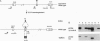Abstract
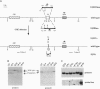
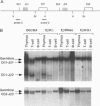
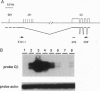
To assess the role of transcriptional enhancers in regulating accessibility of the T-cell receptor beta-chain (TCRbeta) locus, we generated embryonic stem cell lines in which a single allelic copy of the endogenous TCRbeta enhancer (Ebeta) was either deleted or replaced with the immunoglobulin heavy-chain intronic enhancer. We assayed the effects of these mutations on activation of the TCRbeta locus in normal T- and B-lineage cells by RAG-2 (recombination-activating gene 2)-deficient blastocyst complementation. We found that Ebeta is required for rearrangement and germ-line transcription of the TCRbeta locus in T-lineage cells. In the absence of Ebeta, the heavy-chain intronic enhancer partially supported joining region beta-chain rearrangement in T- but not in B-lineage cells. However, ability of the heavy-chain intronic enhancer to induce rearrangements was blocked by linkage to an expressed neomycin-resistance gene (neo(r)). These results demonstrate a critical role for Ebeta in promoting accessibility of the TCRbeta locus and suggest that additional negative elements may cooperate to further modulate this process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Oltz E. M., Young F., Gorman J., Taccioli G., Chen J. VDJ recombination. Immunol Today. 1992 Aug;13(8):306–314. doi: 10.1016/0167-5699(92)90043-7. [DOI] [PubMed] [Google Scholar]
- Anderson S. J., Abraham K. M., Nakayama T., Singer A., Perlmutter R. M. Inhibition of T-cell receptor beta-chain gene rearrangement by overexpression of the non-receptor protein tyrosine kinase p56lck. EMBO J. 1992 Dec;11(13):4877–4886. doi: 10.1002/j.1460-2075.1992.tb05594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Capone M., Watrin F., Fernex C., Horvat B., Krippl B., Wu L., Scollay R., Ferrier P. TCR beta and TCR alpha gene enhancers confer tissue- and stage-specificity on V(D)J recombination events. EMBO J. 1993 Nov;12(11):4335–4346. doi: 10.1002/j.1460-2075.1993.tb06118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Lansford R., Stewart V., Young F., Alt F. W. RAG-2-deficient blastocyst complementation: an assay of gene function in lymphocyte development. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4528–4532. doi: 10.1073/pnas.90.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Young F., Bottaro A., Stewart V., Smith R. K., Alt F. W. Mutations of the intronic IgH enhancer and its flanking sequences differentially affect accessibility of the JH locus. EMBO J. 1993 Dec;12(12):4635–4645. doi: 10.1002/j.1460-2075.1993.tb06152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cogné M., Lansford R., Bottaro A., Zhang J., Gorman J., Young F., Cheng H. L., Alt F. W. A class switch control region at the 3' end of the immunoglobulin heavy chain locus. Cell. 1994 Jun 3;77(5):737–747. doi: 10.1016/0092-8674(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Demengeot J., Oltz E. M., Alt F. W. Promotion of V(D)J recombinational accessibility by the intronic E kappa element: role of the kappa B motif. Int Immunol. 1995 Dec;7(12):1995–2003. doi: 10.1093/intimm/7.12.1995. [DOI] [PubMed] [Google Scholar]
- Ferrier P., Krippl B., Blackwell T. K., Furley A. J., Suh H., Winoto A., Cook W. D., Hood L., Costantini F., Alt F. W. Separate elements control DJ and VDJ rearrangement in a transgenic recombination substrate. EMBO J. 1990 Jan;9(1):117–125. doi: 10.1002/j.1460-2075.1990.tb08087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Godfrey D. I., Kennedy J., Mombaerts P., Tonegawa S., Zlotnik A. Onset of TCR-beta gene rearrangement and role of TCR-beta expression during CD3-CD4-CD8- thymocyte differentiation. J Immunol. 1994 May 15;152(10):4783–4792. [PubMed] [Google Scholar]
- Gu H., Zou Y. R., Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993 Jun 18;73(6):1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- Krimpenfort P., de Jong R., Uematsu Y., Dembic Z., Ryser S., von Boehmer H., Steinmetz M., Berns A. Transcription of T cell receptor beta-chain genes is controlled by a downstream regulatory element. EMBO J. 1988 Mar;7(3):745–750. doi: 10.1002/j.1460-2075.1988.tb02871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauzurica P., Krangel M. S. Enhancer-dependent and -independent steps in the rearrangement of a human T cell receptor delta transgene. J Exp Med. 1994 Jan 1;179(1):43–55. doi: 10.1084/jem.179.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L., Groudine M. Identification of a locus control region in the immunoglobulin heavy-chain locus that deregulates c-myc expression in plasmacytoma and Burkitt's lymphoma cells. Genes Dev. 1994 Sep 15;8(18):2212–2226. doi: 10.1101/gad.8.18.2212. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- McDougall S., Peterson C. L., Calame K. A transcriptional enhancer 3' of C beta 2 in the T cell receptor beta locus. Science. 1988 Jul 8;241(4862):205–208. doi: 10.1126/science.2968651. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Clarke A. R., Rudnicki M. A., Iacomini J., Itohara S., Lafaille J. J., Wang L., Ichikawa Y., Jaenisch R., Hooper M. L. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992 Nov 19;360(6401):225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- Oettinger M. A., Schatz D. G., Gorka C., Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990 Jun 22;248(4962):1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Okada A., Alt F. W. Mechanisms that control antigen receptor variable region gene assembly. Semin Immunol. 1994 Jun;6(3):185–196. doi: 10.1006/smim.1994.1024. [DOI] [PubMed] [Google Scholar]
- Okada A., Mendelsohn M., Alt F. Differential activation of transcription versus recombination of transgenic T cell receptor beta variable region gene segments in B and T lineage cells. J Exp Med. 1994 Jul 1;180(1):261–272. doi: 10.1084/jem.180.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltz E. M., Alt F. W., Lin W. C., Chen J., Taccioli G., Desiderio S., Rathbun G. A V(D)J recombinase-inducible B-cell line: role of transcriptional enhancer elements in directing V(D)J recombination. Mol Cell Biol. 1993 Oct;13(10):6223–6230. doi: 10.1128/mcb.13.10.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B., Henderson N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5166–5170. doi: 10.1073/pnas.85.14.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwe M., Sablitzky F. V(D)J recombination in B cells is impaired but not blocked by targeted deletion of the immunoglobulin heavy chain intron enhancer. EMBO J. 1993 Jun;12(6):2321–2327. doi: 10.1002/j.1460-2075.1993.tb05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y., Koyasu S., Nakayama K., Murphy K. M., Loh D. Y., Reinherz E. L., Alt F. W. Restoration of T cell development in RAG-2-deficient mice by functional TCR transgenes. Science. 1993 Feb 5;259(5096):822–825. doi: 10.1126/science.8430336. [DOI] [PubMed] [Google Scholar]
- Takeda S., Zou Y. R., Bluethmann H., Kitamura D., Muller U., Rajewsky K. Deletion of the immunoglobulin kappa chain intron enhancer abolishes kappa chain gene rearrangement in cis but not lambda chain gene rearrangement in trans. EMBO J. 1993 Jun;12(6):2329–2336. doi: 10.1002/j.1460-2075.1993.tb05887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Tybulewicz V. L., Crawford C. E., Jackson P. K., Bronson R. T., Mulligan R. C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991 Jun 28;65(7):1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Blackwell T. K., Suh H., Hood L., Alt F. W. Introduced T cell receptor variable region gene segments recombine in pre-B cells: evidence that B and T cells use a common recombinase. Cell. 1986 Jan 31;44(2):251–259. doi: 10.1016/0092-8674(86)90759-2. [DOI] [PubMed] [Google Scholar]