Abstract
Background and Objective
Serum leptin measures are associated with radiographic knee osteoarthritis but no studies have examined leptin levels with respect to different measures of knee joint damage from magnetic resonance imaging (MRI).
Methods
Participants in the Michigan Study of Women’s Health Across the Nation underwent bilateral knee MRIs at follow-up visit 11 for assessment of cartilage defects, bone marrow lesions, osteophytes, meniscal tears, synovitis and joint effusion. Serum leptin measures were available from baseline, follow-up visits 1 and 3–7.
Results
Baseline serum leptin levels were associated with greater odds of having more severe knee joint damage at follow-up visit 11 after adjustment for age, smoking status, menopause status and BMI residuals. The greatest effect was observed for osteophytes; a 5 ng/mL increase in baseline leptin was associated with 24% higher odds of having larger osteophytes (95% CI 1.17, 1.32). Correlations with baseline serum leptin were greatest for MRI-assessed osteophytes (r=0.41), followed by followed by effusion (r=0.32), synovitis (r=0.30), cartilage defects (r=0.28), bone marrow lesions (r=0.24) and meniscal abnormalities (r=0.21).
Conclusions
Leptin levels ten years prior to MRI assessment were associated with the presence of cartilage defects, bone marrow lesions, osteophytes, meniscal tears, synovitis and effusion among a population of mid-aged women. Understanding the role that leptin plays in the joint degradation process is critical for development of more targeted interventions for osteoarthritis.
Keywords: Osteoarthritis, obesity, leptin, magnetic resonance imaging, knee
INTRODUCTION
Osteoarthritis (OA) is a highly prevalent joint condition, affecting 37% of adults over age 60.[1] Initially thought to afflict only elderly populations, research has shown that the onset of radiographic OA can begin by age 40 [2] and that the prevalence of magnetic resonance imaging (MRI)-defined knee joint damage is common among mid-life women.[3]
By 2030, nearly one-third of mid-aged adults will have arthritis,[4] given the aging of the population and the increasing prevalence of obesity.[5] Obesity is a major risk factor for radiographic knee OA [6–12] as well as for the presence of knee cartilage defects [13–15] and bone marrow lesions (BMLs).[16,17] However, not all obese persons have joint damage nor are all individuals with joint damage obese. This fact, in addition to the observed association between obesity and radiographic OA in non-weight bearing joints [18–21] suggests that the relationship between body size and OA extends beyond that of mechanical loading, the commonly conceptualized mechanism through which obesity influences onset and progression of OA.
Most studies of OA or joint damage and obesity use body mass index (BMI) as a marker of body size and proxy for mechanical loading. However, fat mass and skeletal muscle mass are better predictors of radiographic knee OA incidence and severity as compared to BMI.[22] Greater skeletal muscle mass is associated with increased cartilage volume [23–25] and is protective against cartilage loss [24,26] whereas greater fat mass is a risk factor for cartilage defects, [26,27] cartilage loss, [28] BMLs [27] and joint replacement.[29]
Adipose tissue is a metabolic endocrine organ which may be involved in joint damage through mechanisms other than increased mechanical loading. Adipokines, metabolically-active agents secreted by adipose tissue have been implicated in a variety of cardiovascular, metabolic, and inflammatory-mediated diseases.[30] Differences in the distribution of the adipokines between the joint and the circulating compartment suggest that the joint is a unique area of adipokine activity.[31–34]
Most efforts to examine adipokines and joint degradation have focused on leptin because of its strong correlation with body size. Synovial fluid leptin levels were correlated with radiographic knee OA severity [35] and serum leptin levels were associated with higher odds of radiographic knee OA in the National Health and Nutrition Examination Survey III [36] and among mid-aged women.[37] Leptin has a demonstrated catabolic role on bovine cartilage through inflammatory mechanisms [38] and greater cartilage degradation indices [13] and less cartilage volume [25] have been associated with higher serum leptin levels in some but not all [39] studies.
Although the joint is vulnerable to damage beyond cartilage degradation, no studies have related leptin levels to other markers of joint damage. This paper examines the relationship of baseline and longitudinal serum leptin measures and MRI-assessed cartilage degradation, BMLs, osteophytes, meniscal abnormalities, joint effusions and synovitis among a cohort of mid-life women.
METHODS
Study population
The Michigan Study of Women’s Health Across the Nation (SWAN) is one of seven sites for SWAN, a multiethnic cohort study characterizing the menopausal transition. The Michigan SWAN population, established in 1996, is a population-based sample of eligible women from two Detroit-area communities [40] including 543 eligible women (325 African American and 218 Caucasian). Baseline eligibility criterion included 42–52 years of age, having an intact uterus, having had at least one menstrual period in the previous 3 months, no use of reproductive hormones in the previous 3 months, and self-identification with the site’s designated race or ethnic group.
Michigan SWAN women have completed annual assessment protocols and specimen collection common to all SWAN sites with 80% retention after 10 years. At follow-up visit 11 (2007), 387 Michigan SWAN women participated in a supplemental OA imaging protocol including knee radiographs and MRI. Women were not included in the MRI protocol if they were ineligible for the MRI (n=10) or if they refused (n=15), leaving 364 women available for this analysis. Women who did not have MRI data did not differ in baseline age, body size, leptin levels, race/ethnicity or menopause status as compared to those with MRIs. The University of Michigan Institutional Review Board approved the study protocol, and written informed consent was obtained from each participant.
Magnetic Resonance Imaging
Knee joints were imaged using a 3T (Model Achieva, Philips Healthcare, Andover, Massachusetts) or 1.5 T (GE Signa, GE Medical Systems, Milwaukee, WI) MR scanner. MR images of each knee were independently scored by two musculoskeletal radiologists, globally and by compartment using a semi-quantitative system for cartilage defects, subchondral BMLs, osteophytes, meniscal tears, joint effusions and synovitis across multiple surfaces and compartments, as appropriate. Information about the MRI and scoring protocol, including specific imaging sequences has been published [3] and is available as Online Supplemental Material.
To ensure reproducibility, a rigorous quality-control program was maintained such that 60% of MRI images were double-read with agreement (intra-class correlation) ranging from 0.60 for effusion to 0.91 for BMLs. Any measures with discrepant scores were resolved by consensus. Data used for this analysis represent each participant’s maximum score for each type of feature across knees and compartments, as appropriate.
Knee OA Status
Knee OA status was based on anterior-posterior radiographs taken in the semi-flexed position (7–10° flexion). Knees were scored using the Kellgren and Lawrence (K–L) grading system where 0=normal; 1=doubtful OA; 2=minimal OA; 3=moderate OA; 4=severe OA.[41] K–L scores ≥ 2 in either knee were considered to have radiographic knee OA. The inter-rater reliability for K–L scores was in excess of 0.85.[42]
Body Size Measures
Measurement of body size including height (cm), weight (kg), waist and hip circumference (cm) was measured annually; height and weight were used to calculate BMI. Body composition was measured using bioelectrical impedance, the technique used in the NHANES body composition assessments.
Leptin Assay
The SWAN specimen collection protocol includes a fasted blood draw to provide samples for a specimen repository that is maintained at −80°C until processing. Serum leptin levels were determined spectrophotometrically using commercially-available colorimetric enzyme immunoassay kits (Millipore, St. Charles, MO) and run according to the manufacturer’s instructions. The coefficient of variation percent for duplicate samples is 3.7% and the lower limit of detection is 0.5 ng/mL. Banked serum specimens from baseline and visits 1 and 3–7were assayed for leptin. Leptin levels were missing for some participant’s visits due to lack of serum collection; 113 women were missing leptin at one visit; 48 at 2 visits; 11 at 3 visits; 6 at 4 visits and only 1 was missing at 6 visits. Missingness was not associated with demographic, body size or joint damage measures.
Other Measures
Participants were asked about their current smoking status at each visit; smoking was considered as a potential confounder given that it has been associated with lower leptin levels among women.[43] Race/ethnicity classification (African American or Caucasian) was determined by self-report at baseline. Age at each visit was calculated as date of visit minus date of birth. Menopause status was ascertained at each annual exam based on questions about bleeding patterns, current hormone use, hysterectomy and oophorectomy. At each visit, participants were categorized as being premenopausal, early perimenopausal, late perimenopausal, postmenopausal, hysterectomy, or unable to determine due to exogenous hormone use.
Statistical Analysis
The prevalence (frequencies and percents) of cartilage defects, BMLs, osteophytes, meniscal tears, synovitis and joint effusions were calculated. Means and standard deviation (SD) or frequencies and percents of leptin, body size, radiographic knee OA status and relevant covariates at baseline were examined overall and by categories of MRI knee joint damage from follow-up visit 11; the statistical significance of these relationships were assessed using analysis of variance or chi-square tests at the α=0.05 level. Spearman correlation coefficients for leptin and each of the measures of MRI knee joint damage were calculated. Multivariable analyses using ordinal logistic regression analysis were conducted to relate each of the knee MRI variables with baseline leptin measures adjusting for relevant covariates. Longitudinal linear mixed models (PROC MIXED) with random intercepts and slopes for age were used to examine the level and rates of change in leptin measures over time, stratified by category of cartilage defects, BMLs, osteophytes, meniscal tears, synovitis and joint effusions. Predicted trajectories of leptin measures with corresponding 95% confidence bands were graphed by MRI severity using PROC SGPLOT.
Given the collinearity between body size and leptin, all multivariable modeling included residuals from the regression of leptin on BMI as the measure of body size confounding. The R-squared for this regression was 0.54. Leptin represents the metabolic component of body size and the residual represents the association of body size and joint damage through other pathways, including mechanical loading. Interactions of the BMI residual and leptin were tested to assess potential effect modification of the relationship between leptin and joint damage by body size and were found to be non-significant.
Model fit and final model selection was evaluated using Akaike’s information criterion and chi-square tests comparing the log likelihood ratios between candidate models. Statistical significance was defined at α<0.05 and all analyses were completed using SAS v9.3 (SAS Institute, Cary, NC).
RESULTS
The prevalence of each of the MRI-assessed measures of knee joint damage is provided in Table 1. Nearly all participants had cartilage defects with the prevalence of full-thickness defects being 24% among this population of mid-aged women. BMLs were also common; 29% of women had a “small” (≤1 cm) BML and 12% had a large/very large BML (> 1 cm). Most women had osteophytes, with 60% less than 5 mm, 28% 5–10 mm and 12% greater than 10 mm in size. More than half of the sample had meniscal tears: 31% of women had displaced or macerated tears. Knee synovitis was present in 33% of the women and approximately one-third of those women had moderate-to-marked synovitis. Joint effusions were very common and 14% of the participants had moderate-large effusions (more than 10 mm in size). As expected, the prevalence of radiographic knee OA was higher among women with more severe MRI-assessed measures of knee joint damage.
Table 1.
Prevalence of Knee Magnetic Resonance Imaging Findings and Average Serum Leptin Levels at Baseline and Follow-Up Visit 7 According to Magnetic Resonance Imaging-Defined Knee Cartilage Defects, Bone Marrow Lesions, Osteophytes, Meniscal Tears, Synovitis, and Joint Effusions Among Michigan Study of Women’s Health Across the Nation (SWAN) Women at Follow-Up Visit 11.
| Overall (n=364) |
Baseline Leptin (ng/mL) |
Visit 7 Leptin (ng/mL) |
|
|---|---|---|---|
| Cartilage defects | N (%) | Mean (SD) | Mean (SD) |
| Normal, internal signal alteration only | 8 (2.2%) | 14.9 (5.7) | 27.0 (9.1) |
| Cartilage defect < 50% thickness | 143 (39.3%) | 27.4 (16.1) | 33.5 (19.1) |
| Cartilage defect 50–99% thickness | 127 (34.9%) | 31.3 (17.4) | 37.1 (19.4) |
| Cartilage defect 100% thickness | 86 (23.6%) | 40.2 (20.4) | 47.1 (22.6) |
| P-value* | <0.0001 | <0.0001 | |
| Bone marrow lesions (BML) | |||
| Normal | 215 (59.1%) | 27.6 (16.6) | 35.0 (19.8) |
| Largest diameter ≤ 1 cm | 104 (28.6%) | 36.6 (19.9) | 40.4 (20.5) |
| Largest diameter > 1 cm | 45 (12.4%) | 37.4 (17.9) | 45.9 (22.8) |
| P-value* | <0.0001 | 0.005 | |
| Osteophytes | |||
| None | 74 (20.3%) | 21.5 (16.2) | 28.8 (17.7) |
| ≤ 5 mm | 174 (47.8%) | 29.5 (15.1) | 34.3 (18.2) |
| 5–10 mm | 82 (22.5%) | 38.7 (20.6) | 46.2 (21.1) |
| > 10 mm | 34 (9.3%) | 45.9 (16.6) | 56.5 (20.6) |
| P-value* | <0.0001 | <0.0001 | |
| Meniscal abnormalities/tears | |||
| Normal | 18 (5.0%) | 27.7 (16.5) | 40.8 (19.8) |
| Intrasubstance meniscal abnormality only | 157 (43.1%) | 27.4 (17.2) | 31.9 (18.2) |
| Non-displaced tear | 77 (21.2%) | 33.7 (17.3) | 40.1 (21.2) |
| Displaced or macerated tear | 112 (30.8%) | 36.3 (19.4) | 44.6 (21.7) |
| P-value* | 0.0007 | <0.0001 | |
| Synovitis | |||
| Normal | 241 (66.2%) | 27.5 (16.3) | 34.3 (19.8) |
| Mild synovitis | 90 (24.7%) | 37.6 (18.4) | 43.3 (19.5) |
| Moderate-to-marked synovitis | 33 (9.1%) | 44.0 (21.9) | 48.4 (23.9) |
| P-value* | <0.0001 | <0.0001 | |
| Effusion | |||
| Physiologic fluid | 61 (16.8%) | 24.5 (15.8) | 29.3 (16.5) |
| Small effusion (≤ 10 mm) | 253 (69.5%) | 29.6 (15.9) | 37.5 (20.8) |
| Moderate and/or large effusion (> 10 mm) | 50 (13.7%) | 48.8 (21.7) | 50.6 (18.9) |
| P-value* | <0.0001 | <0.0001 | |
All p-values are based on global F-test from ANOVA which tests hypothesis that all means across severity groups are equal.
Body size and joint defects
Severity of knee cartilage defects increased with greater baseline body size, including greater weight, BMI, waist circumference, hip circumference waist:hip ratio, fat mass and skeletal muscle mass (Table 2) but did not differ by height, demographic characteristics, menopause status or smoking status. Baseline weight and BMI among women with full-thickness cartilage defects were 25% higher than among women with cartilage defects < 50% thickness. Baseline fat mass was 40% higher. Similar associations with body size were observed for BMLs, osteophytes, meniscal tears, synovitis and joint effusion (see Online Supplemental Data Tables).
Table 2.
Baseline Characteristics of Michigan Study of Women’s Health Across the Nation (SWAN) Women Overall and by Knee Cartilage Defect Severity From Magnetic Resonance Imaging at Follow-Up Visit 11.
| Cartilage Defect Score | ||||||
|---|---|---|---|---|---|---|
| Overall | None, internal signal alteration only |
Defect < 50% thickness |
Defect 50–99% thickness |
Defect 100% thickness |
P-value | |
| Mean (SD) |
Mean (SD) |
Mean (SD) |
Mean (SD) |
Mean (SD) |
||
| Age (years) | 46.1 (2.8) |
46.0 (3.5) |
45.8 (2.6) |
46.3 (3.0) |
46.2 (2.7) |
0.42 |
| Weight (kg) | 86.2 (22.0) |
69.6 (12.7) |
80.0 (20.2) |
85.0 (21.1) |
99.8 (20.8) |
<0.0001 |
| Height (cm) | 163.5 (6.2) |
163.9 (4.8) |
163.7 (5.6) |
163.0 (6.1) |
163.9 (7.1) |
0.67 |
| BMI (kg/m2) | 32.2 (8.1) |
25.9 (4.8) |
29.8 (7.4) |
32.0 (7.5) |
37.3 (7.9) |
<0.0001 |
| Waist circumference (cm) | 94.3 (17.0) |
81.5 (7.3) |
89.3 (15.9) |
94.0 (15.6) |
104.2 (16.9) |
<0.0001 |
| Hip circumference (cm) |
114.0 (16.3) |
102.3 (11.5) |
109.6 (14.7) |
112.8 (15.4) |
124.3 (16.0) |
<0.0001 |
| Waist:hip ratio | 0.82 (0.07) |
0.80 (0.07) |
0.81 (0.07) |
0.83 (0.07) |
0.84 (0.07) |
0.03 |
| Fat mass (kg) | 37.0 (16.7) |
23.6 (9.2) |
33.0 (14.8) |
36.2 (15.8) |
46.1 (17.8) |
<0.0001 |
| Skeletal muscle mass (kg) |
21.7 (3.4) |
20.5 (1.7) |
20.9 (3.3) |
21.5 (3.3) |
23.5 (3.1) |
<0.0001 |
|
n (%) |
n (%) |
n (%) |
n (%) |
|||
| Obese (BMI≥30 kg/m2) | 203 (56.1%) |
1 (12.5%) |
63 (44.4%) |
69 (54.3%) |
70 (82.4%) |
<0.0001 |
| Current Smoker | 87 (24.2%) |
2 (25.0%) |
35 (25.0%) |
36 (28.4%) |
14 (16.7%) |
0.28 |
| Ethnicity | ||||||
| African-American | 227 (62.4%) |
7 (87.5%) |
88 (61.5%) |
79 (62.2%) |
53 (61.6%) |
0.53 |
| Caucasian | 137 (37.6%) |
1 (12.5%) |
55 (38.5%) |
48 (37.8%) |
33 (38.4%) |
|
| Menopause Status | ||||||
| Premenopausal | 182 (50.3%) |
3 (37.5%) |
77 (53.9%) |
64 (50.8%) |
38 (44.7%) |
0.51 |
| Early Perimenopausal |
180 (49.7%) |
5 (62.5%) |
66 (46.2%) |
62 (49.2%) |
47 (55.3%) |
|
Leptin levels and joint defects
As shown in Table 1, leptin levels were strongly associated with having severe cartilage defects, larger bone abnormalities and more meniscal tears, synovitis and effusion. At both baseline and follow-up visit 7 (the last year in which leptin measures were available), a statistically significant increasing trend in leptin levels was observed with greater severity of all measures of knee joint damage. Similar associations were also observed with leptin levels at visits 1, 3, 4, 5, and 6. Baseline leptin levels were most highly correlated with knee osteophytes (r=0.41), followed by effusion (r=0.32), synovitis (r=0.30), cartilage defects (r=0.28), BMLs (r=0.24) and meniscal abnormalities (r=0.21). While leptin levels increased from baseline to follow-up visit 7, the amount of change was not associated with severity of knee joint damage.
As shown in Table 3, higher leptin levels at baseline were associated with greater odds of having more severe knee joint damage at follow-up visit 11 after adjusting for age, smoking status, menopause status and BMI residuals. The odds ratios associated with a 5 ng/mL change in baseline leptin ranged from 1.10 to 1.24. The greatest effect was observed for osteophytes: a 5 ng/mL increase in baseline leptin values was associated with 24% higher odds being in the next severity category of osteophytes (95% CI 1.17, 1.32).
Table 3.
Odds Ratios (95% Confidence Intervals) of Baseline Serum Leptin in Relation to Magnetic Resonance Imaging-Defined Knee Cartilage Defects, Bone Marrow Lesions, Osteophytes, Meniscal Tears, Synovitis, and Joint Effusion Among Michigan Study of Women’s Health Across the Nation (SWAN) Women at Follow-Up Visit 11.*
| Leptin |
||
|---|---|---|
| Odds Ratio† | 95% Confidence Interval | |
| Cartilage defects | 1.15 | 1.08, 1.22 |
| Bone marrow lesions | 1.13 | 1.06, 1.20 |
| Osteophytes | 1.24 | 1.17, 1.32 |
| Meniscal abnormalities/tears | 1.10 | 1.04, 1.16 |
| Synovitis | 1.19 | 1.11, 1.27 |
| Effusion | 1.23 | 1.15, 1.32 |
All models adjusted for age, race/ethnicity, menopause status, smoking status and residuals from regression of leptin on BMI.
Odds ratio represents 5 ng/mL change in leptin.
Leptin trajectories and joint damage
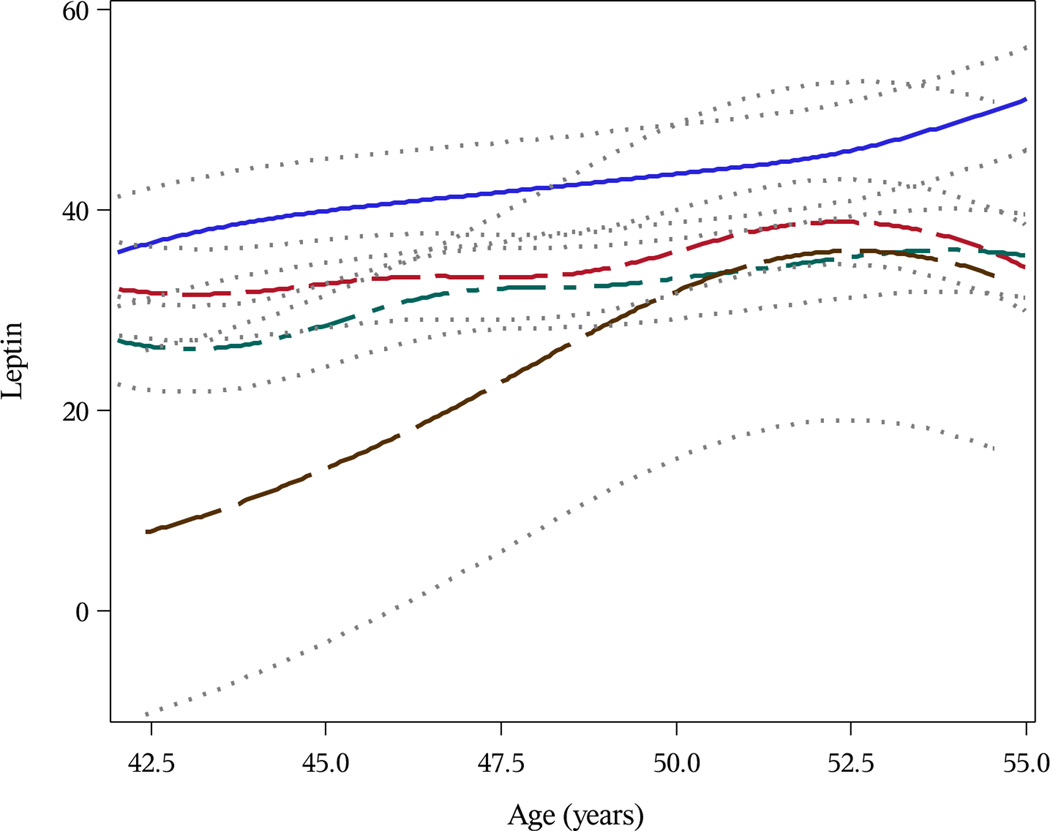
Trajectories of leptin levels from baseline through follow-up visit 7 were modeled and stratified by level of each MRI-assessed marker of knee joint damage. The greatest differences in leptin levels at baseline and over time were observed with respect to cartilage defects, osteophytes, synovitis and joint effusion. As shown in Figure 1, compared to those with no cartilage defects, leptin levels at age 42 years were 20.0 ng/mL higher among women with 50–99% thickness cartilage defects and 34.2 ng/mL higher among women with full-thickness cartilage defects (P=0.03 and P=0.002, respectively). Women with full-thickness cartilage defects had higher leptin levels at age 42 than the highest leptin levels observed among women with less severe cartilage defects. The rate of change in leptin levels over time was attenuated among those with more severe cartilage defects as compared to those with no cartilage defects (P<0.05).
Figure 1.
Predicted Trajectories of Serum Leptin (ng/mL) by Magnetic Resonance Imaging-Defined Knee Cartilage Defects at Follow-Up Visit 11 Among Michigan Study of Women’s Health Across the Nation (SWAN) Participants. Brown Line Represents Women With No Cartilage Defects (Signal Alteration Only); Green Line Represents Women With Cartilage Defects < 50% Thickness; Red Line Represents Women With Cartilage Defects 50–99% Thickness; Blue Line Represents Women With Cartilage Defects 100% Thickness.
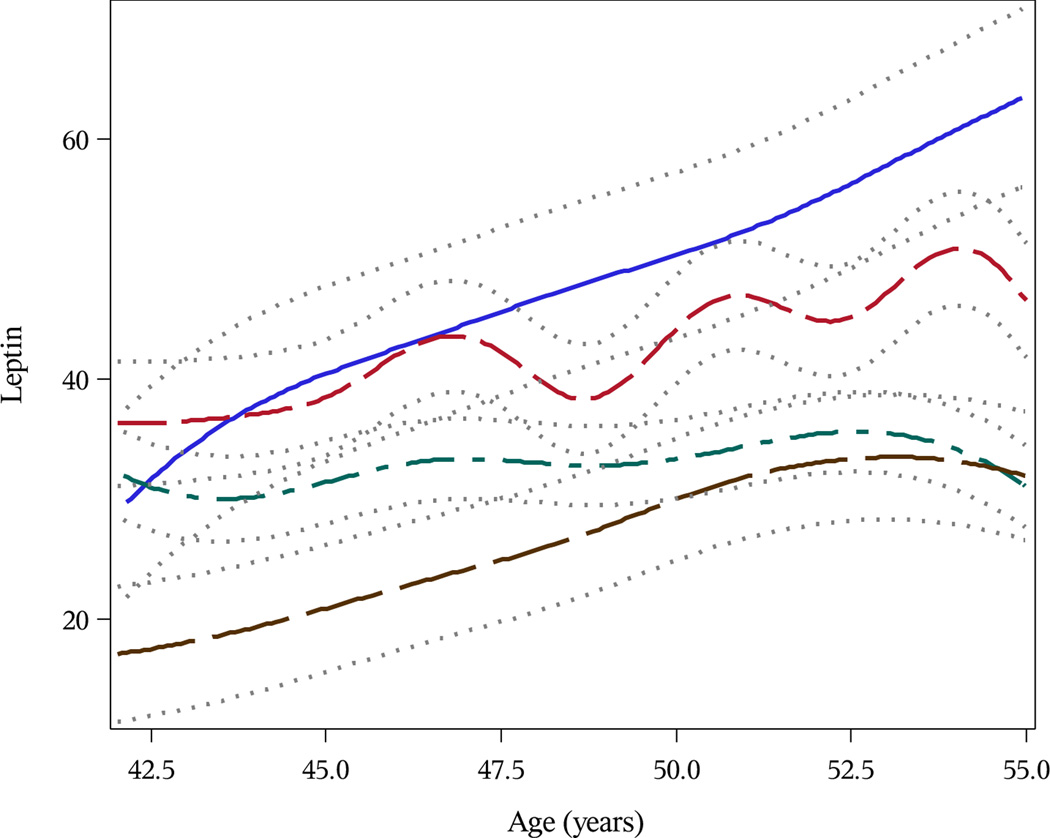
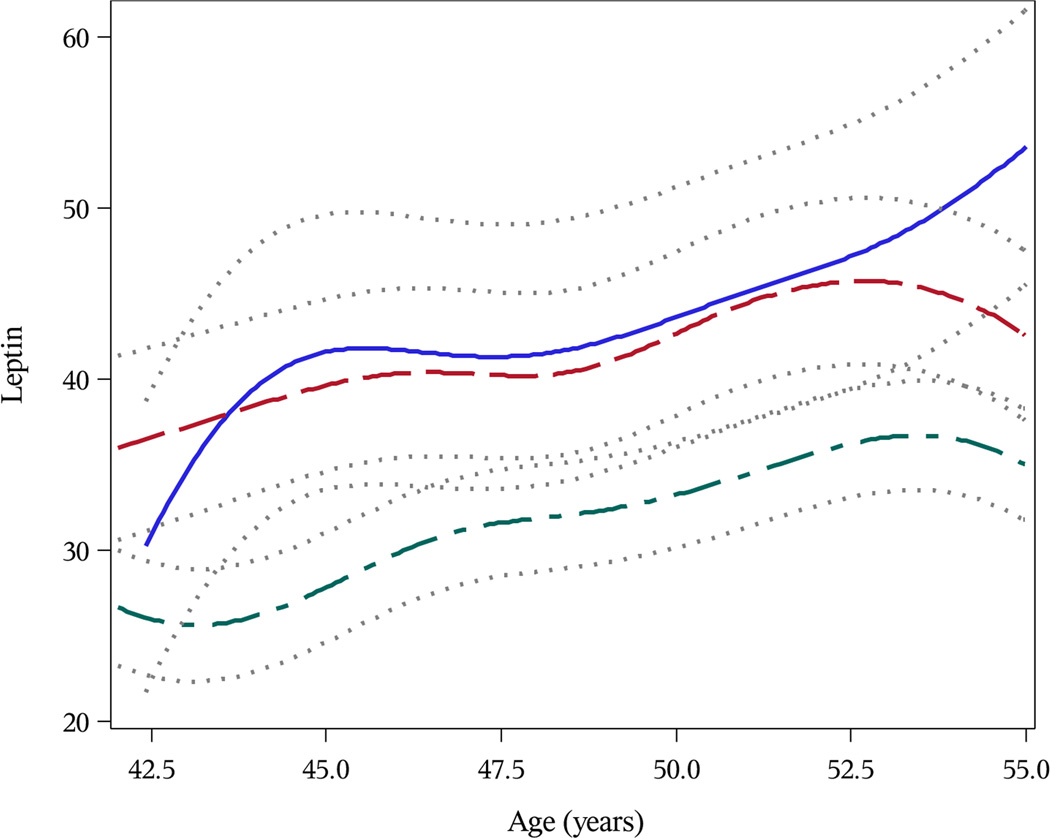
Leptin levels at age 42 years were, on average, 41.4 ng/mL higher among women with osteophytes > 10 mm; 32.8 ng/mL higher for those with ostophytes 5–10 mm; and 14.3 ng/mL higher for those with osteophytes < 5 mm (all P<0.0001). As shown in Figure 2, leptin levels among women with large (> 5 mm) osteophytes were consistently higher than levels among women with small (≤ 5 mm) or no osteophytes. Women with moderate to marked synovitis at follow-up visit 11 had 25.1 ng/mL higher leptin levels at age 42 years as compared to women with no synovitis (P<0.0001) (Figure 3). Similarly, women with moderate to large effusions had leptin levels 33.2 ng/mL higher on average at age 42 as compared to women with only normal physiologic fluid (P<0.0001).
Figure 2.
Predicted Trajectories of Serum Leptin (ng/mL) by Magnetic Resonance Imaging-Defined Knee Osteophytes at Follow-Up Visit 11 Among Michigan Study of Women’s Health Across the Nation (SWAN) Participants. Brown Line Represents Women With No Osteophytes; Green Line Represents Women With Osteophytes ≤ 5 mm; Red Line Represents Women With Osteophytes 5–10 mm; Blue Line Represents Women With Osteophytes > 10 mm.
Figure 3.
Predicted Trajectories of Serum Leptin (ng/mL) by Magnetic Resonance Imaging-Defined Knee Synovitis at Follow-Up Visit 11 Among Michigan Study of Women’s Health Across the Nation (SWAN) Participants. Green Line Represents Women With No Synovitis; Red Line Represents Women With Mild Synovitis; Blue Line Represents Women With Moderate-Marked Synovitis.
DISCUSSION
Leptin is speculated to be part of the biological mechanism which links obesity and joing damage including OA.[44–47] This paper is the first to examine the relationship between leptin levels and multiple characteristics of knee joint damage imaged using MRI. We found that leptin levels ten years prior to MRI assessment were associated with the presence of cartilage defects, BMLs, osteophytes, meniscal tears, synovitis and effusion among a population of mid-aged women.
While leptin levels have been reported to be associated with the prevalence and severity of radiographic knee OA, [35–37] an NHANES study did not find an association between hand OA and leptin.[48] While there is substantial interest in the association of leptin and hand OA, a joint not subjected to mechanical loading, the hand OA data from NHANES was based upon clinical exam and not the preferred method of radiography [49–51], thereby potentially under-estimating the prevalence of disease and biasing findings towards the null. In an analysis relating serum leptin levels to hand OA progression, Yusuf et al. [52] reported slightly greater serum leptin levels among those with progressive hand OA as compared to non-progressive disease (3 ng/mL, p=0.08).
Questions remain about the mechanism by which leptin may modulate joint damage. Leptin may have both an anabolic and catabolic impact on joint tissues. The anabolic effect of leptin on chondrocytes and osteoblasts may be associated with osteophyte development,[31] a hallmark of OA and part of the K–L based scoring system of radiographs. However, leptin is well-known to exhibit pro-inflammatory properties and is associated with increased production of interleukin-1 beta (IL-1β), matrix metallopeptidase 9 (MMP-9) and MMP-13.[32] This pro-inflammatory effect of leptin has been shown to be associated with cartilage catabolism in vitro.[38]
Previous studies of leptin and joint damage have utilized radiographs to characterize OA status using K–L scores [35–37] or have focused on cartilage when using MRI.[13,25] While our data demonstrate that leptin is associated with increased severity of all measures of knee joint damage, leptin measures were most strongly correlated with osteophytes and with the greatest differences in leptin levels observed across categories of osteophyte severity. These findings provide preliminary evidence to suggest that, among mid-aged women relatively early in the disease process, leptin is associated with not only cartilage damaging effects reported by other studies [13,25] but also with multiple measures of knee joint damage. We observed a high prevalence of full-thickness cartilage defects, meniscal tears, synovitis and joint effusion in this population of mid-aged women. We have previously reported [3] the prevalence of these MRI-defined abnormalities on a by-knee and by-compartment basis, where appropriate. In the current analysis, we chose to analyze our data on a by-woman basis and utilized maximum severity scores across knees and compartments given that it is hypothesized that the effect of serum leptin on the knee joint would be systemic. A sensitivity analysis restricted to knee or compartment yielded similar findings. This difference in analytical approaches explains the slightly higher prevalences reported as compared to our previous paper.[3] Other studies have observed cartilage damage, BMLs, and meniscal tears among mid-aged adults.[17,53,54] Guymer et al. reported 13% prevalence of BMLs among mid-aged women without knee OA;[17] the prevalence in our non-osteoarthritic population was 17%. Our reported prevalence of meniscal tears (31%) are slightly higher than those reported among mid-aged women from Framingham, Massachusetts (19%), although the prevalence of radiographic knee OA is notably different (63% vs. 18%).[54]
Our analysis was limited to MRI-assessed measures of knee joint damage and the knee joint may not be the ideal system in which to evaluate the metabolic impact of obesity given the additional impact of mechanical loading. Thus, our analytical approach was complicated by the high correlation of leptin and body size. However, knees are the most common joint assessed in OA studies, particularly when using MRI as the imaging modality. Efforts were made statistically to adjust for the non-metabolic impact of obesity on knee joint damage. Unfortunately, we did not have early MRI measures and so could not determine whether leptin was associated with incident MRI-assessed knee joint damage, thus, no statement about causality can be made. Our analytic population included only women so we cannot comment about associations in men. Given previous findings of a sex dimorphism for leptin and radiographic knee OA [36], it would be of substantial interest to replicate this analysis among men. Further, our population was middle-aged and given that the relationships with leptin may be non-linear, we cannot extend our findings to other age groups.In conclusion, leptin levels were associated with MRI-defined cartilage defects, BMLs, osteophytes, meniscal abnormalities, synovitis and joint effusion and that correlations were strongest with osteophytes. Replication of these findings in other populations with leptin measures and knee MRI data is needed. Understanding the role that leptin plays in the joint degradation process is critical to the development of more targeted interventions for OA with respect to timing of disease onset and mechanisms by which leptin acts on the joint.
Supplementary Material
Acknowledgments
ACKNOWLEDGEMENTS AND FUNDING
Grant Support:
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495, AG017719). This work was additionally supported by AG017104, AG027708 and a research award from the Arthritis Foundation (#5375). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
We thank the study staff at each site and all the women who participated in SWAN.
Appendix
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
SWAN Repository: University of Michigan, Ann Arbor – Dan McConnell 2011; MaryFran Sowers 2000 – 2011.
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, Co-PI 2001 – present; Maria Mori Brooks Co-PI 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
Footnotes
Licence for Publication
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in ARD and any other BMJPGL products and sublicences such use and exploit all subsidiary rights, as set out in our licence (http://group.bmj.com/products/journals/instructions-for-authors/licence-forms).
Financial Disclosure:
None of the authors have any financial conflicts of interests to declare.
REFERENCES
- 1.Dillon CF, Rasch EK, Gu Q, et al. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. J Rheumatol. 2006;33:2271–2279. [PubMed] [Google Scholar]
- 2.Sowers M, Lachance L, Hochberg M, et al. Radiographically defined osteoarthritis of the hand and knee in young and middle-aged African American and Caucasian women. Osteoarthritis Cartilage. 2000;8:69–77. doi: 10.1053/joca.1999.0273. [DOI] [PubMed] [Google Scholar]
- 3.Sowers M, Karvonen-Gutierrez CA, Jacobson JA, et al. Associations of anatomical measures from MRI with radiographically defined knee osteoarthritis score, pain, and physical functioning. J Bone Joint Surg Am. 2011;93:241–251. doi: 10.2106/JBJS.I.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hootman JM, Helmick CG. Projections of US prevalence arthritis and associated activity limitation. Arthritis Rheum. 2006;54:226–229. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 6.Lewis-Faning E, Fletcher E. A statistical study of 1,000 cases of chronic rheumatism-Part III. Postgrad Med J. 1945;21:137–146. doi: 10.1136/pgmj.21.234.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson JJ, Felson DT. Factors associated with osteoarthritis of the knee in the first national Health and Nutrition Examination Survey (HANES I). Evidence for an association with overweight, race, and physical demands of work. Am J Epidemiol. 1988;128:179–189. doi: 10.1093/oxfordjournals.aje.a114939. [DOI] [PubMed] [Google Scholar]
- 8.Davis MA, Ettinger WH, Neuhaus JM. Obesity and osteoarthritis of the knee: evidence from the National Health and Nutrition Examination Survey (NHANES I) Semin Arthritis Rheum. 1990;20:34–41. doi: 10.1016/0049-0172(90)90045-h. [DOI] [PubMed] [Google Scholar]
- 9.Hochberg MC, Lethbridge-Cejku M, Scott WW, Jr., et al. The association of body weight, body fatness and body fat distribution with osteoarthritis of the knee: data from the Baltimore Longitudinal Study of Aging. J Rheumatol. 1995;22:488–493. [PubMed] [Google Scholar]
- 10.Manninen P, Hiihimaki H, Heliövaara M, et al. Overweight, gender and knee osteoarthritis. Int J Obes Relat Metab Disord. 1996;20:595–597. [PubMed] [Google Scholar]
- 11.Coggon D, Reading I, Croft P, et al. Knee osteoarthritis and obesity. Int J Obes Relat Metab Disord. 2001;25:622–627. doi: 10.1038/sj.ijo.0801585. [DOI] [PubMed] [Google Scholar]
- 12.Lachance L, Sowers M, Jamadar D, et al. The experience of pain and emergent osteoarthritis of the knee. Osteoarthritis Cartilage. 2001;9:527–532. doi: 10.1053/joca.2000.0429. [DOI] [PubMed] [Google Scholar]
- 13.Anandacoomarasamy A, Smith G, Leibman S, et al. Cartilage defects are associated with physical disability in obese adults. Rheumatology. 2009;48:1290–1293. doi: 10.1093/rheumatology/kep246. [DOI] [PubMed] [Google Scholar]
- 14.Ding C, Cicuttini F, Scott F, et al. Knee structural alteration and BMI: a cross-sectional study. Obes Res. 2005;13:350–361. doi: 10.1038/oby.2005.47. [DOI] [PubMed] [Google Scholar]
- 15.Ding C, Cicuttini F, Scott F, et al. Natural history of knee cartilage defects and factors affecting change. Arch Intern Med. 2006;166:651–658. doi: 10.1001/archinte.166.6.651. [DOI] [PubMed] [Google Scholar]
- 16.Davies-Tuck ML, Wluka AE, Wang Y, et al. The natural history of bone marrow lesions in community-based adults with no clinical knee osteoarthritis. Ann Rheum Dis. 2009;68:904–908. doi: 10.1136/ard.2008.092973. [DOI] [PubMed] [Google Scholar]
- 17.Guymer E, Baranyay F, Wluka AE, et al. A study of the prevalence and associations of subchondral bone marrow lesions in the knees of healthy, middle-aged women. Osteoarthritis Cartilage. 2007;15:1437–1442. doi: 10.1016/j.joca.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Hart DJ, Spector TD. The relationship of obesity, fat distribution and osteoarthritis in women in the general population: the Chingford Study. J Rheumatol. 1993;20:331–335. [PubMed] [Google Scholar]
- 19.Carman WJ, Sowers M, Hawthorne VM, et al. Obesity as a risk factor for osteoarthritis of the hand and wrist: a prospective study. Am J Epidemiol. 1994;139:119–129. doi: 10.1093/oxfordjournals.aje.a116974. [DOI] [PubMed] [Google Scholar]
- 20.Grotle M, Hagen KB, Natvig B, et al. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9:132. doi: 10.1186/1471-2474-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveria SA, Felson DT, Cirillo PA, et al. Body weight, body mass index, and symptomatic osteoarthritis of the hand, hip and knee. Epidemiology. 1999;10:161–166. [PubMed] [Google Scholar]
- 22.Sowers MF, Yosef M, Jamadar D, BMI vs, et al. body composition and radiographically defined osteoarthritis of the knee in women: a 4-year follow-up study. Osteoarthritis Cartilage. 2008;16:367–372. doi: 10.1016/j.joca.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Wluka AE, English DR, et al. Body composition and knee cartilage properties in healthy, community-based adults. Ann Rheum Dis. 2007;66:1244–1248. doi: 10.1136/ard.2006.064352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cicuttini FM, Teichtahl AJ, Wluka AE, et al. The relationship between body composition and knee cartilage volume in healthy, middle-aged subjects. Arthritis Rheum. 2005;52:461–467. doi: 10.1002/art.20791. [DOI] [PubMed] [Google Scholar]
- 25.Ding C, Parameswaran V, Cicuttini F, et al. Association between leptin, body composition, sex and knee cartilage morphology in older adults: the Tasmanian older adult cohort (TASOAC) study. Ann Rheum Dis. 2008;67:1256–1261. doi: 10.1136/ard.2007.082651. [DOI] [PubMed] [Google Scholar]
- 26.Berry PA, Wluka AE, Davies-Tuck ML, et al. The relationship between body composition and structural changes at the knee. Rheumatology. 2010;49:2362–2369. doi: 10.1093/rheumatology/keq255. [DOI] [PubMed] [Google Scholar]
- 27.Teichtahl AJ, Wang Y, Wluka AE, et al. The longitudinal relationship between body composition and patella cartilage in healthy adults. Obesity. 2008;16:421–427. doi: 10.1038/oby.2007.37. [DOI] [PubMed] [Google Scholar]
- 28.Teichtahl AJ, Wluka AE, Wang Y, et al. Obesity and adiposity are associated with the rate of patella cartilage volume loss over 2 years in adults without knee osteoarthritis. Ann Rheum Dis. 2009;68:909–913. doi: 10.1136/ard.2008.093310. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Simpson JA, Wluka AE, et al. Relationship between body adiposity measures and risk of primary knee and hip replacement for osteoarthritis: a prospective cohort study. Arthritis Res Ther. 2009;11:R31. doi: 10.1186/ar2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gulcelik NE, Usman A, Gurlek A. Role of adipocytokines in predicting the development of diabetes and its late complications. Endocrine. 2009;36:397–403. doi: 10.1007/s12020-009-9234-7. [DOI] [PubMed] [Google Scholar]
- 31.Dumond H, Presle N, Terlain B, et al. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 2003;48:3118–3129. doi: 10.1002/art.11303. [DOI] [PubMed] [Google Scholar]
- 32.Simopoulou T, Malizos KN, Iliopoulos D, et al. Differential expression of leptin and leptin’s receptor isoform (Ob-Rb) mRNA between advanced and minimally affected osteoarthritic cartilage; effect on cartilage metabolism. Osteoarthritis Cartilage. 2007;15:872–883. doi: 10.1016/j.joca.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 33.Gegout PP, Francin PJ, Mainard D, et al. Adipokines in osteoarthritis: friends or foes of cartilage homeostasis? Joint Bone Spine. 2008;75:669–671. doi: 10.1016/j.jbspin.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Presle N, Pottie P, Dumond H, et al. Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production. Osteoarthritis Cartilage. 2006;14:690–695. doi: 10.1016/j.joca.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Ku JH, Lee CK, Joo BS, et al. Correlation of synovial fluid leptin concentrations with the severity of osteoarthritis. Clin Rheumatol. 2009;28:1431–1435. doi: 10.1007/s10067-009-1242-8. [DOI] [PubMed] [Google Scholar]
- 36.Karvonen-Gutierrez CA, Sowers MR, Heeringa SG. Sex dimorphism in the association of cardiometabolic characteristics and osteophytes-defined radiographic knee osteoarthritis among obese and non-obese adults: NHANES III. Osteoarthritis Cartilage. 2012;20:614–621. doi: 10.1016/j.joca.2012.02.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karvonen-Gutierrez CA, Harlow SD, Mancuso P, et al. Leptin levels are associated with radiographic knee osteoarthritis among a cohort of mid-life women. Arthritis Care Res. 2012 doi: 10.1002/acr.21922. Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hui W, Litherland GJ, Elias MS, et al. Leptin produced by white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Ann Rheum Dis. 2012;71:455–462. doi: 10.1136/annrheumdis-2011-200372. [DOI] [PubMed] [Google Scholar]
- 39.Berry PA, Jones SW, Cicuttini FM, et al. Temporal relationship between serum adipokines, biomarkers of bone and cartilage turnover, and cartilage volume loss in a population with clinical knee osteoarthritis. Arthritis Rheum. 2011;63:700–707. doi: 10.1002/art.30182. [DOI] [PubMed] [Google Scholar]
- 40.Sowers MF, Crawford SL, Sternfeld B, et al. SWAN: A Multicenter, Multiethnic, Community-Based Cohort Study of Women and the Menopausal Transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego: Academic Press; 2000. pp. 175–188. Chapter 11. [Google Scholar]
- 41.Kellgren JH, Lawrence JS. The epidemiology of chronic rheumatism. Vol. II. Atlas of standard radiographs of arthritis. Philadelphia: FA Davis; 1963. [Google Scholar]
- 42.Sowers MF, Karvonen-Gutierrez CA, Yosef M, et al. Longitudinal changes of serum COMP and urinary CTX-II predict X-ray defined knee osteoarthritis severity and stiffness in women. Osteoarthritis Cartilage. 2009;17:1609–1614. doi: 10.1016/j.joca.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marques-Vidal P, Bochud M, Paccaud F, et al. Distribution of plasma levels of adiponectin and leptin in an adult Caucasian population. Clin Endocrinol. 2010;72:38–46. doi: 10.1111/j.1365-2265.2009.03628.x. [DOI] [PubMed] [Google Scholar]
- 44.Rai MF, Sandell LJ. Inflammatory mediators: tracing links between obesity and osteoarthritis. Crit Rev Eukaryot Gene Expr. 2011;21:131–142. doi: 10.1615/critreveukargeneexpr.v21.i2.30. [DOI] [PubMed] [Google Scholar]
- 45.Gómez R, Conde J, Scotece M, et al. What’s new in our understanding of the role of adipokines in rheumatic diseases? Nat Rev Rheumatol. 2011;7:528–536. doi: 10.1038/nrrheum.2011.107. [DOI] [PubMed] [Google Scholar]
- 46.Sowers MR, Karvonen-Gutierrez CA. The evolving role of obesity in knee osteoarthritis. Curr Opin Rheumatol. 2010;22:533–537. doi: 10.1097/BOR.0b013e32833b4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lajeunesse D, Pelletier JP, Martel-Pelletier J. Osteoarthritis: a metabolic disease induced by local abnormal leptin activity? Curr Rheumatol Rep. 2005;7:79–81. doi: 10.1007/s11926-005-0057-0. [DOI] [PubMed] [Google Scholar]
- 48.Massengale M, Reichmann WM, Losina E, et al. The relationship between hand osteoarthritis and serum leptin concentration in participants of the Third National Health and Nutrition Examination Survey. Arthritis Res Ther. 2012;14:R132. doi: 10.1186/ar3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dieppe P, Cushnaghan J. The natural course and prognosis of osteoarthritis. In: Moskowitz R, Howell DJ, Goldberg VM, Mankin JH, editors. Osteoarthritis: Diagnosis and Medical Surgical Management. 2nd ed. London: Saunders; 1992. pp. 399–412. [Google Scholar]
- 50.Hart DJ, Spector TD, Egger P, Coggon D, Cooper C. Defining osteoarthritis of the hand for epidemiologic studies. The Chingford Study. Ann Rheum Dis. 1994;53:220–223. doi: 10.1136/ard.53.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valkenburg HA. Clinical versus radiological osteoarthritis in the general population. In: Peyron JG, editor. Epidemiology of Osteoarthritis. Paris: Geigy; 1991. pp. 53–58. [Google Scholar]
- 52.Yusuf E, Ioan-Facsinay A, Bijsterbosch J, et al. Association between leptin, adiponectin and resistin and long-term progression of hand osteoarthritis. Ann Rheum Dis. 2011;70:1282–1284. doi: 10.1136/ard.2010.146282. [DOI] [PubMed] [Google Scholar]
- 53.Ding C, Cicuttini F, Blizzard L, et al. A longitudinal study of the effect of sex and age on change in knee cartilage volume in adults. Rheumatology. 2007;46:273–279. doi: 10.1093/rheumatology/kel243. [DOI] [PubMed] [Google Scholar]
- 54.Englund M, Guermazi A, Gale D, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359:1108–1115. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.