Abstract
It is important to consider whether habitual high phosphorus intake adversely affects bone health, because phosphorus intake has been increasing, whereas calcium intake has been decreasing in dietary patterns. A higher total habitual dietary phosphorus intake has been associated with higher serum parathyroid hormone (PTH) and lower serum calcium concentrations in healthy individuals. Higher serum PTH concentrations have been shown in those who consume foods with phosphorus additives. These findings suggest that long-term dietary phosphorus loads and long-term hyperphosphatemia may have important negative effects on bone health. In contrast, PTH concentrations did not increase as a result of high dietary phosphorus intake when phosphorus was provided with adequate amounts of calcium. Intake of foods with a ratio of calcium to phosphorus close to that found in dairy products led to positive effects on bone health. Several randomized controlled trials have shown positive relations between dairy intake and bone mineral density. In our loading test with a low-calcium, high-phosphorus lunch provided to healthy young men, serum PTH concentrations showed peaks at 1 and 6 h, and serum fibroblast growth factor 23 (FGF23) concentrations increased significantly at 8 h after the meal. In contrast, the high-calcium, high-phosphorus meal suppressed the second PTH and FGF23 elevations until 8 h after the meal. This implies that adequate dietary calcium intake is needed to overcome the interfering effects of high phosphorus intake on PTH and FGF23 secretion. FGF23 acts on the parathyroid gland to decrease PTH mRNA and PTH secretion in rats with normal kidney function. However, increased serum FGF23 is an early alteration of mineral metabolism in chronic kidney disease, causing secondary hyperthyroidism, and implying resistance of the parathyroid gland to the action of FGF23 in chronic kidney disease. These findings suggest that long-term high-phosphorus diets may impair bone health mediated by FGF23 resistance both in chronic kidney disease patients and in the healthy population.
Introduction
Phosphorus intake is necessary for bone growth and mineralization. Phosphorus is abundantly supplied in the diet through meat, grains, and dairy products. In many countries, dietary intake of phosphorus is higher (1, 2) than the recommended daily allowance and phosphorus, as phosphorus salts, is added to foods as food additives. High phosphorus intake has been shown to inhibit the increase in serum 1,25-dihydroxyvitamin D [1,25(OH)2D]6 concentration in response to low dietary calcium intake. Furthermore, phosphorus is considered to be a major dietary source of acid (3). It is an ongoing discussion whether a high phosphorus intake adversely affects bone mass and density. Therefore, the role of excess dietary phosphorus intake from natural foods and processed foods on bone health is discussed in this review.
Current Status of Knowledge
Phosphorus intake and bone health.
Approximately 80–90% of the mineral content of bone is made up of calcium and phosphorus, and 85% of the phosphorus found in the body is in the skeleton. Adequate phosphorus intake is essential for many biologic processes, including skeletal mineralization, but it is thought that excessive intake can have deleterious effects on bone. Phosphorus can directly cause apoptosis in cultured osteoblasts as well as an increase in parathyroid hormone (PTH) concentrations (4). Diets high in phosphorus and low in calcium lead to diminished intestinal calcium absorption, reducing serum calcium concentration and stimulating PTH secretion, which, in turn, causes bone resorption to return serum calcium to homeostatic concentrations. Intermittent administration of human PTH increases bone mass in humans and rats (5, 6), but continuously high concentrations of PTH reduce bone mineral density. High dietary phosphorus has been shown to cause bone loss in animals (7).
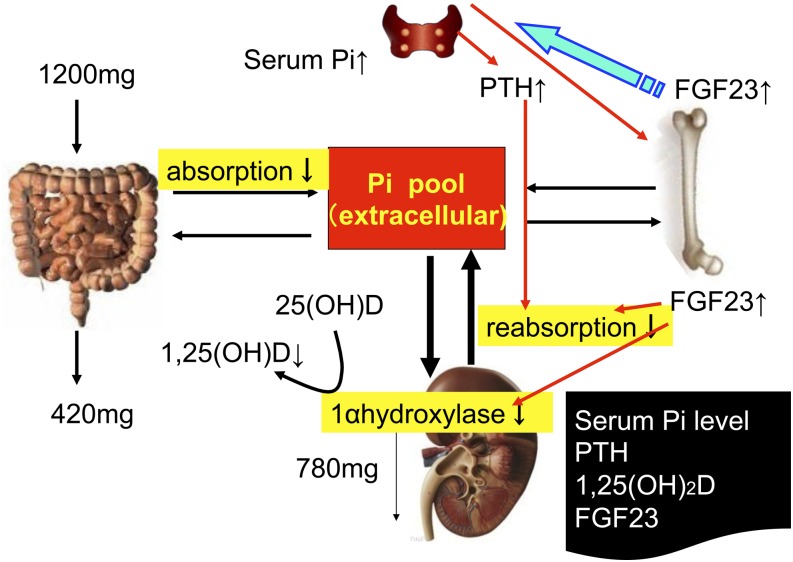
A sustained increase in PTH after the intake of phosphorus has been observed in both animal and human studies (8, 9). In addition to several natural sources of phosphorus in dairy products, meats, whole grains, nuts, and eggs, the use of phosphorus additives in the food industry is common and further increases phosphorus intake (10, 11). Phosphorus from inorganic additives is absorbed almost completely (12) and may have effects that differ from those of natural phosphorus (13, 14). It has been estimated that phosphorus additives may add as much as 1 g of phosphorus to the diet, depending on food choices, in the United States (12). Based on a recent report, a typical hemodialysis patient in Europe consumes at least 100–300 mg of extra phosphorus from additives (15). Phosphorus directly regulates the production of 1,25(OH)2D by kidney cells in culture (16) and in vivo (17) (Fig. 1).
FIGURE 1.
Phosphorus metabolism in humans. High dietary phosphorus intake increases serum phosphorus concentrations, which stimulates PTH secretion and FGF23 secretion. Both PTH and FGF23 induce phosphaturia. PTH and FGF23 activate and inhibit 1,25(OH)2D synthesis, which increases and decreases the efficiency of dietary phosphorus absorption in intestine, respectively. PTH also stimulates FGF23 secretion, whereas FGF23 inhibits PTH secretion. FGF23, fibroblast growth factor 23; Pi, inorganic phosphate; PTH, parathyroid hormone; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
Serum fibroblast growth factor 23 (FGF23) is predominantly secreted by osteocytes and is a major factor in the regulation of phosphorus homeostasis (18, 19). FGF23 achieves its cellular specificity in the kidney and parathyroid glands by binding in the presence of its obligatory transmembrane protein coreceptor Klotho, which increases the affinity of FGF23 for ubiquitously expressed FGF receptors (20). FGF23 acts on the kidney to cause phosphaturia, a decreased synthesis of 1,25(OH)2D, and potentially corrects high phosphorus and 1,25(OH)2D concentrations. It has been shown that the administration of recombinant FGF23 suppresses PTH gene expression and secretion in rats with normal renal function and in vitro in organ cultures of rat parathyroid glands in rats with normal renal function (21).
Phosphorus loading and bone metabolism.
Kemi et al. (22) reported short-term effects of 4 phosphorus doses on calcium and bone metabolism in 14 healthy women, 20–28 y of age, who were randomly assigned to 4 controlled study days. Participants were served control meals containing 495 mg phosphorus and 250 mg calcium and different phosphorus supplement doses of 0 (placebo), 250, 750, or 1500 mg/d. Serum calcium concentration declined in response to phosphorus intake but was significant only after the 1500-mg phosphorus dose. Serum calcium continued to decrease after the 1500-mg phosphorus dose on the following morning compared with the morning fasting value of that study session. Serum PTH concentration increased in a dose-dependent manner in response to phosphorus intake. There was a significant decline in serum bone-specific alkaline phosphatase (BALP) activity, a marker of bone formation, after the 750- and 1500-mg phosphorus doses. The 24-h urinary excretion of N-terminal telopeptide of collagen type I (U-NTx), corrected for creatinine excretion (U-Cr; U-NTx/U-Cr), was affected by phosphorus intake (P = 0.048). With the 1500-mg phosphorus dose, U-NTx/U-Cr was 33% (P = 0.06) above that on the control day. U-NTx/U-Cr tended to correlate with the increase in serum phosphorus AUC values (r = 0.42, P = 0.13) but not with the increase in serum PTH AUC values (P = 0.6).
We examined an acute effect of oral phosphorus loading on PTH and FGF23 to clarify their role in the rapid adjustment of serum phosphorus (23). In the first study, 8 healthy male volunteers were alternately served 1 of 3 test meals containing 200 mg calcium and different phosphorus amounts [400 mg (P400), 800 mg (P800), and 1200 mg (P1200)] as lunch.
Serum phosphorus and urinary phosphorus excretion increased significantly and dose-dependently within 1 h after P400, P800, and P1200 intake. Serum calcium did not change as the intake of phosphorus increased. Similarly, serum calcium declined after an intake of 1995 mg phosphorus but did not change after an intake of 1245 mg phosphorus (22). Serum PTH concentrations significantly increased at 1, 2, and 4 h after the P800 and P1200 meals compared with those after the P400 meal. Serum PTH showed a dual-phase curve at 1–2 and 4–6 h after P1200 intake and was significantly higher than that after P400 intake. Serum FGF23 increased significantly at 8 h after P1200 intake compared with after both P400 and P800 intakes (23). These data indicate that PTH may be associated with rapid adaptation of phosphorus homeostasis but that FGF23 may not be.
Calcium and phosphorus loading and bone metabolism.
Animals fed a diet with a low calcium to phosphorus ratio have been shown to have secondary hyperparathyroidism, loss of bone, and osteopenia (24). Even high calcium intake (1680 mg/d) did not counteract the effect of dietary phosphorus intake in healthy females (1). In contrast, when phosphorus intake was above the dietary guidelines (700 mg/d), oral calcium intake decreased serum PTH concentration and bone resorption, both of which have been induced by increased phosphorus intake (22). In addition, a high calcium to phosphorus ratio was found to be favorable for bone mineralization in adult rats and human participants (25–27). Therefore, the dietary calcium-to-phosphorus ratio is considered to be a marker for the prediction of bone health and/or quality, independent of the absolute intake of both elements separately (1,28).
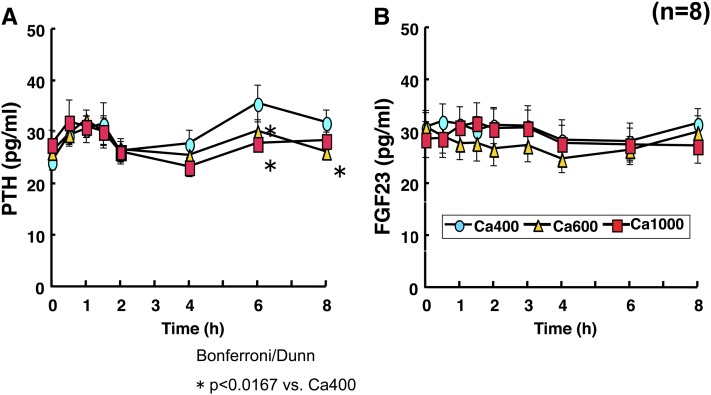
Therefore, in our second study, the effect of varying calcium intake with high phosphorus intake on serum calcium and phosphorus homeostasis was investigated by serving 1 of 3 test meals containing 1200 mg phosphorus and varying calcium intakes [400 mg (Ca400), 600 mg (Ca600), and 1000 mg (Ca1000)] (29). Serum phosphorus increased to the same concentration after ingestion of 3 meals, and high concentrations were maintained for 8 h after meal intakes. There were no significant differences in serum 1,25(OH)2D between the calcium-loaded groups. Time-dependent changes in serum PTH demonstrated a dual-phase curve with peaks at 1 and 6 h after the intake of Ca400. It is interesting that the second peaks at 6 h of PTH after Ca600 and Ca1000 were significantly suppressed and disappeared when compared with that after Ca400 (Fig. 2A). These data contradict those of the previous study, which was conducted in a different ethnic group (1). After the ingestion of each of the 3 meals, serum FGF23 did not change for 8 h (Fig. 2B). This implies that adequate dietary calcium intake is needed to minimize the effects of high phosphorus intake on PTH and FGF23 secretion.
FIGURE 2.
Serum FGF23 and PTH concentrations after phosphate-calcium loading. Healthy men were alternately served 1 of 3 test meals containing 1000 mg phosphorus and varying calcium amounts [400 mg (Ca400), 600 mg (Ca600), and 1000 mg (Ca1000)] provided as lunch. The postprandial changes in serum concentrations of PTH and FGF23 were measured. Intake of 1000 mg calcium with high phosphorus intake (1200 mg) suppressed second peak of serum PTH and FGF23 increase. Data were derived from reference 29. FGF23, fibroblast growth factor 23; PTH, parathyroid hormone.
Short-term high phosphorus intake and bone metabolism.
Ferrari et al. (30) demonstrated that phosphorus loading for 3 d increased serum FGF23 in the morning after fasting despite normal serum phosphorus. They also demonstrated that changes in FGF23were positively correlated with changes in 24-h urinary phosphorus excretion and were negatively correlated with changes in the maximal phosphorus reabsorption, although PTH was not. Additionally, serum FGF23 concentrations were not correlated with fasting morning serum phosphorus.
Recently, Martin et al. (31) demonstrated that the immediate effect of phosphorus on PTH was not dependent on increases in serum phosphorus, suggesting that additional signals arising from the gastrointestinal tract would contribute to the rapid response. These factors may also mediate the first peak of serum PTH in our first study. On the other hand, the second peak was probably mediated by an increase in serum phosphorus after dietary phosphorus loading. Studies that have evaluated the short-term effects of a low-phosphorus diet or phosphorus supplementation have failed to find changes in FGF23 in healthy individuals after 6 h (23) or after 2–3 d (30).
Long-term high phosphorus intake and bone health.
Phosphorus intake has been correlated with bone fragility fractures in the Brazilian population (32). It was demonstrated that for every 100 mg phosphorus, the risk of fractures increased by 9%. Furthermore, higher serum phosphate concentrations and lower hip bone mineral density have been shown to be independent of bone strength and quality and have been associated with elevated vascular calcification scores, supporting the search for effective prevention and treatment strategies that may simultaneously reduce these modifiable risk factors in older adults (33). These observations suggest that long-term dietary phosphorus loading and long-term hyperphosphatemia may be important negative regulators of bone health.
High calcium to phosphorus ratio and bone health.
PTH concentrations did not increase as a result of high dietary phosphorus intake when given together with adequate amounts of calcium, where the decrease in free ionized calcium was compensated for by the diet (32). The higher the calcium intake, the less often serum phosphorus exceeded the upper reference limit. This suggests that phosphorus absorption diminishes with increasing calcium intake, although insufficiently to prevent excessive serum phosphorus concentrations. In studies in which calcium intake was adequate (1000 mg) or high (1995 mg), high phosphorus intake did not increase serum phosphorus concentrations significantly (34, 35). This is probably due to diminished phosphorus absorption because of the formation of calcium phosphate complex in the gut. Basabe Tuero et al. (36) concluded that high calcium consumption (>1000 mg/d) and a calcium to phosphorus ratio >0.74 are associated with better bone mineral densities in young women. These findings are in accordance with earlier epidemiologic studies (37, 38). Thus, an adverse effect on PTH secretion from high phosphorus intake was seen only when calcium intake was inadequate.
Dairy food intake and bone health.
During childhood and adulthood, intake of foods with a calcium to phosphorus ratio close to that of dairy products led to positive effects on bone health (39, 40). Dairy consumption has been positively linked to bone health in observational, retrospective, and intervention studies. Two reviews of the literature found that 25 of 32 observational studies and 11 of 11 randomized controlled trials showed a significantly positive association between dairy food intake and bone mineral density or bone mineral content (41, 42). Milk and cheese, excluding processed cheese, are free of phosphate additives but high in natural phosphate. Those who consumed more milk and cheese had lower mean serum PTH concentration than those who consumed less (14). The high calcium content of milk and cheese probably explains the effect of higher consumption of these products on serum PTH. High calcium intake hinders the absorption of phosphorus in the intestine (43), and calcium supplementation has been found to suppress higher serum PTH concentrations induced by high phosphorus intake in healthy women (1). Consumption of fermented cheese in a short-term controlled diet decreased serum PTH concentration and even decreased bone resorption in healthy young women (13). The long-term beneficial effect of combined calcium-phosphorus salt on fracture prevention could be mediated by a reduction in bone resorption, as observed after short-term consumption of milk or dairy products in healthy postmenopausal women living in the community (44) and in institutionalized elderly persons (45, 46). Four additional randomized controlled trials have shown positive relations between dairy intake and bone mineral density (47, 48).
Processed food intake and bone health.
The amount of phosphorus in the American diet has increased considerably, primarily from phosphorus-containing additives in convenience and fast foods (49). It is estimated that, depending on individual food choices, such additives add as much 1000 mg/d of phosphorus to the diet (12). Moreover, phosphorus in additives is almost entirely absorbed, whereas only 60% of naturally occurring phosphorus is absorbed (50). Higher total habitual dietary phosphorus intake was associated with higher serum PTH and lower serum calcium concentrations in healthy individuals, and phosphate additives containing food had more harmful effects on calcium metabolism than foods containing natural phosphorus. These effects were seen as higher serum PTH concentrations in those who consumed phosphorus additive–containing foods. This difference may be due to the differential bioavailability of phosphorus from phosphorus-containing food additives and natural phosphorus sources. Phosphorus additives are present as phosphate salts. Colas contain phosphoric acid, which has been shown to interfere with calcium absorption and to contribute to imbalances that lead to additional loss of calcium (51). In a large population-based cohort, consistent robust associations were observed between cola consumption and low bone mineral density in women (52, 53). Thus, because of the increasing use of processed foods with phosphorus-containing food additives, in addition to an increase in the incidence of osteoporosis it is important to also focus concern on dietary phosphorus intakes of and calcium to phosphorus ratios in diets consumed by Western populations.
FGF23 resistance by habitual excess phosphorus intake.
The association between previous bone fragility fracture and higher serum phosphate has been described in individuals with renal failure (54, 55). As renal function decreases and chronic kidney disease develops, increased phosphate retention results in an increase in serum phosphorus and FGF23 (56). Meanwhile, a reduction in calcium absorption, caused by decreased 1,25(OH)2D secretion, leads to a decrease in serum calcium and an increase in PTH. Thus, the tendency to develop hyperphosphatemia in chronic kidney disease patients is delayed for a time by high FGF23 and PTH, which compensate by decreasing renal phosphate reabsorption and reducing gut phosphate absorption. Eventually, however, as renal function continues to decrease, frank hyperphosphatemia develops.
In parathyroid organ culture, FGF23 was shown to decrease secreted PTH and PTH mRNA in control or early chronic kidney disease rats but not in those with advanced chronic kidney disease (57). Recombinant FGF23 failed to decrease serum PTH in parathyroid glands of rats with advanced chronic kidney disease (58). Thus, the increased FGF23 failed to decrease PTH in established chronic kidney disease because of a downregulation of its receptor heterodimer complex Klotho-FGFR1c (59). These results demonstrate the resistance of the parathyroid gland to FGF23 in advanced chronic kidney disease.
Conclusions
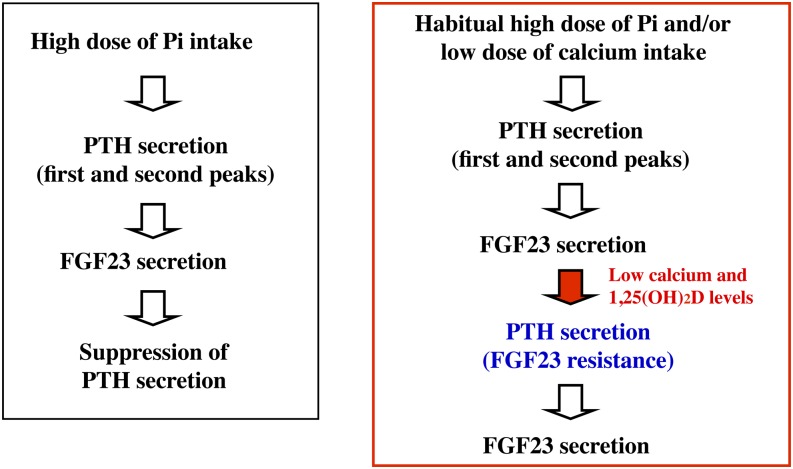
FGF23 suppresses PTH gene expression and secretion in the parathyroid gland of rats with normal renal function (21). Mean serum PTH was almost 2-fold higher and mean serum calcium concentrations lower among the general population with the highest total phosphorus intakes compared with those with the lowest intakes (14). These findings are in accordance with earlier intervention studies (60, 61), suggesting the development of FGF23 resistance by habitual excess phosphorus intake (Fig. 3). Because of the high dietary phosphorus intake and current upward trend in consumption of processed foods in Western countries, these findings have important public health implications. Therefore, it is suggested that excessive dietary phosphorus intake may impair bone health, mediated by FGF23 resistance, in healthy populations as well as in chronic kidney disease patients.
FIGURE 3.
Process leading to FGF23 resistance by habitual high phosphorus and/or low calcium intakes. Excessive dietary phosphorus intake may impair bone health mediated by FGF23 resistance. FGF23, fibroblast growth factor 23; Pi, inorganic phosphate; PTH, parathyroid hormone; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: BALP, bone-specific alkaline phosphatase; FGF23, fibroblast growth factor 23; PTH, parathyroid hormone; U-Cr, urinary creatinine excretion; U-NTx, urinary N-terminal telopeptide of collagen type I; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
Literature Cited
- 1.Kemi VE, Kärkkäinen MU, Karp HJ, Laitinen KA, Lamberg-Allardt CJ. Increased calcium intake does not completely counteract the effects of increased phosphorus intake on bone: an acute dose-response study in healthy females. Br J Nutr. 2008;99:832–9 [DOI] [PubMed] [Google Scholar]
- 2.Takeda E, Sakamoto K, Yokota K, Shinohara M, Taketani Y, Morita K, Yamamoto H, Miyamoto K, Shibayama M. Phosphorus supply per capita from food in Japan between 1960 and 1995. J Nutr Sci Vitaminol (Tokyo). 2002;48:102–8 [DOI] [PubMed] [Google Scholar]
- 3.Remer T, Manz F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr. 1994;59:1356–61 [DOI] [PubMed] [Google Scholar]
- 4.Meleti Z, Shapiro I, Adams C. Inorganic phosphate induces apoptosis of osteoblast-like cells in culture. Bone. 2000;27:359–66 [DOI] [PubMed] [Google Scholar]
- 5.Black DM, Greenspan S, Ensrud K, Palermo L, McGowan J, Lang T, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–15 [DOI] [PubMed] [Google Scholar]
- 6.Arita S, Ikeda S, Sakai A, Okimoto N, Akahoshi S, Nagashima M, Nishida A, Ito M, Nakamura T. Human parathyroid hormone (1–34) increases mass and structure of cortical shell, with resultant increase in lumbar bone strength, in ovariectomized rats. J Bone Miner Metab. 2004;22:530–40 [DOI] [PubMed] [Google Scholar]
- 7.Calvo MS. Dietary phosphorus, calcium metabolism and bone. J Nutr. 1993;123:1627–33 [DOI] [PubMed] [Google Scholar]
- 8.Estepa JC, Aguilera-Tejero E, Lopez I, Almaden Y, Rodriguez M, Felsenfeld AJ. Effect of phosphate on parathyroid hormone secretion in vivo. J Bone Miner Res. 1999;14:1848–54 [DOI] [PubMed] [Google Scholar]
- 9.Bizik BK, Ding W, Cerklewski FL. Evidence that bone resorption of young men is not increased by high dietary phosphorus obtained from milk and cheese. Nutr Res. 1996;16:1143–6 [Google Scholar]
- 10.Kalantar-Zadeh K, Gutekunst L, Mehrotra R, Kovesdy CP, Bross R, Shinaberger CS, Noori N, Hirschberg R, Benner D, Nissenson AR, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:519–30 [DOI] [PubMed] [Google Scholar]
- 11.Murphy-Gutekunst L, Uribarri J. Hidden phosphorus—enhanced meats: part 3. J Ren Nutr. 2005;15:E1–4 [Google Scholar]
- 12.Bell RR, Draper HH, Tzeng DY, Shin HK, Schmidt GR. Physiological responses of human adults to foods containing phosphate additives. J Nutr. 1977;107:42–50 [DOI] [PubMed] [Google Scholar]
- 13.Karp HJ, Vaihia KP, Kärkkäinen MU, Niemistö MJ, Lamberg-Allardt CJ. Acute effects of different phosphorus sources on calcium and bone metabolism in young women: a whole-foods approach. Calcif Tissue Int. 2007;80:251–8 [DOI] [PubMed] [Google Scholar]
- 14.Kemi VE, Rita HJ, Kärkkäinen MU, Viljakainen HT, Laaksonen MM, Outila TA, Lamberg-Allardt CJ. Habitual high phosphorus intakes and foods with phosphate additives negatively affect serum parathyroid hormone concentration: a cross-sectional study on healthy premenopausal women. Public Health Nutr. 2009;12:1885–92 [DOI] [PubMed] [Google Scholar]
- 15.Benini O, D’Alessandro C, Gianfaldoni D, Cupisti A. Extra-phosphate load from food additives in commonly eaten foods: a real and insidious danger for renal patients. J Ren Nutr. 2011;21:303–8 [DOI] [PubMed] [Google Scholar]
- 16.Condamine L, Vrtovsnik F, Friedlander G, Friedlander G, Garabédian M. Local action of phosphate depletion and insulin-like growth factor 1 on in vitro production of 1,25-dihydroxyvitamin D by cultured mammalian kidney cells. J Clin Invest. 1994;94:1673–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Portale AA, Halloran BP, Curtis Morris J. Physiologic regulation of the serum concentration of 1,25-dihydroxyvitamin D by phosphorus in normal men. J Clin Invest. 1989;83:1494–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol 2006;17:1305–15 [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Gupta A, Quarles LD. Emerging role of fibroblast growth factor 23 in a bone-kidney axis regulating systemic phosphate homeostasis and extracellular matrix mineralization. Curr Opin Nephrol Hypertens. 2007;16:329–35 [DOI] [PubMed] [Google Scholar]
- 20.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–4 [DOI] [PubMed] [Google Scholar]
- 21.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kemi VE, Karkkainen MUM, Lamberg-Allardt CJE. High phosphorus intakes acutely and negatively affect calcium and bone metabolism in a dose-dependent manner in healthy young females. Br J Nutr. 2006;96:545–52 [PubMed] [Google Scholar]
- 23.Nishida Y, Taketani Y, Yamanaka-Okumura H, Imamura F, Taniguchi A, Sato T, Shuto E, Nashiki K, Arai H, Yamamoto H, et al. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 2006;70:2141–7 [DOI] [PubMed] [Google Scholar]
- 24.Lutwak L. Metabolic and biochemical consideration of bone. Ann Clin Lab Sci. 1975;5:185–94 [PubMed] [Google Scholar]
- 25.Kristensen M, Jensen M, Kudsk J, Henriksen M, Mølgaard C. Short-term effects on bone turnover of replacing milk with cola beverages: a 10-day interventional study in young men. Osteoporos Int. 2005;16:1803–8 [DOI] [PubMed] [Google Scholar]
- 26.Koshihara M, Katsumata S, Uehara M, Suzuki K. Effects of dietary phosphorus intake on bone mineralization and calcium absorption in adult female rats. Biosci Biotechnol Biochem. 2005;69:1025–8 [DOI] [PubMed] [Google Scholar]
- 27.Huttunen MM, Pietilä PE, Viljakainen HT, Lamberg-Allardt CJ. Prolonged increase in dietary phosphate intake alters bone mineralization in adult male rats. J Nutr Biochem. 2006;17:479–84 [DOI] [PubMed] [Google Scholar]
- 28.Sax L. The Institute of Medicine’s “dietary reference intake” for phosphorus: a critical perspective. J Am Coll Nutr. 2001;20:271–8 [DOI] [PubMed] [Google Scholar]
- 29.Taketani Y, Nishida Y, Yamanaka-Okumura H, Taniguchi A, Syuto E, Sato T, Yamamoto H, Takeda E. Effect of high phosphate and high calcium diet on serum intact FGF23 and PTH levels in adult healthy men. J Bone Miner Res. 2006;21:S150–1 [Google Scholar]
- 30.Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005;90:1519–24 [DOI] [PubMed] [Google Scholar]
- 31.Martin DR, Ritter CS, Slatopolsky E, Brown AJ. Acute regulation of parathyroid hormone by dietary phosphate. Am J Physiol Endocrinol Metab. 2005;289:E729–34 [DOI] [PubMed] [Google Scholar]
- 32.Pinheiro MM, Schuch NJ, Genaro PS, Ciconelli RM, Ferraz MB, Martini LA. Nutrient intakes related to osteoporotic fractures in men and women—the Brazilian Osteoporosis Study (BRAZOS). Nutr J. 2009;8:6–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Figueiredo CP, Rajamannan NM, Lopes JB, Caparbo VF, Takayama L, Kuroishi ME, Oliveira IS, Menezes PR, Scazufca M, Bonfá E, et al. Serum phosphate and hip bone mineral density as additional factors for high vascular calcification scores in a community-dwelling: the São Paulo Ageing & Health Study (SPAH). Bone. 2013;52:354–9 [DOI] [PubMed] [Google Scholar]
- 34.Grimm M, Muller A, Hein G, Funfstuck R, Jahreis G. High phosphorus intake only slightly affects serum minerals, urinary pyrinidium crosslinks and renal function in young women. Eur J Clin Nutr. 2001;55:153–61 [DOI] [PubMed] [Google Scholar]
- 35.Whybro A, Jagger H, Barker M, Eastell R. Phosphate supplementation in young men:lack of effect on calcium homeostasis and bone turnover. Eur J Clin Nutr. 1998;52:29–33 [DOI] [PubMed] [Google Scholar]
- 36.Basabe Tuero B, Mena VMC, Faci VM, Aparicio VA, Lopez SAM, Ortega ARM. The influence of calcium and phosphorus intake on bone mineral density in young women. Arch Latinoam Nutr. 2004;54:203–8 [PubMed] [Google Scholar]
- 37.Metz JA, Anderson JJ, Gallagher PN., Jr Intakes of calcium, phosphorus, and protein, and physical-activity level are related to radial bone mass in young adult women. Am J Clin Nutr. 1993;58:537–42 [DOI] [PubMed] [Google Scholar]
- 38.Teegarden D, Lyle RM, McCabe GP, McCabe LD, Proulx WR, Michon K, Johnston CC, Weaver CM. Dietary calcium, protein, and phosphorus are related to bone mineral density and content in young women. Am J Clin Nutr. 1998;68:749–54 [DOI] [PubMed] [Google Scholar]
- 39.Bonjour JP, Carne AL, Ferrari S, Clavien H, Slosman D, Theintz G, Rizzoli R. Calcium-enriched foods and bone mass growth in prepubertal girls: a randomized, double-blind, placebo-controlled trial. J Clin Invest. 1997;99:1287–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rizzoli R, Bianchi ML, Garabedian M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294–305 [DOI] [PubMed] [Google Scholar]
- 41. U.S. Department of Health and Human Services; U.S. Department of Agriculture. Dietary guidelines for Americans. 6th ed. Washington: U.S. Government Printing Office; 2005. Dietary Guidelines Advisory Committee Report [cited 2012 Apr 23]. Available from: www.health.gov/dietaryguidelines/pubs.asp.
- 42.Heaney RP. Dairy and bone health. J Am Coll Nutr. 2009;28 Suppl 1:82S–90S [DOI] [PubMed] [Google Scholar]
- 43.Nolan CR, Cunibi WY. Calcium salts in the treatment of hyperphosphatemia in hemodialysis patients. Curr Opin Nephrol Hypertens. 2003;12:373–9 [DOI] [PubMed] [Google Scholar]
- 44.Bonjour JP, Brandolini-Bunlon M, Boirie Y, Morel-Laporte F, Braesco V, Bettiere MC, Souberbielle JC. Inhibition of bone turnover by milk intake in postmenopausal women. Br J Nutr. 2008;100:866–74 [DOI] [PubMed] [Google Scholar]
- 45.Bonjour JP, Benoit V, Pourchaire O, Ferry M, Rousseau B, Souberbielle JC. Inhibition of markers of bone resorption by consumption of vitamin D and calcium-fortified soft plain cheese by institutionalised elderly women. Br J Nutr. 2009;102:962–6 [DOI] [PubMed] [Google Scholar]
- 46.Bonjour JP, Benoit V, Pourchaire O, Rousseau B, Souberbielle JC. Nutritional approach for inhibiting bone resorption in institutionalized elderly women with vitamin D deficiency and high prevalence of fracture. J Nutr Health Aging. 2011;15:404–9 [DOI] [PubMed] [Google Scholar]
- 47.Cheng S, Lyytikainen A, Kroger H, Lamberg-Allardt C, Alen M, Koistinen A. Effects of calcium, dairy product, and vitamin D supplementation on bone mass accrual and body composition in 10–12-y-old girls: a 2-y randomized trial. Am J Clin Nutr. 2005;82:1115–26 [DOI] [PubMed] [Google Scholar]
- 48.Moschonis G, Manios Y. Skeletal site-dependent response of bone mineral density and quantitative ultrasound parameters following a 12-month dietary intervention using dairy products fortified with calcium and vitamin D: the Postmenopausal Health Study. Br J Nutr. 2006;96:1140–8 [DOI] [PubMed] [Google Scholar]
- 49.Calvo MS, Park YK. Changing phosphorus content of the U.S diet: potential for adverse effects on bone. J Nutr. 1996;126:1168S–80S [DOI] [PubMed] [Google Scholar]
- 50.Uribarri J, Calvo MS. Hidden sources of phosphorus in the typical American diet: does it matter in nephrology? Semin Dial. 2003;16:186–8 [DOI] [PubMed] [Google Scholar]
- 51.Amato D, Maravilla A, Montoya C, Gaja O, Revilla C, Guerra R, Paniagua R. Acute effects of soft drink intake on calcium and phosphate metabolism in immature and adult rats. Rev Invest Clin. 1998;50:185–9 [PubMed] [Google Scholar]
- 52.Fitzpatrick L, Heaney RP. Got soda? J Bone Miner Res. 2003;18:1570–2 [DOI] [PubMed] [Google Scholar]
- 53.Tucker KL, Morita K, Qiao N, Hannan MT, Cupples LA, Kiel DP. Colas, but not other carbonated beverages, are associated with low bone mineral density in older women: the Framingham Osteoporosis Study. Am J Clin Nutr. 2006;84:936–42 [DOI] [PubMed] [Google Scholar]
- 54.Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg-Gresham JL, Mason N, Prutz KG, Young EW, Pisoni RL. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2006;70:1358–66 [DOI] [PubMed] [Google Scholar]
- 55.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–18 [DOI] [PubMed] [Google Scholar]
- 56.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galitzer H, Ben-Dov IZ, Silver J, Naveh-Many T. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 2010;77:211–8 [DOI] [PubMed] [Google Scholar]
- 58.Canalejo R, Canalejo A, Martinez-Moreno JM, Rodriguez-Ortiz ME, Estepa JC, Mendoza FJ, Munoz-Castaneda JR, Shalhoub V, Almaden Y, Rodriguez M. FGF23 fails to inhibit uremic parathyroid glands. J Am Soc Nephro1. 2010;21(7):1125–35. [DOI] [PMC free article] [PubMed]
- 59.Komaba H, Goto S, Fujii H, Hamada Y, Kobayashi A, Shibuya K, Tominaga Y, Otsuki N, Nibu K, Nakagawa K, Tsugawa N, Okano T, Kitazawa R, Fukagawa M, Kita T. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int. 2010;77:232–8 [DOI] [PubMed] [Google Scholar]
- 60.Calvo MS, Kumar R, Heath H., III Elevated secretion and action of serum parathyroid hormone in young adults consuming high phosphorus, low calcium diets assembled from common foods. J Clin Endocrinol Metab. 1988;66:823–9 [DOI] [PubMed] [Google Scholar]
- 61.Calvo MS, Kumar R, Heath H., III Persistently elevated parathyroid hormone secretion and action in young women after four weeks of ingesting high phosphorus, low calcium diets. J Clin Endocrinol Metab. 1990;70:1334–40 [DOI] [PubMed] [Google Scholar]