Abstract
Several taxa of animals fast completely from food and water during energy-intensive periods such as lactation, breeding, and development. In elephant seals, these behaviors are sustained by high adiposity, high rates of fat mobilization, and reduced oxidation of carbohydrates and proteins. Adiposity and the regulation of lipolysis directly affect lactation energetics, milk composition, and mating success. Long-term fasting induces changes in regulation of lipolysis and lipid metabolism that influence fatty acid (FA) availability and the onset of insulin resistance. Hypoinsulinemia and elevated circulating FAs are also associated with several unique features of carbohydrate metabolism, including elevated plasma glucose, gluconeogenesis, and Cori cycle activity as well as high rates of pyruvate and tricarboxylic acid cycling. Glucose-lactate pools and triacylglycerol-FA cycles may be linked via glyceroneogenesis and this may be an important pathway influencing both fat and carbohydrate metabolism. Together, these features allow a sustained, high intensity, fat-based metabolism without substantial accumulation of ketoacids.
Introduction
Nearly all wild animals experience periods of fasting, usually due to seasonal variation in food availability or because of life-history patterns that limit access to food during critical periods of reproduction or migration. Many species mitigate the loss of body reserves while fasting by decreasing metabolic rate through torpor or hibernation. The most extreme cases of natural fasting are found in species that rely on stored body reserves during periods of high nutrient demands such as mating, lactation, or development. One taxon that routinely undergoes natural energy-intensive fasts is the pinnipeds, seals, and sea lions. The temporal separation of marine feeding and terrestrial reproduction has resulted in natural fasts of variable durations that are concurrent with high rates of energy demand in adults and during critical periods of development in juveniles. Among the pinnipeds, the metabolic adaptations that allow extended high-energy fasts have been studied most extensively in the northern elephant seal (NES)6, Mirounga angustirostris. Coastal access to this species, as well as a unique robustness to research handling, have facilitated application of a diverse set of research tools to understanding the features that allow routine annual loss of up to 40% of body mass with little impact on survival or health. When chemically sedated using dissociative drugs and valium, elephant seals exhibit little stress response or metabolic alteration in response to research handling (1). This has allowed the use and interpretation of experimental approaches and metabolic tracer technologies not typically used in field studies on large animals. In this review, we summarize 2 decades of research on fasting metabolism in elephant seals with an emphasis on features associated with lipid mobilization and use.
Northern Elephant Seals
Northern elephant seals (NESs) spend most of the year foraging widely throughout the northeast Pacific Ocean (2). NES haul out on land twice a year at colonies in California and Mexico, once for breeding and once to undergo a catastrophic molt of their entire pelage (3). During these periods on shore, NESs fast completely from food and water, meeting nutrient demands using stored body reserves and water needs from metabolic water production (4). Both sexes of adults and juveniles spend ~1 mo on shore molting, shedding, and replacing their entire pelage and epidermis (5). NESs return to sea to forage after the molt, accruing body reserves for breeding and gestating a pup for females. Adult males return prior to the pregnant females and compete to establish dominance hierarchies through combat. Male breeding fasts are associated with high rates of energy expenditure; males expend energy at a rate of 195 ± 49 MJ·d−1, which is equivalent to 3.1 times the standard metabolic rate predicted by Kleiber’s equation (6). Increased rates of energy expenditure are associated with high mating success and effective males maintain high metabolic rates for fasting durations as long as 4 mo (6). Adult females spend ~1 mo on shore, giving birth to a single pup and producing >100 L of one of the most nutrient-dense milks found in nature (7). By the end of lactation, NES milk is 50% lipid by mass. Females give an average of ~2200 MJ of milk energy to their pup, tripling its mass during several weeks (7). When maternal metabolism and milk energy are combined, females expend 177 ± 31 MJ·d−1 or 5.7 times the standard metabolic rate predicted by Kleiber’s equation (7). Pups are abruptly weaned and subsequently undergo their own 2- to 2.5-mo fast, during which time they undergo development of respiratory pigments, cardiovascular control, and antioxidant defenses prior to their first foraging migration (8–10).
Current Status of Knowledge
The primary feature that facilitates extended high-energy fasting in NESs is the ability to generate the bulk of ATP through mobilization and oxidation of lipids. Changes in adipose tissue proportions across fasts are shown for selected groups in Table 1. Estimates of lipid oxidation based on either changes in body lipid content across the fasts or respiratory gas measurements are >90% of energy expenditure across all age classes and sexes (6, 7, 11, 12). Protein sparing is efficient, with protein oxidation providing a maximum of 2–8% of energy expenditure for the various study groups as measured by urea turnover or urine collection (11–15). The importance of lipid reserves to elephant seals is evident in strong effects of adiposity on reproductive variables, including reproductive effort, mating success, parental investment, and milk composition (7, 16). Direct impacts of adiposity on the ability to spare protein while fasting have been demonstrated in most study groups (4, 6, 15, 16).
TABLE 1.
Changes in adipose tissue proportions and circulating TG, NEFAs, and NEFA:glycerol ratios early and late in the fast in NESs1
| Breeding females (n = 72) |
Breeding males (n = 62) |
Developing pups (n = 68) |
||||
| Early | Late | Early | Late | Early | Late | |
| Adiposity, % fat | 36.3 ± 1.3 | 23.4 ± 0.9* | 32.5 ± 2.1 | 20.5 ± 2.6* | 46.0 ± 0.5 | 42.1 ± 0.4 |
| TG, mmol/L | 0.66 ± 0.06 | 0.92 ± 0.10* | 0.76 ± 0.13 | 0.60 ± 0.15 | 0.69 ± 0.04 | 0.97 ± 0.05* |
| NEFA, mmol/L | 1.28 ± 0.42 | 3.11 ± 0.99* | 0.78 ± 0.11 | 0.97 ± 0.63 | 0.85 ± 0.10 | 1.95 ± 0.15* |
| NEFA:glycerol ratio | 4.4 ± 0.2 | 6.2 ± 0.4* | 4.3 ± 0.4 | 5.7 ± 0.5* | 4.3 ± 0.4 | 6.4 ± 0.4* |
| Lipolysis, μmol/(kg · min) | 24.1 ± 9.22 | 22.3 ± 17.92 | 10.9 ± 3.63 | |||
Values are means ± SEMs. *Changes across the fast, P < 0.05. Data were compiled from references 25, 26, 27, 32, 36 and D. Crocker, unpublished data. Lipolysis data are from reference 25. NEFA, nonesterified fatty acid; NES, northern elephant seal.
n = 10.
n = 5.
Blubber Composition and Mobilization
In addition to its role in providing fuel substrates during fasting, a blubber layer must be maintained for use in thermoregulation in cold water (17). Phocid seals predominantly store their fat in a subcutaneous blubber layer, with minimal amounts of visceral adipose tissue. It has been reported in many different species of marine mammals that blubber layers are stratified from inner to outer layers (18–20). External layers have a higher proportion of medium-chain (≤18 C) MUFAs, possibly as a homeoviscous adaptation for the purpose of maintaining membrane fluidity at the low temperatures encountered at depth. Interior layers in phocid blubber are more highly enriched in SFAs and long-chain (≥20 C) MUFAs. This inner layer is more heavily metabolized during fasting (19).
Prioritization of lipid oxidation is facilitated by high rates of lipolysis and elevated circulating concentrations of TG and nonesterified fatty acids (NEFAs) (Table 1). Plasma NEFA concentrations are usually >0.7 mmol/L and increase dramatically across the fasts in lactating females and weaned pups and can reach values in excess of 3.2 mmol/L. Serum lipid profiles of fasting adult male elephant seals revealed high concentrations of total cholesterol and LDL cholesterol (LDL-C; 3930 and 1610 mg·L−1, respectively) that significantly decreased during fasting in parallel with adipose tissue reserves. LDL-C concentrations as low as 43 mg·L−1 were measured late in the breeding fast. HDL cholesterol remained consistently elevated (1750 mg·L−1), suggesting that elephant seals defend HDL cholesterol concentrations despite significant depletion of total cholesterol and LDL-C with fasting. This selective depletion of serum LDL-C during fasting suggests the metabolic adaptation to fasting may include alterations in cholesterol metabolism.
The pattern of mobilization of specific fatty acids (FAs) from blubber during fasting in adult females conforms to biochemical predictions based on chain length and saturation (21). Long-chain (>20 C) MUFAs were the least mobilized and PUFAs and SFAs were more highly mobilized from the blubber (21). The mammary gland is capable of synthesizing FAs from glucose or ketones and elephant seals exhibit high plasma glucose concentrations and modest increases in ketone concentrations during fasting. Glucose and ketones, therefore, are available for FA synthesis by the mammary gland. In the mammary gland, FA synthesis from glucose and ketones results in short- and medium-chain FAs containing <12 carbons (22). However, these short- and medium-length FAs have not been detected in elephant seal milk, suggesting that there is little de novo lipid synthesis in the mammary gland (23), i.e., plasma NEFA delivery and uptake by the mammary gland is responsible for milk fat content. In lactating females, the majority of long-chain MUFA mobilized is directed to milk synthesis and the mother may preferentially use PUFA and SFA for her own metabolism (21). Plasma acylcarnitine profiling was used as an index of tissue acyl-CoA status in weaned pups. SFA- and MUFA-carnitines make up 51% and 41%, respectively, of the plasma acylcarnitines in fasting weaned pups, suggesting that the SFA and MUFA mobilized are being directed toward mitochondrial oxidation (24). Acetylcarnitine concentrations, long-chain acylcarnitine concentrations, and indices of incomplete NEFA β-oxidation (C6–C14 carnitines) increased significantly in late-fasted seals (24). Together, these differences suggest alterations in FA oxidation with life history stage and nutrient demand.
Regulation of Lipolysis
As expected, whole-body rates of lipolysis are high in elephant seals but appear to be uncoupled from plasma NEFA concentrations. In lactating females, plasma NEFA concentrations double during the fast despite stable rates of whole-body lipolysis (25). NEFA:glycerol ratios consistently increase across the fasts in all groups, suggesting changes in rates of reesterification with fasting duration that may play a role in regulating NEFA availability.
Circulating concentrations of hormones that potentially influence lipolysis are shown in Table 2. The primary hormonal regulator of lipolysis in elephant seals appears to be maintenance of low insulin concentrations during fasting at all life history stages. In general, there is a decreased reliance on the counter-regulatory dynamics of insulin-glucagon concurrent with reduced oxidation of glucose; although strong relations between insulin concentrations and glucose flux have been observed in pups fasting for 7 wk, the relation does not extend to rates of oxidation (12). Elephant seals are glucose intolerant and exhibit reduced insulin sensitivity in adipose tissue during the fast but maintain limited insulin sensitivity in skeletal muscle (26–29). Insulin values decline during the fast in most study groups and insulin:glucagon molar ratios decline with fasting in all published studies. Correlative analysis in adult females suggests that insulin is the primary hormonal influence of lipolysis, being strongly associated with both circulating NEFA and milk fat content (30).
TABLE 2.
Changes in potential hormone regulators of lipolysis in fasting NESs1
| Breeding females (n = 72) |
Breeding males (n = 62) |
Developing pups (n = 68) |
||||
| Early | Late | Early | Late | Early | Late | |
| Insulin, pmol/L | 14.1 ± 2.9 | 7.8 ± 2.0* | 12.7 ± 2.1 | 11.8 ± 2.6 | 13.4 ± 3.7 | 9.2 ± 3.4* |
| I:G | 1.52 ± 0.51* | 0.96 ± 0.43* | 1.27 ± 0.10* | 0.84 ± 0.06* | 0.83 ± 0.22* | 0.49 ± 0.16* |
| Cortisol, nmol/L | 146.2 ± 54.1 | 285.6 ± 82.5* | 229.0 ± 19.3 | 212.3 ± 24.9 | 177.5 ± 22.7 | 250.7 ± 33.2* |
| Growth hormone, μg/L | 2.5 ± 1.0 | 3.3 ± 1.7* | 2.3 ± 0.3 | 1.3 ± 0.1* | 1.5 ± 0.2 | 1.8 ± 0.3 |
| Epinephrine, nmol/L | 120 ± 11 | 108 ± 8 | 116 ± 10 | 105 ± 10 | 54 ± 7 | 47 ± 13 |
Values are means ± SEMs. *Changes across the fast, P < 0.05. Data are compiled from references 24–27, 32–34, 36, 37, 41, 50 and D. Crocker, unpublished data. I:G, insulin:glucagon molar ratio.
Experimental administration of glucagon has strong positive lipolytic and ketogenic impacts in some study groups but also promotes protein catabolism (31). It is likely for this reason that glucagon is maintained at low concentrations during the fast in most studies (12, 31–33). Cortisol concentrations increase dramatically during the fast in adult females, developing pups, and molting animals (34, 35) and exhibit a significant association with plasma NEFA (30). Experimental elevation of cortisol using adrenocorticotropic hormone results in elevation of plasma NEFA but is also associated with increased protein catabolism (D. Crocker, unpublished data), which is consistent with the acute effects of cortisol on glucose production. Growth hormone increases across the fast in lactating females (36) but is stable or declines during the fast in other groups. Growth hormone concentrations are not associated with NEFA concentrations in any group and experimental administration failed to elevate NEFA (30). Cathecholamines play an important role in circulatory adjustments in elephant seals, which undergo extended terrestrial apneas while fasting and are therefore difficult to meaningfully measure in a field context. However, existing data suggest that baseline catecholamines are stable across the fasts and are not associated with plasma variation in NEFA in any study group (37; D. Crocker, unpublished data).
Essentially all of the FA in circulation during fasting results from TG hydrolysis, as hepatic FA synthesis is greatly reduced (38). Three principle intracellular lipases are responsible for breaking down stored TG molecules to release glycerol and 3 FA molecules. Adipose TG lipase (ATGL), hormone sensitive lipase (HSL), and monoglyceride lipase are the primary lipases and each removes a single FA in a stepwise fashion. TG is converted to diacyglycerol (DAG) by ATGL, followed by conversion to monoacylglycerol by HSL and lastly, conversion to free glycerol by monoglyceride lipase. ATGL is the rate-limiting enzyme in TG hydrolysis and exhibits increased expression during food deprivation in humans (39). Studies on adipocyte lipases in adult females and weaned pups in elephant seals suggest a decreased expression of HSL and an increased expression of ATGL that increases with time fasting (24, 30, 40). AMP-activated protein kinase (AMPK) expression increases in adipocytes during the fast in elephant seals (27, 40, 41). AMPK expression suppresses HSL synthesis and increases ATGL synthesis in vitro (42) and thus may be responsible for the increased importance of ATGL in fasting seals. Inhibition of HSL activity during fasting may help reduce the lipolytic effects of catecholamines, which vary widely during terrestrial apneas in seals. The partial hydrolysis of TG by ATGL is reflected in the consistent increases in plasma NEFA:glycerol ratios in all study groups (Table 1) and in increased intra-adipose DAG:TG ratios (28).
In weaned pups, fasting was associated with a 50% decrease in adipocyte lipase activity and plasma lipase [e.g., lipoprotein lipase (LPL), hepatic lipase, pancreatic lipase] activity increased 2-fold. LPL is the primary enzyme involved in directing TGs mobilized from tissue stores to tissues for utilization. LPL is a membrane-bound enzyme found in the capillary lumen in many tissues that hydrolyzes TGs circulating in the blood, releasing FAs that can be taken in by cells. LPL concentrations decrease in mammary tissue during lactation in elephant seals despite increases in milk lipid content (36). LPL concentrations in adipose tissue are low and decline with fasting in seals (28, 36). Plasma apelin, an inhibitor of cellular lipolysis, also declined during the fast in weaned pups (24).
High rates of TG and FA recycling are typical in fasting mammals with as much as 60% of FA released from lipolysis being reesterified in fasting humans (43). NESs appear to fit this general fasting pattern of excess lipolysis followed by re-esterification. Glycerol and FAs from lipolysis are taken up from circulation by the liver and FAs not directed to β-oxidation for use in the tricarboxylic acid (TCA) cycle or ketone production are re-esterified. Glycerol kinase, expressed in hepatocytes but not in adipose tissue, converts glycerol to glycerol-3-phosphate (G3P) for re-esterification. Both fasting and high circulating NEFAs activate PPARα, an important regulator of hepatic metabolism, which can be chronically activated in obesity (44). PPARα stimulates hepatic uptake of glycerol released from lipolysis by increasing expression of aquaglycoporins (45). A portion of G3P derived from glycerol may be used to re-esterify FAs instead of contributing to hepatic gluconeogenesis. Glyceroneogenesis, the production of G3P from precursors other than glycerol or glucose, may also play an important role in re-esterification and may be active in liver and adipose (46). A 2-cell model for glyceroneogenesis in adipose has been proposed where one glycolytic cell produces lactate while another mature adipocyte converts lactate to G3P for TAG synthesis (47).
Re-esterification of circulating NEFA that is mediated by adipocytes is dependent on FA uptake by those cells. FA transporters are the primary regulators of long-chain FA uptake into cells and potentially have strong impacts on the availability and use of circulating NEFA. Measurements in adipose tissue in elephant seals suggest potentially important changes with fasting that may contribute to regulating circulating NEFA. FA translocase and FA transport protein 1 (FATP1) are 2 of the principal transporters regulating FA uptake by cells. FA translocase and FATP1 expression in adipocytes decreased significantly with fasting in weaned pups (24, 28). Additionally, phosphoenol-pyruvate carboxy kinase protein (PEPCKC), which promotes FA reesterification, declined with fasting in this group (24, 28). FA reesterification is principally mediated by adipocytes, which internalize NEFA, as well as the FA released from VLDL-TG by LPL, which declines with fasting in seals. Finally, accumulation of DAG and monoacylglycerol in adipocytes due to partial hydrolysis may allow reduced reliance on glyceroneogenesis as TG is synthesized using acyltransferases (40). Together, these changes suggest that alterations in re-esterification by adipocytes may help regulate plasma NEFA availability and explain the decoupling of NEFA from rates of whole-body lipolysis. These changes may promote NEFA availability while reducing futile cycling of FAs, preserving lipid stores late in the fast and reducing the commitment of glucose or amino acid carbon to re-esterification.
Adipocytokines
Some experimental studies in NES have shown alterations in metabolic responses that vary directly with adiposity, suggesting an important role for adipose-derived chemical messengers in regulating metabolism. Insulin response to an IV glucose load or glucagon challenge varied directly with adiposity in lactating and molting adult females, being essentially absent when adipose tissue proportions were low at the end of fasts (26, 31). The glucogenic and ureagenic response to glucagon challenge also varied directly with adiposity (31) in adult females. Leptin concentrations in elephant seal weanlings do not change with time fasting or vary with changes in adiposity (27). In adult males, there is a small decrease in leptin with time fasting but no relation to fat mass (32). In pups, systemic activation of the renin-angiotensin system with fasting is associated with decreases in adipose adiponectin (Acrp30) mRNA and protein (29). Insulin sensitivity declines in parallel with adipose PPARλ expression and plasma Acrp30 concentrations (27). Future research on elephant seals will examine the potential roles of adipose nuclear receptors in adipocytokines in altering pancreatic sensitivity and tissue metabolism.
Sustained Fat-Based Metabolism
High concentrations of NEFA availability facilitate sustained use of β-oxidation for energy metabolism in NES. However, an examination of elephant seal blood chemistries revealed several features that suggest important differences from the traditional phase II fasting model. Changes in plasma markers for fuel use compiled from several studies are shown in Table 3. As expected, blood urea nitrogen concentrations are low and either stable or declining during the fast, consistent with low rates of protein catabolism. More surprisingly, despite extended high-energy fasting that relies predominantly on β-oxidation, ketoacid concentrations are consistently very low. β-Hydroxybutyrate concentrations do significantly increase during the fast in most study groups, but these changes are very small compared with other species exposed to moderate or even short fasting durations. Second, despite effective protein sparing, glucose concentrations are high and show no suppression during months of fasting. The metabolic adaptations for extending lipid-based metabolism in elephant seals are associated with several unique features of carbohydrate metabolism (4).
TABLE 3.
Plasma metabolites in fasting elephant seals1
| Breeding females (n = 72) |
Breeding males (n = 62) |
Developing pups (n = 68) |
||||
| Early | Late | Early | Late | Early | Late | |
| Glucose, mmol/L | 7.94 ± 0.11 | 7.72 ± 0.86 | 7.73 ± 0.41 | 7.92 ± 0.52 | 8.61 ± 0.42 | 8.37 ± 0.20 |
| βHB, μmol/L | 264 ± 24* | 345 ± 40* | 234 ± 11* | 365 ± 12* | 267 ± 57* | 598 ± 113* |
| BUN, mmol/L | 8.7 ± 0.9 | 8.0 ± 0.7 | 10.2 ± 0.3 | 10.5 ± 0.3 | 4.9 ± 0.2 | 2.8 ± 0.1* |
Data were compiled from references 12, 24–27, 32–34, 36, 37, 41, 50 and D. Crocker, unpublished data. BUN, blood urea nitrogen; βHB, β-hydroxybutyrate.
Carbohydrate Metabolism
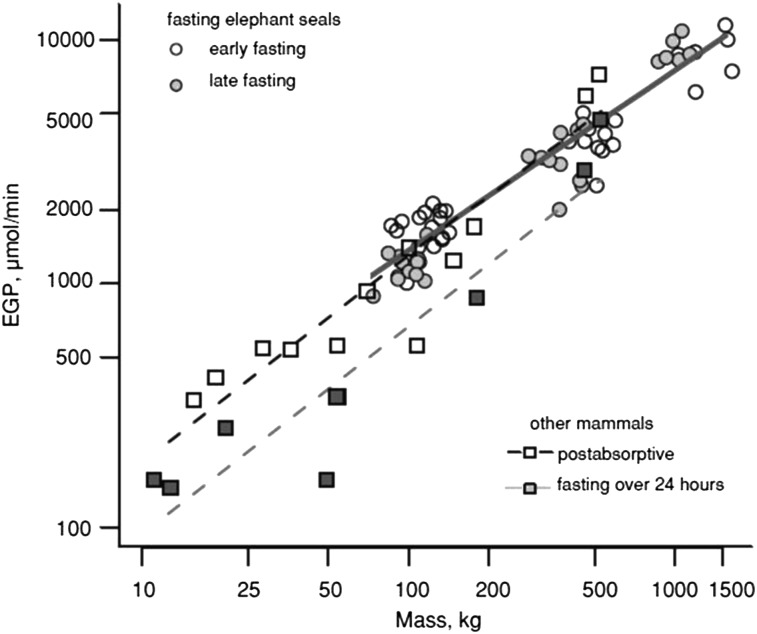
Some tissues, including the central nervous system, erythrocytes, and renal medulla, cannot catabolize FAs and are dependent on carbohydrates or ketone bodies as a fuel source. Erythrocyte mass is unusually high in elephant seals, representing >10% of body mass (48). Carbohydrate stores, primarily in the form of glycogen, are rapidly depleted at the onset of fasting, requiring gluconeogenesis to support glucose-dependent tissues. Studies in nonfasting-adapted species have suggested that suppression of endogenous glucose production (EGP) to reduce gluconeogenesis from amino acids is a key feature related to protein sparing (4). Amino acids directed to gluconeogenesis can lead to lean tissue loss and compromise vital organ function (49). EGP has been measured in most life-history stages of elephant seals and lacked the characteristic suppression with fasting duration found in other species (Fig. 1). It should be noted that most measures of EGP under fasting conditions have been made in domestic species and comparable measures in wild carnivores or fasting-adapted species are largely nonexistent. Despite its abundant availability as a fuel substrate, relatively little of glucose is oxidized. When weaned pups were brought into the laboratory and substrate oxidation assessed through respirometry and urine collection, carbohydrates provided ~8% of energy expenditure (12). Despite low rates of glucose oxidation, glycogen synthesis was negligible during fasting and 98% of glucose produced was committed to glycolysis based on the appearance of glucose label in total body water (12). Because rates of glucose production far exceeded estimated needs of glucose-dependent tissues and measured rates of oxidation, these findings suggested high rates of glucose carbon recycling were occurring in elephant seals (33, 34, 50).
FIGURE 1.
Elephant seals lack the characteristic suppression of EGP during fasting found in most domestic and model species. The relation between EGP and mass is shown with a solid gray line. Rates of EGP from other mammals were drawn from the literature. EGP in NESs fasting for 1–3 mo is similar to EGP in these terrestrial animals in the postabsorptive state. Reproduced with permission from (4). EGP, endogenous glucose production; NES, northern elephant seal.
A likely substrate for EGP was thought to be the glycerol liberated from high rates of lipolysis, because the flux of glycerol was sufficient to account for the high rates of EGP (25). However, studies using a variety of isotopic tracers in weaned pups and adult females, including incorporation of glycerol tracer into glucose and NMR-based positional isotopometry, suggest that glycerol is a minor gluconeogenic substrate, supplying <5% of EGP. Nevertheless, the amount of glucose produced via glycerol gluconeogenesis seems sufficient to meet the metabolic demands of glucose-demanding tissues or at least that of the central nervous system (25). Consistent with low rates of glycogen synthesis, glycogenolysis is essentially absent during fasting. Together with high rates of commitment to glycolysis, these findings suggested that high rates of EGP were a result of futile cycling of glucose carbon, potentially via the Cori cycle, with lipid oxidation providing the ATP to support the energy cost of cycling. Measurements of lactate turnover during fasting revealed high rates of lactate production in resting, eupnic weaned pups (37). Even when no correction factor is applied for dilution of the lactate tracer in the pyruvate pool, lactate accounts for a minimum of ~45% of glucose production during fasting (37). Rates of production of lactate and glucose were strongly correlated and both were associated with plasma insulin concentrations. These data suggested that elephant seal tissues, potentially including erythrocytes and adipocytes, converted glucose to lactate at high rates despite conditions of normoxia. In a fasting state, adipocytes can metabolize the majority of glucose uptake to lactate (51), a process that is enhanced in obese individuals due to their large adipose mass (52). Insulin infusion promotes lactate release from adipose tissue and elevates plasma lactate concentrations in humans (53). The strong relation between insulin and lactate/glucose flux in fasting elephant seal pups may reflect glucose uptake and lactate production by adipocytes with subsequent hepatic conversion of lactate to glucose. High mass-specific erythrocyte content may also contribute to high rates of resting lactate production.
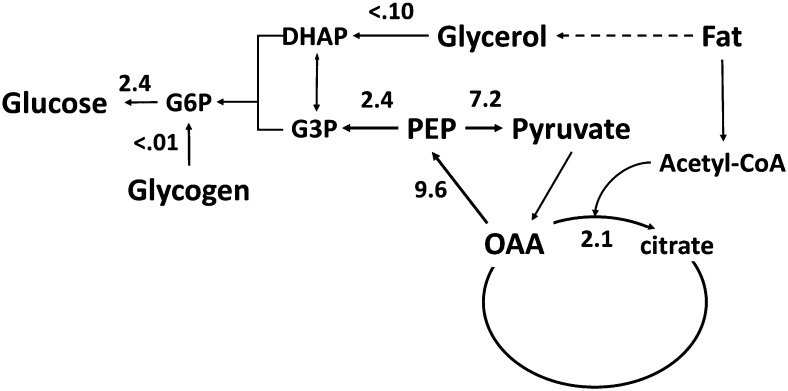
We used NMR-based positional isotopometry to assess the sources of glucose production and pathway flux rates associated with the TCA cycle, specifically flux through PEPCK and pyruvate cycling in fasting elephant seal weanlings (33) (Fig. 2). We found that phosphoenolpyruvate (PEP) was the predominant source of glucose carbon, providing >95% of the carbon used for gluconeogenesis. The fluxes of PEPCK, pyruvate cycling, and citrate synthase were 2–3 times higher in fasting seals than in humans or cats undergoing an overnight fast (33). High rates of TCA cycle activity might be expected in fasting seals, because high rates of fat catabolism support metabolic rates approaching the “metabolic ceilings” proposed for sustained metabolic rates in wildlife. High TCA cycle flux also generates the substrates and ATP necessary for gluconeogenesis throughout the fast. Any carbon loss from the TCA cycle to gluconeogenesis would require replacement from limited gluconeogenic precursors. Carbon loss from the TCA cycle appears to be limited in elephant seals by active pyruvate cycling, with the majority of carbon (~75%) leaving the TCA cycle through PEPCK being recycled back into the TCA cycle. Pyruvate cycling, PEPCK, and citrate synthase flux were all tightly correlated throughout the fast, much more so than has been observed in other species. This tight coupling suggests coordinated regulation of these pathways during the fast in elephant seals. The higher rate of pyruvate cycling relative to the overall rate of gluconeogenesis from PEP is consistent with the model of pathway flux regulation by substrate cycles (50). This possibility has been suggested in similar studies in cats (35) and rats (42). However, the high rate of pyruvate cycling observed in obese rats was due to continued glycogen availability, permitting reduced gluconeogenesis from TCA cycle intermediates while maintaining an appropriate rate of EGP (35). In elephant seals, high rates of pyruvate cycling occur without considerable glycogenolysis.
FIGURE 2.
Flux rates of important features of carbohydrate and energy metabolism in fasting weaned elephant seal pups. Flux rates are in triose units and were measured using NMR-based positional isotopometry. Data are from (33). DHAP, dihydroxyacetone phosphate; G3P, glycerol-3-phosphate; G6P, glucose 6-phosphate; OAA, oxaloacetic acid.
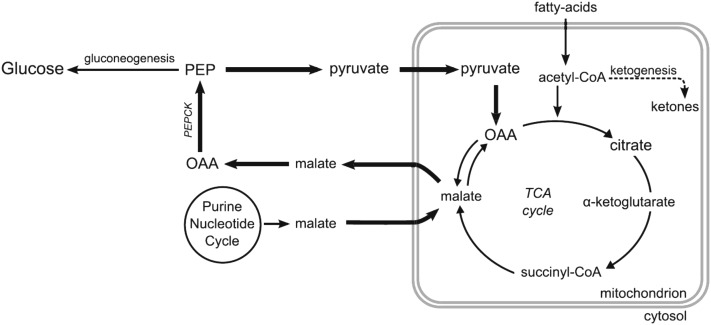
The functional importance of high rates of pyruvate cycling and EGP in elephant seals and their relation to sustained, fat-based metabolism is unknown. Measurement of flux rates suggested that the hepatic energy requirements of gluconeogenesis were small and that PEPCK and pyruvate cycling were far more energetically costly (33). Simultaneous high PEPCK flux and pyruvate cycle activity may provide a regulatory point for EGP and replace TCA cycle intermediates without anaplerosis from amino acids derived from lean tissue stores. Elephant seal fasting metabolism may also reflect changes that occur in response to hypoxia inducible factors (HIFs) that are important regulators of energy homeostasis under low oxygen conditions. HIF-1α alters the expression of numerous genes, including increasing glycolytic enzymes and lactate dehydrogenase and inhibiting pyruvate conversion to acetyl-CoA and its entrance into the TCA cycle (54). The high rates of glucose carbon recycling evident in elephant seals are consistent with upregulation of HIF. HIF-1α mRNA expression increased 3- to 5-fold in muscle and adipose during the fast in weaned elephant seals (55) and HIF-1α protein expression in muscle increased rapidly in response to the terrestrial apneas that seals frequently exhibit while on shore (56). Alternatively, these features may be directly related to the ability to maintain high rates of lipid oxidation during extended fasts. Modification to the anaplerosis of TCA cycle intermediates [e.g., upregulated purine dinucleotide cycle (57)] may facilitate greater TCA cycle flux and oxidation of acetyl-CoA while minimizing production of ketoacids (12) (Fig. 3).
FIGURE 3.
Hypothesized process by which high rates of FA β-oxidation are accommodated without the accumulation of ketoacids. Upregulation of the TCA cycle would require increased availability of TCA cycle intermediates through anaplerosis, potentially increasing gluconeogenesis because of a greater availability of PEP. Reproduced with permission from (12). FA, fatty acid; OAA, oxaloacetic acid; PEP, phosphoenolpyruvate; TCA, tricarboxylic acid.
In conclusion, NESs provide an excellent system with which to examine the metabolic adaptations necessary for extended, high-energy expenditure fasting and the metabolic impacts of natural, adaptive obesity. High adiposity facilitates lipid availability to sustain a predominantly fat-based metabolism. Long-term fasting induces changes in regulation of lipolysis and lipid metabolism that influence FA availability and the onset of insulin resistance. Metabolic adaptation to fasting is associated with several features considered pathological in other species, including hyperlipidemia, hypoinsulemia, and insulin insensitivity, suggesting seals may provide insights into obesity-linked diseases in humans. Fasting is associated with high rates of glucose carbon recycling, elevated PEP and pyruvate flux, and high TCA cycle activity. Together, these features allow sustained, fat-based metabolism without significant accumulation of ketones or protein catabolism. The breadth of investigations into metabolic strategies for extended fasting in NESs is unique among wildlife systems, but researchers have just begun to examine cellular responses in adipose and muscle that likely play critical roles in the regulation of metabolism. Future studies will examine the impact of high circulating NEFA and nuclear receptors (e.g., PPAR) on the regulation of lipid- and tissue-specific metabolism.
Acknowledgments
The authors thank Rudy Ortiz, Dan Costa, and Jose Viscarra for important contributions to the metabolic research on NESs. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: Acrp30, adipose adiponectin; AMPK, AMP-activated protein kinase; ATGL, adipose TG lipase; DAG, diacyglycerol; EGP, endogenous glucose production; FA, fatty acid; FATP1, fatty acid transport protein 1; G3P, glycerol-3-phosphate; HIF, hypoxia inducible factor; HSL, hormone sensitive lipase; LDL-C, LDL cholesterol; LPL, lipoprotein lipase; MGL, monoglyceride lipase; NEFA, nonesterified fatty acid; NES, northern elephant seal; PEP, phosphoenolpyruvate; PEPCK, phosphoenol-pyruvate carboxy kinase; TCA, tricarboxylic acid.
Literature Cited
- 1.Champagne CD, Houser DS, Costa DP, Crocker DE. The effects of handling and anesthetic agents on the stress response and carbohydrate metabolism in northern elephant seals. PLoS ONE. 2012;7:e38442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson PW, Costa DP, Crocker DE, Gallo-Reynoso JP, Champagne CD, Fowler MA, Goetsch C, Goetz KT, Hassrick JL, Hückstädt LA. Foraging behavior and success of a mesopelagic predator in the northeast Pacific Ocean: insights from a data-rich species, the northern elephant seal. PLoS ONE. 2012;7:e36728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Boeuf BJ, Laws RM, editors. Elephant seals: population ecology, behavior, and physiology. Berkeley: University of California Press; 1994
- 4.Champagne C, Crocker D, Fowler M, Houser D. Fasting physiology of the pinnipeds: the challenges of fasting while maintaining high energy expenditure and nutrient delivery for lactation. In: McCue MD, editor. Comparative physiology of fasting, starvation, and food limitation. Berlin, Heidelberg: Springer; 2012. p. 309–36.
- 5.Worthy GAJ, Morris PA, Costa DP, Le Boeuf BJ. Moult energetics of the northern elephant seal (Mirounga angustirostris). J Zool (Lond). 1992;227:257–65 [Google Scholar]
- 6.Crocker DE, Houser DS, Webb PM. Impact of body reserves on energy expenditure, water flux, and mating success in breeding male northern elephant seals. Physiological and biochemical zoology. Physiol Biochem Zool. 2012;85:11–20 [DOI] [PubMed] [Google Scholar]
- 7.Crocker DE, Williams JD, Costa DP, Le Boeuf BJ. Maternal traits and reproductive effort in northern elephant seals. Ecology. 2001;82:3541–55 [Google Scholar]
- 8.Thorson PH, Le Boeuf BJ. Developmental aspects of diving in northern elephant seal pups. In: Le Boeuf BJ, Laws RM, editors. Elephant seals: population ecology, behavior, and physiology. Berkeley: University of California Press; 1994. p. 271–89.
- 9.Vázquez-Medina JP, Crocker DE, Forman HJ, Ortiz RM. Prolonged fasting does not increase oxidative damage or inflammation in postweaned northern elephant seal pups. J Exp Biol. 2010;213:2524–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tift MS, Ranalli EC, Houser DS, Ortiz RM, Crocker DE. Development enhances hypometabolism in northern elephant seal pups (Mirounga angustirostris). Funct Ecol. 2013:27:1155–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelso EJ, Champagne CD, Tift MS, Houser DS, Crocker DE. Sex differences in fuel use and metabolism during development in fasting juvenile northern elephant seals. J Exp Biol. 2012;215:2637–45 [DOI] [PubMed] [Google Scholar]
- 12.Houser DS, Crocker DE, Tift MS, Champagne CD. Glucose oxidation and nonoxidative glucose disposal during prolonged fasts of the northern elephant seal pup (Mirounga angustirostris). Am J Physiol Regul Integr Comp Physiol. 2012;303:R562–70 [DOI] [PubMed] [Google Scholar]
- 13.Adams SH, Costa DP. Water conservation and protein metabolism in northern elephant seal pups during the postweaning fast. J Comp Physiol B. 1993;163:367–73 [DOI] [PubMed] [Google Scholar]
- 14.Houser DS, Costa DP. Protein catabolism in suckling and fasting northern elephant seal pups (Mirounga angustirostris). J Comp Physiol B. 2001;171:635–42 [DOI] [PubMed] [Google Scholar]
- 15.Crocker DE, Webb PM, Costa DP, Le Boeuf BJ. Protein catabolism and renal function in lactating northern elelphant seals. Physiol Zool. 1998;71:485–91 [DOI] [PubMed] [Google Scholar]
- 16.Noren DP, Crocker DE, Williams TM, Costa DP. Energy reserve utilization in northern elephant seal (Mirounga angustirostris) pups during the postweaning fast: size does matter. J Comp Physiol B. 2003;173:443–54 [DOI] [PubMed] [Google Scholar]
- 17.Noren D, Mangel M. Energy reserve allocation in fasting northern elephant seal pups: inter-relationships between body condition and fasting duration. Funct Ecol. 2004;18:233–42 [Google Scholar]
- 18.Best NJ, Bradshaw CJA, Hindell MA, Nichols PD. Vertical stratification of fatty acids in the blubber of southern elephant seals (Mirounga leonina): implications for diet analysis. Comp Biochem Physiol B Biochem Mol Biol. 2003;134:253–63 [DOI] [PubMed] [Google Scholar]
- 19.Strandberg U, Käkelä A, Lydersen C, Kovacs KM, Grahl-Nielsen O, Hyvärinen H, Käkelä R. Stratification, composition, and function of marine mammal blubber: the ecology of fatty acids in marine mammals. Physiol Biochem Zool. 2008;81:473–85 [DOI] [PubMed] [Google Scholar]
- 20.Wheatley KE, Nichols PD, Hindell MA, Harcourt RG, Bradshaw CJ. Temporal variation in the vertical stratification of blubber fatty acids alters diet predictions for lactating Weddell seals. J Exp Mar Biol Ecol. 2007;352:103–13 [Google Scholar]
- 21.Fowler MA, Debier C, Mignolet E, Clementine L, Crocker DE, Costa DP. Fatty acid mobilization and comparison to milk fatty acid content in northern elephant seals. J Comp Physiol B. 2013;Oct 15. (Epub ahead of print; DOI 10.1007/s00360-013-0787-7) [DOI] [PubMed] [Google Scholar]
- 22.Neville MC, Picciano MF. Regulation of milk lipid secretion and composition. Annu Rev Nutr. 1997;17:159–83 [DOI] [PubMed] [Google Scholar]
- 23.Debier C, Kovacs K, Lydersen C, Mignolet E, Larondelle Y. Vitamin E and vitamin A contents, fatty acid profiles, and gross composition of harp and hooded seal milk through lactation. Can J Zool. 1999;77:952–8 [Google Scholar]
- 24.Viscarra JA, Vázquez-Medina JP, Rodriguez R, Champagne CD, Adams SH, Crocker DE, Ortiz RM. Decreased expression of adipose CD36 and FATP1 are associated with increased plasma non-esterified fatty acids during prolonged fasting in northern elephant seal pups (Mirounga angustirostris). J Exp Biol. 2012;215:2455–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houser DS, Champagne CD, Crocker DE. Lipolysis and glycerol gluconeogenesis in simultaneously fasting and lactating northern elephant seals. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2376–81 [DOI] [PubMed] [Google Scholar]
- 26.Fowler MA, Champagne CD, Houser DS, Crocker DE. Hormonal regulation of glucose clearance in lactating northern elephant seals (Mirounga angustirostris). J Exp Biol. 2008;211:2943–9 [DOI] [PubMed] [Google Scholar]
- 27.Viscarra JA, Champagne CD, Crocker DE, Ortiz RM. 5′ AMP-activated protein kinase activity is increased in adipose tissue of northern elephant seal pups during prolonged fasting-induced insulin resistance. J Endocrinol. 2011;209:317–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viscarra JA, Rodriguez R, Vazquez-Medina JP, Lee A, Tift MS, Tavoni SK, Crocker DE, Ortiz RM. Insulin and GLP-1 infusions demonstrate the onset of adipose-specific insulin resistance in a large fasting mammal: potential glucogenic role for GLP-1. Physiol Rep. 2013;1:e00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki M, Vázquez-Medina JP, Viscarra JA, Soñanez-Organis JG, Crocker DE, Rudy OM. Activation of systemic, but not local, renin-angiotensin system is associated with up-regulation of TNF-α during prolonged fasting in northern elephant seal pups. J Exp Biol. 2013;216:3125–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fowler MA. Regulation of lipid metabolism and milk lipid content in northern elephant seals [Ph.D. thesis]. Santa Cruz: University of California; 2012.
- 31.Crocker DE, Fowler MA, Champagne CD, Houser DS. Metabolic response to a glucagon challenge varies with adiposity and life-history stage in fasting northern elephant seals. Gen Comp Endocrinol. In press 2013 [DOI] [PubMed] [Google Scholar]
- 32.Crocker DE, Ortiz RM, Houser DS, Webb PM, Costa DP. Hormone and metabolite changes associated with extended breeding fasts in male northern elephant seals (Mirounga angustirostris). Comp Biochem Physiol A Mol Integr Physiol. 2012;161:388–94 [DOI] [PubMed] [Google Scholar]
- 33.Champagne CD, Houser DS, Fowler MA, Costa DP, Crocker DE. Gluconeogenesis is associated with high rates of tricarboxylic acid and pyruvate cycling in fasting northern elephant seals. Am J Physiol Regul Integr Comp Physiol. 2012;303:R340–52 [DOI] [PubMed] [Google Scholar]
- 34.Champagne CD, Houser DS, Crocker DE. Glucose metabolism during lactation in a fasting animal, the northern elephant seal. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1129–37 [DOI] [PubMed] [Google Scholar]
- 35.Ortiz RM, Wade CE, Ortiz CL. Effects of prolonged fasting on plasma cortisol and TH in postweaned norhtern elephant seal pups. Am J Physiol. 2001;280:R790–5 [DOI] [PubMed] [Google Scholar]
- 36.McDonald BI, Crocker DE. Physiology and behavior influence lactation efficiency in northern elephant seals (Mirounga angustirostris). Physiological and biochemical zoology. Physiol Biochem Zool. 2006;79:484–96 [DOI] [PubMed] [Google Scholar]
- 37.Tavoni SK, Champagne C, Houser D, Crocker D. Lactate flux and gluconeogenesis in fasting, weaned northern elephant seals (Mirounga angustirostris). J Comp Physiol B. 2013;183:537–46 [DOI] [PubMed] [Google Scholar]
- 38.Aarsland A, Wolfe RR. Hepatic secretion of VLDL fatty acids during stimulated lipogenesis in men. J Lipid Res. 1998;39:1280–6 [PubMed] [Google Scholar]
- 39.Nielsen TS, Vendelbo MH, Jessen N, Pedersen SB, Jørgensen JO, Lund S, Møller N. Fasting, but not exercise, increases adipose triglyceride lipase (ATGL) protein and reduces G (0)/G (1) switch gene 2 (G0S2) protein and mRNA content in human adipose tissue. J Clin Endocrinol Metab. 2011;96:E1293–7 [DOI] [PubMed] [Google Scholar]
- 40.Viscarra JA, Ortiz RM. Cellular mechanisms regulating fuel metabolism in mammals: role of adipose tissue and lipids during prolonged food deprivation. Metabolism. 2013;62:889–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viscarra JA, Vázquez-Medina JP, Crocker DE, Ortiz RM. Glut4 is upregulated despite decreased insulin signaling during prolonged fasting in northern elephant seal pups. Am J Physiol Regul Integr Comp Physiol. 2011;300:R150–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaidhu MP, Anthony NM, Patel P, Hawke TJ, Ceddia RB. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: role of ATGL, HSL, and AMPK. Am J Physiol Cell Physiol. 2010;298:C961–71 [DOI] [PubMed] [Google Scholar]
- 43.Jensen MD, Ekberg K, Landau BR. Lipid metabolism during fasting. Am J Physiol Endocrinol Metab. 2001;281:E789–93 [DOI] [PubMed] [Google Scholar]
- 44.Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor α mediates the adaptive response to fasting. J Clin Invest. 1999;103:1489–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maeda N, Funahashi T, Shimomura I. Metabolic impact of adipose and hepatic glycerol channels aquaporin 7 and aquaporin 9. Nat Clin Pract Endocrinol Metab. 2008;4:627–34 [DOI] [PubMed] [Google Scholar]
- 46.Nye C, Kim J, Kalhan SC, Hanson RW. Reassessing triglyceride synthesis in adipose tissue. Trends Endocrinol Metab. 2008;19:356–61 [DOI] [PubMed] [Google Scholar]
- 47.Nye CK, Hanson RW, Kalhan SC. Glyceroneogenesis is the dominant pathway for triglyceride glycerol synthesis in vivo in the rat. J Biol Chem. 2008;283:27565–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hassrick JL, Crocker D, Teutschel N, McDonald B, Robinson P, Simmons S, Costa D. Condition and mass impact oxygen stores and dive duration in adult female northern elephant seals. J Exp Biol. 2010;213:585–92 [DOI] [PubMed] [Google Scholar]
- 49.Owen OE, Smalley KJ, D'Alessio DA, Mozzoli MA, Dawson EK. Protein, fat, and carbohydrate requirements during starvation: anaplerosis and cataplerosis. Am J Clin Nutr. 1998;68:12–34 [DOI] [PubMed] [Google Scholar]
- 50.Champagne CD, Houser DS, Crocker DE. Glucose production and substrate cycle activity in a fasting adapted animal, the northern elephant seal. J Exp Biol. 2005;208:859–68 [DOI] [PubMed] [Google Scholar]
- 51.DiGirolamo M, Newby F, Lovejoy J. Lactate production in adipose tissue: a regulated function with extra-adipose implications. FASEB J. 1992;6:2405–12 [DOI] [PubMed] [Google Scholar]
- 52.Jansson PA, Larsson A, Smith U, Lönnroth P. Lactate release from the subcutaneous tissue in lean and obese men. J Clin Invest. 1994;93:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qvisth V, Hagström-Toft E, Moberg E, Sjöberg S, Bolinder J. Lactate release from adipose tissue and skeletal muscle in vivo: defective insulin regulation in insulin-resistant obese women. Am J Physiol Endocrinol Metab. 2007;292:E709–14 [DOI] [PubMed] [Google Scholar]
- 54.Wheaton WW, Chandel NS. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am J Physiol Cell Physiol. 2011;300:C385–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soñanez-Organis JG, Vázquez-Medina JP, Crocker DE, Ortiz RM. Prolonged fasting activates hypoxia inducible factor-1α,-2α and-3α in a tissue-specific manner in northern elephant seal pups. Gene. 2013;526:155–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vázquez-Medina JP, Zenteno-Savín T, Tift MS, Forman HJ, Crocker DE, Ortiz RM. Apnea stimulates the adaptive response to oxidative stress in elephant seal pups. J Exp Biol. 2011;214:4193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soñanez-Organis JG, Vázquez-Medina JP, Zenteno-Savín T, Aguilar A, Crocker DE, Ortiz RM. Prolonged fasting increases purine recycling in post-weaned northern elephant seals. J Exp Biol. 2012;215:1448–55 [DOI] [PMC free article] [PubMed] [Google Scholar]