Abstract
The significant drawbacks and lack of success associated with current methods to treat critically sized nerve defects have led to increased interest in neural tissue engineering. Conducting polymers show great promise due to their electrical properties, and in the case of polypyrrole (PPY), its cell compatibility as well. Thus, the goal of this study is to synthesize a conducting composite nerve conduit with PPY and poly(D, L-lactic acid) (PDLLA), assess its ability to support the differentiation of rat pheochromocytoma 12 (PC12) cells in vitro, and determine its ability to promote nerve regeneration in vivo. Different amounts of PPY (5%, 10%, and 15%) are used to synthesize the conduits resulting in different conductivities (5.65, 10.40, and 15.56 ms/cm, respectively). When PC12 cells are seeded on these conduits and stimulated with 100 mV for 2 h, there is a marked increase in both the percentage of neurite-bearing cells and the median neurite length as the content of PPY increased. More importantly, when the PPY/PDLLA nerve conduit was used to repair a rat sciatic nerve defect it performed similarly to the gold standard autologous graft. These promising results illustrate the potential that this PPY/PDLLA conducting composite conduit has for neural tissue engineering.
Keywords: Tissue Engineering, Nerve Regeneration, Conducting Polymer, Peripheral Nerve, Animal Model
1. Introduction
In the United States and Europe, approximately 100,000 peripheral nerve repair procedures are performed every year [1]. Peripheral nerve injuries can arise from trauma, cancer, or congenital defects [2, 3], and are challenging clinical issues to address. While short gaps less than 10 mm can be reconnected surgically with microsutures [4] or various nerve guidance channels [5–7], longer defects are more difficult to treat.
Despite the need, current options to regenerate critically-sized nerve defects are limited. The gold standard is the autologous nerve graft [8–10], but there are significant drawbacks including limited donor source, donor site morbidity, multiple surgical sites, and possible size mismatch. Other options include allografts and xenografts, but those run the risk of disease transmission and immune rejection. Thus, there is a significant clinical need to address critical size nerve defects.
Nerve tissue engineering is a promising approach that has shown potential to address this need with synthetic nerve conduits. There has been a significant effort dedicated to developing synthetic nerve conduits that have resulted in encouraging regeneration and some degree of functional recovery of peripheral nerve defects [11–15]. However, more work needs to be done to improve their efficacy compared to autologous nerve grafts.
An important aspect of synthetic nerve grafts is their ability to conduct electricity. Wilson et al. showed that electrical stimulation can significantly promote the regeneration of peripheral nerve injuries after examining a large number of animal experiments [16]. Thus, there has been a large focus on the use of conductive materials in neural tissue engineering. Researchers are hoping to develop a novel material that can meet both the conductivity demands of nerve tissue and the requirements of tissue engineering as a whole. Ideally, this conduit should be biocompatible, biodegradable, and provide an appropriate electrical environment.
As a means to achieve this, researchers have studied electroconducting polymers, such as polypyrrole (PPY), polyaniline, and polyphosphazene. They have been investigated in recent years for a number of applications in the field of microelectronics, polymer batteries, actuators, and biomedical engineering and have been shown to have excellent electrical properties [17, 18]. For biological and medical applications, PPY has been the most widely studied [19]. Not only does it have excellent electrical properties, in vitro and in vivo studies have proven it to have favorable cell and tissue compatibility. In addition, PPY is easy to synthesize, has a readily modifiable surface, and is inexpensive, all of which are incredibly appealing for tissue engineering applications. Thus, PPY has been recognized as a promising scaffold material for nerve tissue engineering and neural prostheses [19, 20]. To demonstrate this, Schmidt et al. first electrically stimulated PC12 cells through PPY films and observed the promotion of neurite outgrowth from the cells, showing the potential use of conducting polymers for nerve tissue engineering scaffolds [21]. Subsequent studies have focused on improving the polymer scaffolds by incorporating various cues, such as neurotrophins [22], cell adhesive molecules [23, 24], and topographical features [25], emphasizing the importance of multiple signals for improved modulation of neuronal responses [26]. For example, Gomez et al. electrochemically synthesized PPY micro-channels to fabricate conductive, topographical substrates for neural interfacing, and found that PPY micro-channels facilitated axon establishment of rat embryonic hippocampal neurons [25]. These studies demonstrated that PPY is a promising candidate for nerve regeneration.
However, the majority of the work done on PPY involves in vitro cell evaluation. Considering its drawbacks, including its poor solubility and degradation profile, more research needs to be done in vivo to confirm the viability of PPY as a scaffold material.
The purpose of this study was to investigate a possible treatment for repairing damaged nerves and to overcome the current shortcomings PPY has in tissue engineering. Poly(d, l-lactic acid) (PDLLA) is widely used in peripheral nerve tissue engineering due to its good biodegradability, non-cytotoxicity, and mechanical properties [27, 28]. In this study, a PPY/PDLLA conductive composite nerve conduit was fabricated by emulsion polymerization to take advantage of the properties of the individual polymers. The material was tested in vitro for its ability to support the neuronal differentiation of PC12 cells in response to electrical stimulation. In addition, the nerve conduits were used to bridge 10 mm defects in the sciatic nerve of Sprague-Dawley rats in vivo.
2. Materials and Methods
2.1. Materials
PDLLA was purchased from Boehringer Ingelheim (Germany). PY was purchased from Sigma-Aldrich (USA). PY was vacuum-distilled at about 130°C until it became a colorless liquid, and then stored at 4°C in the dark until use. All other chemicals were analytical grade reagents.
2.2. Preparation of PPY/PDLLA nerve conduits
PDLLA was dissolved in CHCl3 to obtain a 10% (w/v) solution. For the emulsion polymerization of PPY, PY and 0.7% (w/v) sodium dodecyl sulfate (SDS, 99%, Sigma-Aldrich) aqueous solution were added to the PDLLA/CHCl3 solution under stirring for 2 h. The weight ratio of PY to PDLLA was 5%, 10% and 15%. Then 10% (w/v) FeCl3 aqueous solution (FeCl3∶PY=5.3) was added drop-wise into the mixture under vigorous stirring to trigger oxidative polymerization. The polymerization continued at room temperature for 5 h to obtain a PPY/PDLLA composite emulsion. A cylindrical mandrel was inserted into the PPY/PDLLA composite emulsion for several seconds, and then it was taken into alcohol to remove the CHCl3 and water. This was repeated until a 0.2±0.05 mm thick film on the cylinder mandrel was obtained. After drying, the conduit was removed from the mandrel and stored in a vacuum oven before use. To fabricate the films for cell culture studies, the PPY emulsion polymerization in a PDLLA solution continued at room temperature for 5 h, followed by precipitation with ethyl alcohol and washing in deionized water. The 0.2 mm thick films were obtained by casting the precipitated PPY/PDLLA composite onto a PTFE plate and vacuum-dried for 2 days. Then these films were washed with alcohol and deionized water for three times before being used for cell culture.
2.3. PPY/PDLLA characterization
The chemical compositions were characterized through the ATR-FTIR transmission spectra on a Nicolet Nexus FTIR Spectrometer (Nexus, Thermo Scientific, USA). The conductivity of the PPY/PDLLA conduits was tested by the 4-point probe method using the Hall Effect testing system (HL5500PC, Accent Optical, UK). The nerve conduit surface elemental composition was characterized by using XPS (Escalab MK II, VG Scientific, UK). The morphology at the surface of the PPY/PDLLA conduit was observed using a JSM-5610LV scanning electron microscope (JEOL, Japan) with an accelerating voltage of 10 kV. Prior to observation, the specimens were sputter-coated with gold.
2.4. Sample preparation for in vitro evaluation
Rectangular sections of the PDLLA and PPY/PDLLA films were cut and placed in a device similar to previously described [29]. Briefly, the film was placed on a thin section of polydimethylsiloxane (PDMS, Sylgard 184®, Dow Corning, USA) covering a glass slide. Two wires were placed on either side of the film and covered with a PDMS well to seal the wires from the inner chamber (1 cm × 1 cm × 1 cm dimension). The device was clipped together on two sides and sterilized under UV light for 2 h. The films were then incubated in a 0.1 mg/ml solution of rat tail type I collagen (Sigma) for 24 h at 4°C, washed twice with sterile tissue culture water for 5 min each, and stored in phosphate buffered saline (PBS) at 4°C overnight.
2.5. PC12 cell culture and immunofluorescence
PC12 cells were cultured in F-12K medium (ATCC, USA) supplemented with 15% heat-inactivated horse serum (Gibco®, Life Technologies, USA), 2.5% fetal bovine serum (Gibco®), and 1% Penicillin/Streptomycin solution (Gibco®) and maintained in a humid 37°C incubator with 5% CO2. Three days prior to seeding, the cells were primed with 50 ng/ml nerve growth factor (NGF, PeproTech, USA).
Primed cells were seeded at a density of 2 × 104 cells/cm2 and allowed to adhere for 24 h. A 100 mV potential was then applied across the wires for 2 h and the cells were cultured for an additional 24 h. After a total of two days in culture and 24 h post-stimulation, the cells were fixed in a 4% formaldehyde (Thermo Scientific) in PBS solution for 10 min, permeabilized in a 0.1% Triton X-100 (Sigma) in PBS solution for 6 min, and then blocked in 1% bovine serum albumin (Sigma) in PBS solution for 30 min. The cells were labeled with Alexa Fluor® 555 phalloidin (Life Technologies), excitation at 555 nm and emission at 565 nm, for 20 min and with 4’,6-diamidino-2-phenylindole (DAPI, Vectashield®, Vector Laboratories, USA), excitation at 360 nm and emission at 460 nm, before being sealed under a coverslip and immediately visualized under a confocal fluorescence microscope (Nikon Eclipse C1, Japan). Six samples (n=6) for each film and condition were studied.
2.6. Immunofluorescence image analysis
Image J software (NIH) was used to process and analyze the fluorescent images. The two parameters measured were the percentage of neurite-bearing cells and the median neurite length. The median neurite length was chosen to account for the non-normal distribution and a cut-off of at least a 5 µm neurite length was used [30]. At least 400 PC12 cells were examined for each film and condition.
2.7. Surgical procedure
Adult Sprague-Dawley (SD) rats were purchased from Tongji Medicinal School, Huazhong University of Technology (Wuhan, Hubei, China). All animals were housed under standard conditions, and the experimental procedures involving animals were in accordance with NIH Guidelines for the Care and Use of Laboratory Animals and under the approval of the Administration Committee of Experimental Animals, Hubei Province, China. The surgical procedure was carried out as described previously [31]. Rats weighing 200–250 g were used to evaluate the nerve regeneration performance of PPY/PDLLA conduits. The animals were divided into 3 groups, each with 20 rats. 10-mm defects in the sciatic nerve created by surgical removal of the nerve tissue were connected with PPY/PDLLA conduits, PDLLA conduits, or autologous nerve grafts. The surgical procedure was performed on the sciatic nerve of the right hind limb. The left limb sciatic nerve served as a pairwise control. The animals were anesthetized with 50 mg/kg body weight pentobarbital sodium. The right sciatic nerve was exposed after the skin incision, and the muscles around the nerve tissues were separated using blunt dissection. Subsequently, the right sciatic nerve was severed into proximal and distal segments at the center of the right thigh. Both the proximal and the distal stumps were secured with 8-0 nylon to a depth of 1 mm into the conduits, leaving a 10-mm gap between the stumps. For the autograft group, after a skin incision, the sciatic nerve was exposed through a gluteal muscle-splitting incision, externally dissected to excise a 10-mm nerve, and sutured back to the nerve by epineurial coaptation serving as the autograft control. The muscle layer was re-approximated with 4-0 chromic gut sutures, and the skin was closed with 2-0 silk sutures. Each rat received one implant, which was removed at either 3 or 6 months. Walking track, electrophysiological, and histomorphometric evaluations were performed to evaluate nerve regeneration.
2.8. Walking track analysis
To evaluate the behavior of the rats after nerve injury and repair, walking track analysis was performed at 3 and 6 months post-operatively as described previously[32]. Briefly, the rats were allowed to walk down a wooden walking alley (5.0 × 8.0 × 45 cm3) with a darkened goal box at the end. The floor of the alley was covered with white paper. A thin film of acrylic paint was applied to the rat’s plantar surface to achieve the visualization of important anatomical landmarks of the footprints. As the rats moved down the track, they left footprints on the track and recordings of footprints were made. This process was repeated a minimum of 3 times until clear footmarks were obtained. From the footprints, the following parameters were obtained: distance from the heel to the top of the third toe (print length, PL); distance between the first and the fifth toe (toe spread, TS) and distance from the second to the fourth toe (intermediary toe spread, IT). These measures were made for both the non-operated foot (measurements for this foot are designated as NPL, NTS and NIT) and the operated, experimental foot (measurements for this foot are designated as EPL, ETS and EIT). In the control animals, parameters of the right foot were compared with those from the left foot. In order to calculate the sciatic function index (SFI), these parameters were inserted into an equation used in previous studies [33–35]:
| (1) |
in which PLF = (EPL-NPL)/NPL; TSF = (ETS-NTS)/NTS; and ITF= (EIT-NIT)/NIT. Interpolating identical values of PL, TS, and IT from the right and the left hind feet results in a value close to zero in normal rats. A value of −100 implies total impairment.
2.9. Triceps weight analysis
After performing the electrophysiological evaluation studies at 3 and 6 months, the animals were sacrificed and their triceps surae muscle was dissected. The moist weights of the triceps surae muscle were measured. After gently blotted with absorbent paper to remove any excess blood or serum on the surface of the retrieved tissue, the muscle was promptly weighed. To measure the target muscle reinnervation, the triceps surae muscles from the experimental and contralateral sides (un-operated) were weighed. The relative weights were presented as percentages.
| (2) |
2.10. Electrophysiological assessment
Electrophysiological tests were performed on the animals three and six months after implantation. Under anesthesia, the right sciatic nerve was exposed and nerve stimulation electrodes were placed at the proximal part of the proximal joint access and recording electrodes placed in the lower leg triceps. Nerve conduction velocities (NCVs) were recorded on the lower leg triceps.
2.11. Histological assessment
The implanted conduits were harvested immediately after recording the NCVs. At the same time, the nerve grafts were fixed in a cold buffered 3% glutaraldehyde solution. The nerve grafts were then washed in PBS and the sciatic nerve sections were then taken from the middle regions of the regenerated nerve. After fixation, some tissues in each group were embedded in olefin, cut to 4-mm thickness, and stained with hematoxylin and eosin. The other samples were embedded in Epon 812 epoxy resin and stained with methylene blue. All nerve sections were observed under a light microscope (TE2000-U, Nikon, Japan). An image analysis system (Image-Pro Plus, Media Cybernetics, USA) was used to analyze the photographs to determine the number and areas of individual myelinated axons.
Electron microscopy was also employed to evaluate the myelin sheath regeneration. Ultra-thin sections of the regenerated nerve tissues were stained with lead citrate and uranylacetate, and then were examined under a transmission electron microscope (TEM) (JEM-1200 EX, JEOL, Japan).
2.12. Statistical analysis
All numerical data are given as mean ± standard deviation. Significant differences among groups were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test using SPSS 13.0 software for Windows student version. Statistically significant differences between medians were determined with a Mann-Whitney U test. A statistically significant difference was defined as p<0.05.
3. Results
3.1. Preparation and characterization of PPY/PDLLA conduits
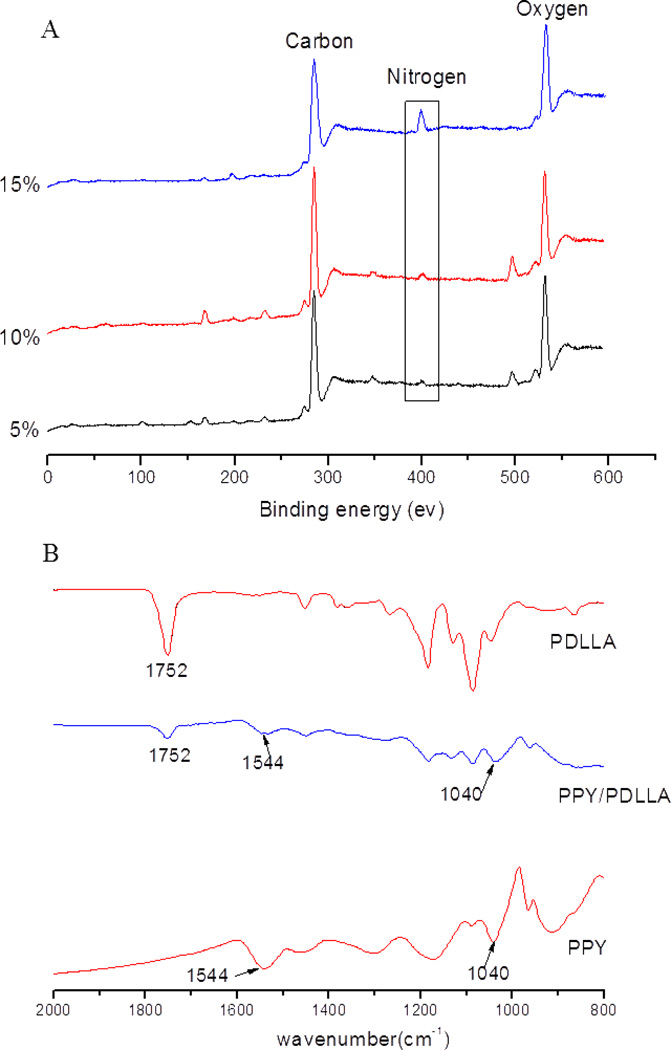
Pyrrole (PY) was oxidized and polymerized to PPY in a PDLLA solution using FeCl3 as an oxidant and doping agent and sodium dodecyl sulfate (SDS) as an emulsifier. Then the PPY/PDLLA emulsion was used to prepare the PPY/PDLLA conduits through a repeated dip coating method with a cylindrical mandrel. Attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) was used to obtain the FTIR spectra of the PDLLA, PPY/PDLLA, and PPY (Fig. 1). In the spectrum of PPY/PDLLA, the band at 1752 cm−1 is a characteristic peak of PDLLA. The two bands at 1544 cm−1 and 1040 cm−1 are characteristic PPY peaks. All the above three peaks are present in the PPY/PDLLA spectrum, verifying that the PPY/PDLLA was successfully synthesized.
Figure 1.
A) ATR-FTIR spectra of PDLLA, PPY/PDLLA and PPY. B) XPS of different ratio PPY/PDLLA conduits.
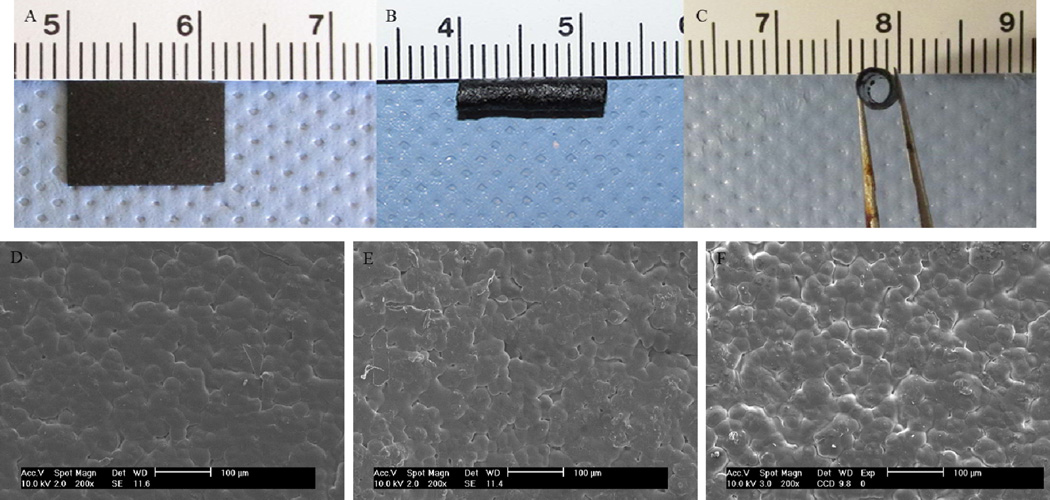
PPY/PDLLA conduits with a length of 12 mm, an inner diameter of 1.6 mm and an outer diameter of 2.0 mm were fabricated. The photographic images and scanning electron micrographs of the PPY/PDLLA films and conduits are shown in Figure 2. Figure 2A shows the PPY/PDLLA film while Figure 2B and Figure 2C show the PPY/PDLLA conduit. Figures 2D, 2E, and 2F show the surface morphology of the 5%, 10%, and 15% PPY/PDLLA conduits, respectively.
Figure 2.
A) Image of the PPY/PDLLA film. B) and C) Image of the PPY/PDLLA nerve conduit. D), E), and F) SEM micrographs of the surface morphology of 5%, 10%, and 15% PPY/PDLLA, respectively.
The surface elemental compositions of the different PPY/PDLLA weight ratio conduits were analyzed using X-ray photoelectron spectroscopy (XPS) to quantify the amount of PY incorporated onto the conduit surface. All the PPY/PDLLA conduits contained C, N and O, and the content of element N was 1.74%, 1.98% and 2.51% for the 5%, 10%, and 15% PPY/PDLLA weight ratios, respectively (Table 1). The measured conductivity of a PPY/PDLLA conduit was higher as the weight ratio of PPY increased (Table 1).
Table 1.
Content of C, N, and O and conductivity test results for different PPY/PDLLA materials.
| Sample | Content (%) | Conductivity (ms/cm) | ||
|---|---|---|---|---|
| C | N | O | ||
| 5% PPY/PDLLA | 74.22 | 1.74 | 24.04 | 5.65 |
| 10% PPY/PDLLA | 75.92 | 1.98 | 22.11 | 10.40 |
| 15% PPY/PDLLA | 73.97 | 2.51 | 23.53 | 15.56 |
3.2. Cell culture evaluation
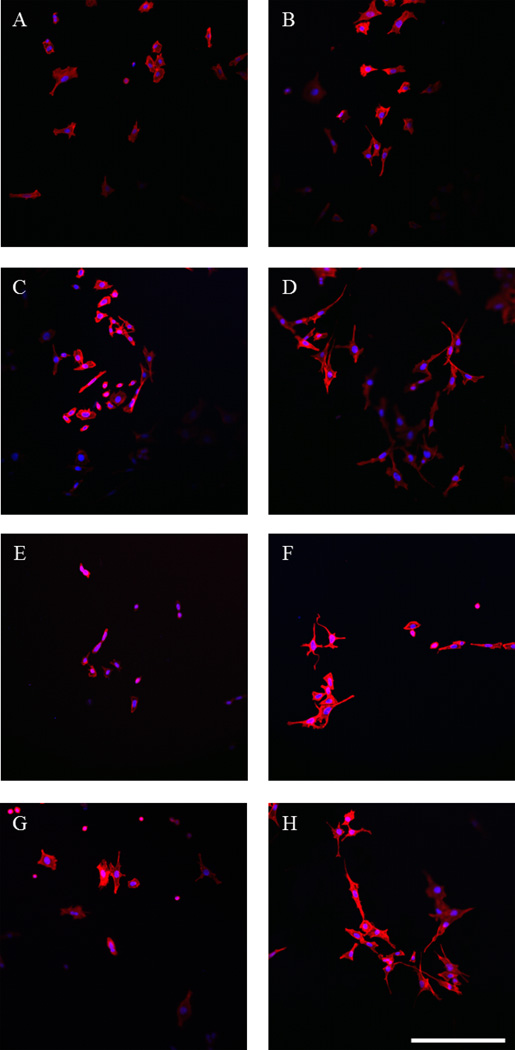
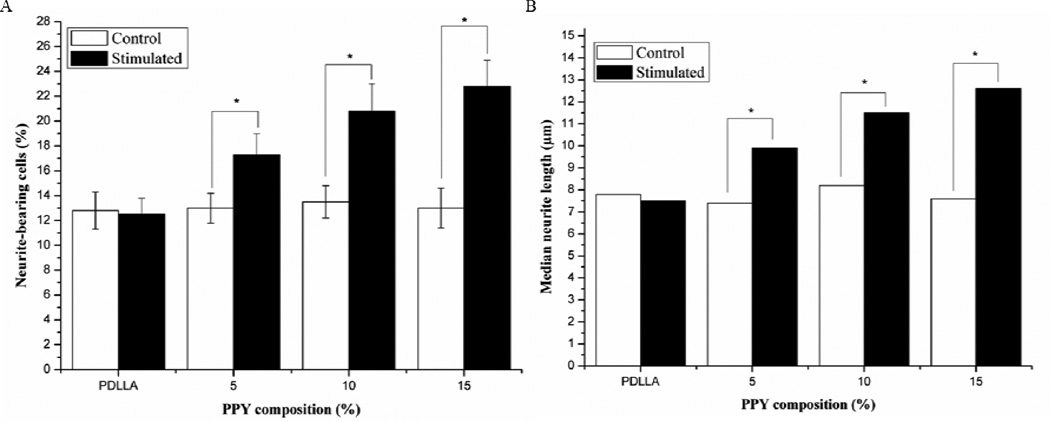
PC12 cells were employed to study the effect that PPY/PDLLA films combined with electrical stimulation had on their behavior. In theory, this would provide an indication of how viable the PPY/PDLLA composite is for use in nerve tissue engineering. The cells were allowed to adhere to the collagen type I coated substrate for 24 h before being stimulated with 100 mV for 2 h [21, 22]. After stimulation, the cells remained in culture for an additional 24 h. PC12 cells were able to adhere to the array of PDLLA and PPY/PDLLA films and were viable throughout culture. There was no significant difference in the cell number throughout the experiments on any of the films (data not shown), indicating that the PPY content did not have any significant effect on adhesion or proliferation. The control cells, where no electrical stimulation was applied, had minimal levels of differentiation across the board. Cells that were stimulated with 100 mV for 2 h exhibited more and longer neurites, as can be seen on the actin-labeled cells (Fig. 3). On all of the control films, approximately 13% of the cells (Fig. 4A) formed neurites with a median length around 8 µm (Fig. 4B). However, there was a clear increase in both the percentage of neurite-bearing cells and the median neurite length for the cells stimulated with 100 mV for 2 h as the PPY content increased. The percentage of neurite-bearing cells increased from 12.5±1.3% on the PDLLA to 17.3±1.7%, 20.8±2.2%, and 22.8±2.1% on the 5%, 10%, and 15% PPY/PDLLA, respectively. The median neurite length increased from 7.5 µm on the PDLLA to 9.9 µm, 11.5 µm, and 12.6 µm on the 5%, 10%, and 15% PPY/PDLLA, respectively.
Figure 3.
Fluorescent images of PC12 cells labeled for actin (red) and nuclei (blue). A) and B) PDLLA. C) and D) 5% PPY/PDLLA. E) and F) 10% PPY/PDLLA. G) and H) 15% PPY/PDLLA. A), C), E), and G) Control cells. B), D), F), and H) Cells stimulated with 100 mV for 2 h. Scale bar: 200 µm.
Figure 4.
A) Percentage of neurite-bearing PC12 cells on PPY/PDLLA composite films with varying PPY composition (n=4, *p<0.05). B) Median neurite length on PPY/PDLLA composite films with varying PPY composition.
3.3. Animal study
A 10 mm defect in the sciatic nerve of adult Sprague-Dawley (SD) rats was used as a model for in vivo nerve regeneration. The defects were repaired with 5% PPY/PDLLA conduits, PDLLA conduits, and the gold standard autografts. Samples were harvested after 3 and 6 months. The 5% PPY/PDLLA conduit was chosen to minimize the amount of PPY since it degrades very slowly.
3.3.1. General observations post-operation
The animals in this study tolerated the anesthetic and operative procedures, and showed no sign of infection at any time. The animals showed none of the complications typically associated with the operation, and all wounds healed without any issues. Moreover, no signs of discomfort were observed throughout the 6 month evaluation period.
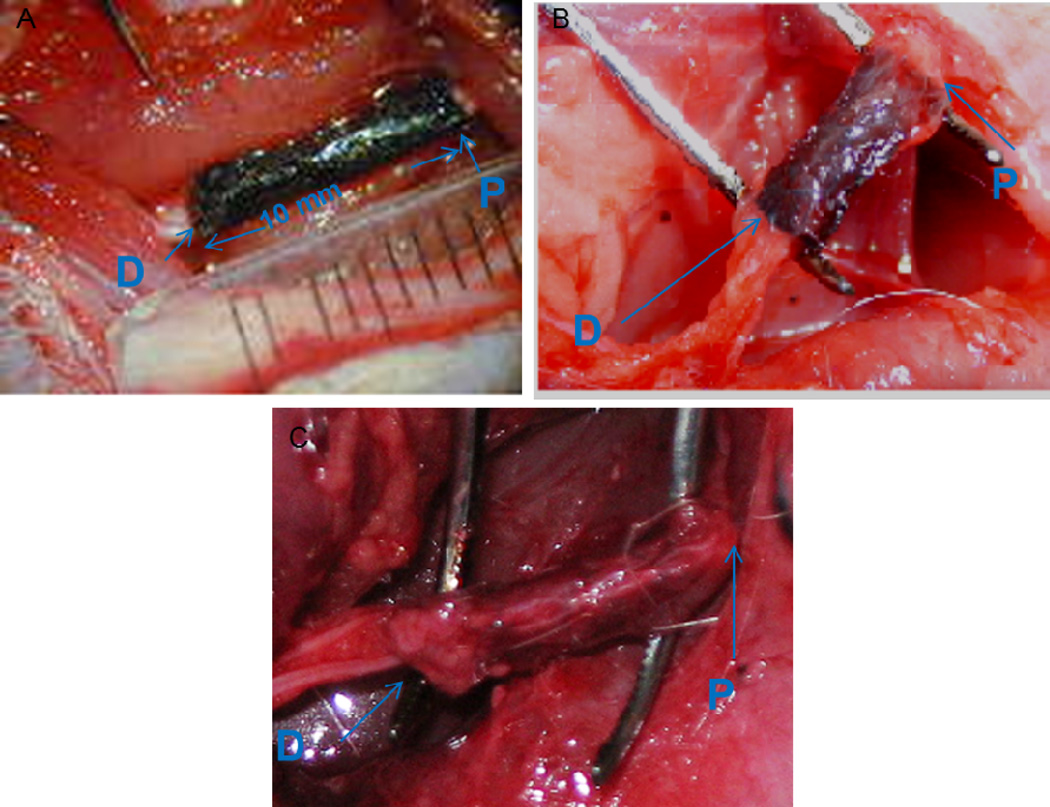
Figure 5 shows the PPY/PDLLA conduit immediately after implantation, 3 months post-surgery, and 6 months post-surgery. Significant levels of degradation can be seen over time, with the conduit becoming thin and crisp after 3 months, but it still maintained lumen and wall integrity. The degradation was even more severe at 6 months, but significant regeneration had occurred, indicating that the conduit had met the demand.
Figure 5.
Intraoperative photographs of the PPY/PDLLA nerve conduits. “P” signifies the proximal end and “D” signifies the distal end. A) Immediately after grafting. B) 3 months postoperatively. C) 6 months postoperatively.
3.3.2. Walking track analysis
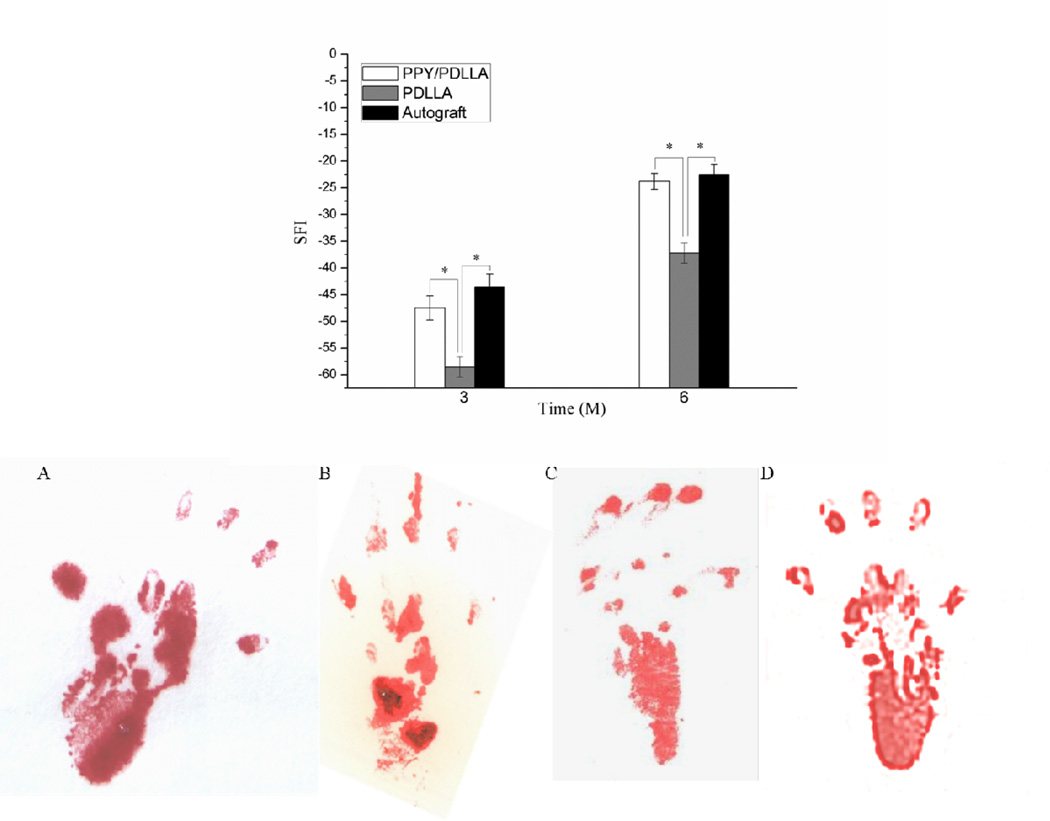
Walking track analysis was used to assess the functional recovery of all operated animals and quantified by calculating the sciatic function index (SFI), a measure of the sciatic nerve function where a value close to 0 indicates normal function and a value close to −100 implies total impairment. Figure 6 (top) demonstrates the recovery of sciatic nerve function 3 and 6 months after the operation. 3 months after implantation, the SFI of the PPY/PDLLA group, PDLLA group, and the autograft group were −47.5±2.3, −58.6±1.9, and −43.6±2.5, respectively. There was a significant difference between the PDLLA group and PPY/PDLLA group. There was also significant difference between the PDLLA group and autograft group (p<0.05), while there was no significant difference between PPY/PDLLA group and autograft group (p>0.05). After 6 months post-operation, the three groups, PPY/PDLLA, PDLLA, and autograft, reached an SFI of −23.8±1.5, −37.2±1.9and −22.5±1.8, respectively. There was a significant difference between the PDLLA group and PPY/PDLLA group (p<0.05), while there was no significant difference between PPY/PDLLA group and autograft group (p>0.05). Figure 6 (bottom) illustrates the footprints from walking track analysis of all nerve conduits after 6 months post-implantation. Footprints in the PPY/PDLLA group showed a greater improvement in toe spreading, and the footprints themselves were shorter and wider than those in the PDLLA group. Impressively, the PPY/PDLLA group had similar results to the autograft group.
Figure 6.
Recovery of sciatic nerve function. Sciatic function index (SFI) as a function of implantation time (top). Footprint stamps in walking track analysis after 6 months of implantation (bottom). A) PPY/PDLLA. B) PDLLA. C) Autograft. D) Normal left leg.
3.3.3. Electrophysiological evaluation
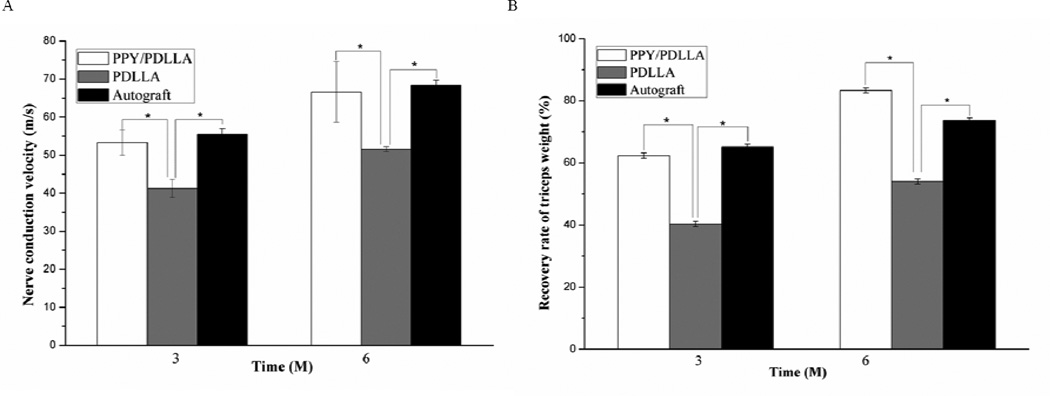
Since the nerve conduction velocity (NCV) can offer important insight for studying the conducting function of a peripheral nerve, NCVs were recorded in the lower leg triceps at postoperative intervals 3 and 6 months. The NCVs of the PPY/PDLLA group were significantly faster than those of PDLLA group (p<0.05) and not significantly different from those of the autograft group (p>0.05) (Fig. 7A). At 6 months after implantation the NCVs of the autograft were found to be 68.39±1.28 m/s, whereas those bridged with the PPY/PDLLA conduits were 66.59±7.97 m/s, and those bridged with the PDLLA conduits were 51.54±0.66 m/s.
Figure 7.
A) Nerve conduction velocities (NCVs) 3 and 6 months after implantation (n=6, *p<0.05). B) Triceps weight (%) 3 and 6 months post-operation (n=6, *p<0.05).
3.3.4. Triceps weight analysis
As an index of muscle atrophy, the triceps surae muscle on the operated leg was compared to that of the healthy leg to calculate a muscle weight ratio. Figure 7B illustrates that the muscle weight ratio of the PPY/PDLLA and autograft groups was significantly higher than that of PDLLA group (p<0.05), while the triceps surae muscle weight ratio of autograft groups was not significantly different from that of PPY/PDLLA group (p>0.05). The improvement in muscle weight indicates that there was significant reinnervation of the target muscle.
3.3.5. Nerve morphological assessment
During the early nerve regeneration process, the structure of PPY/PDLLA conduits remained intact (Fig. 5). After 3 months, the conduit showed signs of degradation and was covered with connective tissue. The degradation was more apparent after 6 months and the presence of PPY was visibly reduced. The 10 mm gap removed by surgery had been bridged by the regenerated tissue 3 months after implantation.
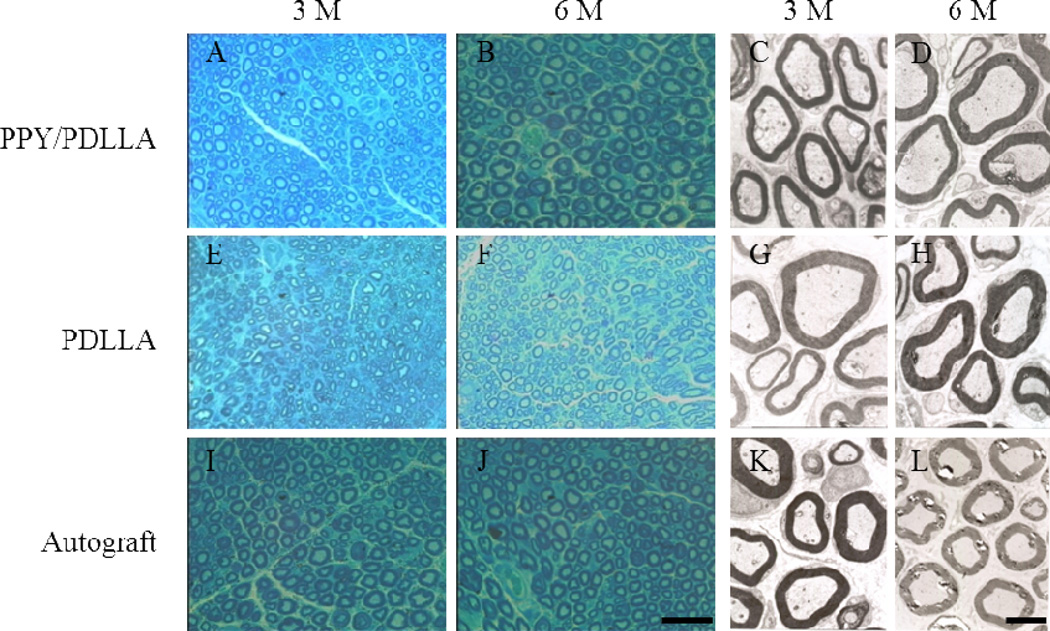
The regenerated nerve samples were removed from the conduits and stained with methylene blue to evaluate the number and diameters of regenerated axons in all of the conduit groups. Numerous bundles of regenerated nerve fibers were clearly identified in the sections of the regenerated tissues (Fig. 8). Transmission electron microscopy (TEM) of the mid-portion of the regenerated nerve tissues 3 months after implantation revealed that the formation of regenerated myelinated fibers occurred at similar levels in both the PPY/PDLLA and autograft groups. In addition, the structure of the myelinated fibers in the regenerated tissues of those two groups was more compact and more uniform than that of the PDLLA group (Fig. 8).
Figure 8.
Histology images stained with methylene blue and transmission electron microscopy (TEM) micrographs. Images represent cross sections of regenerated nerves taken from types of nerve conduits implanted in rats after 3 and 6 months. Histology scale bar: 50 µm. TEM scale bar: 2 µm.
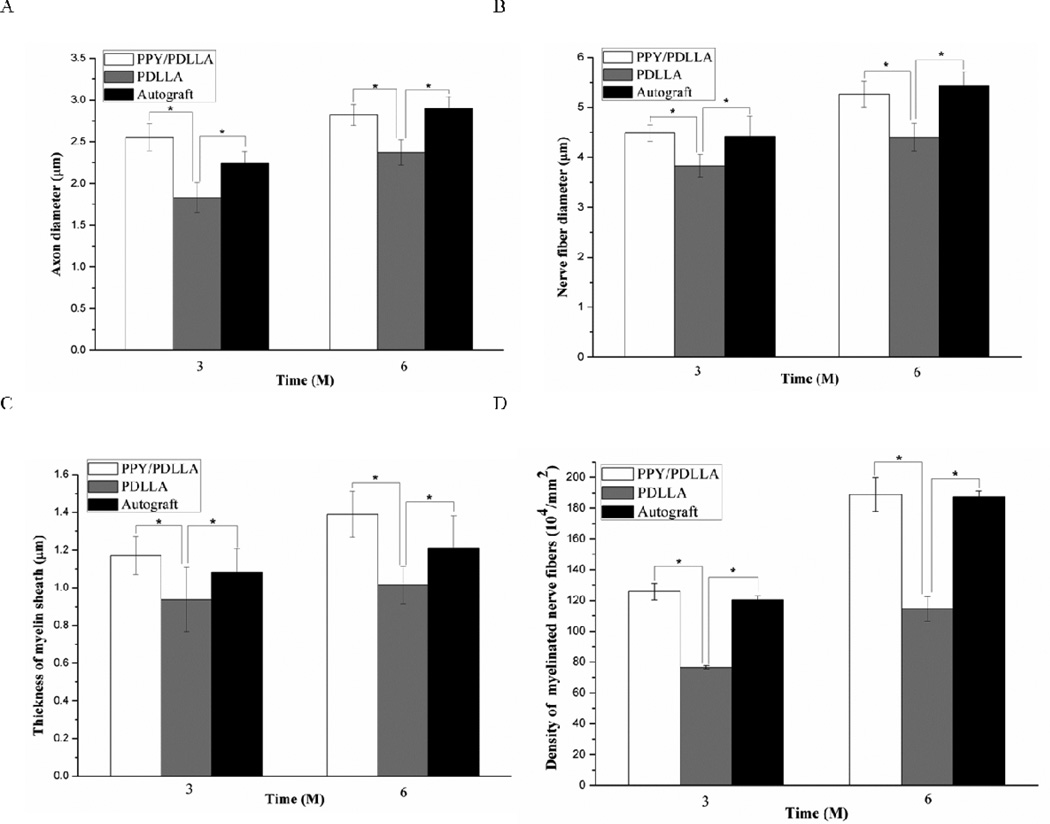
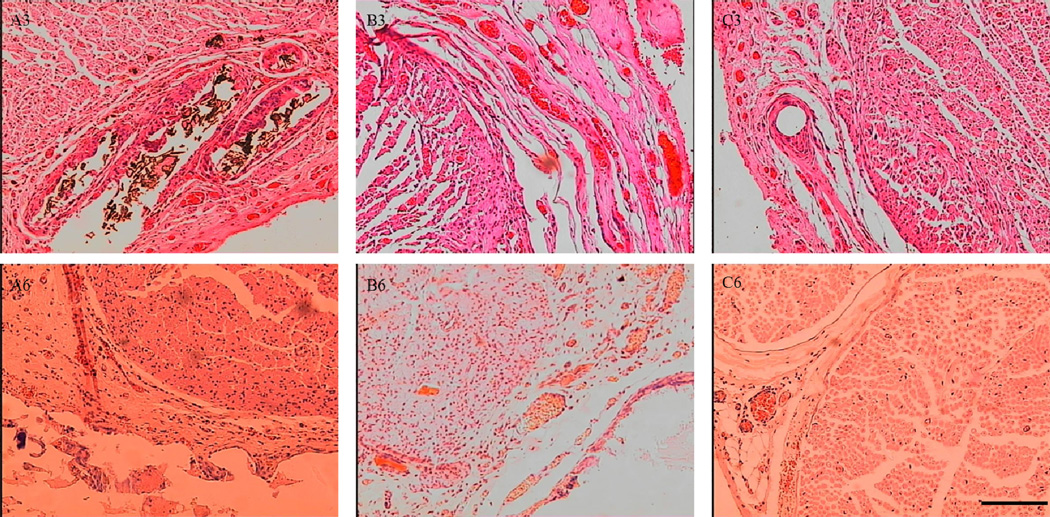
Statistical analysis was performed on the average axon diameter (Fig. 9A), the myelinated fiber diameter (Fig. 9B), the thickness of regenerated myelin sheath (Fig. 9C), and the density of myelinated fibers of the regenerated nerve (Fig. 9D). For all of the parameters measured, there was a significant difference between the PPY/PDLLA and the PDLLA groups (p<0.05). Not only was there a higher density of myelinated fibers in the PPY/PDLLA conduits, comparable to that in the autograft, but the myelin sheath was thicker. To evaluate the ingrowth of connective tissue to the wall of conduits, the regenerated nerves were stained with hematoxylin and eosin (HE). The histological sections demonstrated that the inner layers of PPY/PDLLA group were compact enough to prevent any connective tissue ingrowth, while the PDLLA group was filled with connective tissue (Fig. 10). In addition, the PPY content in the PPY/PDLLA group is visibly reduced from 3 to 6 months, mirroring the gross observations from Figure 4.
Figure 9.
Quantification of the histological assessment of the regenerated nerve fibers 3 and 6 months after implantation. A) Average axon diameter of regenerated myelinated nerve fibers. B) Average diameter of the regenerated nerve fiber. C) Average thickness of regenerated myelinated sheath. D) The average density of regenerated myelinated nerve fibers (n=6, *p<0.05).
Figure 10.
Cross sections of regenerated nerves in rats 3 months and 6 months post-operation and stained with hematoxylin and eosin (HE). Scale bar: 50 µm. A) PPY/PDLLA. B) PDLLA. C) Autograft. (A3, B3 and C3: 3 months after implantation. A6, B6 and C6: 6 months after implantation).
4. Discussion
Tissue engineering combines a scaffold with cells and growth factors to functionally repair tissue defects. Each tissue has its own unique requirements for each component. For nerve, the scaffold must guide the regenerating axons across the gap to connect with the distal end. Only then will signal transduction occur. Moreover, as an electrically conducting tissue, the scaffold should likewise be able to conduct an electrical signal. Most polymers used in tissue engineering are unable to do so, but the addition of a conducting polymer, PPY in our study, greatly increases the electrical properties of the scaffold. Additionally, the scaffold must be non-immunogenic to avoid any adverse response, and biodegradable to allow the newly regenerated nerve to fully develop and eliminate the need for a secondary surgery to remove the implant.
In our study, we attempted to address all of the above requirements using a PPY/PDLLA conduit. The in vitro experiments showed that increasing amounts of PPY resulted in more and longer neurites. Interestingly, this suggests that electrically stimulated differentiation of PC12 cells is mediated by the conductivity of the substrate. While the mechanism behind how electrical stimulation promotes neurite formation is not fully understood, these data may provide insight for further investigation. However, pure PPY is brittle and marginally biodegradable, so we used the minimum amount necessary for the in vivo study. Our in vitro experiments confirmed that 5% PPY/PDLLA was sufficient to induce more and longer neurites while maintaining the mechanical flexibility of PDLLA. This was likely caused by the distribution of the PPY on the surface of the material in micro-domains. Thus, the 5% PPY/PDLLA conduits were used in the in vivo experiments.
Impressively, the PPY/PDLLA group had similar results to the autograft group. There was an encouraging level of functional regeneration for a synthetic nerve conduit. After 6 months, there was no statistical difference in the SFI between the PPY/PDLLA group and the autograft group. NCVs were clearly detected in all nerve conduits groups after 3 months, indicating a rapid functional recovery for the injured nerves. Moreover, 6 months after conduit implantation the NCVs were faster than those after 3 months, showing no deterioration of the conducting function of the regenerated nerve tissues. Conduction velocities of healthy nerve can range from 60 to 70 m/s [36], which compare favorably to the PPY/PDLLA and autograft groups. NCVs are dependent on a variety of factors including the diameter of the axons, myelin sheath thickness, and internode length [37], so they provide important insight into the ability of the nerve to conduct an action potential. Generally, NCVs of regenerated nerves are less than those of healthy nerves. To recover the electrophysiological properties of the nerve and achieve NCVs that are within the range of those of healthy nerve is an important step toward achieving functional regeneration. Histological analysis also showed significant similarities between the PPY/PDLLA conduit and the autograft. Taken together, it is clear that the presence of PPY to make the PPY/PDLLA conduits electrically conductive had a significant positive effect on the axon regeneration and myelination compared to those in the PDLLA conduits.
Previous work has illustrated the beneficial effects a conducting polymer with electrical stimulation has on neurite outgrowth [21, 29]. This study translates that effect to an in vivo system to show the feasibility PPY/PDLLA conduits have for nerve tissue regeneration. By achieving similar results to the gold standard autograft, PPY/PDLLA conduits are a prime candidate for a nerve tissue engineering construct. While this is a great achievement, the ultimate goal would be to create a synthetic nerve conduit that outperforms an autologous graft, not just matches it, and that is the next hurdle to overcome.
5. Conclusions
Nerve tissue engineering offers an innovative and promising approach to treating nerve defects. Conductive polymers have great potential as biomaterials to fabricate synthetic nerve conduits. We fabricated PPY/PDLLA composite nerve conduits using emulsion polymerization and dip coating methods. PC12 cells were used to assess the cell compatibility in vitro, and the cells exhibited more and longer neurites than on PDLLA conduits after being stimulated with 100 mV for 2 h. The 5% PPY/PDLLA was used to fabricate nerve conduits to bridge a 10 mm defect in the sciatic nerve of a rat. After 6 months, the rats with the PPY/PDLLA conduits showed functional recovery similar to that of the gold standard autologous nerve graft and significantly better than that of the PDLLA conduits. While achieving recovery on the level of healthy nerve is the ultimate goal of nerve tissue regeneration, creating a graft that provides the same functional recovery level as an autologous graft is exciting because such a conduit eliminates the drawbacks associated with using an autologous graft, including limited donor source, donor site morbidity, multiple surgery sites, and possible size mismatch. In this study, we have demonstrated that the synthetic PPY/PDLLA conduit has great potential for nerve tissue regeneration.
Acknowledgements
The authors wish to thank the Major State Basic Research Program of China (Grant No. 2011CB606205), the International Cooperation Funding of Hubei Province (Grant No. 2012IHA0120), the Fundamental Research Funds for the Central Universities (Grant No. 2012-IV-029), US National Institutes of Health (NIDCR DE015384, DE017689 and DE022327) and DOD (W81XWH-12-2-0008) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schlosshauer B, Dreesmann L, Schaller HE, Sinis N. Synthetic nerve guide implants in humans: a comprehensive survey. Neurosurgery. 2006;59:740–748. doi: 10.1227/01.NEU.0000235197.36789.42. [DOI] [PubMed] [Google Scholar]
- 2.Evans GR. Peripheral nerve injury: a review and approach to tissue engineered constructs. Anat Rec. 2001;263:396–404. doi: 10.1002/ar.1120. [DOI] [PubMed] [Google Scholar]
- 3.Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus. 2004;16:E1. doi: 10.3171/foc.2004.16.5.2. [DOI] [PubMed] [Google Scholar]
- 4.Scherman P, Kanje M, Dahlin LB. Bridging short nerve defects by direct repair under tension, nerve grafts or longitudinal sutures. Restor Neurol Neurosci. 2004;22:65–72. [PubMed] [Google Scholar]
- 5.Zacchigna S, Giacca M. Chapter 20: Gene therapy perspectives for nerve repair. Int Rev Neurobiol. 2009;87:381–392. doi: 10.1016/S0074-7742(09)87020-7. [DOI] [PubMed] [Google Scholar]
- 6.Strasberg SR, Mackinnon SE, Genden EM, Bain JR, Purcell CM, Hunter DA, et al. Long-segment nerve allograft regeneration in the sheep model: experimental study and review of the literature. J Reconstr Microsurg. 1996;12:529–537. doi: 10.1055/s-2007-1006625. [DOI] [PubMed] [Google Scholar]
- 7.Dahlin L, Johansson F, Lindwall C, Kanje M. Chapter 28: Future perspective in peripheral nerve reconstruction. Int Rev Neurobiol. 2009;87:507–530. doi: 10.1016/S0074-7742(09)87028-1. [DOI] [PubMed] [Google Scholar]
- 8.Ignatiadis IA, Yiannakopoulos CK, Barbitsioti AD, Avram AM, Patralexis HG, Tsolakis CK, et al. Diverse types of epineural conduits for bridging short nerve defects. An experimental study in the rabbit. Microsurgery. 2007;27:98–104. doi: 10.1002/micr.20313. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Hu W, Cao Y, Yao J, Wu J, Gu X. Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft. Brain. 2005;128:1897–1910. doi: 10.1093/brain/awh517. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama K, Takakuda K, Koyama Y, Itoh S, Wang W, Mukai T, et al. Enhancement of peripheral nerve regeneration using bioabsorbable polymer tubes packed with fibrin gel. Artif Organs. 2007;31:500–508. doi: 10.1111/j.1525-1594.2007.00418.x. [DOI] [PubMed] [Google Scholar]
- 11.Schnell E, Klinkhammer K, Balzer S, Brook G, Klee D, Dalton P, et al. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials. 2007;28:3012–3025. doi: 10.1016/j.biomaterials.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Neubauer D, Graham JB, Muir D. Nerve grafts with various sensory and motor fiber compositions are equally effective for the repair of a mixed nerve defect. Exp Neurol. 2010;223:203–206. doi: 10.1016/j.expneurol.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Roganovic Z, Ilic S, Savic M. Radial nerve repair using an autologous denatured muscle graft: comparison with outcomes of nerve graft repair. Acta Neurochir (Wien) 2007;149:1033–1038. doi: 10.1007/s00701-007-1269-z. [DOI] [PubMed] [Google Scholar]
- 14.Fansa H, Keilhoff G, Wolf G, Schneider W. Tissue engineering of peripheral nerves: A comparison of venous and acellular muscle grafts with cultured Schwann cells. Plast Reconstr Surg. 2001;107:485–496. doi: 10.1097/00006534-200102000-00026. [DOI] [PubMed] [Google Scholar]
- 15.Merle M, Dellon AL, Campbell JN, Chang PS. Complications from silicon-polymer intubulation of nerves. Microsurgery. 1989;10:130–123. doi: 10.1002/micr.1920100213. [DOI] [PubMed] [Google Scholar]
- 16.Wilson DH, Jagadeesh P. Experimental regeneration in peripheral nerves and the spinal cord in laboratory animals exposed to a pulsed electromagnetic field. Paraplegia. 1976;14:12–20. doi: 10.1038/sc.1976.2. [DOI] [PubMed] [Google Scholar]
- 17.Gurunathan K, Murugan AV, Marimuthu R, Mulik UP, Amalnerkar DP. Electrochemically synthesised conducting polymeric materials for applications towards technology in electronics, optoelectronics and energy storage devices. Mater Chem Phys. 1999;61:173–191. [Google Scholar]
- 18.Jager EW, Smela E, Inganas O. Microfabricating conjugated polymer actuators. Science. 2000;290:1540–1545. doi: 10.1126/science.290.5496.1540. [DOI] [PubMed] [Google Scholar]
- 19.Guimard NK, Gomez N, Schmidt CE. Conducting polymers in biomedical engineering. Prog Polym Sci. 2007;32:876–921. [Google Scholar]
- 20.Ateh DD, Navsaria HA, Vadgama P. Polypyrrole-based conducting polymers and interactions with biological tissues. J R Soc Interface. 2006;3:741–752. doi: 10.1098/rsif.2006.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt CE, Shastri VR, Vacanti JP, Langer R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc Natl Acad Sci U S A. 1997;94:8948–8953. doi: 10.1073/pnas.94.17.8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez N, Schmidt CE. Nerve growth factor-immobilized polypyrrole: bioactive electrically conducting polymer for enhanced neurite extension. J Biomed Mater Res A. 2007;81:135–149. doi: 10.1002/jbm.a.31047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanghvi AB, Miller KP, Belcher AM, Schmidt CE. Biomaterials functionalization using a novel peptide that selectively binds to a conducting polymer. Nat Mater. 2005;4:496–502. doi: 10.1038/nmat1397. [DOI] [PubMed] [Google Scholar]
- 24.Song HK, Toste B, Ahmann K, Hoffman-Kim D, Palmore GT. Micropatterns of positive guidance cues anchored to polypyrrole doped with polyglutamic acid: a new platform for characterizing neurite extension in complex environments. Biomaterials. 2006;27:473–484. doi: 10.1016/j.biomaterials.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 25.Gomez N, Lee JY, Nickels JD, Schmidt CE. Micropatterned Polypyrrole: A Combination of Electrical and Topographical Characteristics for the Stimulation of Cells. Adv Funct Mater. 2007;17:1645–1653. doi: 10.1002/adfm.200600669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li GN, Hoffman-Kim D. Tissue-engineered platforms of axon guidance. Tissue Eng Part B Rev. 2008;14:33–51. doi: 10.1089/teb.2007.0181. [DOI] [PubMed] [Google Scholar]
- 27.Amado S, Simoes MJ, Armada da Silva PA, Luis AL, Shirosaki Y, Lopes MA, et al. Use of hybrid chitosan membranes and N1E-115 cells for promoting nerve regeneration in an axonotmesis rat model. Biomaterials. 2008;29:4409–4419. doi: 10.1016/j.biomaterials.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 28.Tan X, Lu L, Wang Z. An experimental study of repairing facial nerve defect in rabbit with silk fibroin-chitosan conduit. Int J Oral Maxillofac Surg. 2009;38:500. [Google Scholar]
- 29.Lee JY, Bashur CA, Goldstein AS, Schmidt CE. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials. 2009;30:4325–4335. doi: 10.1016/j.biomaterials.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leach JB, Brown XQ, Jacot JG, Dimilla PA, Wong JY. Neurite outgrowth and branching of PC12 cells on very soft substrates sharply decreases below a threshold of substrate rigidity. J Neural Eng. 2007;4:26–34. doi: 10.1088/1741-2560/4/2/003. [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Yan Y, Li S. PDLLA/chondroitin sulfate/chitosan/NGF conduits for peripheral nerve regeneration. Biomaterials. 2011;32:4506–4516. doi: 10.1016/j.biomaterials.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 32.Ozmen S, Ayhan S, Latifoglu O, Siemionow M. Stamp and paper method: a superior technique for the walking track analysis. Plast Reconstr Surg. 2002;109:1760–1761. doi: 10.1097/00006534-200204150-00064. [DOI] [PubMed] [Google Scholar]
- 33.Dijkstra JR, Meek MF, Robinson PH, Gramsbergen A. Methods to evaluate functional nerve recovery in adult rats: walking track analysis, video analysis and the withdrawal reflex. J Neurosci Methods. 2000;96:89–96. doi: 10.1016/s0165-0270(99)00174-0. [DOI] [PubMed] [Google Scholar]
- 34.Hare GM, Evans PJ, Mackinnon SE, Best TJ, Bain JR, Szalai JP, et al. Walking track analysis: a long-term assessment of peripheral nerve recovery. Plast Reconstr Surg. 1992;89:251–258. [PubMed] [Google Scholar]
- 35.Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83:129–138. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- 36.Ao Q, Fung C-K, Tsui AY-P, Cai S, Zuo H-C, Chan Y-S, et al. The regeneration of transected sciatic nerves of adult rats using chitosan nerve conduits seeded with bone marrow stromal cell-derived Schwann cells. Biomaterials. 2011;32:787–796. doi: 10.1016/j.biomaterials.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto K, Ohnishi K, Kiyotani T, Sekine T, Ueda H, Nakamura T, et al. Peripheral nerve regeneration across an 80-mm gap bridged by a polyglycolic acid (PGA)-collagen tube filled with laminin-coated collagen fibers: a histological and electrophysiological evaluation of regenerated nerves. Brain Res. 2000;868:315–328. doi: 10.1016/s0006-8993(00)02207-1. [DOI] [PubMed] [Google Scholar]