Abstract
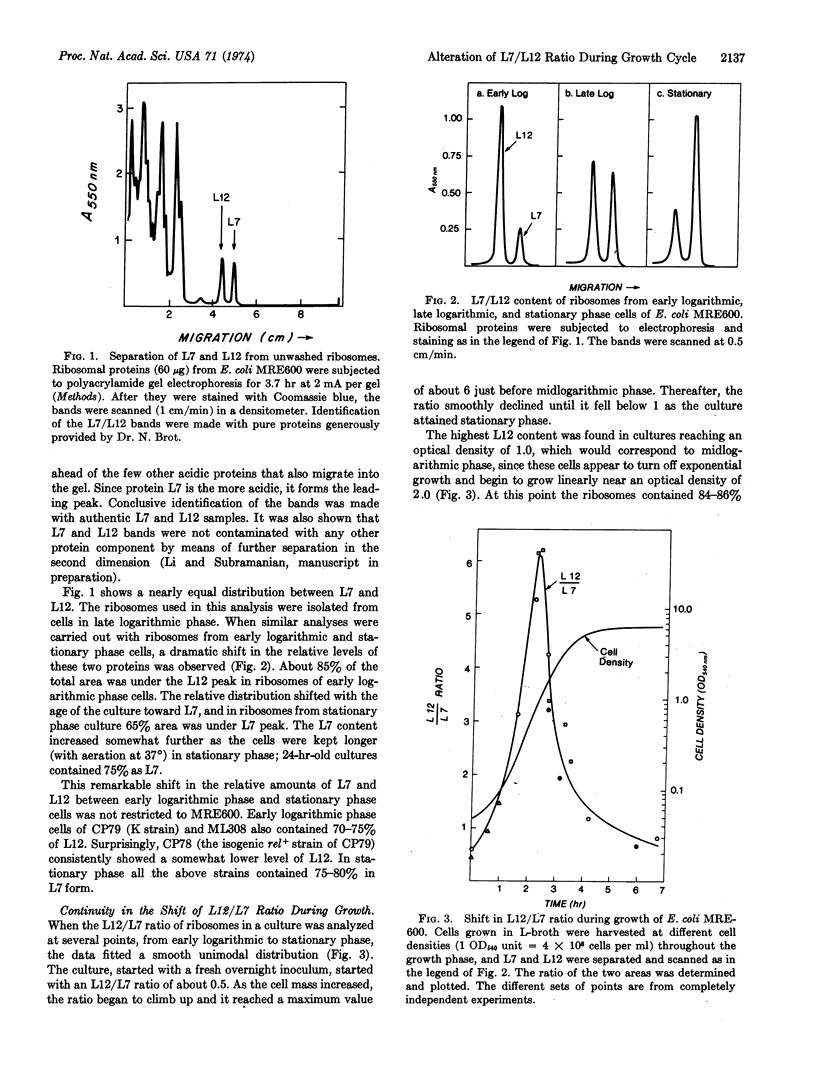
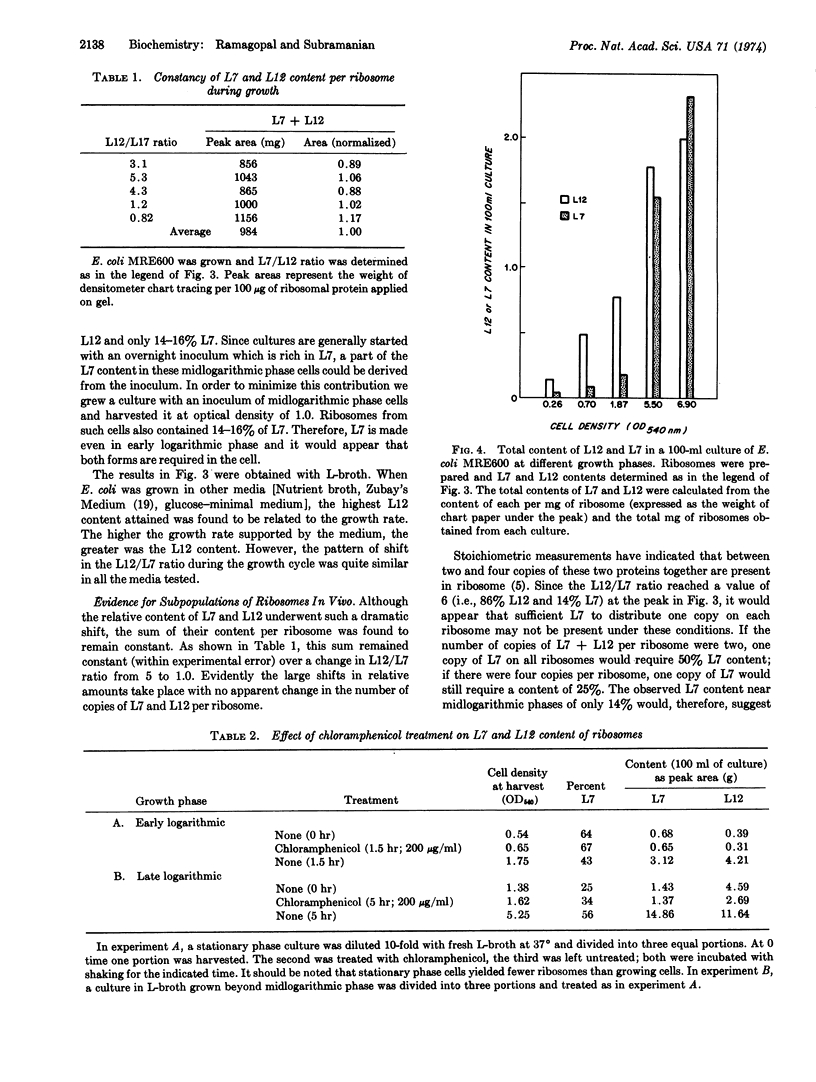
The relative content in ribosomes of L7 and L12, the two forms of a protein in the 50S subunit specifically involved in GTP hydrolysis, is found to undergo a striking shift with the growth phase of E. coli. The content of L12 (nonacetylated form) increases during early logarithmic phase, becoming about 85% of the total before midlogarithmic phase. Thereafter, L7 (N-acetylated form) content begins to increase, eventually becoming 75-80% in stationary phase. The L7 + L12 content per ribosome, however, remained constant during this shift. Our evidence suggests that the shift did not occur through modification of preexisting ribosomes. The data further indicate that the E. coli cell may contain more than one structurally distinct (with regard to L7 or L12 content) 50S subunit population.
Keywords: ribosome heterogeneity, regulation of acetylation, stationary phase adaptation
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brot N., Marcel R., Cupp L., Weissbach H. The enzymatic acetylation of ribosomal bound protein L 12 . Arch Biochem Biophys. 1973 Apr;155(2):475–477. doi: 10.1016/0003-9861(73)90140-9. [DOI] [PubMed] [Google Scholar]
- Brot N., Tate W. P., Caskey C. T., Weissbach H. The requirement for ribosomal proteins L7 and L12 in peptide-chain termination. Proc Natl Acad Sci U S A. 1974 Jan;71(1):89–92. doi: 10.1073/pnas.71.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot N., Weisbach H. The enzymatic acetylation of E. coli ribosomal protein L 12 . Biochem Biophys Res Commun. 1972 Nov 1;49(3):673–679. doi: 10.1016/0006-291x(72)90464-0. [DOI] [PubMed] [Google Scholar]
- Cammack K. A., Wade H. E. The sedimentation behaviour of ribonuclease-active and -inactive ribosomes from bacteria. Biochem J. 1965 Sep;96(3):671–680. doi: 10.1042/bj0960671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Davis B. D. Role of subunits in the ribosome cycle. Nature. 1971 May 21;231(5299):153–157. doi: 10.1038/231153a0. [DOI] [PubMed] [Google Scholar]
- Deusser E. Heterogeneity of ribosomal populations in Escherichia coli cells grown in different media. Mol Gen Genet. 1972;119(3):249–258. doi: 10.1007/BF00333862. [DOI] [PubMed] [Google Scholar]
- Deusser E., Weber J., Maschler R., Stöffler G., Wittmann H. G. Structural and functional evidence for a repeated 50S subunit ribosomal protein. Nat New Biol. 1973 Mar 14;242(115):47–49. doi: 10.1038/newbio242047a0. [DOI] [PubMed] [Google Scholar]
- Fakunding J. L., Traut R. R., Hershey J. W. Dependence of initiation factor IF-2 activity on proteins L7 and L12 from Escherichia coli 50 S ribosomes. J Biol Chem. 1973 Dec 25;248(24):8555–8559. [PubMed] [Google Scholar]
- Forchhammer J., Lindahl L. Growth rate of polypeptide chains as a function of the cell growth rate in a mutant of Escherichia coli 15. J Mol Biol. 1971 Feb 14;55(3):563–568. doi: 10.1016/0022-2836(71)90337-8. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Raab C. In vivo synthesis of tRNA Tyr 1 and tRNA Tyr 2 : differences in "early" and "late log" E. coli MRE 600. Biochem Biophys Res Commun. 1972 Mar 24;46(6):2006–2011. doi: 10.1016/0006-291x(72)90751-6. [DOI] [PubMed] [Google Scholar]
- Hamel E., Koka M., Nakamoto T. Requirement of an Escherichia coli 50 S ribosomal protein component for effective interaction of the ribosome with T and G factors and with guanosine triphosphate. J Biol Chem. 1972 Feb 10;247(3):805–814. [PubMed] [Google Scholar]
- Highland J. H., Bodley J. W., Gordon J., Hasenbank R., Stöffler G. Identity of the ribosomal proteins involved in the interaction with elongation factor G. Proc Natl Acad Sci U S A. 1973 Jan;70(1):147–150. doi: 10.1073/pnas.70.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. XII. Number of proteins in small and large ribosomal subunits of Escherichia coli as determined by two-dimensional gel electrophoresis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1276–1282. doi: 10.1073/pnas.67.3.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A., Sander G., Grunberg-Manago M. Effect of ribosomal protein L12 upon initiation factor IF-2 activities. Biochem Biophys Res Commun. 1973 Apr 16;51(4):979–986. doi: 10.1016/0006-291x(73)90023-5. [DOI] [PubMed] [Google Scholar]
- Kischa K., Möller W., Stöffler G. Reconstitution of a GTPase activity by a 50S ribosomal protein and E. coli. Nat New Biol. 1971 Sep 8;233(36):62–63. doi: 10.1038/newbio233062a0. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Fox J. E., Spears C., Brot N., Weissbach H. Studies on the role of ribosomal proteins L 7 and L 12 in the in vitro synthesis of -galactosidase. J Biol Chem. 1973 Jul 25;248(14):5012–5015. [PubMed] [Google Scholar]
- Lipmann F. Polypeptide chain elongation in protein biosynthesis. Science. 1969 May 30;164(3883):1024–1031. doi: 10.1126/science.164.3883.1024. [DOI] [PubMed] [Google Scholar]
- Möller W., Groene A., Terhorst C., Amons R. 50-S ribosomal proteins. Purification and partial characterization of two acidic proteins, A 1 and A 2, isolated from 50-S ribosomes of Escherichia coli. Eur J Biochem. 1972 Jan 31;25(1):5–12. doi: 10.1111/j.1432-1033.1972.tb01660.x. [DOI] [PubMed] [Google Scholar]
- Raetz C. R., Kennedy E. P. The association of phosphatidylserine synthetase with ribosomes in extracts of Escherichia coli. J Biol Chem. 1972 Apr 10;247(7):2008–2014. [PubMed] [Google Scholar]
- Subramanian A. R., Davis B. D. Release of 70 S ribosomes from polysomes in Escherichia coli. J Mol Biol. 1973 Feb 15;74(1):45–56. doi: 10.1016/0022-2836(73)90353-7. [DOI] [PubMed] [Google Scholar]
- Subramanian A. R. Glutaraldehyde fixation of ribosomes. Its use in the analysis of ribosome dissociation. Biochemistry. 1972 Jul 4;11(14):2710–2714. doi: 10.1021/bi00764a025. [DOI] [PubMed] [Google Scholar]
- Terhorst C., Möller W., Laursen R., Wittmann-Liebold B. The primary structure of an acidic protein from 50-S ribosomes of Escherichia coli which is involved in GTP hydrolysis dependent on elongation factors G and T. Eur J Biochem. 1973 Apr 2;34(1):138–152. doi: 10.1111/j.1432-1033.1973.tb02740.x. [DOI] [PubMed] [Google Scholar]
- Weber H. J. Stoichiometric measurements of 30S and 50S ribosomal proteins from Escherichia coli. Mol Gen Genet. 1972;119(3):233–248. doi: 10.1007/BF00333861. [DOI] [PubMed] [Google Scholar]


