased expression of UCP1 and/or increase in brown versus white adipose tissue could potentially lead to increased energy expenditure in these MNR male fetuses and how to reconcile this possibility with the increased risk of obesity documented in small for gestational age newborn babies7. Brown adipocytes are only present in large numbers during the perinatal and early postnatal periods. It is not known whether these brown adipocytes transdifferentiate into white adipocytes or are lost due to increased cell death. Also, it is not known whether the brown-to-white transdifferentiated adipocytes during early postnatal growth retain a higher expression of β3-adrenoreceptors which appears to be critical for the appearance of brown adipocytes in response to cold stimulation49 or emerge de novo as a new population. Lastly, there is no consensus yet whether the expression of UCP1 or the increased brown versus white adipose tissue is fundamental to body weight regulation50. Further longitudinal studies of the dynamic changes in brown features of postnatal adipocytes are needed to fill in these gaps of knowledge.
In conclusion, the parallel evaluation of white and brown adipogenesis in fetal adipose tissue development suggest that the control of adipogenesis and the establishment of brown/white adipocyte phenotype may be an important target for nutritional reprogramming of adipogenesis and thermogenesis in response to suboptimal nutrition. Our data support the emerging view that challenges in pregnancy can have differential effects in the presence of a male or female fetus as shown here in the different response of the female MNR fetus which adapts while the male fetus continues on the fetal brown adipose tissue track in a relatively non-adaptive fashion. Our findings further reinforce the need to observe and compare responses according to fetal sex when assessing developmental programming of adiposity in response to sub-optimal maternal nutrition.
Acknowledgements
The authors thank Dexter Graves for technical help.
This project is funded by National Institute of Child Health and Human Development (NIH R03-HD 060158 and HD 21350). This work used Genomics and Cell Biology core facilities at the Pennington Center that are supported in part by COBRE (NIH P20-RR021945) and NORC (NIH 1P30-DK072476) center grants from the National Institutes of Health.
Footnotes
Conflict of interest
The authors have no conflict of interest.
References
- 1.Guo Z, Hensrud DD, Johnson CM, Jensen MD. Regional postprandial fatty acid metabolism in different obesity phenotypes. Diabetes. 1999;48(8):1586–1592. doi: 10.2337/diabetes.48.8.1586. [DOI] [PubMed] [Google Scholar]
- 2.Despres JP, Nadeau A, Tremblay A, Ferland M, Moorjani S, Lupien PJ, et al. Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes. 1989;38(3):304–309. doi: 10.2337/diab.38.3.304. [DOI] [PubMed] [Google Scholar]
- 3.Canoy D, Luben R, Welch A, Bingham S, Wareham N, Day N, et al. Fat distribution, body mass index and blood pressure in 22,090 men and women in the Norfolk cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Norfolk) study. J Hypertens. 2004;22(11):2067–2074. doi: 10.1097/00004872-200411000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Snijder MB, Dekker JM, Visser M, Yudkin JS, Stehouwer CD, Bouter LM, et al. Larger thigh and hip circumferences are associated with better glucose tolerance: the Hoorn study. Obesity research. 2003;11(1):104–111. doi: 10.1038/oby.2003.18. [DOI] [PubMed] [Google Scholar]
- 5.Heitmann BL, Frederiksen P, Lissner L. Hip circumference and cardiovascular morbidity and mortality in men and women. Obes Res. 2004;12(3):482–487. doi: 10.1038/oby.2004.54. [DOI] [PubMed] [Google Scholar]
- 6.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341(8850):938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 7.Sarr O, Yang K, Regnault TR. In utero programming of later adiposity: the role of fetal growth restriction. Journal of pregnancy. 2012;2012:134758. doi: 10.1155/2012/134758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crandall DL, Hausman GJ, Kral JG. A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation. 1997;4(2):211–232. doi: 10.3109/10739689709146786. [DOI] [PubMed] [Google Scholar]
- 9.Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci. 2004;82(3):925–934. doi: 10.2527/2004.823925x. [DOI] [PubMed] [Google Scholar]
- 10.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322(5901):583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pope M, Budge H, Symonds ME. The developmental transition of ovine adipose tissue through early life. Acta Physiol (Oxf) 2013 doi: 10.1111/apha.12053. [DOI] [PubMed] [Google Scholar]
- 12.Li C, McDonald TJ, Wu G, Nijland MJ, Nathanielsz PW. Intrauterine growth restriction alters term fetal baboon hypothalamic appetitive peptide balance. J Endocrinol. 2013;217(3):275–282. doi: 10.1530/JOE-13-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi J, Li C, McDonald TJ, Comuzzie A, Mattern V, Nathanielsz PW. Emergence of insulin resistance in juvenile baboon offspring of mothers exposed to moderate maternal nutrient reduction. Am J Physiol Regul Integr Comp Physiol. 2011;301(3):R757–R762. doi: 10.1152/ajpregu.00051.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlabritz-Loutsevitch NE, Howell K, Rice K, Glover EJ, Nevill CH, Jenkins SL, et al. Development of a system for individual feeding of baboons maintained in an outdoor group social environment. J Med Primatol. 2004;33(3):117–126. doi: 10.1111/j.1600-0684.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- 15.Cox LA, Nijland MJ, Gilbert JS, Schlabritz-Loutsevitch NE, Hubbard GB, McDonald TJ, et al. Effect of 30 per cent maternal nutrient restriction from 0.16 to 0.5 gestation on fetal baboon kidney gene expression. J Physiol. 2006;572(Pt 1):67–85. doi: 10.1113/jphysiol.2006.106872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlabritz-Loutsevitch NE, Hubbard GB, Dammann MJ, Jenkins SL, Frost PA, McDonald TJ, et al. Normal concentrations of essential and toxic elements in pregnant baboons and fetuses (Papio species) J Med Primatol. 2004;33(3):152–162. doi: 10.1111/j.1600-0684.2004.00066.x. [DOI] [PubMed] [Google Scholar]
- 17.Tchoukalova YD, Nathanielsz PW, Conover CA, Smith SR, Ravussin E. Regional variation in adipogenesis and IGF regulatory proteins in the fetal baboon. Biochem Biophys Res Commun. 2009;380(3):679–683. doi: 10.1016/j.bbrc.2009.01.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Commins SP, Watson PM, Padgett MA, Dudley A, Argyropoulos G, Gettys TW. Induction of uncoupling protein expression in brown and white adipose tissue by leptin. Endocrinology. 1999;140(1):292–300. doi: 10.1210/endo.140.1.6399. [DOI] [PubMed] [Google Scholar]
- 19.Parker AJ, Davies P, Mayho AM, Newton JR. The ultrasound estimation of sex-related variations of intrauterine growth. Am J Obstet Gynecol. 1984;149(6):665–669. doi: 10.1016/0002-9378(84)90255-2. [DOI] [PubMed] [Google Scholar]
- 20.Padoan A, Rigano S, Ferrazzi E, Beaty BL, Battaglia FC, Galan HL. Differences in fat and lean mass proportions in normal and growth-restricted fetuses. American journal of obstetrics and gynecology. 2004;191(4):1459–1464. doi: 10.1016/j.ajog.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 21.Larciprete G, Valensise H, Di Pierro G, Vasapollo B, Casalino B, Arduini D, et al. Intrauterine growth restriction and fetal body composition. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2005;26(3):258–262. doi: 10.1002/uog.1980. [DOI] [PubMed] [Google Scholar]
- 22.Aubert R, Suquet JP, Lemonnier D. Long-term morphological and metabolic effects of early under- and over-nutrition in mice. J Nutr. 1980;110(4):649–661. doi: 10.1093/jn/110.4.649. [DOI] [PubMed] [Google Scholar]
- 23.Johnson PR, Stern JS, Greenwood MR, Zucker LM, Hirsch J. Effect of early nutrition on adipose cellularity and pancreatic insulin release in the Zucker rat. J Nutr. 1973;103(5):738–743. doi: 10.1093/jn/103.5.738. [DOI] [PubMed] [Google Scholar]
- 24.Xue B, Coulter A, Rim JS, Koza RA, Kozak LP. Transcriptional synergy and the regulation of Ucp1 during brown adipocyte induction in white fat depots. Molecular and cellular biology. 2005;25(18):8311–8322. doi: 10.1128/MCB.25.18.8311-8322.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 26.Nadal-Casellas A, Bauza-Thorbrugge M, Proenza AM, Gianotti M, Llado I. Sex-dependent differences in rat brown adipose tissue mitochondrial biogenesis and insulin signaling parameters in response to an obesogenic diet. Molecular and cellular biochemistry. 2013;373(1-2):125–135. doi: 10.1007/s11010-012-1481-x. [DOI] [PubMed] [Google Scholar]
- 27.Giordano A, Frontini A, Murano I, Tonello C, Marino MA, Carruba MO, et al. Regional-dependent increase of sympathetic innervation in rat white adipose tissue during prolonged fasting. J Histochem Cytochem. 2005;53(6):679–687. doi: 10.1369/jhc.4A6566.2005. [DOI] [PubMed] [Google Scholar]
- 28.Lafontan M, Berlan M. Fat cell adrenergic receptors and the control of white and brown fat cell function. Journal of lipid research. 1993;34(7):1057–1091. [PubMed] [Google Scholar]
- 29.Kamat A, Nijland MJ, McDonald TJ, Cox LA, Nathanielsz PW, Li C. Moderate global reduction in maternal nutrition has differential stage of gestation specific effects on {beta}1- and {beta}2-adrenergic receptors in the fetal baboon liver. Reprod Sci. 2011;18(4):398–405. doi: 10.1177/1933719110386496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monjo M, Pujol E, Roca P. alpha2- to beta3-Adrenoceptor switch in 3T3-L1 preadipocytes and adipocytes: modulation by testosterone, 17beta-estradiol, and progesterone. American journal of physiology. Endocrinology and metabolism. 2005;289(1):E145–E150. doi: 10.1152/ajpendo.00563.2004. [DOI] [PubMed] [Google Scholar]
- 31.Rae MT, Rhind SM, Fowler PA, Miller DW, Kyle CE, Brooks AN. Effect of maternal undernutrition on fetal testicular steroidogenesis during the CNS androgen-responsive period in male sheep fetuses. Reproduction. 2002;124(1):33–39. [PubMed] [Google Scholar]
- 32.Fowden AL, Giussani DA, Forhead AJ. Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda) 2006;21:29–37. doi: 10.1152/physiol.00050.2005. [DOI] [PubMed] [Google Scholar]
- 33.Li C, Ramahi E, Nijland MJ, Choi J, Myers DA, Nathanielsz PW, et al. Up-regulation of the Fetal Baboon Hypothalamo-Pituitary-Adrenal Axis in Intrauterine Growth Restriction: Coincidence with Hypothalamic Glucocorticoid Receptor Insensitivity and Leptin Receptor Down-regulation. Endocrinology. 2013 doi: 10.1210/en.2012-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo C, Li C, Myatt L, Nathanielsz PW, Sun K. Sexually dimorphic effects of maternal nutrient reduction on expression of genes regulating cortisol metabolism in fetal baboon adipose and liver tissues. Diabetes. 2013;62(4):1175–1185. doi: 10.2337/db12-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu ZK, Wright JT, Hausman GJ. Preadipocyte recruitment in stromal vascular cultures after depletion of committed preadipocytes by immunocytotoxicity. Obes Res. 1997;5(1):9–15. doi: 10.1002/j.1550-8528.1997.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 36.Tchoukalova YD, Hausman DB, Dean RG, Hausman GJ. Enhancing effect of troglitazone on porcine adipocyte differentiation in primary culture: a comparison with dexamethasone. Obes Res. 2000;8(9):664–672. doi: 10.1038/oby.2000.85. [DOI] [PubMed] [Google Scholar]
- 37.Cantile M, Procino A, D'Armiento M, Cindolo L, Cillo C. HOX gene network is involved in the transcriptional regulation of in vivo human adipogenesis. J Cell Physiol. 2003;194(2):225–236. doi: 10.1002/jcp.10210. [DOI] [PubMed] [Google Scholar]
- 38.Vohl MC, Sladek R, Robitaille J, Gurd S, Marceau P, Richard D, et al. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes Res. 2004;12(8):1217–1222. doi: 10.1038/oby.2004.153. [DOI] [PubMed] [Google Scholar]
- 39.Gesta S, Bluher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(17):6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tchkonia T, Giorgadze N, Pirtskhalava T, Thomou T, DePonte M, Koo A, et al. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes. 2006;55(9):2571–2578. doi: 10.2337/db06-0540. [DOI] [PubMed] [Google Scholar]
- 41.Gburcik V, Cawthorn WP, Nedergaard J, Timmons JA, Cannon B. An essential role for Tbx15 in the differentiation of brown and "brite" but not white adipocytes. American journal of physiology. Endocrinology and metabolism. 2012;303(8):E1053–E1060. doi: 10.1152/ajpendo.00104.2012. [DOI] [PubMed] [Google Scholar]
- 42.Nookaew I, Svensson PA, Jacobson P, Jernas M, Taube M, Larsson I, et al. Adipose tissue resting energy expenditure and expression of genes involved in mitochondrial function are higher in women than in men. The Journal of clinical endocrinology and metabolism. 2013;98(2):E370–E378. doi: 10.1210/jc.2012-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karastergiou K, Fried SK, Xie H, Lee MJ, Divoux A, Rosencrantz MA, et al. Distinct Developmental Signatures of Human Abdominal and Gluteal Subcutaneous Adipose Tissue Depots. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2012-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56(12):2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 45.MacKellar J, Cushman SW, Periwal V. Waves of adipose tissue growth in the genetically obese Zucker fatty rat. PLoS One. 2010;5(1):e8197. doi: 10.1371/journal.pone.0008197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gustafson B, Gogg S, Hedjazifar S, Jenndahl L, Hammarstedt A, Smith U. Inflammation and impaired adipogenesis in hypertrophic obesity in man. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00377.2009. [DOI] [PubMed] [Google Scholar]
- 47.Yanney M, Marlow N. Paediatric consequences of fetal growth restriction. Seminars in fetal & neonatal medicine. 2004;9(5):411–418. doi: 10.1016/j.siny.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Sharkey D, Symonds ME, Budge H. Adipose tissue inflammation: developmental ontogeny and consequences of gestational nutrient restriction in offspring. Endocrinology. 2009;150(8):3913–3920. doi: 10.1210/en.2008-1784. [DOI] [PubMed] [Google Scholar]
- 49.Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. American journal of physiology. Endocrinology and metabolism. 2010;298(6):E1244–E1253. doi: 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- 50.Kozak LP, Koza RA, Anunciado-Koza R. Brown fat thermogenesis and body weight regulation in mice: relevance to humans. Int J Obes (Lond) 2010;34(Suppl 1):S23–S27. doi: 10.1038/ijo.2010.179. [DOI] [PubMed] [Google Scholar]


