Abstract
Background
Cervical cancer is a major public health problem in resource-limited settings, particularly among HIV-infected women. Given the challenges of cytology-based approaches, the efficiency of new screening programs need to be assessed.
Setting
Community and hospital-based clinics in Gaborone, Botswana.
Objective
To determine the feasibility, and efficiency of the “See and Treat” approach using Visual Inspection Acetic Acid (VIA) and Enhanced Digital Imaging (EDI) for cervical cancer prevention in HIV-infected women.
Methods
A two-tier community-based cervical cancer prevention program was implemented. HIV-infected women were screened by nurses at the community using the VIA/EDI approach. Low-grade lesions were treated with cryotherapy on the same visit.
Women with complex lesions were referred to our second tier, specialized clinic for evaluation. Weekly quality control assessments were performed by a specialist in collaboration with the nurses on all pictures taken.
Results
From March 2009 through January 2011, 2,175 patients were screened for cervical cancer at our community-based clinic. 253 (11.6%) were found to have low-grade lesions and received same-day cryotherapy. 1,347 (61.9%) women were considered to have a normal examination and 575 (27.3%) were referred for further evaluation and treatment. Of the 1,347 women initially considered to have normal exams, 267 (19.8%) were recalled based on weekly quality control assessments. 210 (78.6%) of the 267 recalled women and 499 (86.8%) of the 575 referred women were seen at the referral clinic. Of these 709 women, 506 (71.4%) required additional treatment. Overall, 264 CIN stage 2 or 3 were identified and treated, and six micro-invasive cancers identified were referred for further management.
Conclusions
Our “See and Treat” cervical cancer prevention program using the VIA/EDI approach is a feasible, high-output and high-efficiency program, worthy of considering as an additional cervical cancer screening method in Botswana, especially for women with limited access to the current cytology-based screening services.
Keywords: Cervical cancer, HPV, VIA, EDI, Africa, prevention
INTRODUCTION
An estimated 16 million women are infected with HIV worldwide, with the majority living in Sub-Saharan Africa1. Cervical cancer is a preventable disease caused by human papilloma virus (HPV), and is a leading cause of cancer deaths among women in low-income countries1–4. In spite of the large burden of disease, only 20–40% of HIV-infected women are screened for cervical cancer. Although 10–30% are found to have lesions requiring treatment, less than 10% receive appropriate therapy1,2,5–7. The reasons behind the failure to implement effective cervical cancer prevention programs in HIV-infected women are multiple. Prevention of cervical cancer requires widespread screening, accurate diagnosis of precursor lesions, appropriate triaging and therapy1,7. Cervical cytology, the screening test most commonly used in developed countries, requires multiple visits by clients, screening at regular intervals because of low sensitivity, and excellent laboratory infrastructure, including cytology and pathology6,8–11. Cervical cancer prevention programs targeting HIV-infected women face the additional challenge of identifying and treating a disease that appears to have a more rapid progression and higher rates of recurrences than in the general population12–15.
Prevalence rates for HIV in Botswana are currently approximately 28% in women in the 15–49 age group16–19. By extrapolation, a large proportion of Batswana women can be assumed to be at increased risk of pre-cervical cancer lesions and possible invasive cervical cancer, thus making this a significant public health concern in Botswana20,21. Like in many resource-limited settings, medical and technical resources are strained in Botswana, resulting in challenges in cytology-based screening1,7.
Visual inspection with acetic acid (VIA) has recently emerged as an inexpensive, practical alternative to cytology-based screening5,11,22–27. Application of 4 or 5% acetic acid to the cervix results in dysplastic and neoplastic epithelium transiently appearing white, with normal cervical squamous epithelium assuming a pink colour. VIA is more sensitive but less specific than the Pap smear, with estimated sensitivity of 66–99% or 55–90%, and specificity of 64–98% or 65–92%5,6,11,24,25,28. VIA can be performed by midwives, nurses and other health care workers, which decreases barriers regarding staff shortages26,29–32.
Women with abnormal Pap smears or VIA results generally are referred for colposcopy, which includes magnified visual inspection of the cervix after application of acetic acid. Colposcopy has been extremely successful as a diagnostic modality for cervical pre-cancer lesions and provides increased sensitivity compared with VIA5,27. However, use of this technology in developing nations is severely limited by the cost of the colposcope1. To overcome this limitation, lower-cost methods to provide easily interpretable, magnified images of the cervix were developed. One such method is cervicography in which the cervix is inspected through magnified photographic images1,27. Cervicography has been shown in multiple studies to be a reliable method of detecting precancerous cervical lesions25,28,33. Digital cameras have the advantage of easy portability and relative cost-effectiveness compared with colposcopy. These cameras have been shown to produce quality, high resolution images, which may be used to perform magnified examination of the cervix and vagina (enhanced digital imaging, EDI)27,30,31,34.
To take advantage of the potential benefits of VIA/EDI, we implemented a two-tier community-based cervical cancer prevention program for HIV-infected women in Botswana. We describe the program and report the outcomes of the first 23 months (March 2009 through January 2011) of its implementation.
DESIGN AND METHODS
Training
The initial phase of this program required training of staff in VIA/EDI and cryotherapy (“See and Treat”)1. As part of on-going capacity building other health practitioners were also trained. The community-based clinic was located at a primary clinic in Gaborone, the capital city. HIV antiretroviral therapy and minimal access to cytology-based screening was available at this clinic prior to our pilot. All providers were licensed nurses or nurse-midwives who were trained to perform VIA, EDI and cryotherapy. Nurses were chosen as the program’s primary providers because they are more available than physicians in Botswana, and many are familiar with performing pelvic exams and function independently in clinic settings. A three-day didactic training session took place in Zambia in January 2009, where a similar project has been running since 2006. Following the didactic training, nurses spent two weeks in clinics gaining hands-on experience in VIA, EDI, cryotherapy, and indications for referral, under the guidance of the Zambian team. The training continued for six weeks in Botswana under the guidance of our program gynaecologist. At the end of the practicum, each nurse had successfully performed a minimum of 100 VIA examinations, 100 EDI photographs, and 35 cryotherapies.
Patient population
The community clinic was set up within the same facility that has an Adult HIV clinic as part of the National HIV program. The HIV clinic acted as a referral centre for five other local clinics. Both men and women were referred to this clinic once tested positive for HIV. The services provided at this clinic include assessment for initiation of HIV treatment, follow up of HIV infected adults from the catchment area both on treatment, and those not needing treatment yet. Any HIV-infected woman enrolled in this clinic who self-referred or was referred by a health care worker, and chose to have cervical cancer screening at our clinic, was cared for in our program.
Procedures at the community-based clinic
Women presenting to the “See and Treat” clinic were counselled regarding cervical cancer prevention and consented to allow cervical photography, and cryotherapy if needed. During speculum inspection, women were assessed for lesions suspicious of cervical cancer (raised, ulcerative lesions with contact bleeding, bizarre blood vessel patterns); these were referred to the tertiary hospital immediately. Women were also assessed for sexually transmitted infections, and appropriate national treatment guidelines followed. VIA using white household vinegar was immediately followed by EDI with a digital camera, and the results were categorized as positive based on:
Observation of a well-defined, opaque aceto-white area close to the squamous-cervical junction
Observation of dense aceto-whitening of a cervical growth
VIA-negative women were offered reassurance. Cryotherapy at the community-based clinic was only performed on women diagnosed with low-grade lesions meeting all of the following criteria:
An opaque lesion involving less than 3 quadrants of the transformation zone
No extension of the lesion into the endocervical canal or onto the vaginal wall
The entire lesion could be covered by the cryotherapy probe
No clinical evidence of severe lesion or invasive cancer
Cryotherapy was performed using liquid nitrogen and a 15 to 24 mm ectocervical cryoprobe tip (probe size varied depending on lesion size) with a shallow nipple. A single freeze for seven minutes was applied. No local anaesthesia or analgesics were used prior to the procedure. Women with abnormal VIA or EDI that did not meet the criteria for immediate treatment were referred to our colposcopy/loop electrosurgical excision procedure (LEEP) clinic at the Princess Marina Hospital for further evaluation.
Colposcopy and treatment of pre-cancerous lesions
Colposcopy was performed in women with high grade lesions according to VIA/EDI, or those whose lesions were inappropriate for cryotherapy. Unlike in traditional colposcopy examination, punch biopsies were not obtained from abnormal areas on the cervix. The “See and Treat” approach was followed here as well, with lesions either treated with LEEP or cautery.
Women with extensive lesions reaching the vaginal wall were referred to the gynaecology department for cone biopsy or hysterectomy. Excised tissue specimens were processed in the pathology laboratory. Women were given home-care instructions, asked to avoid sexual intercourse for six weeks, and instructed to return to the clinic if they had fever for more than two days, were passing blood clots or had severe lower abdominal pain. Women with suspected invasive cancer were referred to the Gynaecology Department at the Princess Marina Hospital for surgery and/or radiotherapy.
Quality Control and Nursing Education
Weekly, nurses participated in an EDI quality-control meeting that involved reviewing all images of cervigrams from the prior week on a large screen using a projector. Discrepancies between the nurse and the gynaecologist expert consultant were assessed with the final decision based on the opinion of the gynaecologist. Weekly reviews included an analysis of the correlation between the digital image and histology if available, as a means of increasing the understanding of the myriad visual manifestations of cervicitis, squamous metaplasia, nabothian gland cysts, endocervical polyps, glandular hyperplasia, cervical hyperplasia, keratosis, CIN1/HPV, CIN2, CIN3, microinvasive cancer, and invasive cancer.
Data and statistical analysis
Data entry was performed at the treatment site using Access, while data analysis was carried out using STATA 11.0. Comparisons were performed using a Mann-Whitney or Student’s t test according to the distribution of the variables. The sensitivity, specificity, negative and positive predictive values of nurse’s assessments were calculated using the gynaecologist evaluation as the gold standard. In addition, inter- and intra-subject variability was determined by asking the nurses to diagnose a selected set of 100 high-quality pictures from our patients. Pictures showing normal and abnormal cervixes were included in the set and the answers were not provided to the nurses. We used the gynaecologist diagnosis (for normal cervix and low grade lesions requiring cryotherapy but not referral) and pathology (for an abnormal cervix). The test was administered 2 weeks later showing the same set of pictures in a different order. Intra- and inter-subject variability was calculated using the kappa coefficient.
Ethics Statement
This was set up as a public health care program to provide service, with a monitoring and evaluation component. The Health Research and Development Committee of the Botswana Ministry of Health approved the program protocol and data collection for programmatic evaluation, including consent for imaging.
RESULTS
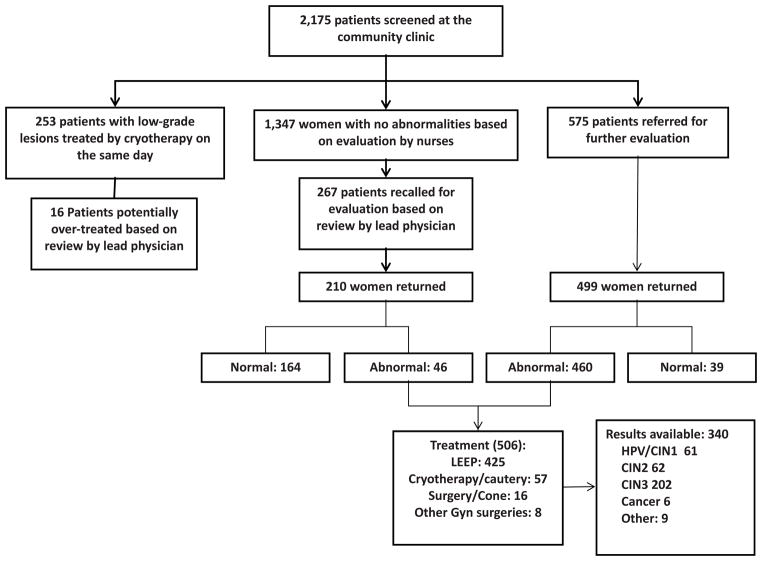
During the first 23 months 2175 HIV-infected women were evaluated (Figure 1). Our population was included predominantly young (mean age 34.9, 95% confidence interval [CI], 34.6 – 35.2) single women from lower socio-economical strata (as indicated by their average monthly household income; Table 1). The average CD4 T cell count was 405 cells/mm2 and the 68.8% of the women were on anti-retroviral therapy (ART).
Figure 1.
Distribution of the number of HIV-infected women screened and treated by a “See-and-Treat” cervical prevention program, Botswana 2007–2011
TABLE 1.
Demographics and Baseline Characteristics of the Participants
| Number/Mean | % or 95%CI | |
|---|---|---|
| Age | 34.9 | 34.6 to 35.2 |
| Education | ||
| No formal education | 278 | 12.8% |
| Primary | 451 | 20.8% |
| Secondary | 1352 | 62.2% |
| Graduate | 94 | 4.3% |
| Monthly income (1 US $ = ~ 6.5 Pula) | ||
| Less than P1000 | 744 | 34.2% |
| Between P1000–2500 | 643 | 29.6% |
| Between P2500–5000 | 556 | 25.6% |
| Between P5000–10,000 | 154 | 7.1% |
| Over P10,000 | 78 | 3.6% |
| Occupation | ||
| Unemployed | 681 | 31.3% |
| Formal sector | 1163 | 53.5% |
| Informal sector | 220 | 10.1% |
| Other | 111 | 5.1% |
| Marital status | ||
| Single | 1,454 | 66.9% |
| Cohabit | 323 | 14.9% |
| Married, husband lives at home | 281 | 12.9% |
| Married, husband does not live at home | 15 | 0.7% |
| Separated/Divorced | 46 | 2.1% |
| Widow | 56 | 2.6% |
| Smoker | ||
| No | 2047 | 94.1% |
| Yes | 128 | 5.9% |
| Age of first sexual encounter | 18.2 | 18.0 to 18.4 |
| Number of lifetime partners | 7.2 | 6.8 to 7.6 |
| Number of partners last month | 0.9 | 0.8 to 1.0 |
| Age of menarche | 15.7 | 15.6 to 15.8 |
| Age of menopause | 40.5 | 37.8 to 43.2 |
| Pap smear in the past | 679 | 31.2% |
| If yes, the patient knows the results | 572 | 84.3% |
| If yes, the PAP smear was normal | 461 | 80.6% |
| Initial CD4 cell count | 308.8 | 298.4 to 319.1 |
| Last CD4 cell count | 405.5 | 374.7 to 395.3 |
| On ARTs | ||
| No | 668 | 30.7% |
| Yes, is currently taking | 1492 | 68.6% |
| Yes, in the past (not now) | 15 | 0.7% |
Two hundred and fifty three of 2,175 women screened (11.6%) were found to have low-grade lesions and received same-day cryotherapy. 1,347 (61.9%women were considered to have a normal examination and 575 (27.3%were referred for further treatment (Figure 1). Of the 1,347 women initially considered to have normal exams, 267 (19.8%were recalled when the gynaecologist was unable to confirm the initial decision of the nurse either because the images were of poor quality (68, 25.6%; Table 2) or because a possible lesion could not be excluded (199, 74.4%; Table 2). Thus, a total of 1333 (1,347 + 253 – 267) (61.3%women received appropriate same-day screening and treatment without the need for recall or referral. 210 (78.6%) of the 267 recalled, and 499 (86.8%of the 575 referred women were seen at the referral clinic (overall follow up 709 of 842, 84.2%). Reasons for recall and referral are shown in Table 2.
TABLE 2.
Indications for Recall and Referral of Patients and Number Showing up for Follow up
| Reason/Indication | Recall*
|
Referral
|
||
|---|---|---|---|---|
| Total | Followed Up | Total | Followed Up | |
| Suspicion for low grade lesion not treated at the community-based clinic | 106 | 90 (84.9%) | NA | — |
| Suspicion for high-grade lesion or icc† | 17 | 17 (100.0%) | 236 | 212 (90.0%) |
| Lesion extending into the os or vaginal walls | 45 | 39 (86.7%) | 137 | 119 (86.9%) |
| Lesion too big for cryotherapy | 24 | 17 (70.8%) | 150 | 133 (88.7%) |
| Digital image unclear | 68 | 45 (66.2%) | NA | – |
| Other | 7 | 2 (28.6%) | 52 | 35 (67.3%) |
| Total | 267 | 210 (78.7%) | 575 | 499 (86.8%) |
Reasons for recall as indicated by the specialist by review of the pictures during the quality assurance meetings.
Includes patients with suspicion for high-grade lesions identified at the weekly quality control sessions that were either treated with cryotherapy or not treated.
NA, not applicable.
Five Hundred and six (71.4% of the 709 women; 210 recall plus 499 referred) required treatment. None of the women recalled were found to have high grade lesions (Tables 2 and 3). Overall, our program identified and treated 264 pre-cancerous lesions (CIN 2 or 3), and identified six micro-invasive cancers which were referred for further management (Tables 2 and 3).
TABLE 3.
Types of Lesions Identified and Treated at the Referral Clinic by Recall and Referral
| Total | Recalls | Referrals | |
|---|---|---|---|
| Pathology not available (260)* | |||
| No treatment | 203 | 164 | 39 |
| Cryotherapy/cautery | 57 | 25 | 32 |
| Pathology available (340)† | |||
| CIN1/HPV-related | 61 | 12 | 49 |
| CIN2 | 62 | 0 | 62 |
| CIN3 | 202 | 0 | 202 |
| Cancer | 6 | 0 | 6 |
| Other | 9 | 9 | 0 |
Pathology was not available because treatment was not required or treatment with cryotherapy or cautery does not involve collection of tissue.
Pathology results of the 340 reports available at the time of analysis (of 425 LEEP procedures).
CIN, cervical intraepithelial neoplasia.
Of the 210 women that were recalled and followed-up by the time of this analysis, 164 required no treatment and 25 required cryotherapy/cautery. Tissue was obtained from 46 other recalled women and results were available on 12 of them, all of whom showed changes compatible with CIN1 lesions. Of the 460 women who were referred and followed-up, 39 required no treatment and 32 underwent cryotherapy/cautery for low-grade lesions. Of the 319 remaining ones who were referred and had pathological results by the time of analysis, 49 had CIN1, 62 had CIN2, 202 had CIN 3 and 6 had micro-invasive cancer. The remaining 9 women had lesions unrelated to HPV infection (Table 3).
We based the calculations of the sensitivity and specificity of the program on the performance of peripheral clinic and its capacity to appropriately identify patients that were normal, patients that could be treated on-site and patients that needed referral. Using this approach, the 267 patients recalled for evaluation were considered to be “undertreated” by the first-tier of the program. Although the majority of those patients were found to have no lesions (164 of the 210 patients who followed up), we believe our approach provides a better understanding of the overall performance of the system. The nurses at the community-based clinic diagnosed 1080 (1347 – 267) women were confirmed to be negative, 237 were declared appropriately diagnosed and treated, 16 were “potentially over-treated (considered “misdiagnosed” for the purpose of this analyses) and 575 were appropriately referred. Thus, the sensitivity and specificity of the nurses’ assessment (and, therefore, of the first-tier of our program) were 75.3% (95% CI, 72.5 – 77.8%) and 98.5% (95% CI, 97.6 – 99.1%) respectively.
The level of agreement between nurses and the gynaecologist in the evaluation of digital pictures (agreement on 83.3% of the observations, kappa= 0.63, 95% CI 0.48 to 0.77, p<0.01). Although there were some differences in the inter-subject agreement between nurses (agreement on 86.5% of the observations, kappa= 0.69, 95% CI, 0.54 – 0.83, p<0.01), the intra-subject agreement was substantially higher (agreement on 91.2% of the observations, kappa= 0.72, 95% CI, 0.64 – 0.80, p<0.001).
DISCUSSION
Our results demonstrate that community-based “See and Treat” programs using VIA/EDI can be valuable additions to screening programs for cervical cancer prevention in HIV-infected women in settings like Botswana. To our knowledge, this is the first community-based VIA/EDI screening program targeting exclusively HIV-infected women. Our approach was efficient and had a high throughput. In addition, visualizing lesions on the digital images by the patients serves as a form of patient education and may lead to improved follow up rates. The digital images also allowed the implementation of quality control measures that further improve safety34. Although a significant percentage of women required a second visit to receive definitive treatment, the large majority of women were able to be screened and treated on a single visit.
Consistent with our findings, digital cameras, with their advantageous portability, ease of use, and relative cost-effectiveness, have been shown to produce quality, high resolution images, which may be used to perform magnified examination of the cervix and vagina. Mobile telemedicine phones with high resolution camera handset technology has the added advantage of not requiring internet connections or electricity, and allows images to be transmitted immediately. This “simultaneous telemedicine” technology could provide the opportunity for evaluation by an expert at a distant location while the patient is being evaluated in clinic34.
Our results are comparable to those reported by similar VIA-based prevention programs from resource-limited settings1,5,29,33. Among the patients screened through our program, only a third of women had been screened for cervical cancer by Pap smear in the past. Of interest, the large majority of them knew their results (85%). However, in spite of abnormalities in approximately 20%, follow up or treatment was never provided, highlighting some of the barriers to care within cytology-based programs. The potential benefit of treating early HPV-lesions in HIV-infected women are important advantages of the VIA/EDI approach. Our program provided successful treatment for 85% of the patients who needed it, a reasonable number of them on the same day. Referral for LEEP required a second visit, but multiple visits were minimized by providing “See and LEEP”. Providing VIA/EDI/Cryotherapy, as well as LEEP at the same facility can eliminate the need for second visit for those patients not legible for cryotherapy.
Concerns have been raised about the safety of the See and Treat approach. In particular, concern that lesions may be missed by nurses performing point-of-care evaluation and treatment has limited the uptake of this approach. In our program, during the quality assurance evaluations performed weekly, only 12% (267/2,175) of the women assessed at the community clinic were recalled for further evaluation by the specialist. The main reason for recall was the poor quality of the picture, or the inability to completely rule out the possibility of a lesion by the gynaecologist based only on the picture. It is highly reassuring that missed lesions were found only in 3.5% (7/210 who showed up) of the women recalled. Importantly, 164 of 210 (78%) patients recalled did not need any treatment and those that did require treatment had either low-grade lesions (12) or other lesions (9) not related to HPV infection.
Although we detected 264 women with CIN 2 or 3, the magnitude of the impact that such programs will have on cervical cancer can only be established through long-term prospective studies. The 264 pre-malignant lesions identified and treated during the 23 months of our program represent nearly 70% of all the premalignant lesions treated in the region over the same period. “See and Treat” may offer a viable alternative to reach women living in distant and/or underserved regions of the country (e.g. rural), while cytology-based screening programs may still play a role in areas where the appropriate infrastructure and human resources are available.
CONCLUSIONS
This program has shown that screening and treating women using a See-and Treat approach of VIA/EDI followed by cryotherapy and referral for LEEP therapy is feasible, effective, and has a significant impact on the identification and treatment of precancerous and micro-invasive cancerous lesions in HIV-infected women in Botswana. In addition, we showed that including telemedicine technology is feasible in these settings and could add to the overall benefit of these programs. In resource-limited settings, “See and Treat” approaches are highly likely to reduce the risks of a common and preventable cancer in women, especially if both cryotherapy and LEEP can be provided at the same facility to truly allow same day treatment for all lesions.
Acknowledgments
Financial Support: This work was supported by the NIH CFAR Grant NIH IP30 AI 45008 and Health Services and Human Research grant 1U2GPS001949 (President’s Emergency Plan for AIDS Relief
We thank the staff of the Botswana-UPenn Partnership Women’s Health clinic for their admirable devotion to the care of the patients enrolled in this study. We thank the women participating in the program for their invaluable contribution.
Footnotes
Financial Disclosures: None
References
- 1.Report of a WHO consultation. World Health Organization; Geneva: 2002. Cervical cancer screening in developing countries. [Google Scholar]
- 2.World Health Organization. Geneva: 2005. [Google Scholar]
- 3.Hawes SE, Critchlow CW, Faye Niang MA, et al. Increased risk of high-grade cervical squamous intraepithelial lesions and invasive cervical cancer among African women with human immunodeficiency virus type 1 and 2 infections. J Infect Dis. 2003 Aug 15;188(4):555–563. doi: 10.1086/376996. [DOI] [PubMed] [Google Scholar]
- 4.Firnhaber C, Evans D, Friedman-Khalili R, et al. Seroprevalence of HPV vaccine types 6, 11, 16 and 18 in HIV-infected women from South Africa, Brazil and Botswana. J Clin Virol. 2011 Nov;52(3):265–268. doi: 10.1016/j.jcv.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Arbyn M, Sankaranarayanan R, Muwonge R, et al. Pooled analysis of the accuracy of five cervical cancer screening tests assessed in eleven studies in Africa and India. Int J Cancer. 2008 Jul 1;123(1):153–160. doi: 10.1002/ijc.23489. [DOI] [PubMed] [Google Scholar]
- 6.Denny L, Quinn M, Sankaranarayanan R. Chapter 8: Screening for cervical cancer in developing countries. Vaccine. 2006 Aug 31;24(Suppl 3):S3/71–77. doi: 10.1016/j.vaccine.2006.05.121. [DOI] [PubMed] [Google Scholar]
- 7.Finn A. HPV prevention: waiting for screening services. Lancet. May 15;375(9727):1694. doi: 10.1016/S0140-6736(10)60738-6. [DOI] [PubMed] [Google Scholar]
- 8.Danso D, Lyons F, Bradbeer C. Cervical screening and management of cervical intraepithelial neoplasia in HIV-positive women. Int J STD AIDS. 2006 Sep;17(9):579–584. doi: 10.1258/095646206778113087. quiz 585–577. [DOI] [PubMed] [Google Scholar]
- 9.Kim JJ, Brisson M, Edmunds WJ, Goldie SJ. Modeling cervical cancer prevention in developed countries. Vaccine. 2008 Aug 19;26( Suppl 10):K76–86. doi: 10.1016/j.vaccine.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leinonen M, Nieminen P, Kotaniemi-Talonen L, et al. Age-specific evaluation of primary human papillomavirus screening vs conventional cytology in a randomized setting. J Natl Cancer Inst. 2009 Dec 2;101(23):1612–1623. doi: 10.1093/jnci/djp367. [DOI] [PubMed] [Google Scholar]
- 11.Sankaranarayanan R, Budukh AM, Rajkumar R. Effective screening programmes for cervical cancer in low- and middle-income developing countries. Bull World Health Organ. 2001;79(10):954–962. [PMC free article] [PubMed] [Google Scholar]
- 12.De Vuyst H, Franceschi S. Human papillomavirus vaccines in HIV-positive men and women. Curr Opin Oncol. 2007 Sep;19(5):470–475. doi: 10.1097/CCO.0b013e3282c8c8fc. [DOI] [PubMed] [Google Scholar]
- 13.De Vuyst H, Lillo F, Broutet N, Smith JS. HIV, human papillomavirus, and cervical neoplasia and cancer in the era of highly active antiretroviral therapy. Eur J Cancer Prev. 2008 Nov;17(6):545–554. doi: 10.1097/CEJ.0b013e3282f75ea1. [DOI] [PubMed] [Google Scholar]
- 14.Strickler HD, Burk RD, Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005 Apr 20;97(8):577–586. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 15.van der Burg SH, Palefsky JM. Human Immunodeficiency Virus and Human Papilloma Virus - why HPV-induced lesions do not spontaneously resolve and why therapeutic vaccination can be successful. J Transl Med. 2009;7:108. doi: 10.1186/1479-5876-7-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Central Statistics Office. Botswana: 2007. [Google Scholar]
- 17.Trends in HIV prevalence and sexual behaviour among young people aged 15–24 years in countries most affected by HIV. Sex Transm Infect. 2010 Dec;86(Suppl 2):ii72–ii83. doi: 10.1136/sti.2010.044933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bussmann H, de la Hoz Gomez F, Roels TH, et al. Prevalence of transmitted HIV drug resistance in Botswana: lessons learned from the HIVDR-Threshold Survey conducted among women presenting for routine antenatal care as part of the 2007 national sentinel survey. AIDS Res Hum Retroviruses. 2011 Apr;27(4):365–372. doi: 10.1089/aid.2009.0299. [DOI] [PubMed] [Google Scholar]
- 19.Riviello ED, Sterling TR, Shepherd B, Fantan T, Makhema J. HIV in the workplace in Botswana: incidence, prevalence, and disease severity. AIDS Res Hum Retroviruses. 2007 Dec;23(12):1453–1460. doi: 10.1089/aid.2007.0132. [DOI] [PubMed] [Google Scholar]
- 20.Macleod IJ, O’Donnell B, Moyo S, et al. Prevalence of human papillomavirus genotypes and associated cervical squamous intraepithelial lesions in HIV-infected women in Botswana. J Med Virol. 2011 Oct;83(10):1689–1695. doi: 10.1002/jmv.22178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramogola-Masire D, McGrath CM, Barnhart KT, Friedman HM, Zetola NM. Subtype Distribution of Human Papillomavirus in HIV-Infected Women With Cervical Intraepithelial Neoplasia Stages 2 and 3 in Botswana. Int J Gynecol Pathol. 2011 Nov;30(6):591–596. doi: 10.1097/PGP.0b013e31821bf2a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sankaranarayanan R, Esmy PO, Rajkumar R, et al. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. Lancet. 2007 Aug 4;370(9585):398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- 23.Sankaranarayanan R, Mathew B, Jacob BJ, et al. Early findings from a community-based, cluster-randomized, controlled oral cancer screening trial in Kerala, India. The Trivandrum Oral Cancer Screening Study Group. Cancer. 2000 Feb 1;88(3):664–673. [PubMed] [Google Scholar]
- 24.Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009 Apr 2;360(14):1385–1394. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 25.Sankaranarayanan R, Shyamalakumary B, Wesley R, Sreedevi Amma N, Parkin DM, Nair MK. Visual inspection with acetic acid in the early detection of cervical cancer and precursors. Int J Cancer. 1999 Jan 5;80(1):161–163. doi: 10.1002/(sici)1097-0215(19990105)80:1<161::aid-ijc28>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Nene BM, Deshpande S, Jayant K, et al. Early detection of cervical cancer by visual inspection: a population-based study in rural India. Int J Cancer. 1996 Dec 11;68(6):770–773. doi: 10.1002/(SICI)1097-0215(19961211)68:6<770::AID-IJC14>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Berkhof J, de Bruijne MC, Zielinski GD, et al. Evaluation of cervical screening strategies with adjunct high-risk human papillomavirus testing for women with borderline or mild dyskaryosis. Int J Cancer. 2006 Apr 1;118(7):1759–1768. doi: 10.1002/ijc.21513. [DOI] [PubMed] [Google Scholar]
- 28.Sankaranarayanan R, Nene BM, Dinshaw K, et al. Early detection of cervical cancer with visual inspection methods: a summary of completed and on-going studies in India. Salud Publica Mex. 2003;45( Suppl 3):S399–407. doi: 10.1590/s0036-36342003000900014. [DOI] [PubMed] [Google Scholar]
- 29.Nene BM, Hiremath PS, Kane S, Fayette JM, Shastri SS, Sankaranarayanan R. Effectiveness, safety, and acceptability of cryotherapy by midwives for cervical intraepithelial neoplasia in Maharashtra, India. Int J Gynaecol Obstet. 2008 Dec;103(3):232–236. doi: 10.1016/j.ijgo.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Gage JC, Rodriguez AC, Schiffman M, et al. An evaluation by midwives and gynecologists of treatability of cervical lesions by cryotherapy among human papillomavirus-positive women. Int J Gynecol Cancer. 2009 May;19(4):728–733. doi: 10.1111/IGC.0b013e3181a48b99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gage JC, Rodriguez AC, Schiffman M, et al. Treatability by cryotherapy in a screen-and-treat strategy. J Low Genit Tract Dis. 2009 Jul;13(3):174–181. doi: 10.1097/LGT.0b013e3181909f30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blumenthal PD, Lauterbach M, Sellors JW, Sankaranarayanan R. Training for cervical cancer prevention programs in low-resource settings: focus on visual inspection with acetic acid and cryotherapy. Int J Gynaecol Obstet. 2005 May;89( Suppl 2):S30–37. doi: 10.1016/j.ijgo.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Cuzick J, Arbyn M, Sankaranarayanan R, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008 Aug 19;26( Suppl 10):K29–41. doi: 10.1016/j.vaccine.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 34.Quinley KE, Gormley RH, Ratcliffe SJ, et al. Use of mobile telemedicine for cervical cancer screening. J Telemed Telecare. 2011;17(4):203–209. doi: 10.1258/jtt.2011.101008. [DOI] [PMC free article] [PubMed] [Google Scholar]