Abstract
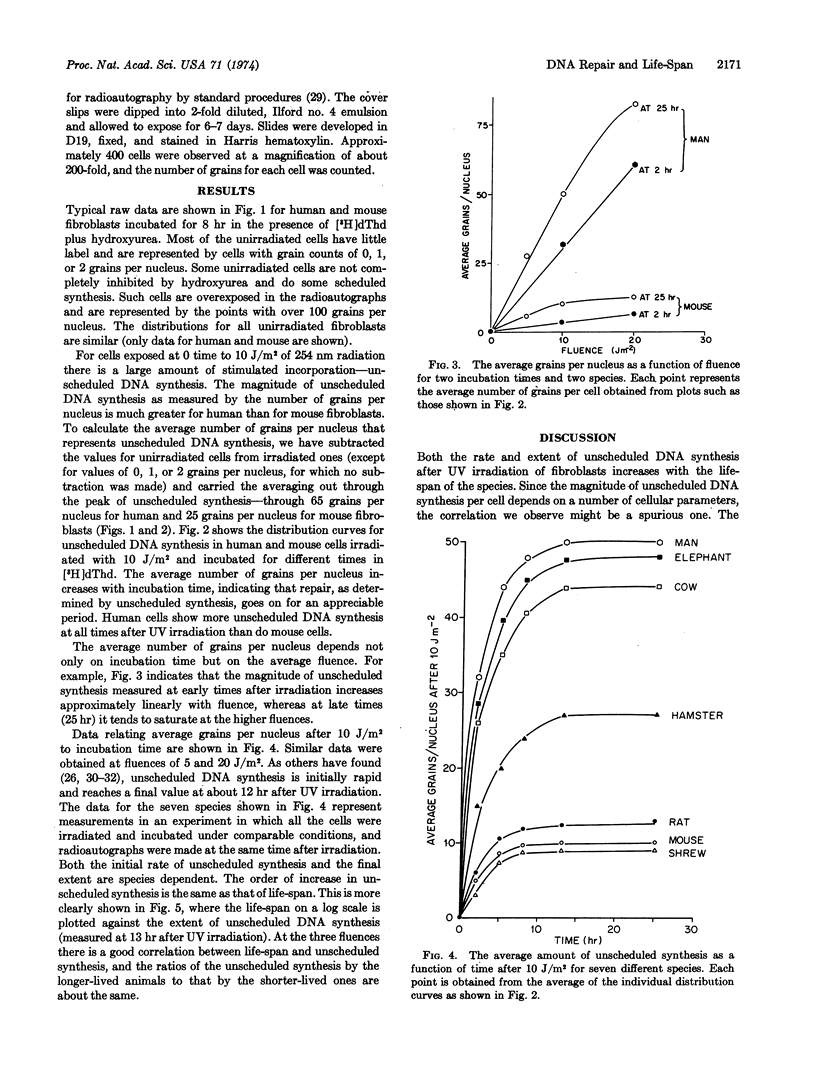
The ability of fibroblasts to perform unscheduled DNA synthesis (a measure of excision-repair) after UV irradiation was measured radioautographically for seven species at several times after several UV fluences. Both the initial rate and the maximum incorporation of [3H]dThd increased with the life-span of the species (shrew, mouse, rat, hamster, cow, elephant, man). Unscheduled DNA synthesis was approximately proportional to the logarithm of life-span.
Keywords: aging, UV irradiation, unscheduled DNA synthesis
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bootsma D., Mulder M. P., Cohen J. A., Pot F. Different inherited levels of DNA repair replication in xeroderma pigmentosum cell strains after exposure to ultraviolet irradiation. Mutat Res. 1970 May;9(5):507–516. doi: 10.1016/0027-5107(70)90035-7. [DOI] [PubMed] [Google Scholar]
- Clarkson J. M., Evans H. J. Unscheduled DNA synthesis in human leucocytes after exposure to UV light, -rays and chemical mutagens. Mutat Res. 1972 Apr;14(4):413–430. doi: 10.1016/0027-5107(72)90139-x. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. DNA damage and repair in light-sensitive human skin disease. J Invest Dermatol. 1970 Mar;54(3):181–195. doi: 10.1111/1523-1747.ep12280225. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Repair replication in Chinese hamster cells after damage from ultraviolet light. Photochem Photobiol. 1970 Jul;12(1):17–28. doi: 10.1111/j.1751-1097.1970.tb06033.x. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Repair replication of mammalian cell DNA: effects of compounds that inhibit DNA synthesis or dark repair. Radiat Res. 1969 Feb;37(2):334–348. [PubMed] [Google Scholar]
- Cleaver J. E., Thomas G. H., Trosko J. E., Lett J. T. Excision repair (dimer excision, strand breakage and repair replication) in primary cultures of eukaryotic (bovine) cells. Exp Cell Res. 1972 Sep;74(1):67–80. doi: 10.1016/0014-4827(72)90482-x. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Trosko J. E. Absence of excision of ultraviolet-induced cyclobutane dimers in xeroderma pigmentosum. Photochem Photobiol. 1970 Jun;11(6):547–550. doi: 10.1111/j.1751-1097.1970.tb06025.x. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Hanawalt P. C. The timecourse of DNA repair replication in ultraviolet-irradiated HeLa cells. Biochim Biophys Acta. 1973 Oct 12;324(2):206–217. doi: 10.1016/0005-2787(73)90138-x. [DOI] [PubMed] [Google Scholar]
- Epstein J., Williams J. R., Little J. B. Deficient DNA repair in human progeroid cells. Proc Natl Acad Sci U S A. 1973 Apr;70(4):977–981. doi: 10.1073/pnas.70.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. G., Norman A. Unscheduled incorporation of thymidine in ultraviolet-irradiated human lymphocytes. Radiat Res. 1968 Nov;36(2):287–298. [PubMed] [Google Scholar]
- Fox M., Fox B. W. Repair replication after U.V.-irradiation in rodent cell-lines of different sensitivity. Int J Radiat Biol Relat Stud Phys Chem Med. 1973 Apr;23(4):359–376. doi: 10.1080/09553007314550421. [DOI] [PubMed] [Google Scholar]
- Goldstein S. The biology of aging. N Engl J Med. 1971 Nov;285(20):1120–1129. doi: 10.1056/NEJM197111112852005. [DOI] [PubMed] [Google Scholar]
- Hahn G. M., King D., Yang S. J. Quantitative changes in unscheduled DNA synthesis in rat muscle cells after differentiation. Nat New Biol. 1971 Apr 21;230(16):242–244. doi: 10.1038/newbio230242a0. [DOI] [PubMed] [Google Scholar]
- Hayflick L. The biology of human aging. Am J Med Sci. 1973 Jun;265(6):432–445. doi: 10.1097/00000441-197306000-00001. [DOI] [PubMed] [Google Scholar]
- JAGGER J. A small and inexpensive ultraviolet dose-rate meter useful in biological experiements. Radiat Res. 1961 Apr;14:394–403. [PubMed] [Google Scholar]
- Karran P., Ormerod M. G. Is the ability to repair damage to DNA related to the proliferative capacity of a cell? The rejoining of X-ray-produced strand breaks. Biochim Biophys Acta. 1973 Feb 23;299(1):54–64. doi: 10.1016/0005-2787(73)90397-3. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Andersson A. Rate of chain breakage at apurinic sites in double-stranded deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3618–3623. doi: 10.1021/bi00769a019. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- Martin G. M., Sprague C. A., Epstein C. J. Replicative life-span of cultivated human cells. Effects of donor's age, tissue, and genotype. Lab Invest. 1970 Jul;23(1):86–92. [PubMed] [Google Scholar]
- Modak S. P., Price G. B. Exogenous DNA polymerase-catalysed incorporation of deoxyribonucleotide monophosphates in nuclei of fixed mouse-brain cells. Exp Cell Res. 1971 Apr;65(2):289–296. doi: 10.1016/0014-4827(71)90004-8. [DOI] [PubMed] [Google Scholar]
- ORGEL L. E. The maintenance of the accuracy of protein synthesis and its relevance to ageing. Proc Natl Acad Sci U S A. 1963 Apr;49:517–521. doi: 10.1073/pnas.49.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. B., Clarkson J. M., Young B. R. Letter: Ultraviolet-induced repair replication in aging diploid human cells (WI-38). Radiat Res. 1973 Dec;56(3):560–564. [PubMed] [Google Scholar]
- Painter R. B., Cleaver J. E. Repair replication, unscheduled DNA synthesis, and the repair of mammalian DNA. Radiat Res. 1969 Mar;37(3):451–466. [PubMed] [Google Scholar]
- Painter R. B. The action of ultraviolet light on mammalian cells. Photophysiology. 1970;5:169–189. [PubMed] [Google Scholar]
- Price G. B., Modak S. P., Makinodan T. Age-associated changes in the DNA of mouse tissue. Science. 1971 Mar 5;171(3974):917–920. doi: 10.1126/science.171.3974.917. [DOI] [PubMed] [Google Scholar]
- Rasmussen R. E., Painter R. B. Radiation-stimulated DNA synthesis in cultured mammalian cells. J Cell Biol. 1966 Apr;29(1):11–19. doi: 10.1083/jcb.29.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B., Ley R. D. Normal and defective repair of damaged DNA in human cells: a sensitive assay utilizing the photolysis of bromodeoxyuridine. Proc Natl Acad Sci U S A. 1971 Apr;68(4):708–712. doi: 10.1073/pnas.68.4.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEFANSSON V. Eskimo longevity in Northern Alaska. Science. 1958 Jan 3;127(3288):16–19. doi: 10.1126/science.127.3288.16. [DOI] [PubMed] [Google Scholar]
- Setlow R. B., Regan J. D., German J., Carrier W. L. Evidence that xeroderma pigmentosum cells do not perform the first step in the repair of ultraviolet damage to their DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1035–1041. doi: 10.1073/pnas.64.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B., Setlow J. K. Effects of radiation on polynucleotides. Annu Rev Biophys Bioeng. 1972;1:293–346. doi: 10.1146/annurev.bb.01.060172.001453. [DOI] [PubMed] [Google Scholar]
- Setlow R. B. The photochemistry, photobiology, and repair of polynucleotides. Prog Nucleic Acid Res Mol Biol. 1968;8:257–295. doi: 10.1016/s0079-6603(08)60548-6. [DOI] [PubMed] [Google Scholar]
- Storer J. B. Longevity and gross pathology at death in 22 inbred mouse strains. J Gerontol. 1966 Jul;21(3):404–409. doi: 10.1093/geronj/21.3.404. [DOI] [PubMed] [Google Scholar]
- Verly W. G., Paquette Y., Thibodeau L. Nuclease for DNA apurinic sites may be involved in the maintenance of DNA in normal cells. Nat New Biol. 1973 Jul 18;244(133):67–69. doi: 10.1038/newbio244067a0. [DOI] [PubMed] [Google Scholar]
- Wheeler K. T., Lett J. T. Formation and rejoining of DNA strand breaks in irradiated neurons: in vivo. Radiat Res. 1972 Oct;52(1):59–67. [PubMed] [Google Scholar]
- Wheeler K. T., Sheridan R. E., Pautler E. L., Lett J. T. In vivo restitution of the DNA structure in gamma irradiated rabbit retinas. Radiat Res. 1973 Mar;53(3):414–427. [PubMed] [Google Scholar]


