Abstract
The Western dietary pattern of intake common to many Americans is high in fat, refined carbohydrates, sodium, and phosphorus, all of which are associated with processed food consumption and higher risk of life-threatening chronic diseases. In this review, we focus on the available information on current phosphorus intake with this Western dietary pattern, and new knowledge of how the disruption of phosphorus homeostasis can occur when intake of phosphorus far exceeds nutrient needs and calcium intake is limited. Elevation of extracellular phosphorus, even when phosphorus intake is seemingly modest, but excessive relative to need and calcium intake, may disrupt the endocrine regulation of phosphorus balance in healthy individuals, as it is known to do in renal disease. This elevation in serum phosphate, whether episodic or chronically sustained, may trigger the secretion of regulatory hormones, whose actions can damage tissue, leading to the development of cardiovascular disease, renal impairment, and bone loss. Therefore, we assessed the health impact of excess phosphorus intake in the context of specific issues that reflect changes over time in the U.S. food supply and patterns of intake. Important issues include food processing and food preferences, the need to evaluate phosphorus intake in relation to calcium intake and phosphorus bioavailability, the accuracy of various approaches used to assess phosphorus intake, and the difficulties encountered in evaluating the relations of phosphorus intake to chronic disease markers or incident disease.
Introduction
Clinical interest in potential adverse health effects of high-phosphorus intake began >40 y ago, when low bone mass and secondary hyperparathyroidism were observed in animals fed high-phosphorus, low-calcium diets, along with the clinical finding that oral phosphate loading in young adults stimulated parathyroid hormone (PTH )7 secretion (1–3). Then, as now, many believed that the phosphorus content of the American diet was increasing as a result of the growing availability of and preference for processed foods and the widespread use of phosphate additives by food processors (4–6). Recently, this interest has been rekindled, sparked by new knowledge about the tight endocrine regulation that maintains phosphorus balance and newly gained understanding of how this balance can be disrupted by acute or prolonged excessive intakes of phosphorus (7).
Much of our understanding of the risks associated with high-phosphorus intake comes from studying the events associated with the gradual rise in serum phosphate in chronic kidney disease patients, in which serum phosphate concentration has been shown to be significantly associated with cardiovascular disease and increased mortality (8–10). Accumulating evidence from studies in healthy populations suggests that mild elevations of serum phosphate within the normal range are also associated with cardiovascular disease risk (11–14). Although the pathophysiologic factors triggering disordered phosphorus metabolism are unclear, the compensatory hormonal changes that follow dietary phosphorus loading and the tissue damage that is associated with these elevated hormones present a plausible mechanism whereby modest increases in serum phosphate within the normal range may initiate atherosclerosis, left ventricular hypertrophy, and other disease risks. Important regulatory hormones that respond to oral phosphorus loading include fibroblast growth factor-23 (FGF-23) (15), which is secreted by the osteocytes in bone, and PTH, which is secreted by the parathyroid gland (16). Both of these hormones influence the renal synthesis and circulating concentration of the active metabolite of vitamin D, 1,25-dihydroxyvitamin D3 (calcitriol), but in an opposing manner. FGF-23 inhibits synthesis and PTH stimulates 1-α hydroxylation of 25-hydroxyvitamin D (17, 18). Both PTH and FGF-23 have been shown to be associated with left ventricular cardiac hypertrophy and other cardiovascular disease risk factors, independent of serum phosphate concentration or dietary phosphorus intake (19–21). However, evidence that links high-phosphorus intake to elevated serum phosphate concentration in the general population with healthy renal function remains limited (22). In this review, we focus on the importance of this area of research and current limitations in data availability and suggest that data and research need to better assess risks associated with increasing phosphorus exposure.
Phosphorus in the Food Supply and Contributions to Total Phosphorus Intake
The phosphorus intake in the United States, as well as the intake of other essential nutrients, is monitored in the National Health and Nutrition Education Surveys (NHANES), which are nationally representative surveys conducted and analyzed in 2-y waves since 2000. The “usual” intake of phosphorus by gender/age groups was last published for the 2005–2006 wave of NHANES. The estimates of usual phosphorus intake are based on information from 24-h recall data, using a validated method, with second-day recalls from a subset of the surveyed population to assess intra-individual variability (23–25). The usual mean daily intake of phosphorus and the Dietary Reference Intake guidelines [estimated average requirement (EAR), recommended dietary allowance (RDA), and tolerable upper intake levels for age- and gender-specific groups] are shown in Table 1. For all age/gender groups, the mean usual phosphorus intake exceeds the EAR and RDA, with the single exception of growing girls (9–18 y) who are actively accreting bone. Traditionally, the median usual intakes of a nutrient (intake at the 50th percentile) compared with the EAR are used to determine the intake adequacy of the nutrient in a population (23). The EAR represents the mean phosphorus required by each specific age/gender group. For all but growing young men and women, the EAR for phosphorus was lowered from the previous guidelines to the level shown in Table 1 by the Institute of Medicine in 1997 (23).
TABLE 1.
Usual mean daily phosphorus intake and dietary recommended intake for phosphorus by gender and age1
| Dietary recommended intake |
||||
| Age | Usual phosphorus intake | EAR | UL | RDA |
| y | mg/d | mg/d | ||
| Men | ||||
| 1–3 | 1030 ± 26.3 | 380 | 3000 | 460 |
| 4–8 | 1145 ± 27.4 | 405 | 3000 | 500 |
| 9–13 | 1321 ± 35.4 | 1055 | 4000 | 1250 |
| 14–18 | 1681 ± 61.5 | 1055 | 4000 | 1250 |
| 19–30 | 1656 ± 53.4 | 580 | 4000 | 700 |
| 31–50 | 1727 ± 25.0 | 580 | 4000 | 700 |
| 51–70 | 1492 ± 30.0 | 580 | 4000 | 700 |
| ≥71 | 1270 ± 27.6 | 580 | 3000 | 700 |
| Women | ||||
| 1–3 | 1030 ± 26.3 | 380 | 3000 | 460 |
| 4–8 | 1145 ± 27.4 | 405 | 3000 | 500 |
| 9–13 | 1176 ± 57.5 | 1055 | 4000 | 1250 |
| 14–18 | 1067 ± 29.8 | 1055 | 4000 | 1250 |
| 19–30 | 1120 ± 40.8 | 580 | 4000 | 700 |
| 31–50 | 1197 ± 25.0 | 580 | 4000 | 700 |
| 51–70 | 1106 ± 34.0 | 580 | 4000 | 700 |
| ≥71 | 985 ± 28.8 | 580 | 3000 | 700 |
Usual daily phosphorus intake data from What We Eat in America, NHANES 2005–2006 (unpublished data from Alanna Moshfegh, U.S. Department of Agriculture) are expressed as means ± SEs. The dietary recommended intake levels for phosphorus were established by the Institute of Medicine, Food, and Nutrition Board in1997 (23). EAR, estimated average requirements; UL, tolerable upper intake levels.
Twenty-four-hour recall data from the most recently completed NHANES (2009–2010) were used to show the percentage contribution of phosphorus from various food categories (Table 2). Milk and dairy were the greatest contributors, followed by meat and poultry contributions. This may be because the USDA nutrient database values for some foods in these categories, which contain phosphate additives, have been updated recently. However, many of the preferred foods in each of these categories in Table 2 are processed with added phosphorus ingredients to achieve the desired texture, taste, color, or other characteristic.
TABLE 2.
Contribution of food categories to phosphorus intake and examples of phosphorus containing generally recognized as safe ingredients frequently used in processing foods in each category1
| Food Category | % Contribution to Phosphorus Intake | Examples of Phosphorus Ingredients Used in Processing Foods in Each Category2 |
| Milk and dairy | 20.9 | Phosphoric acid, sodium phosphate, calcium phosphate, potassium tripolyphosphate |
| Mixed dishes: grain-based | 10.1 | Modified food starch, sodium acid pyrophosphate, disodium phosphate |
| Breads, rolls, and tortillas | 5.8 | Sodium aluminum phosphate, mono-calcium phosphate, sodium acid pyrophosphate |
| Quick breads, bread products, sweet bakery products | 5.2 | Sodium acid pyrophosphate, sodium aluminum phosphate, mono-calcium phosphate, dicalcium phosphate, calcium acid pyrophosphate |
| Poultry | 5.1 | Sodium tripolyphosphate, sodium tripoly/sodium hexa-meta-phosphate blends, sodium acid pyrophosphate, tetrasodium pyrophosphate |
| Pizza | 4.8 | Disodium phosphate, tricalcium phosphate, tetrasodium pyrophosphate, sodium acid pyrophosphate |
| Vegetables | 4.8 | Mono-calcium phosphate, sodium phosphate, disodium phosphate, sodium acid pyrophosphate, disodium hydrogen pyrophosphate |
| Mixed dishes: meat, poultry, seafood | 4.5 | Sodium tripolyphosphate, sodium acid pyrophosphate, tricalcium phosphate, trisodium phosphate |
| Cured meats and poultry | 4.4 | Sodium tripolyphosphate, tetrasodium pyrophosphate, sodium acid pyrophosphate |
| Meats | 4.2 | Potassium tripolyphosphate, tetrapotassium pyrophosphate, sodium hexa-meta-phosphate |
| Plant-based protein foods | 3.7 | Sodium hexa-meta-phosphate, sodium tripolyphosphate |
| Cereals | 3.2 | Disodium phosphate, tricalcium phosphate, trisodium phosphate |
| Eggs | 2.8 | Sodium hexa-meta-phosphate, tetrasodium pyrophosphate, mono-sodium phosphate |
| Seafood | 2.5 | Sodium acid pyrophosphate, potassium tripolyphosphate, tetrapotassium pyrophosphate, sodium tripolyphosphate |
| All other food categories | 18 | |
| Savory snacks, crackers, snack/meal bars | <2.5 | Calcium phosphate, sodium hexa-meta-phosphate, tricalcium phosphate |
| Other desserts | <2.5 | Calcium phosphate, modified corn starch, disodium phosphate, tetrasodium pyrophosphate |
| Candy (chocolate) | <2.5 | Lecithin |
| Sugar sweetened/diet beverages/alcoholic beverages | <2.5 | Phosphoric acid |
| 100% juice | <2.5 | Calcium phosphate |
| Fruits | <2.5 | Mono-calcium phosphate |
| Soups | <2.5 | Mono-potassium phosphate |
| Cooked grains | <2.5 | Disodium phosphate, tricalcium phosphate |
| Condiments/sauces | <2.5 | Phosphoric acid, disodium phosphate, modified food starch, sodium hexa-mono-phosphate |
| Fats and oils | <2.5 | None found |
Unpublished data source: Alanna Moshfegh, U.S. Department of Agriculture, What We Eat in America, NHANES 2009–2010. The U.S. Food and Drug Administration considers the phosphate-containing ingredients shown in this table to be generally recognized as safe under conditions of their intended use in foods.
All of these phosphorus-containing ingredients were granted generally recognized as safe status between 1975 and 1980. Data source: Ingredients labels on products of processed foods currently in the marketplace.
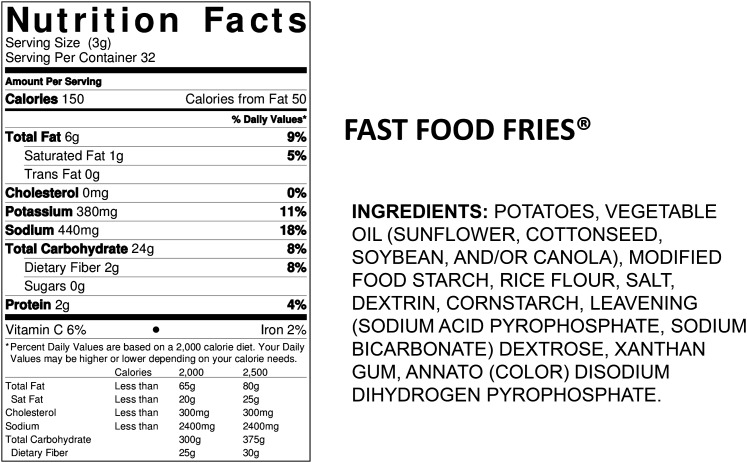
We used the ingredient list found on the food label of products currently in the marketplace to determine examples of phosphate additives commonly used in processed foods found in each category (Table 2). An example of the Nutrition Facts Label and Ingredients List required on all processed foods in the United States is shown in Figure 1. The only minerals required on this label are sodium, calcium, and iron. Although the Food and Drug Administration does not require phosphorus content labeling, phosphorus additives must be listed in the ingredients list. Therefore, consumers may identify which food products contain added phosphorus, but they are not able to quantify how much phosphorus the food is contributing to total daily intake. When manufacturers volunteer to include the phosphorus content of the products on the Nutrition Facts Panel, it is quantified as a percentage of the Daily Value (DV). The percentage of the DV is a dietary guideline developed by the Food and Drug Administration to assist consumers in knowing how much of a particular nutrient they would be consuming in a serving. The DV (also termed the Reference Daily Intake) is 1000 mg for phosphorus. For individuals who need to limit phosphorus intake, this can be a serious source of confusion, because the Nutrition Facts Label guideline is 300 mg higher than the adult RDA and 480 mg greater than the average adult requirement (EAR).
FIGURE 1.
Label information redrawn from the label on fast food fries frozen potatoes. The nutrition facts panel shows no phosphorus content information, which is voluntary for the manufacturer and is not commonly found in the nutrition facts panel of processed foods. The ingredients list is the other mandatory component of the FDA label, and this example shows that the product contains 3 phosphorus-containing additives, although modified food starch is not specified as being one of the possible phosphate-containing modified starch additives, which include acetylated distarch phosphate, hydropropyl distarch phosphate, and monostarch phosphate.
The phosphorus content of the U.S. food supply continues to increase as food manufacturers find new and effective ways to improve taste, speed of preparation, shelf life, or convenience of products through the addition of phosphate ingredients. This growing use of phosphate additives is captured in the nutrient database as foods are reanalyzed for their nutrient composition. An example of such a product can be seen in Figure 1, where the ingredients list shows 3 phosphate additives used to process fast food fries (modified food starch, sodium acid pyrophosphate, and disodium dihydrogen pyrophosphate). The growth in availability and intake of convenience and fast foods is contributing to the changing phosphorus content of the U.S. food supply and to increased intake of phosphorus by those individuals consuming more of these foods, essentially without their knowledge or understanding. For example, few consumers would consider french fries to be a source of phosphorus additives.
Indeed, many other lines of evidence support increased phosphorus content of foods. Several studies comparing estimated dietary phosphorus intake from nutrient databases with direct chemical analyses showed significant underestimation of phosphorus intake, suggesting inaccuracies in the nutrient content databases that serve as the basis for the dietary intake estimates shown in Table 3 (26–29). This underestimation is presumably attributable to the failure to account for the use of phosphorus additives in processing. The likely misclassification of phosphorus intake associated with the observed 25–30% underestimation in existing nutrient databases is likely to obscure associations between dietary phosphorus intake and chronic disease risk.
TABLE 3.
Evidence supporting underestimation of phosphorus intake from food
| Study | Approach and Method of Phosphorus Intake Estimation | Underestimation of Phosphorus Intake |
| Oenning et al., 1988 (26) | Duplicate meals, direct chemical analyses vs. Nutritionist II and III software and hand calculation | 25–30% |
| Sullivan et al., 2007 (27) | Direct chemical analyses vs. ESHA Food Processor SQL version 9.8 software of Midwestern grocery market chicken products (n = 38) | ~34% |
| Mean of 84 mg of underestimated phosphorus/100 g of chicken | ||
| Sherman and Mehta, 2009 (28) | Direct chemical analyses of enhanced meat products vs. natural meat and poultry products expressed as milligrams per gram phosphorus protein ratio | 28% higher phosphorus:protein ratio in enhanced meat/poultry products compared with additive-free meat/poultry |
| Benini et al., 2011 (29) | Direct chemical analyses of 60 foods, 30 containing declared phosphorus additives and 30 similar foods without additives | Additive containing products had nearly 70% higher phosphorus content, contributing an average of >100 mg of phosphorus/100 g of protein |
Despite the evidence of increased phosphorus content of the food supply, and therefore intake, successive waves of the NHANES since 2000 show little change in mean phosphorus intake for men or women, with the exception of the last survey wave conducted in 2009–2010 (Table 4). In that wave, phosphorus intake increased by 90 mg for men and 64 mg for women. Apparently, recent direct chemical analyses of specific products in the USDA Nutrient Content database contributed to this change in the 2009–2010 intake estimates (30). These newly analyzed products included frozen chicken and pizza, for which phosphorus additive use in processing has increased over the past decade, as has consumption of these items (6, 30). This observed increase at the population level for phosphorus intake with changes in the phosphorus content of popular foods underscores the need for reanalyses of additional categories of processed foods.
TABLE 4.
Estimated phosphorus intakes from food1
| NHANES Years | Phosphorus Intake |
| mg/d | |
| Men | |
| 2001–2002 | 1565 ± 24.7 |
| 2003–2004 | 1559 ± 22.4 |
| 2005–2006 | 1600 ± 24.9 |
| 2007–2008 | 1500 ± 26.1 |
| 2009–2010 | 1655 ± 18.7 |
| 10 y change | 90 mg increase |
| Women | |
| 2001–2002 | 1126 ± 18.2 |
| 2003–2004 | 1126 ± 17.0 |
| 2005–2006 | 1148 ± 22.0 |
| 2007–2008 | 1123 ± 22.5 |
| 2009–2010 | 1190 ± 11.5 |
| 10 y change | 64 mg increase |
Values are expressed as the 1-day means ± SEs amount consumed per individual (aged ≥20 y) unless noted otherwise. Unpublished data source: Alanna Moshfegh, U.S. Department of Agriculture, What We Eat in America, NHANES over the past decade, individuals aged ≥20 y (excluding breast-fed children, day-1 dietary weighted, U.S. Department of Agriculture Research Service, Beltsville Human Nutrition Research Center, Food Surveys Research Group; available at: http://www.ars.usda.gov/ba/bhnrc/fsrg).
Other, less considered, sources of dietary phosphorus include dietary supplements. Vitamin and mineral daily supplements include, on average, ~108 mg/d phosphorus. In addition, frequently used over-the-counter and prescription drug products may contribute to phosphorus intakes in currently unknown quantities (31).
Phosphorus Bioavailability from Natural (Organic) and Added (Inorganic) Phosphorus
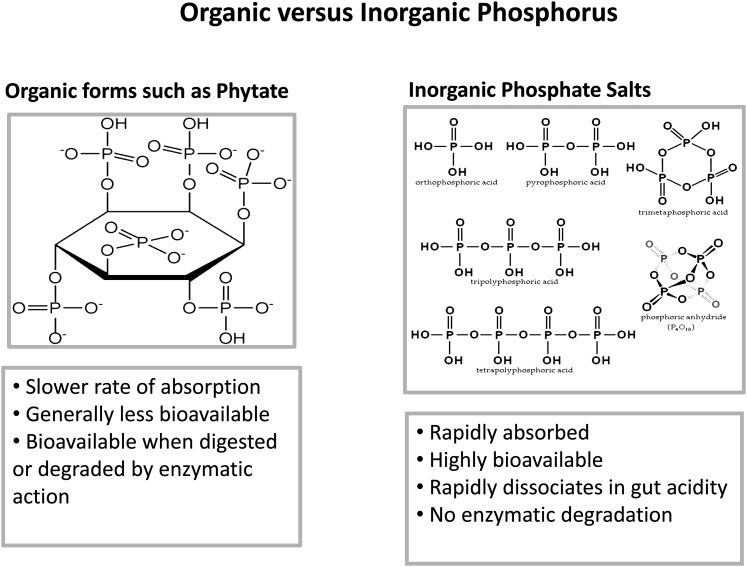
The chemical nature and physiologic characteristics of absorption are also important for understanding how dietary phosphorus may affect hormones that regulate phosphorus and calcium balance. There are basically 2 types of phosphorus in our food supply, natural and added, often referred to as organic and inorganic, and they behave differently in rates and efficiency of absorption (Fig. 2). Natural or organic sources of phosphorus are slowly and less efficiently absorbed, because they are dependent on enzymatic digestion or degradation to release phosphorus from its carbon component. Conversely, inorganic phosphorus added to foods during preparation or processing are primarily inorganic salts requiring no enzymatic digestion, dissociating rapidly in the acid environment of the stomach. Unlike organic sources, inorganic phosphate salts are rapidly and efficiently absorbed—between 80% and 100%. Phosphorus absorption from organic sources ranges from 40% to 60%, with that from animal sources more completely absorbed than that from plants. Phytate phosphorus has the lowest bioavailability in the absence of phytase treatment of whole-grain foods (31).
FIGURE 2.
The figure illustrates the chemical difference between organic phosphorus or the natural phosphorus component of food and inorganic phosphorus, which is primarily added to food as phosphate salts. The figure summarizes the differences in the body’s physiologic handling of these 2 different dietary sources of phosphorus. Ca, calcium; iPTH, intact parathyroid hormone; Pi, inorganic phosphorus.
There is an important knowledge gap in our understanding of the differences in absorption and influence on serum phosphate between these 2 basic sources of dietary phosphorus. This information is fundamental to establishing evidence that may implicate phosphorus intake in the development of cardiovascular and other diseases. We need better understanding of the physiologic response to these different sources of phosphorus. Although we expect differential actions, we know of no long-term studies that have shown different endocrine responses for natural phosphorus relative to enhanced phosphorus foods. However, acute, short-term or cross-sectional studies have been conducted (32–35). In one recent study, which examined physiologic response to meals based on different natural whole foods or a phosphorus supplement, peak serum phosphate concentration, measured over 24 h, was significantly elevated relative to the control in those consuming a whole-grain–based meal (P = 0.006), meat- and cheese-based meals, and an organic phosphate supplement (P = 0.0001). Despite this, serum PTH was only elevated (P = 0.031) with the inorganic phosphate supplement (32). This study demonstrates important differences in the rate of absorption that may trigger spikes in serum phosphate that could affect cellular function or trigger hormone response. Using a randomized crossover design, Moe et al. (34) compared consumption of meat-based and plant-based diets with natural sources of phosphorus that were very similar in total protein and phosphorus content. These investigators found significantly lower serum phosphate and FGF-23 concentrations after 1 wk of consuming the plant-based diet compared with the meat-based diets. The significantly lower phosphorus in 24-h urine samples during the plant-based diet shows the lower rate and efficiency of phosphorus absorption with plant-based dietary patterns.
It is more difficult to measure the effects of food additive phosphate, because these additives are not quantified in the ingredients list of the food label. One recent cross-sectional study in Finland attempted to evaluate the relation between their estimate of food additive phosphate intake and carotid intima-media thickness (33). These investigators reported a significant positive linear trend between estimated food additive phosphorus intake and carotid intima-media thickness, notably among women. More work is needed to determine accurate estimates of the global use of phosphorus additives in food processing, as well as estimates of the cumulative contribution to the total intake of phosphorus from these additives.
Phosphate salts make up the majority of phosphate-containing food additives used in food processing for a host of desirable functions that improve the taste, texture, color, cooking time, and many other properties in processed food (31). The rate and efficiency of absorption of these phosphate salts is dependent on the mineral component to which they are bound, which will affect the rate and efficiency of absorption to varying degrees. One of the most commonly used phosphorus-containing additives, phosphoric acid, is rapidly absorbed and 100% bioavailable. Phosphoric acid in cola soft drinks is consumed by millions worldwide on a daily basis, often without food—which increases the potential for periodic repetitive spikes in serum phosphate. Kristensen et al. (35) demonstrated the potential problems that could arise with consumption of phosphoric acid–containing colas in the absence of an adequate dietary source of calcium. They used a randomized crossover design to examine daily intakes of either 2.5 L of cola or 2.5 L of skimmed milk for 10 d with a 10-d washout between. They reported significant increases in serum phosphate (P < 0.001), PTH (P = 0.046), calcitriol (P < 0.001), and several serum and urine biomarkers of bone turnover (P < 0.001), showing that overconsumption of phosphoric acid–containing cola has the potential to disrupt the endocrine regulation of calcium and phosphorus, which may adversely affect multiple organ systems. Few epidemiologic studies have examined this, but in one, the Framingham Osteoporosis Study, cola intakes of all kinds were shown to be associated with lower bone mineral density in women, with a strong dose–response relation (36).
Unbalanced Phosphorus Intake Relative to Calcium Intake Affects Health
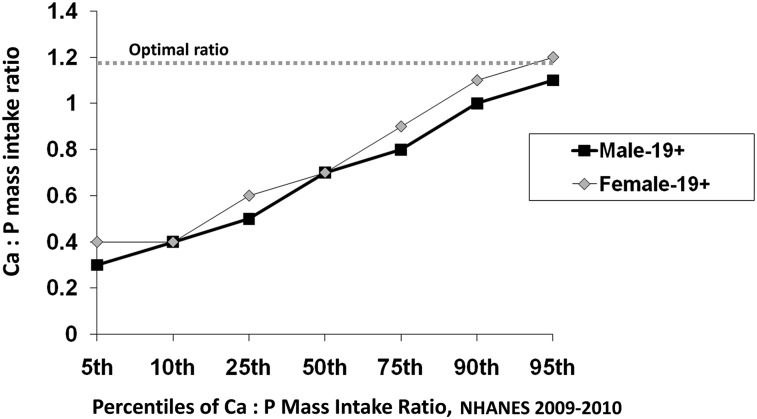
The typical dietary pattern of many Americans is high in phosphorus relative to calcium intake, and it is well established that the physiologic reaction to excess phosphorus intake is significantly influenced by its balance with calcium. Animal studies have shown that high dietary phosphorus relative to calcium can induce secondary hyperparathyroidism, bone resorption, lower peak bone mass, and fragile bones in young and old animals (3, 6). Dietary guidelines recommend relative intakes of these minerals at 1:1 molar intake ratios or 1.5:1 mass intake ratios [calcium-to-phosphorus (Ca:P) ratio, both in milligrams] (6, 23). In reality, the Ca:P ratio in the typical U.S. diet is well below the recommended guidelines. Figure 3 shows the mean mass ratios of Ca:P intake for men and women >19 y of age at selected percentiles of intake estimated in the NHANES 2009–2010. For 25% of the U.S. population, these ratios are <0.6. In animal studies, mass intake ratios ≤0.5 have been shown to be detrimental to bone, even when calcium intake was considered adequate (6).
FIGURE 3.
Daily individual calcium-to-phosphorus (Ca:P) mass intake ratios for men (n = 2880) and women (n = 3038) aged ≥19 y for selected percentiles of intake from day-1 dietary intake estimates of NHANES 2009–2010. Unpublished data were provided by Alanna Moshfegh, U.S. Department of Agriculture, What We Eat in America, NHANES 2009–2010. Ca, calcium; P, phosphorus.
Pettifor et al. (37) noted the importance of balancing intake between these minerals in a chronic feeding study in young vitamin D–replete baboons. Baboons were fed 1 of 3 experimental diets, each with adequate phosphorus, but high, normal, or low calcium levels, containing 1:0.78, 1:2.2, and 1:7.7 Ca:P mass ratios, respectively, and a fourth diet low in both minerals with a mass ratio of 1:2.3. By 16 mo, baboons fed the low-calcium, normal-phosphorus diet (1:7.7) showed histologic evidence of hyperparathyroidism and bone loss, whereas baboons fed the low-calcium, low-phosphorus diet (1:2.3) showed only histologic features of osteomalacia (poorly calcified bone). These findings suggest that the balance in intake between these 2 minerals may have greater influence than the absolute level of phosphorus. This appears to be true for human populations as well, as shown in a cross-sectional study of young Finnish women (38). The calcium intake of the women was greater than the RDA in all but 1 quartile, whereas phosphorus intake was greater than twice the RDA in all quartiles. In the quartile with the lowest intake of calcium, the Ca:P intake ratio was 0.56 and the mean serum PTH concentration was significantly higher compared with the other quartiles, whose mass intake ratios were >0.7. Similar to animal models, high dietary phosphorus–induced, persistently elevated PTH has been shown to adversely affect peak bone mass and bone fragility with aging (6).
Recent clinical evidence from the Gambia stresses the importance of balance in the Ca:P intake ratio in children (39). Braithwaite et al. showed that such an imbalance was present in Gambian children with active rickets who consumed natural food diets containing adequate dietary phosphorus but deficient in calcium (Ca:P molar ratio of 0.26–0.27). These children had higher FGF-23 (65 vs. 54 RU/mL) than did local children without rickets consuming diets with higher molar intake ratios (0.32–0.49, P < 0.0008). In children, the skeletal effects of calcium deficiency may be exacerbated with greater imbalance in calcium and phosphorus intakes, even when phosphorus intake is low or moderate. In the Finnish study (38), adequate intake of calcium did not correct this imbalance when phosphorus intake was in great excess relative to calcium. Both natural and added sources of phosphorus appear to influence the Ca:P intake ratio. If either source of phosphorus is in excess relative to calcium, disordered homeostasis through an elevation in PTH and/or FGF-23 can occur. These data suggest that the Ca:P intake ratio should be factored into analyses of the effect of high-phosphorus intake on chronic disease development.
Masking of Diet-Related Changes in Serum Phosphorus, PTH, and FGF-23 by Inherent Circadian Rhythms and Usual Timing of Blood Sampling
Large cross-sectional population studies have shown higher serum PTH with habitual high-phosphorus intake (40), but this relation has not always been evident in cross-sectional population studies (22). Interestingly, fasting blood samples in the Gambian children’s study showed no significant differences between children with active rickets and normal local community children, for serum phosphate, ionized calcium, 25-hydroxyvitamin D, calcitriol, FGF-23, total alkaline phosphatase, or PTH, despite differences in phosphorus and calcium intake (39).
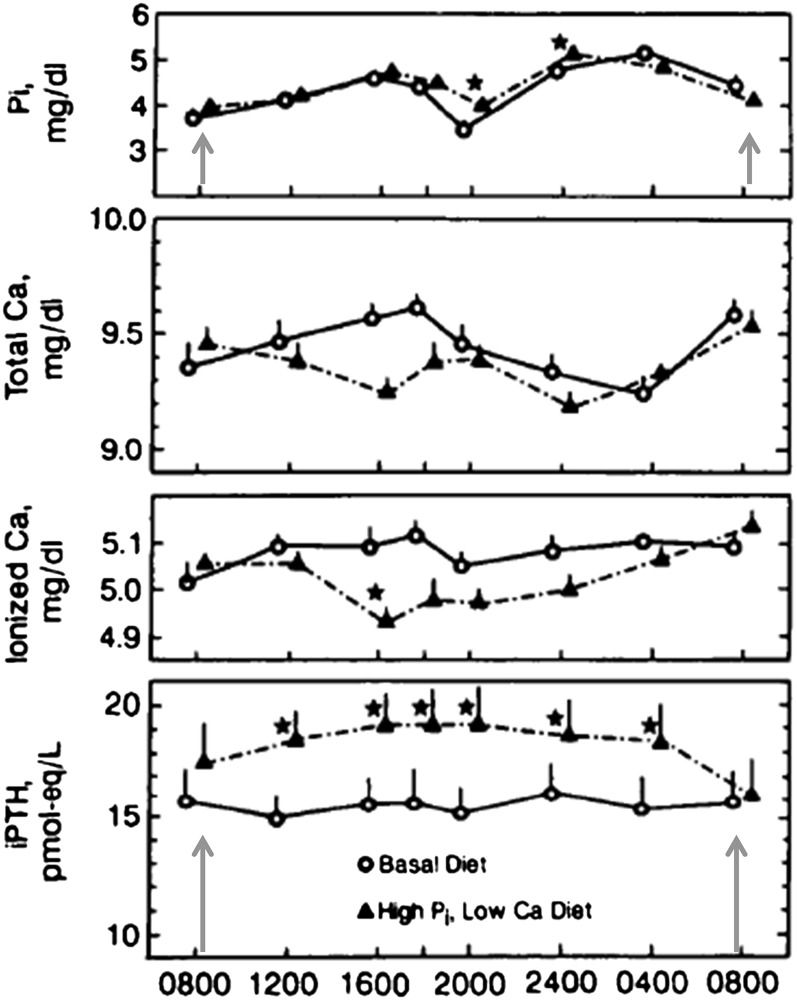
This raises another issue as to why it is often difficult to make the association between phosphorus intake, serum phosphate, and the dysregulation of phosphorus balance associated with cardiovascular, bone, and kidney diseases. A similar failure to observe changes in fasting serum phosphate and PTH was observed in a crossover diet study in young women fed an experimental diet with a Ca:P mass intake ratio of 0.33, achieved with grocery-purchased foods high in phosphorus additives compared with a control diet with a mass intake ratio of 0.98, containing very few market-purchased foods with phosphate additives (16). When both of these groups of investigators (16, 39, 41) used circadian designs taking multiple blood samples over a 24 h period, significant differences in serum phosphate, PTH, and FGF-23 were associated with higher phosphorus intake in samples taken later in the day. Serum PTH follows an inherent circadian variation that closely parallels serum phosphate (42, 43). Figure 4 illustrates how both serum phosphate and PTH fluctuate with an inherent biphasic pattern, peaking in the afternoon and late evening but readjusting to the morning fasting concentration of the previous day (indicated by the arrows). This phenomenon of readjusting to the morning fasting concentration of the previous day has been shown for serum phosphate (16, 42–44), PTH (16, 43), and, although less convincingly, FGF-23 (41, 45). The need for multiple serum sampling to monitor dietary phosphorus effects on calcium and phosphorus balance was first suggested by Smith and Nordin in 1964 (42), when they were unable to demonstrate a change in serum calcium or phosphate concentrations in response to phosphorus intake using a single daily blood sampling. Response to phosphate loading was only demonstrated when they drew 4 daily blood samples.
FIGURE 4.
Hormone and mineral response of young women to diets high in phosphorus additives from market-purchased food compared with control diets made from similar market-purchased foods containing little to no phosphorus additives. Each participant served as their own control in an 8-wk crossover study design. Participants consumed a control diet containing ~900 mg/d phosphorus (calcium-to-phosphorus ratio of 0.9) for the first 4 wk, followed by consumption of a high-phosphorus additive or test diet containing ~1700 mg/d (calcium-to-phosphorus ratio of 0.3) for the remaining 4 wk. Individual lines indicate 24-h hormonal response to each diet. After 4 wk of consuming the high-phosphorus foods, serum phosphate was significantly different from the control dietary period only in the evening samples, whereas serum PTH remained elevated throughout the day after the morning fast. The arrows indicate realignment of both PTH and serum phosphate concentrations to the morning fasting levels despite differences in dietary phosphorus. To determine the effects of dietary intake on serum phosphate concentration, multiple blood draws are needed in the study design. Adapted from reference 16 with permission. iPTH, intact parathyroid hormone; Pi, inorganic phosphorus.
The time of blood sampling appears to be critical in determining the effect of phosphate intake on serum phosphate, PTH, and FGF-23. Sampling during usual morning hours when study participants are in a fasted state, as is commonly done, will impair the ability to determine associations between dietary intake, phosphaturic hormones, and cardiovascular, renal, or skeletal disease biomarkers (16, 34, 41–44).
Data Needs and Study Designs for Accurate Estimates of Total Phosphorus Intake and Physiologic Effect
Nutrient databases currently provide phosphorus intake as total phosphorus measured from foods. As noted above, many of the foods in the database were measured in the past and do not include all of the phosphorus ingredients currently used or new product formulations, meaning that these intake measures underestimate actual phosphorus exposure. Continued updating of the national nutrient database is needed to optimize these measures. However, error will be difficult to eliminate entirely, because the phosphorus content of specific foods may vary by their preparation and packaging methods. For example, most dietary assessments will not ask the respondent to identify whether or not the roast turkey or chicken they consumed was fresh or packaged with basting liquid. Because the latter is a major source of phosphorus, this will be a source of error for individuals. Similar sources of misclassification will exist for a wide variety of prepared products, which may or may not contain added phosphorus. Attention to these variations is needed to better capture the effects of the processed food supply, but this will be challenging because many individuals will not be aware of the type of processing for some items consumed, particularly those consumed outside the home.
Even with optimal assessment of total phosphorus intake, the use of total phosphorus as a variable may be misleading. As discussed above, we know that absorption and metabolism differ for phosphorus embedded in the food matrix with other nutrients from that of added phosphorus compounds. More research is needed to better quantify these differences and the potential differential effects they may have on health outcomes. One place to begin is with our knowledge of differences in absorption. Because added phosphorus is almost fully absorbed, although estimates suggest that only ~40–60% of food-bound phosphorus is absorbed, it is important to be able to classify intakes by these 2 categories at a minimum. Similar calculations have been made available recently for vitamin B12 and folic acid, both of which are also much better absorbed as supplements or stable compounds added to fortified foods. The USDA nutrient data group now provides variables for added B12 and folic acid and, in the case of the latter, a new variable that incorporates what we know about folate bioavailability to estimate total folate equivalents (46). Another example is heme and non-heme iron, with additional algorithms for effects on absorption from co-consumed foods, including less absorption in the presence of phytates and polyphenolic compounds, coffee, tea, soy products, eggs, and calcium and enhanced absorption with vitamin C, meat, poultry, fish and seafood, and alcohol (47).
Because of the risk of hyperphosphatemia, most research in this area has been conducted in relation to kidney disease, in which patients are instructed to avoid foods high in phosphorus and are prescribed phosphorus binders (31). Still, it is only recently that much consideration has been given to the bioavailability of phosphorus (31, 34, 48). Because both phosphorus intake and kidney disease are increasing in the U.S. diet and around the world, it is likely that consideration of these complex balances will become more important for the general population (49).
More research is needed to quantify absorption of differing phosphorus compounds and to identify and quantify effects of co-consumed foods and nutrients, such as calcium, on phosphorus bioavailability. The evidence of periodicity in relation to long-term effects must be considered, because co-timing of exposure may be more important than the overall Ca:P ratio. Inclusion of supplements and phosphorus in frequently used over-the-counter medications, such as antacids and laxatives, must also be considered in estimates of phosphorus exposure.
Conclusions
The evidence of increasing phosphorus intake is clear, with more compounds being added to the food supply and more foods consumed as processed or pre-prepared, and the risk of exceeding the current upper intake level is feasible for large segments of the population. Beyond that, there is accumulating evidence that both the high intakes and the poor balance of intake with other nutrients may place individuals at risk of kidney disease, bone loss, cardiovascular disease, and other chronic health conditions. However, evidence linking these in the general population remains weak at this time, likely attributable to poor assessment of true phosphorus exposure and bioavailability.
Based on evidence of differences in bioavailability of differing forms of phosphorus compounds and effects of co-nutrient modification, there is a need for the development of algorithms for use in modeling the phosphorus impact in human health. A first step is to continue to improve nutrient database estimates of phosphorus in processed foods and to capture these more effectively in dietary assessment. A second step is to create a new variable in the database that identifies added phosphorus separately from natural food phosphorus. With this information, consideration of bioavailability of plant vs. animal sources, co-consumed foods and Ca:P ratios at meals, and other acquired information on phosphorus absorption and bioavailability can be used to better estimate meaningful exposure. Improved data and research designs will allow researchers to identify potential risks on chronic disease outcomes so that health professionals can better advise patients on healthy diet and health maintenance.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: Ca:P, calcium-to-phosphorus intake ratio; DV, daily value; calcitriol, 1,25-dihydroxyvitamin D3; EAR, estimated average requirement; FGF-23, fibroblast growth factor-23; NHANES, National Health and Nutrition Education Surveys; PTH, parathyroid hormone; RDA, recommended dietary allowance.
Literature Cited
- 1.Laflamme GH, Jowsey J. Bone and soft tissue changes with oral phosphate supplements. J Clin Invest. 1972;51:2834–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldsmith RS, Jowsey J, Dube WJ, Riggs BL, Arnaud CD, Kelly PJ. Effects of phosphorus supplementation on serum parathyroid hormone and bone morphology in osteoporosis. J Clin Endocrinol Metab. 1976;43:523–32 [DOI] [PubMed] [Google Scholar]
- 3.Calvo MS. The effects of high phosphorus intake on calcium homeostasis. Adv Nutr Res. 1994;9:183–207 [DOI] [PubMed] [Google Scholar]
- 4.Bell RR, Draper HH, Tzeng DYM, Shin HK, Schmidt GR. Physiological responses of human adults to foods containing phosphate additives. J Nutr. 1977;107:42–50 [DOI] [PubMed] [Google Scholar]
- 5.Greger J, Krystofiak M. Phosphorus intake of Americans. Food Technol. 1982;36:78–84 [Google Scholar]
- 6.Calvo MS, Park YK. Changing phosphorus content of the US diet: potential for adverse effects on bone. J Nutr. 1996;126(suppl):1168S–80S [DOI] [PubMed] [Google Scholar]
- 7.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118:3820–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–17 [DOI] [PubMed] [Google Scholar]
- 9.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger S, Young B, Sherrad DJ, Andress DJ. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520–8 [DOI] [PubMed] [Google Scholar]
- 10.Slinin Y, Foley RN, Collins AJ. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: the USRDS waves 1,3, and 4 study. J Am Soc Nephrol. 2005;16:1788–93 [DOI] [PubMed] [Google Scholar]
- 11.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol. 2009;20:397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhingra R, Sullivan LM, Fox CS, Wang TG, D’Agostino RB, Sr, Gaziamo JM, Vasan RS. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–85 [DOI] [PubMed] [Google Scholar]
- 13.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relations between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–33 [DOI] [PubMed] [Google Scholar]
- 14.Larsson TE, Olauson H, Hagström E, Ingelsson E, Arnlö J, Lind L, Sundström J. Conjoint effects of serum calcium and phosphate on risk of total, cardiovascular, and noncardiovascular mortality in the community. Arterioscler Thromb Vasc Biol. 2010;30:333–9 [DOI] [PubMed] [Google Scholar]
- 15.Ito N, Fukumoto S, Takeuchi Y, Takeda S, Suzuki H, Yamashita T, Tujita T. Effects of acute changes of serum phosphate on fibroblast growth factor (FGF)23 levels in humans. J Bone Miner Metab. 2007;25:419–22 [DOI] [PubMed] [Google Scholar]
- 16.Calvo MS, Kumar R, Heath H. Persistently elevated parathyroid hormone secretion and action in young women after four weeks of ingesting high phosphorus, low calcium diets. J Clin Endocrinol Metab. 1990;70:1334–40 [DOI] [PubMed] [Google Scholar]
- 17.Silverberg SJ, Shane E, de la Cruz L, Segre GV, Clemens TL, Bilezikan JP. Abnormalities in parathyroid hormone secretion and 1,25dihydroxyvitamin D formation in women with osteoporosis. N Engl J Med. 1989;320:277–81 [DOI] [PubMed] [Google Scholar]
- 18.Quarles LD. Role of FGF23 in vitamin D and phosphate metabolism: implications in chronic kidney disease. Exp Cell Res. 2012;318:1040–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Ballegooijen AJ, Reinders I, Visser M, Brouwer IA. Parathyroid hormone and cardiovascular disease events: a systematic review and meta-analyses of prospective studies. Am Heart J. 2013;165:655–64 [DOI] [PubMed] [Google Scholar]
- 20.Buizert PJ, van Schoor NM, Simsek S, Lips P, Heijboer AC, Deeg J, Eekhoft EM. PTH: a new target in arteriosclerosis? J Clin Endocrinol Metab. 2013;98:E1583-90. [DOI] [PubMed] [Google Scholar]
- 21.Yamaoto KT, Robinson-Cohen C, de Oliveira MC, Kostina A, Nettleton JA, Ix JH, Ha N, Eng J, Lima JAC, Siscovick DS, Weiss NS, Kestenbaum B. Dietary phosphorus is associated with greater left ventricular mass. Kidney Int. 2013; 83:707–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calvo MS, Uribarri J. Public health impact of dietary phosphorus excess on bone and cardiovascular health in the general population. Am J Clin Nutr. 2013;98:6–15 [DOI] [PubMed] [Google Scholar]
- 23.Institute of Medicine Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D and fluoride. Washington, DC: National Academies Press; 1997 [PubMed] [Google Scholar]
- 24.Moshfegh A, Goldman J, Ahuja JK, Rhodes D, La Comb R. What we eat in America. NHANES 2005–2006. In: Usual nutrient intakes from food and water compared to 1997 Dietary Reference Intake for vitamin D, calcium, phosphorus and magnesium. Washington, DC: USDA/Agricultural Research Service; 2009 [Google Scholar]
- 25.Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuezynski KJ, Ingwersen LA, et al. The US Department of Agriculture automated multiple-pass method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88:324–32 [DOI] [PubMed] [Google Scholar]
- 26.Oenning LL, Vogel J, Calvo MS. Accuracy of methods estimating calcium and phosphorus intake in daily diets. J Am Diet Assoc. 1988;88:1076–80 [PubMed] [Google Scholar]
- 27.Sullivan CM, Leon JB, Sehgal AR. Phosphorus-containing food additives and the accuracy of nutrient databases: implications for renal patients. J Ren Nutr. 2007;17:350–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherman RA, Mehta O. Dietary phosphorus in dialysis patients: potential impact of processed meat, poultry, and fish products as protein sources. Am J Kidney Dis. 2009;54:18–23 [DOI] [PubMed] [Google Scholar]
- 29. Benini O, D’Alessandro C, Gianfaldoni D, Cupisti A. Extra-phosphate load from food additives in commonly eaten foods: a real insidious danger from renal patients. J Ren Nutr. 2011;21:303–8. [DOI] [PubMed]
- 30.Ahja JKA, Montville JB, Omolewa-Tomobi G, Heendeniya KY, Martin CL, Steinfeldt LC, Anand J, Adler ME, La Comb RB, Moshfegh AJ. USDA Food and Nutrition Database for Dietary Studies, 5.0. Beltsville, MD: US Department of Agriculture Research Service, Food Surveys Research Group; 2012 [Google Scholar]
- 31.Calvo MS, Uribarri J. Contributions to total phosphorus intake: all sources considered. Semin Dial. 2013;26:54–61 [DOI] [PubMed] [Google Scholar]
- 32.Karp HJ, Vaihia KP, Kärkkäinen MUM, Niemisto MJ, Lamberg-Allardt CJE. Acute effects of different phosphorus sources on calcium and bone metabolism in young women: a whole-foods approach. Calcif Tissue Int. 2007;80:251–8 [DOI] [PubMed] [Google Scholar]
- 33.Itkonen S, Karp HJ, Kemi V, Kokkonen E, Saarnio EM, Pekkinen MH, Kärkkäinen MUM, Laitinen EK, Turanlahti M, Lamberg-Allardt CJE. Associations among total and food additive phosphorus intake and carotid intima-media thickness—a cross-sectional study in a middle-aged population in Southern Finland. Nutr J. 2013;12:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moe SM, Zidesharai MP, Chanmbers MA. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:257–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kristensen M, Jensen M, Kudsk J, Henriksen M, Molgaard C. Short-term effects on bone turnover of replacing milk with colas beverages: a 10-day interventional study in young men. Osteoporos Int. 2005;16:1803–8 [DOI] [PubMed] [Google Scholar]
- 36.Tucker KL, Morita K, Qiao N, Hannan MT, Cupples LA, Kiel DP. Colas, but not other carbonated beverages, are associated with lowbone mineral density in older women: the Framingham Osteoporosis Study. Am J Clin Nutr. 2006;84:936–42 [DOI] [PubMed] [Google Scholar]
- 37.Pettifor JM, Marie PJ, Sly MR, du Bruyn DB, Ross F, Isdale JM, Dekerk W, Van der Walt WH. The effects of differing dietary calcium and phosphorus contents on mineral metabolism and bone histomorphometry in young vitamin D-replete baboons. Calcif Tissue Int. 1984;36:668–76 [DOI] [PubMed] [Google Scholar]
- 38.Kemi VE, Kärkkäinen MUA, Rita HJ, Laaksonen MM, Outila TA, Lamberg-Allardt CJ. Low calcium:phosphorus ratio in habitual diets affects serum parathyroid hormone concentration and calcium metabolism in healthy women with adequate calcium intake. Br J Nutr. 2010;103:561–8 [DOI] [PubMed] [Google Scholar]
- 39.Braithwaite V, Jarjou LMA, Goldberg GR, Jones H, Pettifor JM, Prentice A. A follow-up study of Gambian children with rickets-like bone deformities and elevated plasma FGF-23: possible aetiological factors. Bone. 2012;50:218–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kemi VE, Rita HJ, Kärkkäinen MUM, Viljakainen HT, Laaksonen MM, Outila TA, Lamberg-Allardt CJ. Habitual high phosphorus intakes and foods with phosphate additives negatively affect serum parathyroid hormone concentration: cross-sectional study of healthy pre-menopausal women. Public Health Nutr. 2009;12:1885–92 [DOI] [PubMed] [Google Scholar]
- 41.Redmond J, Prentice A, Schoenmakers I. The circadian rhythm of 1alpha, 25(OH)2D in Caucasian and black Gambiam subjects. Abstracts, 15th Vitamin D Workshop. 2012;98.
- 42.Smith DA, Nordin BEC. The effect of high phosphorus intake on total and ultra-filtrable plasma calcium and phosphate clearance. Clin Sci. 1964;26:479–86 [PubMed] [Google Scholar]
- 43.Calvo MS, Eastell R, Offord KP, Berkstrahl EJ, Burritt MF. Circadian variation in ionized calcium and intact parathyroid hormone: evidence for sex differences in calcium homeostasis. J Clin Endocrinol Metab. 1991;72:69–76 [DOI] [PubMed] [Google Scholar]
- 44.Portale AA, Halloran BP, Morris RC., Jr Dietary intake of phosphorus modulates the circadian rhythm in serum concentration of phosphorus. Implications for renal productions for the renal production of 1,25-dihydroxyvitamin D. J Clin Invest. 1987;80:1147–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vervloet MG, van Ittersum FJ, Buttler RM, Heijboer AC, Blankenstein MA, ter Woe PM. Effects of dietary phosphate and calcium intake on fibroblast growth factor-23. Clin J Am Soc Nephrol. 2011;6:383–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gebhardt SE, Holden JM. Consequences of changes in the Dietary Reference Intakes for nutrient databases. J Food Compost Anal. 2006;19:S91–5 [Google Scholar]
- 47.Hallberg L, Hulthén L. Prediction of dietary iron absorption: an algorithm for calculating absorption and bioavailability of dietary iron. Am J Clin Nutr. 2000;71:1147–60 [DOI] [PubMed] [Google Scholar]
- 48.Fukagawa M, Komaba H, Miyamoto K. Source matters: from phosphorus load to bioavailability. Clin J Am Soc Nephrol. 2011;6:239–40 [DOI] [PubMed] [Google Scholar]
- 49.Stevens LA, Viswanathan G, Weiner DE. Chronic kidney disease and end-stage renal disease in the elderly population: current prevalence, future projections, and clinical significance. Adv Chronic Kidney Dis. 2010;17:293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]