Abstract
Sulforaphane (SFN) is a natural compound that has been investigated as a chemopreventive agent. SFN has been shown to inhibit the activator-protein-1 (AP-1) transcription factor and may be effective for inhibition of ultraviolet (UV) induced skin carcinogenesis. This study was designed to investigate the stability of SFN as a function of pH, temperature and in various solvents and formulations. SFN was determined to undergo apparent first order degradation kinetics for the conditions explored. It was observed that SFN undergoes base catalyzed degradation. Buffer species and solvent type impacts stability as well. SFN was found to be very sensitive to temperature with degradation rate changing by a factor of nearly 3.1 for every 10°C change in temperature (at pH 4.0). SFN completely degraded after 30 days in a conventional pharmaceutical cream formulation. Improved stability was observed in organic formulation components. Stability studies were conducted on two non-aqueous topical formulations, a polyethylene glycol (PEG) ointment base and an organic oleaginous base. Topically applied SFN in the PEG base formulation significantly reduced AP-1 activation after UV stimulation in the skin of a transgenic mouse model, indicating that SFN in this formulation retains efficacy in vivo.
Keywords: Isothiocyanate, degradation, hydrolysis, base catalysis, activator-protein-1, UV-B, in-vivo
Introduction
Diagnosed skin cancer cases exceed over one million a year and account for roughly 40% of new cancer cases1. UV radiation (UVR) is the primary factor responsible for the majority of skin cancers. This is mainly a result of direct exposure to the skin leading to DNA damage, inflammation, erythema and edema2. Because of the increasing incidence of skin cancer, safe and effective chemopreventive therapies are being developed in an effort to complement primary skin cancer prevention treatments. Topical delivery of chemopreventive agents for skin cancer provides distinct advantages over other routes as the direct application to the target tissue (skin) can minimize systemic exposure and possible systemic side effects.
Sulforaphane (SFN) (Figure 1), a naturally occurring isothiocyanate (1-Isothiocyanato-4 methylsulfinylbutane) found in cruciferous vegetables such as broccoli, has been investigated in preclinical and clinical studies as a chemopreventive agent that may show effectiveness against ultraviolet (UV) induced skin carcinogenesis3. SFN is commonly known as a strong inducer of cytoprotective enzymes through induction of the phase II detoxification response and antioxidants; SFN activates the Nf-E2-related factor 2 (Nrf2) transcription factor, which binds to the Antioxidant Response Element (ARE) site in the promoter of many genes involved in cellular protection. Through the regulation of transcription linked to the ARE, Nrf2 can also regulate inflammation, phase I metabolism enzymes, the cell cycle and apoptosis4. Another mechanism of action of SFN is its ability to inhibit the activity of the activator-protein-1 (AP-1) transcription factor. AP-1 is strongly activated by treatment of keratinocytes with ultraviolet irradiation, particularly UVB5. Dysregulation of AP-1 signaling during cancer progression can lead to alterations in cellular proliferation, apoptotic responses and differentiation. Inhibition of AP-1 greatly reduces UVB-induced skin carcinogenesis in mice6–8. Recently it has been shown that SFN inhibits AP-1 activity by directly binding to its DNA binding domain, therefore preventing the protein from interacting with its response element, the TRE, in the promoters of AP-1 regulated genes3. It has also been shown that SFN effectively inhibits UVB-induced skin carcinogenesis in mice3, 9.
Figure 1.
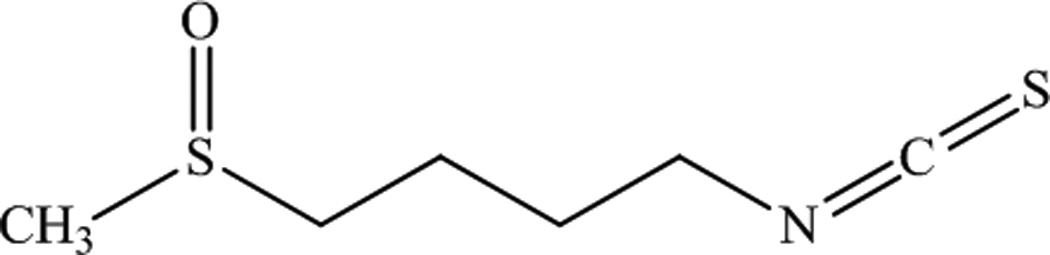
Chemical structure for Sulforaphane (C6H11NOS2, Mol. Wt. 177.29, LogP: 0.23).
Despite evidence provided in the literature demonstrating SFN’s promise as a chemopreventive for skin cancer, few studies have been conducted investigating potential topical formulations. So far, all topical interventions using SFN on mice and human subjects have involved dissolving SFN in acetone or an acetone/water mixture10–11. In order to translate these findings into longer-term clinical trials a topical formulation that is easily applied to the skin and does not cause irritation is required. The formulation needs to efficiently deliver SFN to the epidermis. It must also afford stability over the long term. There is little data available concerning the stability of SFN as a function of pH, temperature and in various solvents that would prove beneficial in developing a suitable formulation for preclinical and clinical trials.
SFN is a viscous yellow liquid at room temperature and has a calculated LogP of 0.2312. Because it has been observed to be highly unstable in water at elevated temperatures13, traditional topical vehicles which usually contain high water content are likely not feasible vehicles for SFN. Thus, non-aqueous environments need to be explored. This study investigates the stability of SFN under various conditions of pH, temperature, solvent vehicle and in two non-aqueous formulations, thus evaluating their potential as lead topical formulations. Following the determination of SFN stability and identifying a prototype formulation, the efficacy of that formulation was evaluated in vivo in an AP-1 luciferase mouse model.
Methods
High performance liquid chromatography (HPLC)
Samples were analyzed with a Waters System consisting of a 2690 separation module, coupled with a 996 Photodiode Array Detector (Waters Corporation, Milford, MA). Separation was achieved using a 150mm × 3.9mm Altima C18 5µm column maintained at 30°C. Ultraviolet detection was done at 241nm. A reverse phase HPLC method was developed to analyze drug concentration from a variety of experimental conditions. Aqueous samples and solvent stability utilized an isocratic acetonitrile:water (76:24) run for 14 minutes. Topical formulations used one of two gradient methods to ensure residual formulation components were removed from the column. Polyethylene glycol (PEG) formulations followed the initial isocratic conditions described above followed by a linear ramp to acetonitrile:water (10:90) over 10 minutes proceeded by a 2 minute linear ramp returning to the original isocratic conditions. Organic oleaginous formulations also utilized the isocratic conditions followed with a linear ramp to 100% isopropyl alcohol for 10 minutes and then a linear ramp of 2 minutes resuming to the initial mobile phase conditions. Total run time was 35 minutes for analysis of both topical formulations. Standards were prepared from roughly 500µg/ml stock solution of SFN in 100% acetonitrile (ACN) and ranged from 2.5–200 µg/ml. The retention time for SFN was approximately 8 minutes for all formulations. Samples were run with a constant flow rate of 0.45mL/min and target injection volume of 10µL. All quantitations were based on peak area.
Mass Spectrometry (MS)
Analysis of SFN was carried out on a TSQ Quantum triple quadrupole LC-MS with a Surveyor MS pump and Surveyor autosampler (Thermo Finnigan, San Jose, CA). Chromatographic separation was achieved using the isocratic method described above with the exception of a Waters Symmetry column (150mm × 3.9mm C18 5µm) used in place of an Altima. The mass spectrometer was operated in electrospray positive ionization mode with a spray voltage of 4500V, sheath gas flow of 20 arbitrary units (au), auxiliary gas flow of 3au and ion transfer capillary temperature of 350°C. Detection was by full scan MS mode, scanning from 150 to 1000 m/z.
Effect of pH
The effect of pH on the stability of SFN was determined using a citrate buffer (0.1M) in the pH range of 4.0–5.0, a phosphate buffer (0.1M) in the pH range 5.0–8.0 and a borate buffer (0.1M) in the pH range of 9.0. Buffers were pH adjusted with NaOH and HCl as needed and samples (n=2) were prepared with ACN as a cosolvent at 20% (V/V). The target concentration of SFN was 200µg/ml and degradation was observed over four weeks at 4, 26, 37 and 47°C. Stability was determined using HPLC isocratic conditions mentioned above.
Effect of solvents
The influence of organic solvents on the stability of SFN was tested via exposure to an elevated temperature of 37°C in order to observer drug degradation in an escalated manner. A target concentration (200µg/ml) of SFN was introduced to several organic solvent environments, including acetonitrile (ACN), ethanol (EtOH), isopropanol (IPA), methanol (MeOH), and triglyme.
Additionally, the degradation rate of SFN was observed in water (H2O, pH 8.0) as well as in a neat solution of pure SFN. In an attempt to stabilize degradation the effect of a chelating agent, EDTA (0.05M, pH 8.0), was also studied. Degradation was analyzed over a week long period and samples (n=2) were assayed using HPLC isocratic conditions stated above.
Stability in topical vehicles
Initial formulation stability was conducted with a variety of topical vehicles at room temperature. Initially, SFN stability was observed in isopropyl palmitate, PEG 400, bath oil composed of cotton seed oil and alkyl aryl polyether alcohol, as well as in a more conventional oil-in-water cream14. Samples (n=2) were prepared at a target concentration of 1% wt/wt and observed over the course of five months.
Effect of PEG chain length
The relationship between stability and PEG chain length was investigated. A target concentration (200µg/ml) of SFN was introduced into pure PEG formulations that included a series of PEG 200, 300, 400, and 600. To understand the potential effect of the polymer chain length, the stability of SFN in the structural subunit ethylene glycol was also investigated in a similar manner. Furthermore, to understand the potential impact of water in the formulation, a series of PEG 400 that contained additional water (0.1% and 1.0% wt/wt) was investigated. Stability was conducted at an elevated temperature of 37°C for one week (n=2).
Topical formulations: preparation
Based on resultant data from the initial formulation screening, two non-aqueous topical formulations were considered for further evaluation. A PEG ointment base (60% PEG 400 and 40% PEG 3350) and an organic oleaginous base composed of the organic components from the previously mentioned oil-in-water cream (39% stearic acid, 35% isopropyl palmitate, 17% mineral oil, 9% PPG-2 myristyl ether propionate) were utilized. These formulations were prepared by manually compounding roughly 4.4 mg of SFN per gram of base formulation. Once mixing was achieved stock material was then aliquoted into three equal portions. Additionally, the effects of antioxidants on stability were studied by adding either 0.1% ascorbic acid or butylated hydroxytoluene (BHT) to separate aliquots of formulation. Stability was conducted at 4, 26, 37 and 47°C.
Effect of temperature
The impact of temperature on the degradation rates of SFN were observed at four different temperatures (4, 26, 37, and 47°C) in two non-aqueous topical formulations. Analysis of the degradation rates of SFN at different temperatures allows for the estimation of activation energy.
Sampling frequency was dependent upon temperature exposure. Formulations at higher temperatures (37°C and 47°C) were sampled every few days for up to six weeks or until drug was depleted. At lower temperatures (4°C and 26°C) formulations were sampled every few weeks, often times only once a month. Due to the higher drug concentration that remained under these conditions, stability was observed over a six month period.
Drug content was analyzed by sampling 7–10µg of formulation and placing it directly into an HPLC vial (n=2). One milliliter of solvent, isopropyl alcohol for the organic oleaginous formulation and oil-in-water cream, and acetonitrile for the PEG formulation and remaining pre-formulation samples, was added to the HPLC vials. Vials where shaken to ensure complete dissolution then analyzed by the HPLC conditions mentioned above.
In Vivo Studies
Mice were housed in accordance with The University of Arizona Animal Care and Use Committee standards. Female SKH-1 mice expressing TRE-driven luciferase (AP-1 luciferase mice)6, 15 were separated into two groups of 10 animals each. The skin of both ears per mouse was treated with the PEG ointment base with or without the addition of SFN four times (day 1, 3, 5 and 8). Punches were taken from the right ear on day 9 (pre-UV samples). Mice were all treated with an acute dose of 2.75 kJ/m2 UVB on day 10 and then treated with vehicle or drug again about 30min after UVB. The source of UVB was a bank of six FS40T12 UVB lamps (National Biological Corporation). Forty-eight hours after UVB treatment (day 12), all mice were sacrificed and punches were taken from the left ear of each mouse (post-UV samples). Ear punches were flash frozen in liquid nitrogen and stored at −80°C until processing for the luciferase assay as described previously16. Ten micrograms of protein per sample was used to perform the luciferase assay. Post-UV luciferase activity was divided by Pre-UV luciferase activity for each individual to calculate fold activation. Outliers were dropped if their post/pre luciferase ratio was more than two standard deviations away from the mean (final n = 8 for both groups).
Results and Discussion
Mass spectrometry analysis
Initial analytical standard solutions were prepared in methanol. However, an additional peak, at 5.5 minutes, was observed in the HPLC chromatograms of samples in methanol, thereby prompting further analysis. Mass spectrometry analysis of these samples identified a methanol solvolysis product with a mass to charge ratio (m/z) of 209.91; SFN eluted at 10.3 minutes with m/z = 177.90. In addition, the presence of the thiourea, N,N’-di-(methylsulfinyl) butyl thiourea, previously discussed13 was also confirmed in these samples (m/z = 312.92) at 2.9 minutes.
Effect of pH
Drug stability can be altered by different pH levels, catalyzed by either hydrogen ions or hydroxide ions and in some cases both. Stability of SFN was tested under five different pH conditions ranging from 4.0–9.0 and utilizing a variety of buffer species (citrate, phosphate, borate), at 26°C and a constant ionic strength of 0.1M. From the kinetic rate profiles (Figure 2) it is evident that the degradation of SFN undergoes apparent first-order kinetics in the pH conditions presented. The degradation rate constants (κ) were calculated from the slope of the linear best fit line from the relationship between the logarithm percent drug remaining versus time. Rate constants were used to plot a pH rate profile (Figure 3) for SFN at 26°C that distinctly indicates that SFN undergoes base catalyzed degradation. These results are consistent with those reported which monitored stability as a function of pH for 2 hours at elevated temperature17. Interestingly, the slope from the pH 6.0 to pH 9.0 (Figure 3) is 1.29, potentially indicating that in addition to base catalysis there may be other mechanisms occurring. For example, in addition to solvolysis, mechanisms directly attributed to thermal degradation have been reported13. It is clear based on the pH 4.0 and pH 5.0 data that buffer species can have an impact on SFN degradation. The data for pH 4.0 and 5.0 in citrate buffer are not included in Figure 3 and the results are discussed below in Effect of buffer species. No pH change was noted in any of the samples over the course of the study.
Figure 2.
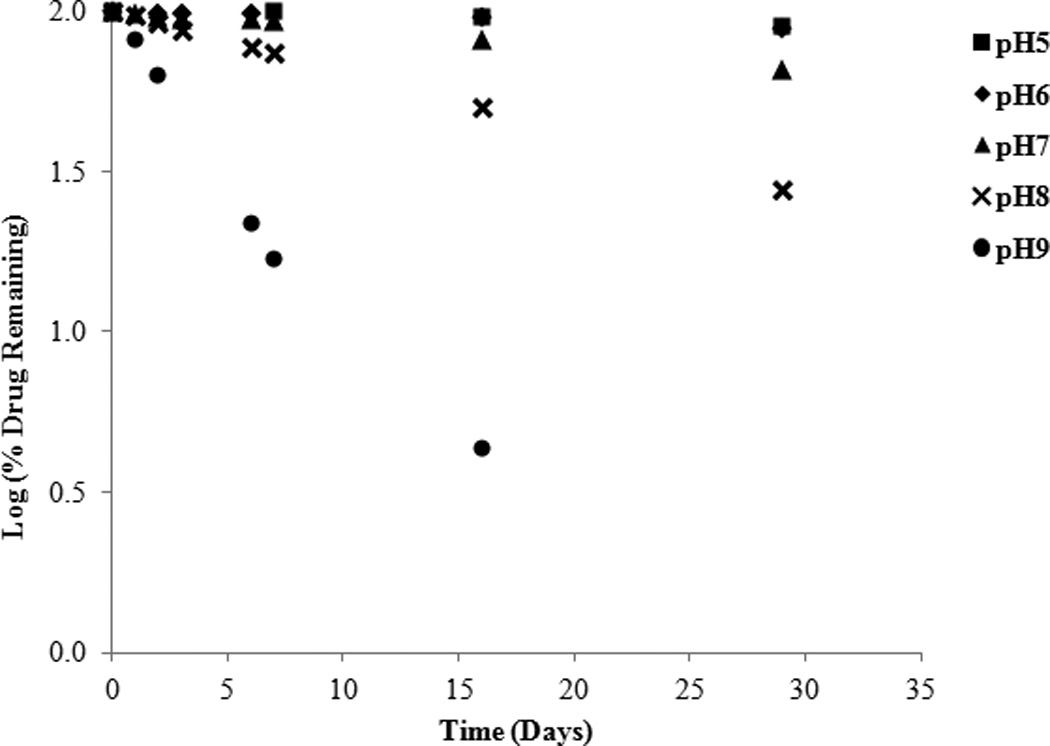
Log percent (%) drug remaining versus time at various pHs and 26°C.
Figure 3.
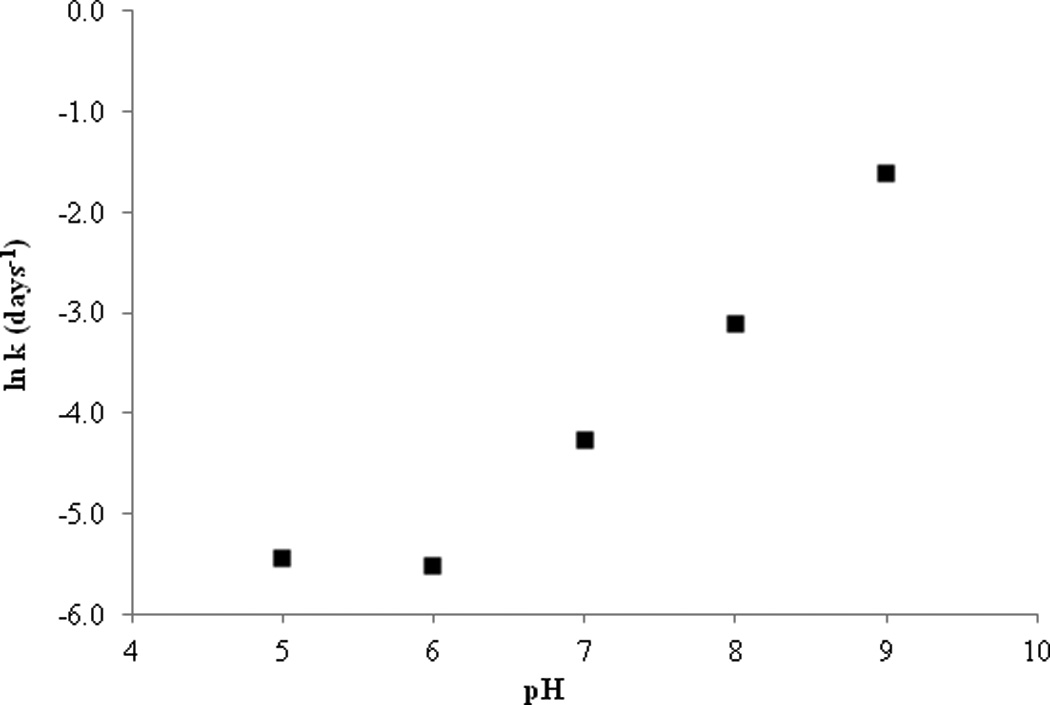
pH rate profile: natural logarithm of degradation rate constant (κ) versus pH for SFN at 26°C.
Effect of buffer species
The effect of buffer species was determined at pH 5.0 under the conditions of 0.1M citrate and 0.1M phosphate buffers at 26°C (ionic strength of 0.1M) (Figure 4). SFN was observed to undergo apparent first-order degradation regardless of buffer species. After 30 days of buffer exposure approximately 89.55% of SFN remained in the phosphate buffer with a κ value of 0.0019 (per day) and a T90 of 16.26 days. In comparison, roughly 12.85% of SFN remained in the citrate buffer after 30 days with a κ value of 0.0309 (per day) and a T90 of 3.39 days. Additionally, when SFN was exposed to a citrate buffer of pH 4.0 (ionic strength of 0.1M), similar trends were seen as compared to the pH 5.0 citrate buffer. At pH 4.0, 28.72% of SFN remained after 30 days with a κ value of 0.019 (per day) and a T90 of 5.52 days. Thus it appears that the SFN degradation rate remains relatively constant between pH 4.0 and 5.0, however it was clear from this observation that SFN is more stable in phosphate buffer compared to citrate buffer. The drastic difference in stability as a function of buffer species supports the idea that SFN undergoes general base catalysis.
Figure 4.
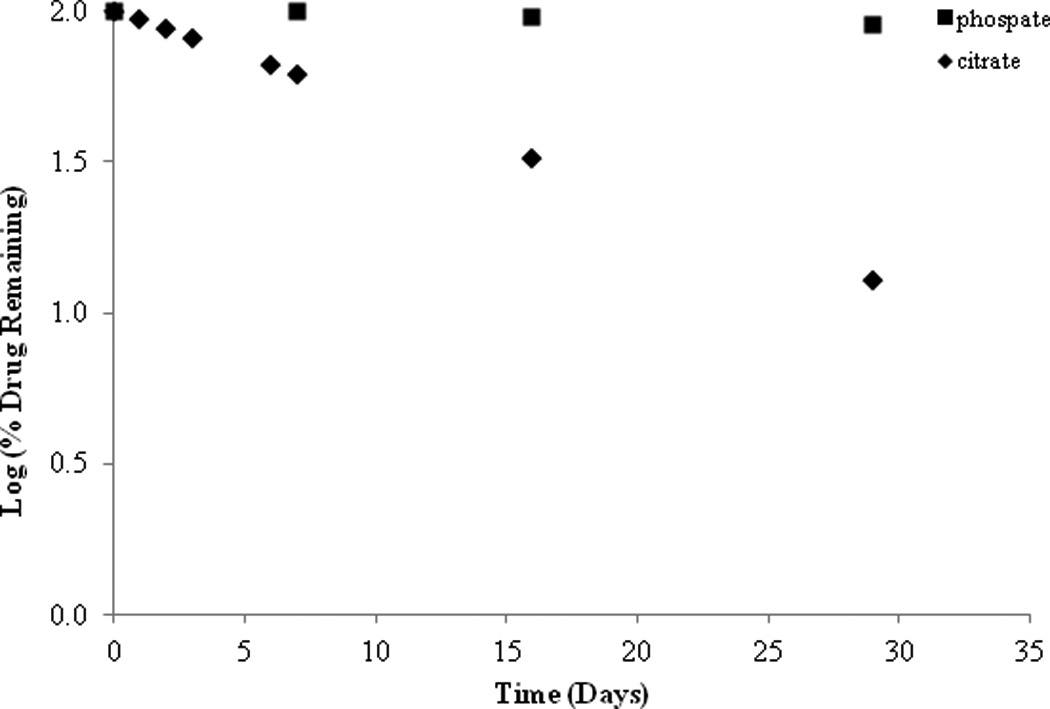
Effect of buffer species on SFN stability at 26°C, pH of both species is 5.0.
Effect of temperature
The degradation rate constants are related to temperature by the Arrhenius equation:
ln κ = ln A− Ea/RT
where κ is the degradation constant, A is the collision factor, Ea is the energy of activation, R is the gas constant and T is the temperature in Kelvin. Buffered samples of SFN were analyzed at pH 4.0 (in citrate buffer) and pH 8.0 (in phosphate buffer) at four different temperatures (4, 26, 37 and 47°C). The natural logarithms of the degradation constants were plotted versus the inverse of temperature in Kelvin (Figure 5). The data shows an interesting relationship between the two buffers tested. At higher temperatures of 37°C and 47°C it is the citrate buffer of pH 4.0 that appears more stable compared to the phosphate buffer of pH 8.0. These results correspond with previously reported data that observed the influence of heat at different pHs over a 24hr time period17. However, further data indicate that at 4°C the buffer at pH 8.0 (T90= 33.87 days) is more stable than the buffer at pH 4.0 (T90= 17.21 days). Interestingly, at room temperature the two buffers have nearly identical degradation constants with a T90 of 2.38 days for pH 8.0 and a T90 of 2.39 days for pH 4.0. Energies of activation were calculated from the slopes and are listed in Figure 5.
Figure 5.
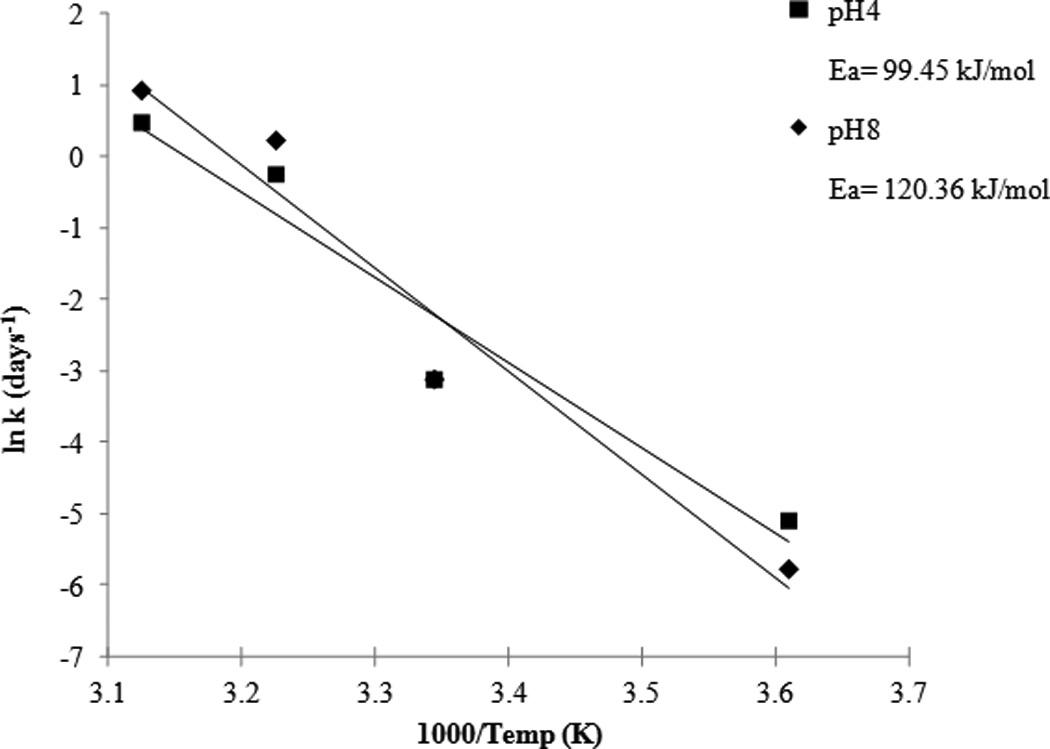
Arrhenius plot: temperature dependence of the degradation rate constants at pH 4.0 and 8.0.
This data indicates that SFN degradation is strongly dependent on temperature, and based on the slopes (Figure 5), the temperature coefficient (Q10) can be calculated by the expression:
Q10= R2/R1 ^10/T2−T1
where R is the rate and T is the temperature in Celsius. Q10 was calculated to be 3.15 for pH 4.0 and 4.0 for pH 8.0 for every 10°C change in temperature.
Effect of solvents
The stability of SFN was investigated in five different organic solvents; ACN, EtOH, IPA, MeOH, and Triglyme. In addition, stability was conducted in H2O, H2O with EDTA, and a neat solution of pure SFN. These samples were evaluated at 37°C over the course of one week and the results are given in Figure 6. From the data it can be observed that SFN is relatively stable in ACN, IPA and Triglyme, less stable in EtOH, H2O and in the pure neat sample and SFN degraded the fastest in the EDTA solution and MeOH. This data indicate that SFN degradation is influenced by the type of polar protic solvent. The two polar aprotic solvents, ACN and Triglyme, were two of the three solvents in which SFN had the greatest stability. Indeed, this is relative as the ACN sample still had 83.48% remaining and triglyme had 30.0% remaining after 6 days. The third solvent in which stability was relatively higher was the tertiary alcohol IPA. However, as the polarity of the alcohol series increased, from IPA to EtOH to MeOH, the stability of SFN dramatically decreased. Comparatively, samples in IPA had 77.74 % remaining after six days and EtOH had 22.30% drug remaining after six days while samples in MeOH only had 2.48% remaining. Since SFN significantly degrades in pure organic environments, it is clear that mechanisms other than simple hydrolysis are important to consider. Attempts to identify some of the degradation products from the organic stability samples were not successful at this time, however some possible mechanistic pathways proposing that SFN degrades to a thiourea compound via hydrolysis have been previously reported13.
Figure 6.
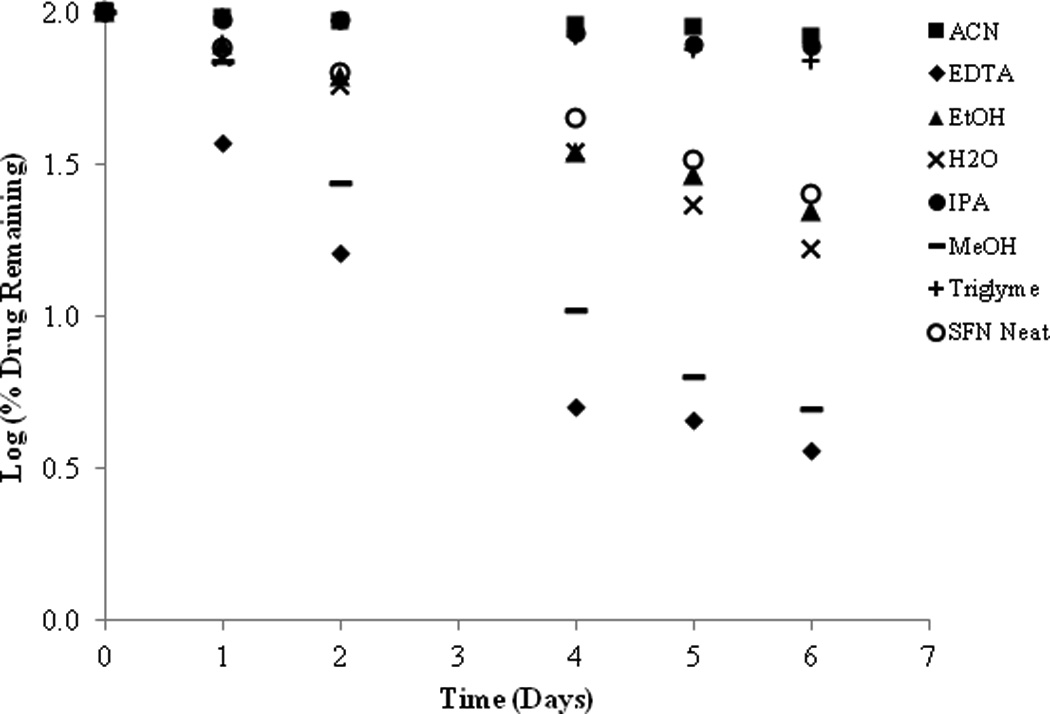
Log percent (%) of drug remaining of SFN in a variety of solvents, water and in a neat solution at 37°C.
In the presence of H2O (pH 8.0, no buffer), approximately 57.49% of SFN remained after two days and had a calculated T90 of 0.84 days whereas, H2O with EDTA (0.05M, pH 8.0) yielded 16.25% SFN remaining after two days and a T90 of 0.44 days. Interestingly, while those H2O samples displayed high levels of degradation, SFN stability conducted in phosphate buffer (pH 8.0) demonstrated even greater instability with 6.37% drug remaining after two days and a T90 of 0.19 days. Furthermore, the inherent instability of SFN is evident by the degradation observed by the neat SFN sample. The pure SFN alone degraded 74.88% after 6 days at 37°C.
Stability in topical formulation vehicles
The preformulation studies indicate that SFN is very unstable not only in aqueous and protic solvents, but also in polar aprotic environments. As such, attempts to formulate SFN in an oil–in-water cream formulation were unsuccessful. In fact, as illustrated in Figure 7, SFN completely degraded after 30 days in a conventional pharmaceutical cream formulation. However, improved stability was observed in organic formulation components (Figure 7). Following the initial observations of stability in PEG 400, SFN stability was further investigated using a variety of PEG chain lengths (Figure 8). It was observed that SFN has greater stability in longer polyethylene glycol chain lengths such as PEG 600 and PEG 400 as compared to the shorter chain lengths of PEG 200 and PEG 300. Approximately 83.13% of drug was remaining in PEG 400 samples after 6 days at 37°C. In comparison, 50.46% of drug was remaining in PEG 300 samples under similar conditions. Consistent with the above, SFN degraded rapidly (8.71% remaining after 6 days at 37°C) in the polar protic solvent ethylene glycol, which is the structural repeating unit of the PEG series.
Figure 7.
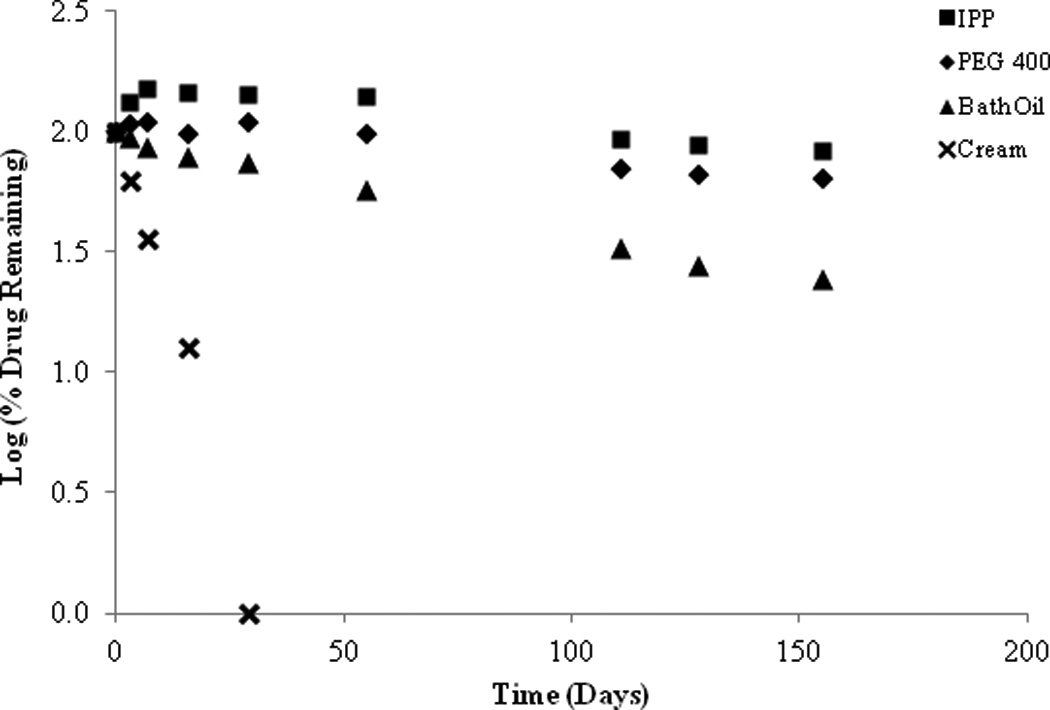
SFN preformulation stability studies (26°C) in isopropyl palmitate (IPP), PEG 400, bath oil and an oil-in-water cream.
Figure 8.
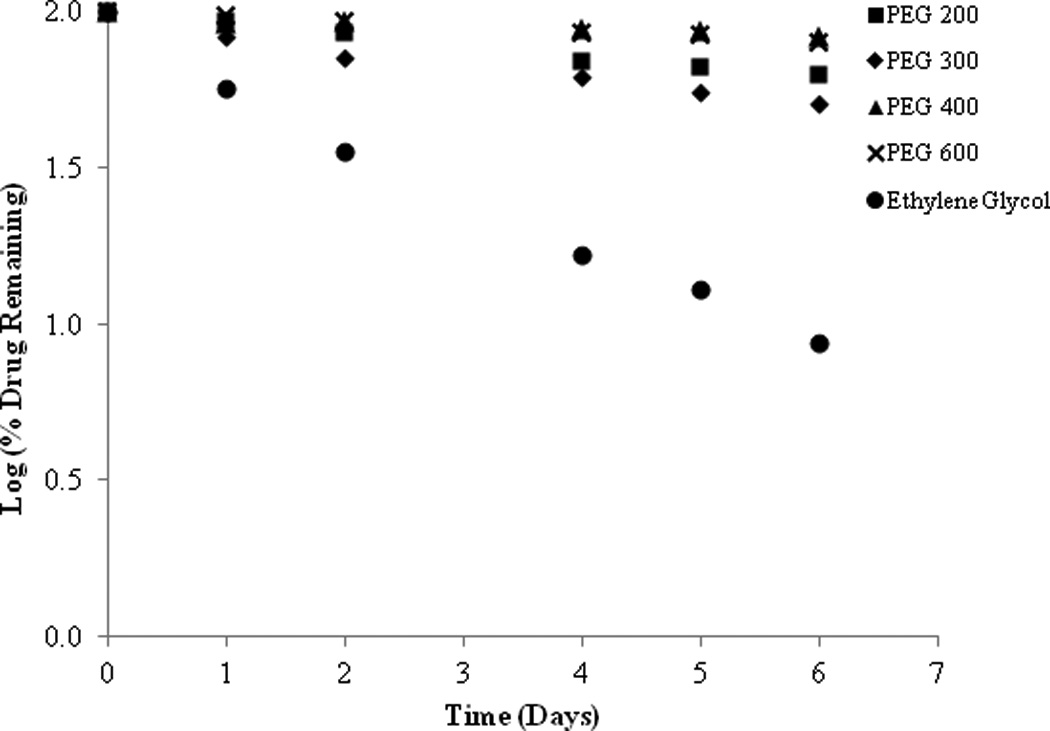
SFN stability in series of PEG with increasing chain length at 37°C.
To understand the influence that H20 may have on degradation of SFN when it is present in an organic solvent as an impurity, a water spiking study was conducted. PEG 400 samples were spiked with 0.1% and 1.0% water. Figure 9 shows that as water content increased, degradation rate also increased, albeit with minimal impact over the course of this particular study. After 6 days, the increase of water content from 0% to 1% increased the degradation from 14.92% to 19.33% at 37°C.
Figure 9.
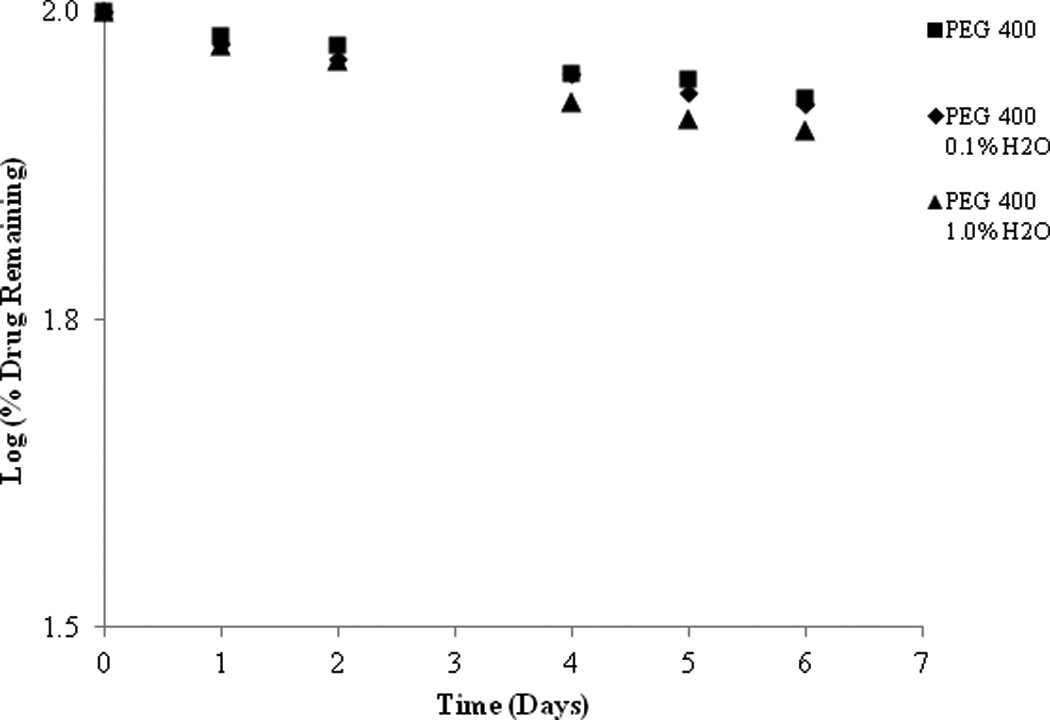
SFN stability in a series of PEG 400 with increasing water content at 37°C.
Stability in topical formulations
As a result of the preliminary stability studies, a PEG ointment base and an organic oleaginous base were selected for further stability investigations. Once again it was found that the stability of SFN is highly temperature dependent with rapid degradation observed at higher temperatures in both formulations. However, an increase in stability was observed at lower temperatures (26°C and 4°C) (Figure 10 & 11). At 26°C, approximately 73.11% SFN was remaining in the organic oleaginous base after 193 days compared to 42.84% remaining in the PEG base. At 4°C, roughly 71.93% SFN was remained in the organic oleaginous base after 193 days compared to 92.63% remaining in the PEG base. The addition of the anti-oxidant ascorbic acid significantly improved the stability of the PEG formulation particularly at lower temperatures, 95.33% remaining after 193 days at 4°C, but had no effect on the organic oleaginous base formulation. However the addition of the anti-oxidant BHT provided no improved stability in either formulation (data not shown). Although there is SFN degradation in these non-aqueous formulations, they are appreciably more stable than the solution studies previously described.
Figure 10.
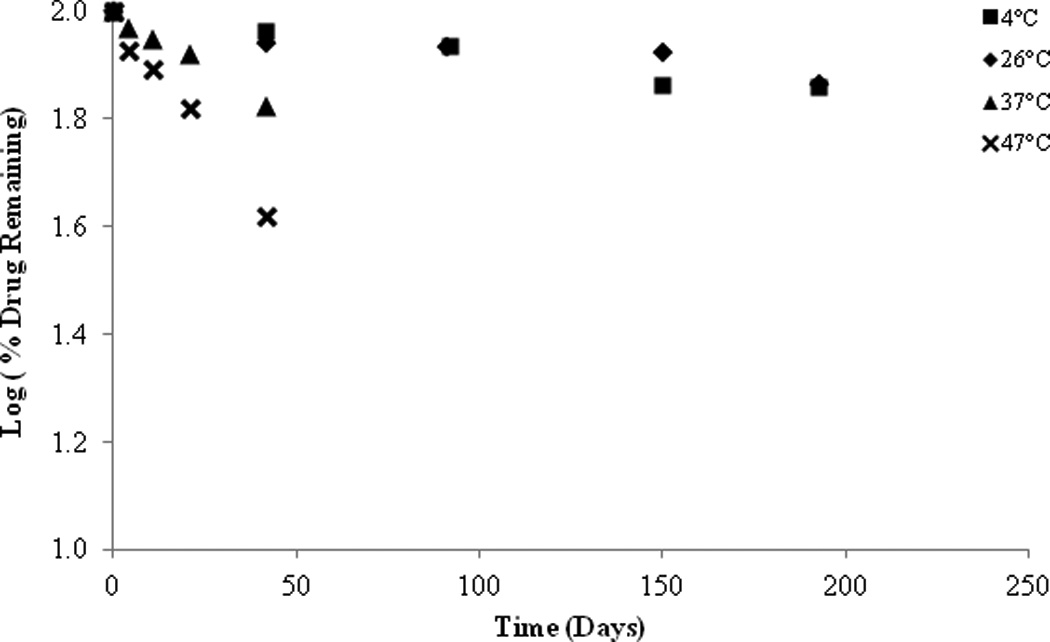
SFN in organic formulation, stability at various temperatures.
Figure 11.
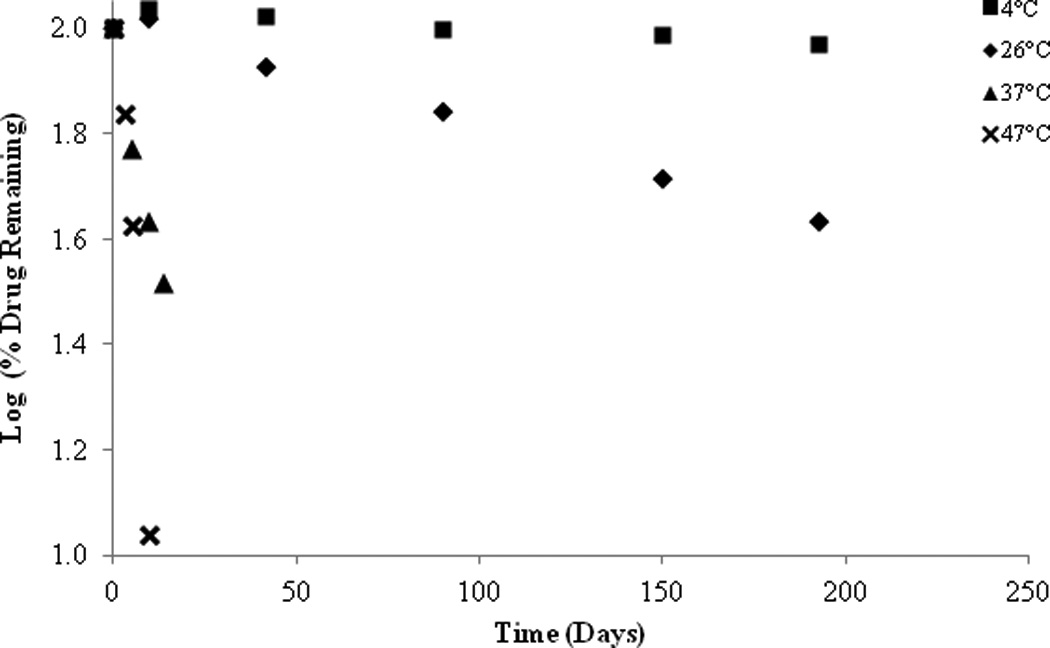
SFN in PEG formulation, stability at various temperatures.
The Arrhenius relationship (Figure 12) demonstrates the difference in degradation rate of the two formulations as a function of temperature. At high temperatures (37°C and 47°C) a larger percent of drug remained within the organic oleaginous base formulation compared to the PEG base formulation. Interestingly, the results are reversed at lower temperatures (4°C) with a greater percent of drug remaining in the PEG formulation. As a result, this corresponds to much higher activation energy for the PEG formulation. SFN in PEG base alone had a calculated Ea of 104 kJ/mol and a T90 of 175 days at 4°C. In comparison, SFN in the organic oleaginous base had a calculated Ea of 41 kJ/mol and T90 of 58 days at 4°C. While activation energy is a useful metric to interpret stability data, in this instance careful consideration should be made when drawing definitive conclusions. Throughout the course of the study it was observed that both the PEG ointment base and organic oleaginous base had changes to the physical nature depending on the given temperature that they were stored. The PEG formulations become significantly less viscous at higher temperatures, whereas the oleaginous base became semi-solid at 4°C.
Figure 12.
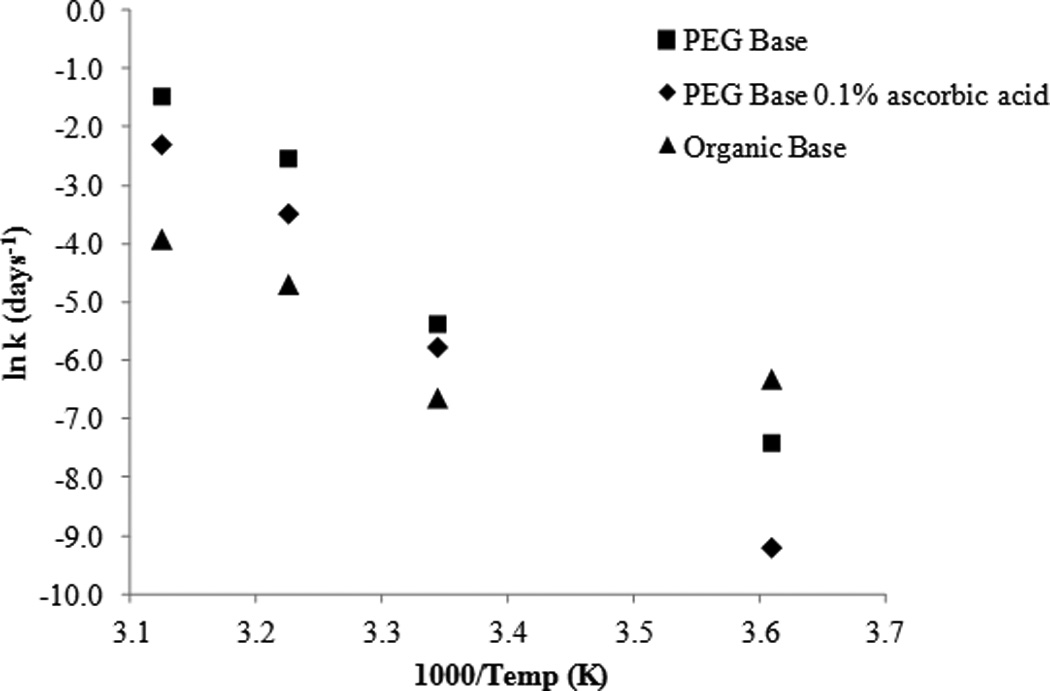
Arrhenius plot: temperature dependence of SFN formulations and the effect of ascorbic acid.
In vivo experiments
To test the ability of the above formulations to affect targets in vivo, the PEG ointment base was tested on the skin of hairless AP-1 luciferase mice. As has been previously reported, acute UVB treatment of AP-1 luciferase mice yielded strong activation of the luciferase transgene in the skin of placebo treated controls3. This stimulation was diminished ~2.5 fold in mice pretreated with the ointment containing SFN, a significant reduction in signaling (p = 0.02) (Figure 13). No adverse reactions were noted with treatment of placebo or SFN-containing PEG ointment base. This suggests that SFN in this formulation is functional in vivo.
Figure 13.
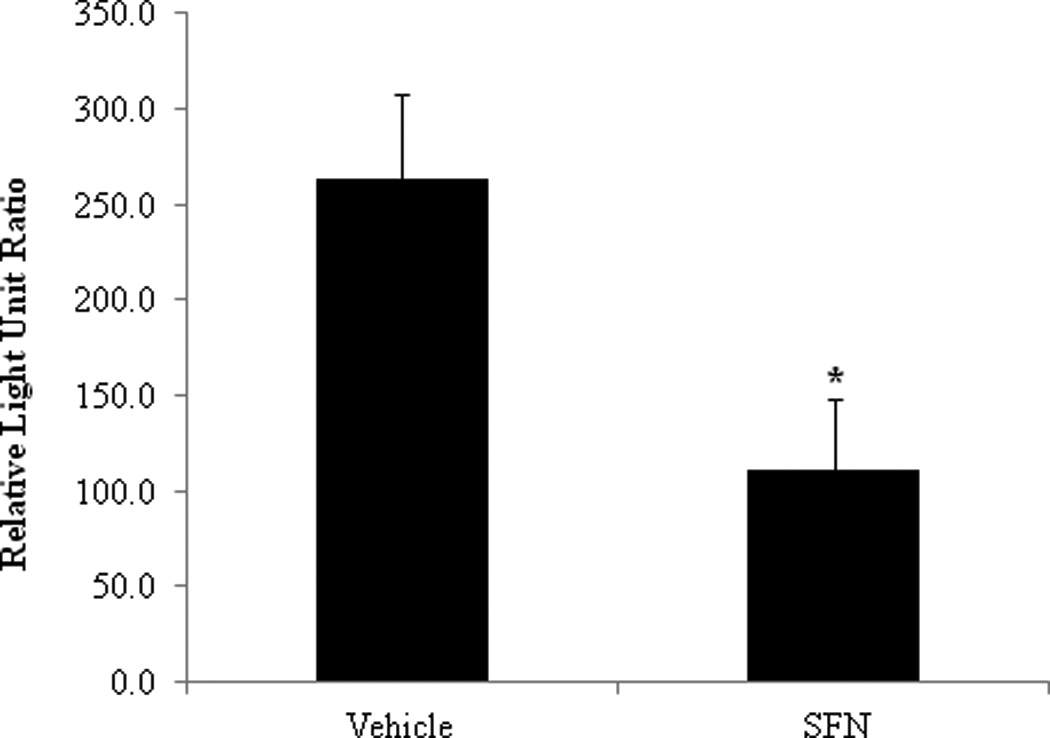
SFN in PEG ointment base affects molecular targets as expected in vivo. The ears of hairless mice transgenic for an AP-1 driven luciferase gene were pretreated with either PEG ointment (vehicle) or the ointment containing SFN 4 times prior to and 1 time 30 minutes after an acute treatment with UVB. Ear punches were taken 24hr before and 48hr after UVB treatment. Fold luciferase readings indicate that UVB significantly activated luciferase, and that this was significantly inhibited by treatment with SFN in the topical formulation (p = 0.02).
Conclusion
Preformulation studies have shown that SFN is chemically unstable not only in aqueous and protic solvents, but also in polar aprotic environments. It has been shown that SFN undergoes rapid apparent first order degradation under basic pH conditions as well with methanol solvolysis. At pH 5.0 and 26°C, SFN was found to be more stable in a phosphate buffer as compared to a citrate buffer system. SFN degradation is strongly dependent on temperature as is evident by changes in the degradation rate as a function of temperature. For every 10°C change in temperature the degradation rate of SFN changed by a factor of 3.15 in a pH 4.0 solution and a factor of 4.0 for a pH 8.0 solution.
While SFN completely degraded after 30 days in a conventional pharmaceutical cream formulation, improved stability was seen in the organic formulation component PEG 400. It was observed that SFN stability in PEG is related to chain length with greater stability seen in longer PEG chain lengths. Long term stability studies were conducted on two non-aqueous topical formulations, a PEG ointment base and an organic oleaginous base. At 4°C, 92.63% of SFN remained in the PEG based formulation after 193 days. Topically applied SFN in the PEG formulation was then tested for efficacy and led to a significant decrease of luciferase transgene activation in an AP-1 luciferase mouse model compared to a vehicle control.
Acknowledgments
The project described was supported by Award Numbers P01CA027502 and K07CA132956, both from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Declaration of interest
The authors report no conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer Statistics. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Clydesdale GJ, Dandie G, Mueller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol Cell Biol. 2001;79:547–568. doi: 10.1046/j.1440-1711.2001.01047.x. [DOI] [PubMed] [Google Scholar]
- 3.Dickinson SE, Melton TF, Olson ER, Zhang J, Saboda K, Bowden GT. Inhibition of activator protein-1 by sulforaphane involves interaction with cysteine in the cFos DNA-binding domain: implications for chemoprevention of UVB-induced skin cancer. Cancer Res. 2009;69:7103–7110. doi: 10.1158/0008-5472.CAN-09-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;64:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W, Borchers AH, Dong Z, Powell MB, Bowden GT. UVB irradiation-induced activator protein-1 activation correlates with increased c-fos gene expression in a human keratinocyte cell line. J Biol Chem. 1998;273:32176–32181. doi: 10.1074/jbc.273.48.32176. [DOI] [PubMed] [Google Scholar]
- 6.Cooper SJ, MacGowan J, Ranger-Moore J, Young MR, Colburn NH, Bowden GT. Expression of dominant negative c-jun inhibits ultraviolet B-induced squamous cell carcinoma number and size in an SKH-1 hairless mouse model. Mol Cancer Res. 2003;11:848–854. [PubMed] [Google Scholar]
- 7.Barthelman M, Chen W, Gensler HL, Huang C, Dong Z, Bowden GT. Inhibitory effects of perillyl alcohol on UVB-induced murine skin cancer and AP-1 transactivation. Cancer Res. 1998;58:711–716. [PubMed] [Google Scholar]
- 8.Li JJ, Dong Z, Dawson MI, Colburn NN. Inhibition of tumor promoter-induced transformation by retinoids that transrepress AP-1 without transactivating retinoic acid response element. Cancer Res. 1996;56:483–489. [PubMed] [Google Scholar]
- 9.Dinkova-Kostova AT, Jenkins SN, Fahey JW, Ye L, Wehage SL, Liby KT, Stephenson KK, Wade KL, Talalay P. Protection against UV-light-induced skin carcinogenesis in SKH-1 high-risk mice by sulforaphane-containing broccoli sprout extracts. Cancer Lett. 2006;240:243–252. doi: 10.1016/j.canlet.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Dinkova-Kostova AT, Fahey JW, Wade KL, Jenkins SN, Shapiro TA, Fuchs EJ, Kerns ML, Talalay P. Induction of the phase 2 response in mouse and human skin by sulforaphane-containing broccoli sprout extracts. Cancer Epidemiol Biomarkers Prev. 2007;16:847–851. doi: 10.1158/1055-9965.EPI-06-0934. [DOI] [PubMed] [Google Scholar]
- 11.Talalay P, Fahey JW, Healy ZR, Wehage SL, Benedict AL, Min C, Dinkova-Kostova AT. Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc Natl Acad Sci U S A. 2007;104:17500–17505. doi: 10.1073/pnas.0708710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ACD/Chemsketch for Windows, Version 12.0. Advanced Chemistry Development, Inc.; [Google Scholar]
- 13.Jin Y, Wang M, Rosen RT, Ho CT. Thermal Degradation of Sulforaphane in Aqueous Solution. J Agric Food Chem. 1999;47:3121–3123. doi: 10.1021/jf990082e. [DOI] [PubMed] [Google Scholar]
- 14.Gupta A, Myrdal PB. Development of a perillyl alcohol topical cream formulation. Int J Pharm. 2004;269:373–383. doi: 10.1016/j.ijpharm.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Rincon M, Flavell RA. AP-1 transcriptional activity requires both T-cell receptor-mediated and co-stimulatory signals in primary T lymphocytes. Embo J. 1994;13:4370–4381. doi: 10.1002/j.1460-2075.1994.tb06757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachelor MA, Cooper SJ, Sikorski ET, Bowden GT. Inhibition of p38 mitogen-activated protein kinase and phosphatidylinositol 3-kinase decreases UVB-induced activator protein-1 and cyclooxygenase-2 in a SKH-1 hairless mouse model. Mol Cancer Res. 2005;3:90–99. doi: 10.1158/1541-7786.MCR-04-0065. [DOI] [PubMed] [Google Scholar]
- 17.Xiao Q, Liang H, Yuan Q-p. Effect of Temperature, pH and Light on the Stability of Sulforaphane Solution. Chin Pharm J. 2009;43:193–196. [Google Scholar]


