Abstract
Temperament atypicalities have been documented in infancy and early development in children who develop autism spectrum disorders (ASD). The current study investigates whether there are differences in developmental trajectories of temperament between infants and toddlers with and without ASD. Parents of infant siblings of children with autism completed the Carey Temperament Scales about their child at 6, 12, 18, 24, and 36 months of age. Temperament trajectories of children with ASD reflected increases over time in activity level, and decreasing adaptability and approach behaviors relative to high-risk typically developing children. This study is the first to compare temperament trajectories between high-risk typically developing infants and infants subsequently diagnosed with ASD in the developmental window when overt symptoms of ASD first emerge.
Keywords: temperament, autism, parent perception, infants, toddlers
Introduction
Temperament has been defined as relatively stable individual differences in behavioral tendencies that exert bidirectional influences upon the social environment (Thomas and Chess 1977). Cross-sectional comparisons have indicated that temperament profiles differ between individuals with Autism Spectrum Disorder (ASD) and their typically developing (TD) peers at multiple points in development from infancy through adolescence (Bailey et al. 2000; Bolton et al. 2012; Brock et al. 2012; Clifford et al. 2013; De Pauw et al. 2011; Hepburn and Stone 2006; Schwartz et al. 2009; Zwaigenbaum et al. 2005). The current study is the first to examine whether changes in temperament across time also distinguish infants and toddlers with and without ASD. Improved understanding of patterns of change in temperament and parents’ awareness of changes in their child’s behavior may help to improve early detection and treatment of ASD.
The current report focuses on temperament trajectories among infant siblings of children with autism from 6 to 36 months of age because these infants are at heightened risk of being diagnosed with ASD (Ozonoff et al. 2011). Symptoms of ASD emerge during this developmental period (Ozonoff et al. 2010; Zwaigenbaum 2005) and temperament may also coalesce during the same period (Rothbart et al. 2000). Indeed, temperament may interact with symptoms of ASD to contribute to individual differences among individuals with ASD (Mundy, Henderson, Inge, & Coman 2007). While ASD is defined by a set of core behavioral symptoms, individual differences among people with ASD are often pronounced. Thus, temperament may be both a potential early marker of ASD and a way to understand how children with ASD influence and are influenced by their environment. Infants who subsequently receive a diagnosis of ASD may exhibit temperament constellations, such as increased negative affect or atypical sensory responses (Bryson et al. 2007; Clifford et al. 2013), which may reduce social opportunities, exacerbate symptoms of the disorder, and complicate efforts to treat those symptoms.
Infant Temperament in ASD
Atypicalities of temperament may be apparent within the first year after birth among infants who are later diagnosed with autism (Bryson et al. 2007; Clifford et al. 2013; Zwaigenbaum et al. 2005). Zwaigenbaum and colleagues (2005) found that infant siblings of children with autism who met criteria for ASD at 24 months (n = 6) were rated as lower in activity level at 6 months of age than high-risk infants who did not meet criteria for ASD (n = 22) and low-risk infants (n = 12). At 12 months of age, high-risk infants later diagnosed with ASD (n = 10) were described as more frequently and intensely distressed and more likely to fixate on objects relative to other high-risk infants (n = 29) and low-risk controls (n = 19).
Clifford and colleagues (2013) found increased perceptual sensitivity at 7 and 14 months of age among high-risk infants who were diagnosed with ASD at 36 months of age (n = 17) relative to high-risk TD infants (n = 23) but not high-risk children with other developmental concerns (n = 12) or low-risk TD children (n = 47). Toddlers with ASD were rated as smiling and laughing less frequently at 14 months, and as having more negative affect at 24 months relative to low-risk controls (Clifford et al. 2013). At 24 months, two dimensions (comprising negative affect, shyness, and soothability) distinguished toddlers with ASD from high-risk TD toddlers, high-risk children with other developmental concerns, and low-risk controls. Effortful control (the ability to regulate attention and behavior) was also reduced in children with ASD relative to both low-risk controls and high-risk children with other developmental concerns (but not high-risk TD infants) at 14 months and relative to low-risk controls at 24 months.
Investigating the same infant sibling sample as described by Zwaigenbaum and colleagues (2005), Garon et al. (2009) prospectively studied whether temperament profiles at 24 months of age distinguished children who went on to develop ASD (n = 34) from low-risk (n = 73) and high-risk TD toddlers (n = 104). They found that two temperament profiles were associated with a subsequent ASD diagnosis. The first profile indicated that low behavioral approach at 24 months (reductions in the goal-oriented aspects of extraversion), characterized by low positive anticipation, high activity level, and low attention shifting, distinguished high-risk toddlers who were diagnosed with ASD by 36 months from both high-risk toddlers without ASD and low-risk toddlers (Garon et al. 2009). Effortful emotion regulation, characterized by low positive affect, poor regulation of negative emotions and difficulty with attention control, differentiated both high-risk groups (infant siblings with and without ASD) from low-risk toddlers.
Similarly, a large, prospective study of a general population sample involving more than 14,000 children found increased activity levels and reduced distractibility at 24 months differentiated toddlers with ASD from TD controls (Bolton et al. 2012). They also reported associations between autistic traits and temperament characteristics such that elevated autistic traits at 6 months of age were associated with less activity and intensity while elevated autistic traits at 24 months of age were associated with more activity and intensity. In contrast to the studies discussed above that focused upon the infant siblings of children with autism, Bolton and colleagues found no evidence that temperament atypicalities distinguished between infants with and without autism in the first year of life in a general population sample. A distinctive feature of the study by Bolton and colleagues was the extensive battery of measures analyzed. Temperament comprised nine of two hundred forty-one potential early features of ASD. Accordingly, this statistical approach may have masked temperament effects.
Temperament in Children with ASD
Atypicalities of temperament are also apparent among older children with ASD. Children with ASD between 3 and 8 years of age were rated as exhibiting reduced adaptability, persistence and sensory responsiveness (Hepburn and Stone 2006) and decreased attention focusing, inhibitory control, and soothability (Konstantareas and Stewart 2006) relative to TD controls. Among children between 3 and 8 years of age, those with ASD and Fragile X Syndrome were described as slower to adapt, less apt to approach both social and non-social novel stimuli, and less persistent than TD children (Bailey et al. 2000). However, only children with ASD were rated as demonstrating less intensity, rhythmicity, sensory reactivity, and distractibility relative to TD children. In another study (Adamek et al. 2011), parents described their children (ages 2–8 years old) as more angry and frustrated and as having a greater liking for both high-intensity pleasure and low-intensity pleasure activities than TD controls. Children with ASD as a group demonstrated poorer inhibitory control and attentional focusing than TD controls (Adamek et al. 2011).
Three-to-seven-year-old children with ASD have also been found to be more active and withdrawn, and less adaptive, intense, sensitive, rhythmic, and distractible than typical children in Thomas and Chess’s original normative sample (Brock et al. 2012). In the same study, children with ASD were also compared to a developmentally delayed sample. Both decreased approach and distractibility differentiated children with ASD from those with developmental delays. De Pauw et al. (2011) reported that children with ASD between about 8–12 years of age were rated higher in negative affect, and lower on effortful control and surgency than typical controls.
Findings thus far suggest that temperament differs between individuals with and without ASD across childhood. However, given variability in the measures used to assess temperament, and in the models used to describe temperament even when using the same measure, the developmental pattern of these differences remains unclear.
Assessing Temperament in ASD
Discerning clear patterns of temperament that distinguish individuals with ASD from TD peers is complicated by the use of different tools to measure temperament across studies. Research examining temperament in children with ASD has generally relied on parent report (e.g., Bailey, Hatton, Mesibov, Ament, & Skinner 2000; Bryson et al. 2007; Bolton et al. 2012; Brock et al. 2012; Clifford et al. 2013; De Pauw et al. 2011; Garon et al. 2009; Konstantareas and Stewart 2006) or self report (Schwartz et al. 2009), and a variety of measures of temperament have been used. The most commonly used measures are derived from either the theoretical approach to temperament of Thomas and Chess, such as the Carey Temperament Scales (e.g., Carey and McDevitt 1995), or the conceptually related but distinct theoretical approach of Rothbart and colleagues, such as the Early Childhood Behavior Questionnaire (Putnam et al., 2006). Thomas and Chess defined temperament as behavioral style, or the “how” of behavior, and emphasized the dynamic interplay between an individual’s temperament and the environment (Goldsmith et al. 1987). In contrast, Rothbart and colleagues defined temperament in terms of biologically based individual differences in tendencies to react in certain ways (Goldsmith et al. 1987).
Although other prospective studies of infant siblings used measures derived from Rothbart’s approach to temperament (Bryson et al. 2007; Clifford et al. 2013; Garon et al. 2009; Zwaigenbaum et al. 2005), some research conducted with older individuals with autism used the Carey Temperament Scales (Bailey et al. 2000; Brock et al. 2012; Hepburn & Stone 2006). Indeed, the largest prospective, longitudinal study to examine temperament between 6 and 24 months of age in autism used the Carey Temperament Scales (Bolton et al. 2012). Thus, use of the CTS facilitates comparison between the high-risk sample examined in the current study, a large community sample (Bolton et al. 2012), and previous research regarding temperament in older individuals with ASD (Bailey et al. 2000; Hepburn & Stone 2006). Given the theoretical emphasis of Thomas and Chess’s model upon dynamic relations between an individual and his or her environment, we selected the Carey Temperament Scales to characterize change in temperament across infant and toddler development in children with ASD.
Study Aims and Hypotheses
The present study is a prospective, longitudinal investigation of parent ratings of temperament in infant siblings of children with autistic disorder (high-risk infants). The study’s aims were to determine whether temperament differences at five time points in infancy and toddlerhood, as well as overall temperament trajectories, distinguished high-risk infants who were subsequently diagnosed with ASD (Sibs-ASD) from high-risk infants who demonstrated typical patterns of development (Sibs-TD). We hypothesized that parents’ ratings of their child’s temperament would reflect differences between diagnostic groups, and that these differences would already be evident when their infants were 6 months old. Temperament differences between later-classified ASD and TD groups have been found as early as 6 and 7 months (Clifford et al. 2013; Zwaigenbaum et al. 2005). Given previous work suggesting that temperament may become more atypical over time for infant siblings later diagnosed with ASD (e.g., Clifford et al. 2013; Zwaigenbaum et al. 2005), we expected that the overall temperament trajectories would be different between the later classified TD and ASD groups. We also hypothesized that, relative to ratings of TD children, parent ratings regarding children later diagnosed with ASD would reflect increased negative affect (e.g., De Pauw et al. 2011, Schwartz et al. 2009), increased activity level and intensity (Bolton et al. 2012), reduced adaptability, persistence, and sensory responsiveness (Hepburn and Stone 2006; Bailey et al. 2000), as well as decreased approach behaviors (Maestro et al. 2002) and distractibility (Bailey et al. 2000; Bolton et al. 2012).
Method
Ethical approval for the study was obtained from the Institutional Review Board of the [University]. All parents provided informed consent for their own and their infant’s participation.
Participants
Participants were recruited by means of referrals from the [University] Autism Evaluation Clinic, other autism studies at [University], and the broader community offering support for children with ASD. Parent reports on child temperament were collected regarding infant siblings of children with autistic disorder at 6, 12, 18, 24, and 36 months of age. See Table 1 for a summary of sample characteristics. Temperament data were provided for a total of 54 children. However, a different number of parents completed the questionnaire at each time point (See Table 2). Despite efforts to collect temperament data from all participating parents at every time point, the rate of parental response was inconsistent. Depending on time point, sample sizes ranged from 10–16 in the Sibs-ASD group and 7–27 in the Sibs-TD group.
Table 1.
Sample Characteristics
| Sibs-ASD | Sibs-TD | Groups Differ | |
|---|---|---|---|
| Gender (% Male) | 85.7% | 51.5% | * |
| Race (% Caucasian) | 33.3% | 69.7% | * |
| Maternal Education | |||
| High school/some college | 11.8% | 16.7% | |
| College degree | 64.7% | 53.3% | |
| Graduate degree | 23.5% | 30.0% | |
| Family Income | |||
| Less than $50K annually | 25.0% | 10.0% | |
| $50–$100K annually | 12.5% | 30.0% | |
| More than $100K annually | 62.5% | 60.0% | |
| 6 month time point | |||
| Chronological age | 6.5 (.9) | 6.0 (.4) | |
| Verbal Mental age | 5.6 (.8) | 5.1 (.8) | |
| Nonverbal Mental age | 5.6 (.7) | 6.6 (1.2) | + |
| 12 month time point | |||
| Chronological age | 12.4 (.6) | 12.6 (.6) | |
| Verbal Mental age | 10.5 (2.2) | 10.4 (2.2) | |
| Nonverbal Mental age | 12.6 (1.8) | 12.5 (1.9) | |
| 18 month time point | |||
| Chronological age | 18.4 (.4) | 18.6 (.6) | |
| Verbal Mental age | 14.9 (5.1) | 17.6 (3.0) | |
| Nonverbal Mental age | 17.6 (2.1) | 18.8 (2.1) | |
| 24 month time point | |||
| Chronological age | 24.4 (.6) | 24.6 (.6) | |
| Verbal Mental age | 20.5 (5.6) | 25.6 (2.8) | * |
| Nonverbal Mental age | 20.5 (1.9) | 25.8 (4.0) | ** |
| ASD Severity Rating | 4.8 (2.2) | 1.3 (.5) | ** |
| 36 month time point | |||
| Chronological age | 37.8 (4.0) | 36.6 (.5) | ** |
| Verbal Mental age | 33.8 (5.8) | 38.6 (3.9) | * |
| Nonverbal Mental age | 33.6 (7.4) | 40.7 (4.3) | * |
| ASD Severity Rating | 6.1 (2.0) | 1.8 (1.4) | ** |
Assessment related data are presented as Mean (Standard Deviation). Chronological age and mental age data are reported in months. Chi-squared analyses and T-tests indicate that the groups differ as represented by the following symbols:
p < .05;
p < .01;
p < .001.
Table 2.
Sample Size By Outcome Group and Time Point
| Time Point | Sibs-ASD | Sibs-TD |
|---|---|---|
| 6 months | 11 | 7 |
|
| ||
| 12 months | 16 | 13 |
| 18 months | 10 | 15 |
| 24 months | 10 | 18 |
| 36 months | 10 | 27 |
Clinical psychologists at the [University] Autism Evaluation Clinic confirmed diagnosis of Autistic Disorder in older siblings using the Autism Diagnostic Observation Schedule (ADOS; Lord et al. 2000), the Autism Diagnostic Interview-Revised (ADI-R; Lord, Rutter, and Le Couteur 1994), and Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV-TR) criteria. All participants in this study were considered high-risk infants by virtue of having an older sibling with autistic disorder (AD). In an effort to constrain heterogeneity among participants, infants whose older sibling was diagnosed with Pervasive Developmental Disorder--Not Otherwise Specified (PDD-NOS) or Asperger’s Disorder were not enrolled in the study.
Infants were evaluated at 6, 12, 18, 24, and 36 months of age, within 2 weeks of reaching the target age. At the 36-month time point, participants were sub-classified into one of three outcome groups: an ASD group (Sibs-ASD), a group of participants exhibiting non-autistic developmental atypicalities (Sibs-Concerns), and those who did not raise concerns and thus appeared to be developing typically (Sibs-TD). A clinician classified participants based on information from the ADOS, the Mullen Scales of Early Learning (MSEL, Mullen 1995), the Vineland Adaptive Behavior Scales (Sparrow, Cicchetti, & Balla 2005), the Social Communication Questionnaire (Rutter et al. 2003), and DSM-IV-TR criteria for the ASDs.
Participants classified in the Sibs-Concerns group met one or more of the following criteria: elevated ADOS scores (within one point of the cut-off for ASD), scores 2 SD below the mean on one of the scales of the MSEL, or scores 1.5 SD below the mean on two or more MSEL scales, indicating developmental delays. Additional participants who did not meet these criteria but raised clinicians’ concerns with respect to their developmental progress (e.g., difficulty with language pragmatics) were also included in the Sibs-Concerns group. The heterogeneity and size of this group, which consisted of as few as 2 participants at some time points, rendered evaluation of its temperament trajectories difficult to analyze. Thus, analyses reported below are based on two comparison groups: infant siblings of children with autism who met criteria for ASD (Sibs-ASD), and those who raised no concerns about developmental progress (Sibs-TD).
Seven subjects who met DSM criteria for ASD at 24 months, but who have not yet returned for a 36-month assessment, were included in the analyses reported below. While ADOS classifications at 24 months of age may (Chawarska et al. 2007, 2009; LeCouteur et al. 2008) or may not be stable predictors of later diagnosis (Charman et al. 2005; Turner and Stone 2007), the number and severity of autism-related symptoms presented by these subjects at 24 months yielded a high confidence rating from the clinician with respect to the ASD classification. The Sibs-TD group included participants who neither met criteria for ASD nor Concerns. This outcome grouping strategy has been described in papers examining other domains of development among infant siblings (Hutman et al. 2012; Young et al. 2011).
Measures
Carey Temperament Scales
Parents completed the Carey Temperament Scales (CTS; Carey and McDevitt 1995) within the target window for each assessment. The CTS is a 107-item parent-report questionnaire that measures nine scales of temperament defined by Thomas and Chess (Thomas et al. 1963; 1968; Chess and Thomas 1996). CTS scales are defined in Table 3.
Table 3.
Interpretation of Carey Scales
| Lower score | Higher score | |
|---|---|---|
| Activity | Inactive | Active |
| Adaptability | Quick | Gradual |
| Approach | Approaching | Cautious |
| Mood | Positive | Negative |
| Intensity | Mild | Intense |
| Distractibility | Rarely | Often |
| Persistence | Often | Rarely |
| Sensory Reactivity | Nonreactive | Sensitive |
| Rhythmicity | Predictable | Not predictable |
Three different versions of the CTS were employed in this study. Parents completed the Revised Infant Temperament Questionnaire (RITQ) when subjects were 6 months old, the Toddler Temperament Questionnaire (TTQ) when subjects were between 12–24 months old, and the Behavior Style Questionnaire (BSQ) at 36 months. All questionnaires have good test-retest reliability and high internal consistency in typically developing samples (McDevitt and Carey 1978; Fullard et al. 1984). The BSQ has also demonstrated good test-retest reliability and high internal consistency in an ASD sample (Hepburn and Stone 2006). The questionnaires present a series of statements that the respondent rates from 1 (“almost never describes my child”) to 6 (“almost always describes my child”). Mean and z-scores for each scale were calculated with scoring software obtained from the publisher. Mean raw scores, the focus of our primary analyses, indicate an individual’s average score for all items on each scale (reverse-scored items are corrected). Z-scores are standardized so that a score of 0 represents the mean score for a child’s age group and gender, and positive and negative scores represent deviation from that mean in either direction.
Table 3 clarifies the interpretation of lower and higher mean scores. Mean scores, standard deviations, and effect sizes for statistically significant group differences in the current sample are reported for the Sibs-ASD and Sibs-TD groups for each scale at each time point in Table 4. Table 5 lists internal consistencies for the scales at each time point for the current sample. An alpha level over .7 indicates adequate internal consistency. Generally, alpha levels were lower at 6 and 12 months and higher at later time points. It is possible that this is an effect of the smaller sample sizes at the earlier time points. Nonetheless, most scales were consistently at or close to .7 across time points. Notably, the Sensory Reactivity scale showed very poor to poor alpha levels at all time points (range: −1.784 to 0.436).
Table 4.
Mean Temperament Scores by Group and Time Point
| Scale | Group | 6 months | 12 months | 18 months | 24 months | 36 months |
|---|---|---|---|---|---|---|
| Activity | Sibs-ASD | 3.776 (.442) | 3.594 (.782) | 3.892 (.448) | 3.832 (.656) | 3.730 (.978) |
| Sibs-TD | 4.275 (.435) | 4.062 (.701) | 3.807 (.830) | 3.739 (.884) | 3.434 (.702) | |
| Adaptability | Sibs-ASD | 2.030 (.417) | 3.157 (.542) | 3.523 (.588) | 3.141 (.817) | 3.084 (.872) |
| Sibs-TD | 2.660 (.492) | 3.592 (.697) | 2.988 (.844) | 2.845 (.669) | 2.206 (.662) | |
| Cohen’s d | −1.380** | −0.697+ | 0.396+ | 1.134** | ||
| Approach | Sibs-ASD | 2.359 (.373) | 3.033 (.971) | 3.574 (.909) | 3.616 (.935) | 3.919 (.734) |
| Sibs-TD | 2.767 (.478) | 3.264 (.975) | 3.472 (.723) | 3.087 (.734) | 2.912 (.729) | |
| Cohen’s d | −0.952* | 0.629* | 1.377** | |||
| Mood | Sibs-ASD | 3.104 (.658) | 3.180 (.600) | 3.361 (.751) | 3.132 (.396) | 3.185 (.925) |
| Sibs-TD | 3.192 (.530) | 3.377 (.910) | 3.092 (.621) | 3.026 (.790) | 3.042 (.689) | |
| Intensity | Sibs-ASD | 2.763 (.661) | 3.672 (.920) | 4.270 (.542) | 4.270 (.643) | 3.900 (.662) |
| Sibs-TD | 3.428 (.825) | 3.761 (.883) | 3.853 (.853) | 3.782 (.832) | 4.025 (.684) | |
| Distractibility | Sibs-ASD | 2.043 (.577) | 4.133 (.770) | 4.380 (.758) | 4.395 (.540) | 3.960 (.781) |
| Sibs-TD | 2.250 (.505) | 4.562 (.708) | 4.466 (.807) | 4.589 (.636) | 4.398 (.628) | |
| Persistence | Sibs-ASD | 3.579 (.504) | 4.320 (.587) | 4.055 (.752) | 3.953 (.690) | 3.360 (.721) |
| Sibs-TD | 4.232 (.831) | 4.472 (.780) | 3.701 (1.142) | 3.559 (.877) | 3.313 (.478) | |
| Sensory Reactivity | Sibs-ASD | 3.436 (.800) | 3.819 (.426) | 4.080 (.695) | 4.224 (.482) | 3.500 (.498) |
| Sibs-TD | 3.453 (.809) | 3.762 (.597) | 4.363 (.512) | 4.479 (.651) | 3.410 (.614) | |
| Rhythmicity | Sibs-ASD | 2.225 (.637) | 2.569 (.625) | 2.451 (.647) | 2.546 (.518) | 2.699 (.543) |
| Sibs-TD | 3.152 (.523) | 2.576 (.744) | 2.504 (.761) | 2.488 (.701) | 3.044 (.660) |
Group scores are presented as Mean (Standard Deviation). Effect sizes (Cohen’s d) are given for group contrasts where significant differences were observed. The groups differ as represented by the following symbols:
p < .05;
p < .01;
p < .001.
Table 5.
Internal Consistencies of CTS Scales by Time Point
| Scale | 6 month | 12 month | 18 month | 24 month | 36 month |
|---|---|---|---|---|---|
| Activity | 0.744 | 0.731 | 0.687 | 0.775 | 0.807 |
| Adaptability | 0.536 | 0.278 | 0.619 | 0.615 | 0.807 |
| Approach | 0.449 | 0.821 | 0.788 | 0.882 | 0.745 |
| Mood | 0.514 | 0.730 | 0.708 | 0.718 | 0.764 |
| Intensity | 0.637 | 0.604 | 0.708 | 0.707 | 0.708 |
| Distractibility | 0.578 | 0.847 | 0.814 | 0.748 | 0.741 |
| Persistence | 0.455 | 0.680 | 0.741 | 0.806 | 0.435 |
| Sensory Reactivity | −0.179 | −1.784 | 0.436 | 0.420 | 0.317 |
| Rhythmicity | 0.680 | 0.687 | 0.748 | 0.675 | 0.482 |
Cronbach’s alpha values in bold type are at greater than adequate levels of internal consistency (> .7). Italicized alpha values are close to adequate levels of internal consistency (.6 – .7).
Mullen Scales of Early Learning
The Mullen Scales of Early Learning (MSEL; Mullen, 1995) were administered at each visit. The MSEL measures cognitive and motor abilities in children aged 0–68 months in the following domains: Gross Motor (GM), Fine Motor (FM), Visual Reception (VR), Receptive Language (RL), and Expressive Language (EL). T-scores, age equivalents, and percentile scores are calculated for each scale. For the purposes of sample characterization reported in Table 1, age equivalent scores on the VR and FM scales were averaged to yield an estimate of nonverbal mental age. The RL and EL scales were also averaged to yield an estimate of verbal mental age.
Autism Diagnostic Observation Schedule
The Autism Diagnostic Observation Schedule (ADOS; Lord et al. 2000) was administered to all participants at the 24- and 36-month time points. The ADOS is a semi-structured, play-based observation that elicits social and communication behaviors relevant to ASD diagnosis. ADOS cut-off scores reliably distinguish between children with ASD, TD children, and other developmentally disabled groups. Examiners were deemed reliable on administration and scoring of the ADOS according to its authors’ specifications. ASD-classification is based on the ADOS at 36 months when available (n = 37), and on the 24-month ADOS for those children who have not completed the 36-month assessment (n = 7).
Statistical Analyses
First, we tested effects of temperament scale, time (centered at 6 months), and outcome classification (Sibs-ASD versus Sibs-TD), as well as all two-way and three-way interactions among the constructs in a linear mixed model (LMM) predicting CTS mean raw scores. Thus, the three-way interaction is a temperament scale by time by outcome group design. Gender was controlled in these analyses as it would be if z-scores were used as the dependent measure. Reference coding was used in the linear mixed model. Results of the LMM indicate effects relative to a reference category. The reference group for these analyses was Sibs-TD and the reference scale was Rhythmicity. Including all levels of a variable in a model results in perfect multi-collinearity. Omitting one of the groups from a regression analysis makes the omitted group the reference category against which the other categories are compared (Hardy, 1993). An advantage of using a mixed model design in longitudinal research is that this model utilizes all available data while dropping missing data points, without excluding the participant entirely (Raudenbush & Bryk, 2002; Singer & Willett, 2003).
Consistent with our hypothesis of different trajectories on the CTS scales between groups, we expected to observe a significant three-way interaction among temperament, time, and outcome group on the full LMM. Once observed, three-way interactions were decomposed post-hoc by calculating the differences in average slopes across all five time points (“diff slope”) between outcome groups on each scale over time and determining the scales for which slopes differed between outcome groups.
In a second step of post-hoc analyses, we calculated differences (“diff”) between the outcome groups’ predicted mean values on each temperament scale at each time point. This is a variation on a test of simple effects. The contrasts identify the time points at which group means were significantly different for each scale indicated in the LMM and slope analyses. LMM and post-hoc analyses were run using Stata statistical software, Release 12.1 (Statacorp LP).
Results
Linear Mixed Model Testing Three-Way Interaction: Scale X Group X Time
The LMM predicting mean raw scores on the nine CTS scales revealed a significant three-way interaction among outcome group (Sibs-ASD versus Sibs-TD), the CTS scales, and time (X2(8) = 23.20, p = .003). Outcome group-by-time interactions were observed for the Adaptability (z = 3.62, SE = .017, p < .001) and Approach (z = 3.42, SE = .017, p = .001) scales.
Post-Hoc Analysis of Average Slopes from 6–36 Months
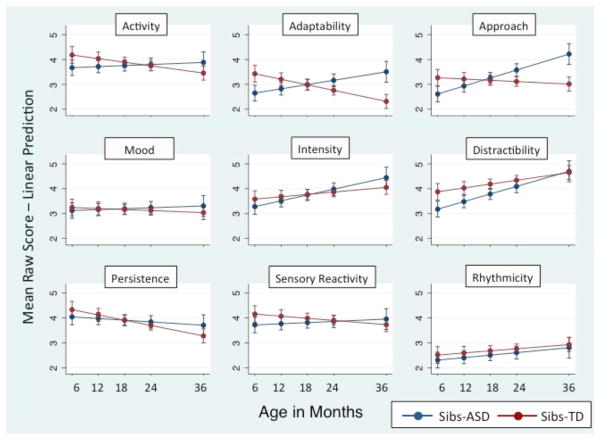
Slopes differed significantly between outcome groups on the Activity, Adaptability, and Approach scales: Activity (diff slope = −.031, SE = .013, p = .015), Adaptability (diff slope = −.066, SE = .013, p < .001), Approach (diff slope = −.062, SE = .013, p < .001). Negative diff slope scores indicate that the Sibs-TD trajectories are flatter (Activity and Approach scales) or slope downward (Adaptability scale) relative to the Sibs-ASD group. Estimated differences in slope between groups on these scales are summarized in Table 6. Figure 1 depicts linear estimates of trajectories by group for all nine CTS scales. Graphing linear estimates does not reflect curvilinearity such as that detected in the Intensity Scale, where means for Sibs-ASD were marginally lower than those reported for Sibs-TD at 6 and 36 months, but differences were in the opposite direction at 18 and 24 months. Subtler changes in direction of raw difference scores were evident for the Sensory Reactivity scale.
Table 6.
Estimated Slope Differences Between Groups by Scale and Time Point
| Activity | Adaptability | Approach | ||||
|---|---|---|---|---|---|---|
| Time Point | Slope Difference | 95% CI | Slope Difference | 95% CI | Slope Difference | 95% CI |
| 6 months | −.508 | −.971, −.046 | −.777 | −1.240, −.315 | −.654 | −1.117, −.192 |
| 12 months | −.321 | −.683, .041 | −.384 | −.746, −.022 | −.282 | −.644, .080 |
| 18 months | −.134 | −.439, .172 | .010 | −.296, .316 | .090 | −.216, .396 |
| 24 months | .054 | −.266, .373 | .403 | .084, .723 | .462 | .142, .782 |
| 36 months | .428 | −.078, .934 | 1.190 | .684, 1.696 | 1.206 | .700, 1.712 |
Fig. 1.
Predicted linear slopes based on mean raw scores for the nine temperament scales by group and time
Although an outcome group-by-time interaction was not observed on the Activity scale in the analysis of mean scores, the slopes on this scale differed significantly between the outcome groups in post-hoc analyses. Additionally, an outcome-group-by-time interaction was evident for the Activity scale in analysis involving z-scores (Appendix 1).
Post-Hoc Analysis of Group Means at Each Time Point
Between-group contrasts by time point for scales with significant three-way interactions are as follows: on the Adaptability scale, Sibs-ASD < Sibs-TD at 6 (diff = −.777, SE = .236, p = .001) and 12 months (diff = −.384, SE = .185, p = .038); Sibs-ASD > Sibs-TD at 24 (diff = .403, SE = .163, p = .013) and 36 months (diff = 1.190, SE = .258, p < .001). On the Approach scale, Sibs-ASD < Sibs-TD at 6 months (diff = −.654, SE = .236, p = .006); Sibs-ASD > Sibs-TD at 24 (diff = .462, SE = .163, p = .005) and 36 months (diff = 1.206, SE = .258, p < .001).
An identical set of analyses was run using z-scores from the nine CTS scales as dependent variables. Z-scores generated by proprietary scoring software are normed for gender and age in TD children. Analysis of these data was undertaken to confirm that effects observed in analysis of raw scores were not driven by norming constructs. Results of these analyses are presented in an Appendix to this article. Effects reported above for the Adaptability and Approach scales were still in evidence. A three-way interaction for Activity was observed in the analysis of z-scores: relative to Sibs-TD, Sibs-ASD demonstrated a significantly less active behavior profile at 6 and 12 months. Over time, Sibs-ASD became more active and thus more similar to Sibs-TD in the current sample and more similar to the normative sample. Details about the z-score analyses are presented in Appendix 1.
Discussion
This study examined whether parent report of temperament trajectories early in development distinguishes high-risk infants who were subsequently diagnosed with ASD from high-risk infants demonstrating typical patterns of development. Several prospective studies have documented temperament differences between infants who were and were not later diagnosed with ASD (Bolton et al. 2012; Clifford et al. 2013; Garon et al. 2009; Zwaigenbaum et al. 2005). The current study is the first to examine differences in temperament ratings at and across multiple time points between high-risk infants with and without ASD.
Key Temperament Differences in Infants with ASD
We expected to find different temperament trajectories between outcome groups. Indeed, we found significantly different slopes between high-risk children with and without ASD on two of the nine temperament scales. Children with ASD had temperament trajectories indicative of decreasing adaptability and approach behaviors relative to TD children who also had at least one sibling with autistic disorder. These temperament trajectories are broadly consistent with past work on temperament in ASD. Temperament studies of infants and older children with ASD suggest that they are less adaptable (Bailey et al. 2000; Hepburn and Stone 2006) and that they are less likely to approach new people, objects, and activities (e.g., Garon et al. 2009) than TD children.
In analyses using z-scores adjusted for child’s age and gender rather than mean raw scores, the effects reported above were replicated and group differences were also observed in the Activity scale such that Sibs-ASD were significantly less active than Sibs-TD at 6 and 12 months of age (See Appendix 1). In that Activity scale effects were inconsistent between z-score and raw-score analyses, they should be interpreted with caution. Still, increasing activity levels are consistent with changing relations between autistic traits and temperament characteristics between 6 and 24 months of age in a large general population sample of children with and without ASD (Bolton et al. 2012).
We hypothesized that parents would detect and report temperament differences related to subsequent ASD diagnosis at the earliest time point in the study. In support of this hypothesis, we first observed significant differences between diagnostic groups at 6 months on the Adaptability, Approach, and Activity scales. During the first year of life, parents rated infants who developed ASD as more adaptable and more inclined to approach than Sibs-TD. Also in the first year, parents rated infants who developed ASD as having lower activity levels than Sibs-TD. Given that reduced social interest and insistence on sameness are among the diagnostic criteria for ASD (APA 2000), and increased activity is frequently reported among young people with ASD (e.g., Aman 2004; Brock et al. 2012), these findings were initially surprising. Notably, findings were in the expected direction in later toddlerhood, suggesting that temperament patterns associated with ASD do not only emerge during toddlerhood, but they appear to change direction between 6 and 24 months relative to Sibs-TD. Parents of children with ASD rated them as slower to adapt and less prone to approach novel social and non-social targets at 24 and 36 months than their high-risk TD peers.
Based on the diagnostic features of ASD noted above and research indicating low levels of adaptability in older children with ASD (Bailey et al. 2000; Hepburn and Stone 2006), we expected to find decreased adaptability in infants later diagnosed with ASD. We were surprised to find that, at 6 and 12 months, parents rated Sibs-ASD as more adaptable than Sibs-TD. This may reflect parents’ anecdotal reports of having an “easy baby” and observations of passivity at earlier ages (Bryson et al., 2007). While decreasing adaptability was observed among Sibs-ASD, a pattern of increasing adaptability was evident among Sibs-TD. Indeed, we found that children with ASD were slower to adapt than high-risk TD toddlers at 24 and 36 months. The latest time point in the current study coincides with the youngest age at which children were evaluated in the aforementioned studies (Bailey et al. 2000; Hepburn and Stone 2006).
Contrary to our hypotheses, children with ASD received significantly lower scores on the Approach scale at 6 months than Sibs-TD. Lower scores on the CTS Approach scale indicate a greater tendency to approach to both social targets (e.g., a new babysitter) and non-social targets (e.g., new surroundings, such as a store or new play area). They also reflect decreased caution in the face of novel elements in the environment. Therefore, the effect observed here may be driven by low levels of inhibition rather than heightened social interest.
By 6 months of age, decreased approach toward social targets has been documented retrospectively (Maestro et al. 2002) and reduced interest in a pre-recorded video of a model has been documented prospectively (Chawarska et al. in press) among infants with ASD. However, 4-month old infants at-risk for ASD were more likely to orient to their mothers calling their names than low-risk infants (Yirmiya, et al., 2006) and 6-month-old infants with ASD allocated marginally more attention toward the faces of their mothers (Rozga et al. 2011) and examiners (Ozonoff et al. 2010) relative to TD infants. Parent reports that their children were initially sociable before symptoms of autism became apparent are not uncommon in autism research (e.g. Bryson et al., 2007). Thus, both behavioral evidence and parent reports suggest enhanced sociability at 6 months of age among at least some infants who are subsequently diagnosed with ASD. This period in development may be associated with faster than normal growth of the brain among infants who go on to develop ASD (Courchesne, Carper, & Akshoomoff, 2003). Greater approach behaviors in autism may reflect more activity in the left frontal lobe and may delay parental recognition of symptoms of autism (see Mundy et al., 2007). Thus, the current findings may reflect changes in the lateralized activity of the developing brain among a subset of individuals with autism.
Still, trajectories of approach behaviors indicate a decrease among participants with ASD relative to TD participants over time. At 24 and 36 months of age, children with ASD in our sample showed reduced approach behaviors compared to Sibs-TD. The endpoint of the trajectory observed here is consistent with previous work wherein parents’ ratings of Sibs-ASD reflected less behavioral approach than typical toddlers at 24 months (Garon et al. 2009).
Zwaigenbaum and colleagues (2005) reported lower activity levels in 6-month olds subsequently diagnosed with ASD. Similarly, we observed lower ratings of activity at 6 and 12 months in Sibs-ASD. Although differences between the outcome groups were not significant from 18 to 36 months, comparisons between groups at individual time points may present an incomplete picture. In z-score analyses, the trajectories indicate increasing activity levels in the Sibs-ASD group relative to the Sibs-TD group over time. Several studies have found increased activity level in children with ASD: children diagnosed with ASD had temperament profiles marked by increased activity levels at 24 months of age (Bolton et al. 2012; Garon et al. 2009). Brock et al. (2012) also found increased activity levels in children with ASD between the ages of 3–7 years. In contrast, Bostrom et al. (2010) found decreased activity levels in children with ASD ranging from 5–79 months of age. Thus, findings regarding activity level in children with ASD are less conclusive than those of adaptability and approach. Nonetheless, the findings of the current study suggest a temperament pattern of reduced activity level at and under 12 months, followed by increasing activity levels in toddlers with ASD.
The unexpected findings in infancy coupled with patterns that are more consistent with previous research later in development highlight the importance of examining autism from a developmental perspective. While autism is defined by key symptoms, including reduced sociability and decreased adaptability, these symptoms may manifest differently depending on developmental level. However, similar surprising effects for the Adaptability, Approach, and Activity scales at the earlier time points may reflect methodological artifacts, e.g., smaller sample sizes at six and twelve months and the use of different instruments to measure temperament across time points. Given that our sample is small and its size varies across time points, the results reported here should be interpreted with caution and should be replicated in future studies involving larger samples.
Overlap in Temperament between Participants with and without ASD
This study provides further evidence that parents of high-risk infants and toddlers later diagnosed with ASD recognize atypicalities, including unusual patterns of temperament, very early in their child’s development (Ozonoff et al., 2009). However, several of our hypotheses were not supported. We hypothesized that parent ratings of children with ASD would reflect more negative affect (Schwartz et al. 2009), increased intensity (Bolton et al. 2012), reduced sensory responsiveness (Hepburn and Stone 2006; Bailey et al. 2000), as well as reduced distractibility (Bailey et al. 2000; Bolton et al. 2012). At 36 months, children with ASD exhibited slightly higher scores than Sibs-TD on the Mood, Intensity, and Persistence scales, indicating more negative affect, more intense response, and reduced persistence. Although these findings were not significant in the LMM, mean raw scores fell in the expected direction at 36 months. Means for both outcome groups were essentially identical on the Distractibility and Sensory Reactivity scales. These results were surprising given previous findings regarding distractibility (Bailey et al. 2000; Bolton et al. 2012) and the diagnostic relevance of atypical sensory responsiveness in ASD (APA, 2013). On the other hand, temperament is not consistently linked with profiles of sensory response in ASD (Brock et al., 2012). Furthermore, our examination of internal consistencies for this sample indicates that the Sensory Reactivity Scale fared poorly across time points. Nonetheless, these results are consistent with evidence that some temperament profiles are characteristic of high-risk toddlers with and without ASD and distinct from those of low-risk TD toddlers (Garon et al. 2009).
Study Limitations
The findings reported here should be interpreted with caution, particularly at the 6-month time point, as our sample was smallest at that time point. Although studies regarding older children with ASD generally have adequate sample sizes (e.g., Hepburn and Stone, 2006; Schwartz et al. 2009; Adamek et al., 2011), small samples are a common limitation in understanding temperament in ASD early in development (Zwaigenbaum et al. 2005; Clifford et al. 2013; Garon et al. 2009).
Assessing temperament with parent report is complicated by the fact that parent report may reflect characteristics of the parent as well as the child (e.g., Mangelsdorf et al., 1990). Furthermore, shared genetic and environmental factors likely lead to similarities in temperament between parents and their children. Parents’ ratings of child temperament may be moderated by factors such as parental stress, which are frequently elevated in parents of children with ASD (e.g., Davis & Carter, 2008), but moderating effects on parent report have not been evaluated in the current study. Still, evidence of convergent validity between parent reports and laboratory observations of infant temperament (Matheny et al., 1984) suggests that parents’ familiarity with a child’s behavior over time and across contexts may make them the best possible source of relevant information.
The lack of a low-risk control group in the current study precludes comparisons with children who do not have a high-risk sibling. On the other hand, comparing high-risk infants who are and who are not subsequently diagnosed with ASD offers the advantage of controlling for parental concern regarding genetic risk for ASD. All parents who provided temperament ratings for this study shared the experience of raising at least one child with autistic disorder and all were aware that the child participating in the study was at elevated risk for ASD. One previous study found that parent report using the CTS and observational measures of temperament were more similar for children with ASD than for TD and intellectually disabled children (Kasari & Sigman 1997). This finding endorses the relative accuracy of reports on child temperament by parents of children with ASD.
While the temperament measure used in this study allows us to compare our results with a range of other relevant studies, it is not without shortcomings. Few studies have evaluated the stability of the Thomas and Chess dimensions of temperament in typical development. Guerin and Gottfried (1994) found that many of the CTS scales were only moderately stable over time in a TD sample. A meta-analysis of temperament and personality studies found only low to moderate levels of longitudinal trait consistency in measures of temperament that used the Thomas and Chess model (Roberts and Delvecchio 2000). The claim that the CTS tests the dynamic interplay between the individual and his environment (Goldsmith et al. 1987) is difficult to evaluate longitudinally -- and has not been validated longitudinally -- because of the relativistic nature of the claim.
Recent work examining temperament stability in typical development has emphasized the Rothbart model (Putnam et al. 2006; Casalin et al. 2012). These studies report stability in Rothbart temperament factors from infancy to toddlerhood (8–13 months through 30–36 months). The Rothbart scales and corresponding instruments are conceptually and empirically related to the Thomas and Chess model. Mervielde and De Pauw (2012) demonstrated that the TTS Activity and Persistence scales were moderately correlated to the Anger Proneness and Activity scale in a comparable Rothbart instrument, and the BSQ Activity and Persistence scales were correlated to both Surgency and Effortful Control. Despite conceptual overlap between these models, longitudinal analyses discussed above suggest that Rotbhart’s model has greater stability over time.
The internal consistency of the CTS measures is also variable across scales and time points. This variability is apparent in the statistics reported in Table 5 although the small sample size at some time points of this study should be taken into account when interpreting these estimates (Javali, Gudaganavar & Shodan 2011). The generally high alpha levels of the activity scale for the current sample suggests that the pattern of results reported for this scale may reflect changes in a more cohesive construct than the pattern of results in other scales.
Another measurement-related limitation is that the nine dimensions of temperament proposed by Thomas and Chess have not been supported by factor analyses (e.g., Martin and Presley 1994). Although the three CTS measures employed in this study are designed to capture the same nine dimensions of temperament, the CTS questionnaires are necessarily adapted to capture behavioral repertoires at different points in development. In the RITQ, which is tailored to infants, one example of an Approach domain item is, “The infant’s initial reaction to a new babysitter is rejection.” In the BSQ, for children ages 3–7 year, an example of an Approach item is, “The child had trouble leaving the mother the first three days when he/she entered school.” Although the items at each time point are conceptually similar, the dynamic interplay between the individual and the environment necessarily explores different behaviors in different settings, so content heterogeneity likely affects continuity and comparability across the three different CTS measures employed in this study. This issue may well apply to other measures of temperament as well.
Future Directions
Future research examining temperament trajectories in ASD should include both a high-risk and a low-risk typically developing comparison group. In order to examine the specificity of atypical temperament trajectories to ASD, future research would benefit from comparing children with ASD to children with a different developmental disability such as Down syndrome. Future research should compare parent-report measures that are commonly employed in the autism literature and test for associations with observational assessments of temperament in order to elucidate which effects are driven by change over time and which are artifacts of measurement.
We propose that improved understanding of the interplay between parent and infant temperament may be useful for family-based intervention. Intervention of this kind may support the development of effective emotion regulation strategies, decrease externalizing behaviors, and improve the quality of a child’s later social skills, in light of documented relations between temperament and adaptive skills among adolescents with ASD (Schwartz et al. 2009). In the likely event that temperament profiles moderate treatment effects, understanding growth trajectories of temperament in autism may enhance the benefits of treatment for more children with ASD.
Supplementary Material
Acknowledgments
We are deeply grateful to all the families who participated in this study, the research assistants who worked hard to collect and prepare the data, and the consultants at UCLA Statistical Consulting. The work that led to this article was supported by the following grants: NIMH U54-MH-068172 (Sigman, PI); NICHD 1P50-HD-55784 (Sigman, Bookheimer, PIs); NICHD R01-HD40432 (Johnson, PI).
Footnotes
The authors declare that they have no conflict of interest.
References
- Adamek L, Nichols S, Tetenbaum JB, Ponzio C, Carr EG. Individual Temperament and Problem Behavior in Children with Autism Spectrum Disorders. Focus on Autism and Other Developmental Disabilities. 2011;26:173–184. [Google Scholar]
- Allen K, Prior M. Assessment of the validity of easy and difficult temperament through observed mother-child behaviours. International Journal of Behavioral Development. 1995;18 (4):609–640. [Google Scholar]
- Aman MG. Seminars in Pediatric Neurology. 3. Vol. 11. WB Saunders; 2004. Sep, Management of hyperactivity and other acting-out problems in patients with autism spectrum disorder; pp. 225–228. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: 2000. text revision. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: 2013. [Google Scholar]
- Bailey DB, Jr, Hatton D, Mesibov G, Ament N, Skinner M. Early development, temperament, and functional impairment in autism and fragile X syndrome. Journal of Autism and Developmental Disorders. 2000;30(1):49–59. doi: 10.1023/a:1005412111706. [DOI] [PubMed] [Google Scholar]
- Baranek G. Autism during infancy: a retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. Journal of Autism and Developmental Disorders. 1999;29(3):213–224. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- Bebko JM, Konstantareas MM, Springer J. Parent and professional evaluations of family stress associated with characteristics of autism. Journal of Autism and Developmental Disorders. 1987;17(4):565–576. doi: 10.1007/BF01486971. [DOI] [PubMed] [Google Scholar]
- Bolton P, Golding J, Emond A, Steer C. Autism spectrum disorder and autistic traits in the Avon longitudinal study of parents and children: Precursors and early signs. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(3):249–260. doi: 10.1016/j.jaac.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Bostrom P, Broberg N, Hwang CP. Different, difficult or distinct? Mothers’ and fathers’ perceptions of temperament in children with and without intellectual disabilities. Journal of Intellectual Disability Research. 2010;54(9):806–819. doi: 10.1111/j.1365-2788.2010.01309.x. [DOI] [PubMed] [Google Scholar]
- Brock ME, Freuler GT, Baranek GT, Watson LR, Poe MD, Sabatino A. Temperament and sensory features of children with Autism. Journal of Autism and Developmental Disorders. 2012;42:2271–2284. doi: 10.1007/s10803-012-1472-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson SE, Zwaigenbaum L, Brian J, Roberts W, Szatmari P, Rombough V, et al. A prospective case series of high-risk infants who developed autism. Journal of Autism and Developmental Disorders. 2007;37(1):12–24. doi: 10.1007/s10803-006-0328-2. [DOI] [PubMed] [Google Scholar]
- Carey WB, McDevitt SC. The Carey temperament scales. Scottsdale, AZ: Behavioral-Developmental Initiatives; 1995. [Google Scholar]
- Carey WB, McDevitt SC. Revision of the infant temperament questionnaire. Pediatrics. 1978;61:735–739. [PubMed] [Google Scholar]
- Casalin S, Luyten P, Vliegen N, Meurs P. The structure and stability of temperament from infancy to toddlerhood: A one-year prospective study. Infant Behavior and Development. 2012;35:94–108. doi: 10.1016/j.infbeh.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Charman T, Taylor E, Drew A, Cockerill H, Brown JA, Baird G. Outcome at 7 years of children diagnosed with autism at age 2: predictive validity of assessments conducted at 2 and 3 years of age and pattern of symptom change over time. Journal of Child Psychology and Psychiatry. 2005;46(5):500–513. doi: 10.1111/j.1469-7610.2004.00377.x. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Paul R, Macari S, Volkmar F. A prospective study of toddlers with ASD: short-term diagnostic and cognitive outcomes. Journal of Child Psychology and Psychiatry. 2009;50(10):1235–1245. doi: 10.1111/j.1469-7610.2009.02101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Macari S, Shic F. Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with Autism Spectrum Disorders. Biological Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.11.022. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Paul R, Klin A, Hannigen S, Dichtel LE, Volkmar F. Parental recognition of developmental problems in toddlers with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(1):62–72. doi: 10.1007/s10803-006-0330-8. [DOI] [PubMed] [Google Scholar]
- Chess S, Thomas A. Temperament: Theory and practice. New York: Bruner Mazel; 1996. [Google Scholar]
- Clifford S, Hudry K, Elsabbagh M, Charman T, Johnson M. Temperament in the first 2 years of life in infants at high-risk for autism spectrum disorders. Journal of Autism Developmental Disorders. 2013;43(3):673–686. doi: 10.1007/s10803-012-1612-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Clifford S, Young R, Williamson P. Assessing the early characteristics of autistic disorder using video analysis. Journal of Autism and Devt Disorders. 2007;37:301–313. doi: 10.1007/s10803-006-0160-8. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA: Jrnl of the American Medical Association. 2003;290(3):337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Davis NO, Carter AS. Parenting stress in mothers and fathers of toddlers with Autism Spectrum Disorders: associations with child characteristics. Journal of Autism and Developmental Disorders. 2008;38(7):1278–1291. doi: 10.1007/s10803-007-0512-z. [DOI] [PubMed] [Google Scholar]
- De Pauw SSW, Mervielde I, Van Leeuwen KG, De Clercq BJ. How Temperament and personality contribute to the maladjustment of children with Autism. Journal of Autism and Developmental Disorders. 2011;41:196–212. doi: 10.1007/s10803-010-1043-6. [DOI] [PubMed] [Google Scholar]
- Fullard W, McDevitt SC, Carey WB. Assessing temperament in one- to three-year-old children. Journal of Pediatric Psychology. 1984;9(2):205–217. doi: 10.1093/jpepsy/9.2.205. [DOI] [PubMed] [Google Scholar]
- Garon N, Bryson SE, Zwaigenbaum L, Smith IM, Brian J, Roberts W, et al. Temperament and its relationship to autistic symptoms in a high-risk infant sib cohort. Journal of Abnormal Child Psychology. 2009;37:59–78. doi: 10.1007/s10802-008-9258-0. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Buss AH, Plomin R, Rothbart MK, Thomas A, Chess S, et al. Roundtable: What is temperament? Four approaches. Child Development. 1987;58(2):505–529. [PubMed] [Google Scholar]
- Gomez C, Baird S. Identifying early indicators for autism in self-regulation difficulties. Focus on Autism and Other Developmental Disabilities. 2005;20(2):106–116. [Google Scholar]
- Guerin DW, Gottfried AW. Developmental stability and change in parent reports of temperament: a ten-year longitudinal investigation from infancy through preadolescence. Merrill-Palmer Quarterly. 1994;40 (3):334–355. [Google Scholar]
- Hepburn SL, Stone WL. Using Carey Temperament Scales to assess behavioral style in children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2006;36:637–642. doi: 10.1007/s10803-006-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutman T, Chela M, Gillespie-Lynch K, Sigman M. Selective visual attention at twelve months: signs of autism in early social interactions. Journal of Autism and Developmental Disorders. 2012;42:487–498. doi: 10.1007/s10803-011-1262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasari C, Sigman M. Linking parental perceptions to actions in young children with autism. Journal of Autism and Developmental Disorders. 1997;27(1):39–57. doi: 10.1023/a:1025869105208. [DOI] [PubMed] [Google Scholar]
- Konstantareas MM, Stewart K. Affect regulation and temperament in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2006;36(2):143–154. doi: 10.1007/s10803-005-0051-4. [DOI] [PubMed] [Google Scholar]
- Konstantearas MM, Papageorgiou V. Effects of temperament, symptom severity and level of functioning on maternal stress in Greek youth and children with ASD. Autism. 2006;10(6):593–607. doi: 10.1177/1362361306068511. [DOI] [PubMed] [Google Scholar]
- Le Couteur A, Haden G, Hammal D, McConachie H. Diagnosing autism spectrum disorders in pre-school children using two standardised assessment instruments: the ADI-R and the ADOS. Journal of Autism and Developmental Disorders. 2008;38(2):362–72. doi: 10.1007/s10803-007-0403-3. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule–Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview – Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Jrnl of Autism and Devt Disorders. 1994;34:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Maestro S, Muratori F, Cavallaro MC, Pei F, Stern D, Golse B, et al. Attentional skills during the first 6 months of age in autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(10):1239–1245. doi: 10.1097/00004583-200210000-00014. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf S, Gunnar M, Kestenbaum R, Lang S, Andreas D. Infant proneness-to-distress temperament, maternal personality, and mother-infant attachment: associations and goodness of fit. Child Development. 1990;61(3):820–831. [PubMed] [Google Scholar]
- Matheny A, Jr, Wilson R, Nuss SM. Toddler temperament: stability across settings and over ages. Child Development. 1984;55(4):1200–1211. [Google Scholar]
- McDevitt SC, Carey WB. The measurement of temperament in 3–7 year old children. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1978;19:245–253. doi: 10.1111/j.1469-7610.1978.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Mervielde I, De Pauw SSW. Models of Child Temperament. In: Zentner M, Shiner RL, editors. Handbook of Temperament. 1. The Guilford Press; 2012. pp. 21–40. [Google Scholar]
- Mullen EM. Mullen scales of early learning manual. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- Mundy P, Henderson HA, Inge AP, Coman D. The modifier model of autism and social development in higher functioning children. Res Pract Persons Severe Disabl. 2007;32(2):124–139. doi: 10.2511/rpsd.32.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard EE, Smith L, Torgersen AM. Temperament in children with Down syndrome and in prematurely born children. Scandinavian Jrnl of Psychology. 2002;43(1):61–71. doi: 10.1111/1467-9450.00269. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Iosif A-M, Baguio F, Cook IC, Hill MM, Hutman T, Young GS. A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Adolescence and Psychiatry. 2010;49(3):256–266. [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Steinfeld MB, Hill MM, Cook I, Hutman T, Sigman M. How early do parent concerns predict later autism diagnosis? Journal of developmental and behavioral pediatrics: JDBP. 2009;30(5):367. doi: 10.1097/dbp.0b013e3181ba0fcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young G, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, et al. Recurrence risk for autism spectrum disorders: a baby siblings research consortium study. Pediatrics. 2011;128(3):488–495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley R, Martin RP. Toward a structure of preschool temperament: factor structure of the temperament assessment battery for children. Jrnl of Personality. 1994;62 (3):415–448. doi: 10.1111/j.1467-6494.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Gartstein MA, Rothbart MK. Measurement of fine-grained aspects of toddler temperament: the early childhood behavior questionnaire. Infant Behavior and Development. 2006;29:386–401. doi: 10.1016/j.infbeh.2006.01.004. et al 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods (Advanced Quantitative Techniques in the Social Sciences) 2 Sage Publications, Inc; 2001. [Google Scholar]
- Roberts BW, Delvecchio WF. The rank-order consistency of personality traits from childhood to old age: a quantitative review of longitudinal studies. Psychological Bulletin. 2000;126 (1):3–25. doi: 10.1037/0033-2909.126.1.3. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn SL, Wehner E. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. Journal of Autism and Developmental Disorders. 2003;33:631–642. doi: 10.1023/b:jadd.0000006000.38991.a7. [DOI] [PubMed] [Google Scholar]
- Rogers S, Ozonoff S. Annotation: What do we know about sensory dysfunction in autism? A critical review of the empirical evidence. Journal of Child Psychology and Psychiatry. 2005;46(12):1255–1268. doi: 10.1111/j.1469-7610.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Evans DE. Temperament and personality: Origins and outcomes. Journal of Personality and Social Psychology. 2000;78:122–135. doi: 10.1037//0022-3514.78.1.122. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ellis LK, Rueda MR, Posner MI. Developing mechanisms of temperamental effortful control. Journal of Personality. 2003;71:1113–1143. doi: 10.1111/1467-6494.7106009. [DOI] [PubMed] [Google Scholar]
- Rozga A, Hutman T, Young GS, Rogers SJ, Ozonoff S, Dapretto M, et al. Behavioral profiles of affected and unaffected siblings of children with autism: Contribution of measures of mother-infant interaction and nonverbal communication. Journal of Autism and Development Disorders. 2011;41:287–301. doi: 10.1007/s10803-010-1051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social communication questionnaire. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Schwartz CB, Henderson HA, Inge AP, Zahka NE, Coman DC, Kojkowski NM, et al. Temperament as a predictor of symptomatology and adaptive functioning in adolescents with high-functioning autism. Journal of Autism and Development Disorders. 2009;39:842–855. doi: 10.1007/s10803-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willet JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford University Press; 2003. [Google Scholar]
- Sparrow SS, Cicchetti D, Balla DA. Vineland Adaptive Behavior Scales - 2nd Edition manual. Minneapolis, MN: NCS Pearson, Inc; 2005. [Google Scholar]
- Thomas A, Chess S. Temperament and Development. Oxford, England: Brunner/Mazel; 1977. [Google Scholar]
- Thomas A, Chess S, Birch H. Temperament and behavior disorders in children. New York: New York University Press; 1968. [Google Scholar]
- Thomas A, Chess S, Birch HG, Hertzig ME, Korn S. Behavioral individuality in early childhood. New York: New York University Press; 1963. [Google Scholar]
- Turner LM, Stone WL. Variability in outcome for children with an ASD diagnosis at age 2. Journal of Child Psychology and Psychiatry. 2007;48(8):793–802. doi: 10.1111/j.1469-7610.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- Watson L, Baranek G, Crais E, Reznick S, Dykstra J, Perryman T. The first year inventory: retrospective parent responses to a questionnaire designed to identify one-year-olds at risk for autism. Journal of Autism and Developmental Disorders. 2007;37(1):49–61. doi: 10.1007/s10803-006-0334-4. [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Gamliel I, Pilowsky T, Feldman R, Baron-Cohen S, Sigman M. The development of siblings of children with autism at 4 and 14 months: Social engagement, communication, and cognition. Jrnl of Child Psychology and Psychiatry. 2006;47(5):511–523. doi: 10.1111/j.1469-7610.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- Young GS, Rogers S, Hutman T, Rozga A, Sigman M, et al. Imitation from 12 to 24 months in autism and typical development: a longitudinal Rasch analysis. Developmental Psychology. 2011;47(6):1565–1578. doi: 10.1037/a0025418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.