Abstract
Tryptamine is an endogenous and dietary indoleamine-based trace amine implicated in cardiovascular pathologies, including hypertension, migraine and myocardial infarction. This study aimed at identifying the signalling pathways for the vasoconstrictor response to tryptamine in rat isolated perfused mesenteric arterial beds and co-released vasodilator modulators of tryptamine-mediated vasoconstriction. Tryptamine caused concentration-dependent vasoconstriction of the mesenteric bed, measured as increases in perfusion pressure. These were inhibited by the 5-HT2A receptor antagonist, ritanserin, indicating mediation via 5-HT2A receptors. The response was inhibited by the phospholipase C (PLC) and phospholipase A2 (iPLA2) inhibitors, U-73122 and PACOCF3, suggesting involvement of phospholipase pathways. Activation of these pathways by tryptamine releases cyclooxygenase (COX) products since indomethacin (non-selective inhibitor of COX-1/2) and nimesulide (selective COX-2 inhibitor) reduced the vasoconstriction. The most likely COX vasoconstrictor product was prostaglandin PGE2 since the responses to tryptamine were reduced by AH-6809, a non-selective EP1 receptor antagonist. Involvement of the Rho-kinase pathway in the tryptamine-evoked vasoconstriction was also indicated by its reduction by the Rho-kinase inhibitors, Y-27,632 and fasudil. The tryptamine vasoconstriction is modulated by the co-released endothelial vasodilator, nitric oxide. Thus, circulating tryptamine can regulate mesenteric blood flow through a cascade of signalling pathways secondary to stimulation of 5-HT2A receptors.
Abbreviations: COX, cyclooxygenase; DAG, diacylglycerol; DRC, dose–response curve; 5-HT, 5-hydroxytryptamine; IP, inositol phosphate; IP3, inositol 1,4,5-trisphosphate; MAO, monoamine oxidase; l-NAME, Nω-nitro-l-arginine methyl ester; NO, nitric oxide; NOS, nitric oxide synthase; PAF, platelet activating factor; PIP2, phosphatidylinositol 4,5-bisphosphate; PLA2, phospholipase A2; PLC, phospholipase C; PKC, protein kinase C; TAAR, trace amine-associated receptor; TxA2, thromboxane A2
Keywords: Tryptamine, Rat mesenteric artery, Vasoconstriction, 5-HT2A receptors, Nitric oxide
Graphical abstract
1. Introduction
Tryptamine is a biogenic amine structurally related to 5-hydroxytryptamine (5-HT) that is generated in the body by neural and peripheral tissues. It is also formed by the microflora in the gastrointestinal tract and is a component of many food items. Tryptamine is implicated in various cardiovascular pathologies, including hypertension, myocardial infarction and migraine. Migraine cluster headaches have been shown to be relieved by psilocybin, a component of magic mushrooms, whose active metabolite, psilocin is a trypamine analogue (N,N-dimethyltryptamine). This action is at a subhallucinogenic dose and likely due to cardiovascular actions (Sewell et al., 2006). The concentration of tryptamine in serum is correlated with that of its precursor l-tryptophan (Wollman et al., 1985), which is metabolised into tryptamine by aromatic l-amino acid decarboxylase. Tryptamine is deaminated by monoamine oxidase (MAO) types A and B (Tipton et al., 2004) to indole-3-acetaldehyde, which is subsequently reduced by aldehyde dehydrogenase to indole-3-acetic acid (Weissbach et al., 1959).
Tryptamine increases blood pressure (Eble, 1965), an effect that has long been held to be due to indirect sympathomimetic actions, since it may be regarded as a trace amine (Zucchi et al., 2006). Indirect sympathomimetic amines release noradrenaline from sympathetic neurones onto vascular α-adrenoceptors to cause vasoconstriction (Trendelenburg, 1972). However, in isolated vascular tissues, tryptamine has been shown to cause vasoconstriction of rabbit aorta not by an indirect mechanism but by direct stimulation of both α-adrenoceptors and 5-HT receptors (Stollak and Furchgott, 1983). Vasoconstriction by tryptamine has also been demonstrated in rat mesenteric arteries (Watts et al., 1994), rat caudal arteries (Hicks and Langer, 1983, Bradley et al., 1985) and rat aorta (Fehler et al., 2010). Hicks and Langer (1983) suggested that specific tryptaminergic receptors mediated the vasoconstriction by tryptamine in rat tail arteries. More recently, the vasoconstriction of rat aorta by tryptamine and other trace amines has been shown to be resistant to blockade by α-adrenoceptor and 5-HT antagonists (Broadley et al., 2009, Fehler et al., 2010). The vasoconstrictor response was attributed (Broadley et al., 2009, Broadley, 2010) to the recently described trace amine-associated receptors (TAARs) (Borowsky et al., 2001, Bunzow et al., 2001).
Given the importance of both dietary and endogenous tryptamine in health and disease, there is a paucity of data on the receptors activated by this amine and their signalling pathways in vascular smooth muscle. The few mechanistic studies that do exist have focussed on the phospholipids. In rat cortical slices, tryptamine-induced stimulation of inositol phosphate (IP) accumulation was insensitive to atropine, cyproheptadine, haloperidol, phenoxybenzamine and propranolol indicating that classical neurotransmitter receptors were not involved (Osborne et al., 1986). In a later study, tryptamine activation of primary cultures of rat cerebellar granule cells increased IP turnover, which was not counteracted by atropine, ketanserin and prazosin (Ishitani et al., 1994). Subsequently, it was shown that in NIH3T3 fibroblasts stably expressing the 5-HT2A receptor, tryptamine activated the phospholipase C (PLC) and phospholipase A2 (PLA2) signalling pathways (Kurrasch-Orbaugh et al., 2003).
In the rat mesentery, we have recently demonstrated that tryptamine mediates both vasopressor and vasodepressor responses. The vasoconstrictor response was blocked by the 5-HT antagonists, ritanserin and ketanserin, and is therefore mediated predominantly via 5-HT2A receptors (Anwar et al., 2012). However, there is a clear lack of data on the mechanisms of tryptamine-induced changes in vascular tone, specifically in the resistance size arteries of the mesentery. Based on the knowledge from our mesenteric arterial network studies and the above-mentioned cellular and tissue experiments, we undertook the present investigation to assess the contributions made by selected contractile transduction pathways in tryptamine-derived vascular tone. We also determined the possible roles of co-released vasodepressor transducers in modulating tryptamine-evoked vasoconstriction.
Preliminary accounts of some of these findings have been reported to the British Pharmacological Society (Anwar et al., 2006) and the European Microcirculation Society (Anwar et al., 2008).
2. Materials and methods
2.1. Animal care
Male Sprague–Dawley rats (250–350 g body weight; Harlan, Bicester, Oxfordshire, U.K.) were housed in temperature (22 ± 1 °C) and humidity (50%) controlled quarters on a 12 h light–dark cycle (07.00–19.00 h light and 19.00–07.00 h dark), 4 animals to a cage, and provided with food and water ad libitum. They were killed by cervical dislocation following stunning in accordance with the Home Office Guidance on the operation of The Animals (Scientific Procedures) Act 1986 (H.M.S.O.), and after local ethical review by the Animal Care and Use Committee of Cardiff University.
2.2. Isolated mesenteric arterial bed
The mesenteric vascular bed was exposed through a midline laparotomy incision; the superior mesenteric artery was cleaned of adipose and connective tissues. The artery was cannulated, close to the junction with the abdominal aorta, with a PE-50 polyethylene (BD Intramedic, Oxford, U.K.) cannula, which was ligated and secured with cotton ties, and the mesenteric vascular bed was immediately perfused with Krebs'–Henseleit-bicarbonate solution, composition in mM: NaCl 118.0, KC1 4.7, NaHCO3 25.0, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, and glucose 11 (osmolality ≅ 292 mosmol/kg H2O), warmed to 37 °C and gassed continuously with 5% CO2/95% O2 to maintain the pH at 7.4. The initial flow rate was set at 2 ml per minute using a Gilson peristaltic pump to remove blood and metabolites. Simultaneously, the mesenteric bed was excised by gently separating from the gastrointestinal tract (stomach to anterior of rectum), placed in a thermostatic perfusion chamber, and the arterial network was perfused at a constant flow rate (4 ml min− 1) that was maintained throughout the experimental procedure (McGregor, 1965). Changes in perfusion pressure, a reflection of vascular resistance, were monitored by a pressure transducer (Elcomatic EM 750, Elcomatic Ltd., Glasgow, U.K.) located immediately proximal to the inflow cannula. The transducer was coupled to a PowerLab/4SP computerised data acquisition system (AD Instruments, Charlgrove, Oxon, U.K.) and Chart version 5 software (AD Instruments, U.K.) to display and analyse data. A bubble trap, distal to the cannulated mesentery and proximal to the perfusate solution, removed any air bubbles in the perfusate and also dampened pulses in flow.
2.3. Experimental protocols
After an equilibration period of 1 h, each arterial preparation was subjected to one of the following experimental protocols. Concentrations of signalling pathway inhibitors were chosen based on previously published data. Dose–response curves (DRC) to tryptamine by bolus injection (100 μl volume) were constructed in logarithmic increments in the absence and repeated in the presence of continuous infusion of inhibitors in the same preparation. The response of the preceding dose was permitted to return to the base line before the start of the next incremental dose. Each inhibitor was infused for approximately 20 min prior to the commencement of the subsequent DRC. A 30 min washout interval was allowed between successive dose–response curves.
2.3.1. Effects of shear stress
Shear stress, the frictional force generated by blood flow acting on the luminal wall, is known to have a profound effect on the endothelium, and therefore its influence on vascular reactivity (Davies, 1995). Consequently, flow–pressure relationships were determined by incrementally ramping flow rate to achieve a perfusion pressure of 45 mm Hg (Fulep et al., 2002). The flow rate–perfusion pressure relationship was examined in the absence and presence of l-NAME (100 μM).
2.3.2. Drug interventions
To elucidate whether the endothelium could modulate tryptamine-associated vasoconstriction, endothelium denudation was achieved by perfusing distilled water for 4 min through the mesenteric arterial network, followed by perfusion with Kreb's solution for 30 min. The extent of endothelium disruption was confirmed by acetylcholine-induced (10− 7 M) relaxation of pre-constricted vascular bed (10 μM phenylephrine). Tissues exhibiting inhibition of the acetylcholine-induced vasodilatation by more than 50% were considered acceptable for inclusion in the study.
To examine the roles of 5-HT2A receptors, nitric oxide (NO), monoamine transporters, monoamine oxidases A and B (MAOA/B), phospholipase C (PLC), phospholipase A2 (PLA2), Rho-kinases (ROCK), cyclooxygenases 1 and 2 (COX1 and COX2), prostanoid receptors (EP1 and TP) and prostacyclin synthase (PGI2 synthase), DRCs for tryptamine were constructed in the absence and presence of ritanserin (100 pM), l-NAME (100 μM), cocaine (10 μM), U73122 (synthetic aminosteroid compound, 10 μM), PACOCF3 (calcium-independent PLA2 antagonist, 10 μM); indomethacin (a non-selective (COX) inhibitor, 10 μM), nimesulide (selective COX-2 antagonist, 10 μM), tranylcypromine (a prostacyclin synthase and non-specific MAOA and MAOB inhibitor, 10 μM), AH 6809 (PGE2 receptor (EP1 and EP2)/less selective PGD2 receptor (DP) antagonist, 10 μM), ICI 192,605 (TP receptor antagonist; 10 μM), and the ROCK inhibitors, Y-27,632 (10 μM) and fasudil (also known as HA-1077, 20 μM).
2.4. Data and statistical analysis
Responses to each dose of tryptamine were measured as the increase in perfusion pressure from the baseline immediately preceding the first dose. Data are expressed as mean ± S.E.M. n indicates the number of animals used. Individual DRCs were plotted as mean increase in perfusion pressure (mm Hg), and the dose–response curves were fitted to a four parameter logistic model to calculate ED50 values (the concentration of agonist which produces a response halfway between the baseline and maximum response, Emax) using FigP (Biosoft, Cambridge, U.K.). From these quantities, geometric means of ED50 and Emax with 95% confidence limits were computed.
Linear correlation analysis and significances of differences between control and paired Emax and ED50 values were obtained by paired Student's t-test. Comparisons of Emax and ED50 values between different tissues were made by Student's unpaired t-test, and comparisons between more than two groups were made by ANOVA followed by Tukey's multiple comparison test. A P value of < 0.05 was considered to be statistically significant.
2.5. Drugs and chemicals
The following drugs were used and were purchased from Tocris (Bristol, U.K.): AH-6809, fasudil, 1-[6-[[(17b)-3-methoxyestra-1,3,5(10)-trie-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione, ICI-192,605 (4-(Z)-6-(2-o-Chlorophenyl-4-o-hydroxyphenyl-1,3-dioxan-cis-5-yl)hexenoic acid), nimesulide, PACOCF3 (palmitoyl trifluoromethyl ketone), ritanserin, U73122 and Y-27,632. The following drugs were acquired from Sigma-Aldrich (Poole, UK): acetylcholine, cocaine hydrochloride, 5-hydroxytryptamine (5-HT) hydrochloride, indomethacin [1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indole-3-acetic acid], l-NAME (Nω-nitro-l-arginine methyl ester), pargyline, tranylcypromine (trans-2-phenyl-cyclopropylamine hydrochloride), tryptamine hydrochloride, U-46619 (9,11-dideoxy-9a, 11a-methanoepoxy prostaglandin F2a).
All agonists and inhibitors were prepared in distilled water, except indomethacin, U73122, ICI 192,605, ritanserin and PACOCF3 which were dissolved in ethanol and AH6809 which was dissolved in 1.1 eq of NaOH. The stock solutions were stored frozen in aliquots, and when required were thawed and diluted. All drug dilutions were made using Krebs' solution. To eliminate any possible effect of the vehicle on vascular reactivity, the concentration of ethanol used when required was ≤ 0.1% (vol/vol) in the perfusion fluid (Moreau et al., 1997).
3. Results
Basal perfusion pressure was 21.2 ± 0.5 mm Hg for n = 75 animals; unless otherwise indicated, there was no effect of inhibitors on basal perfusion pressure.
3.1. Responses to tryptamine
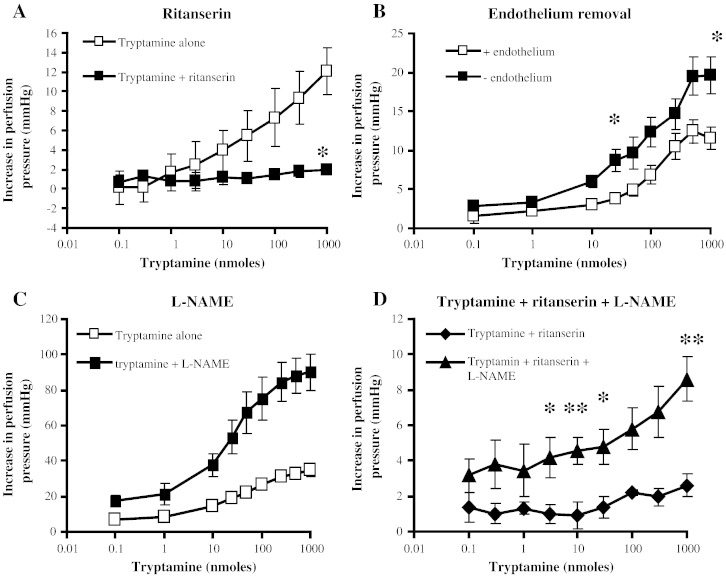
Tryptamine caused dose-related increases in perfusion pressure. These responses were inhibited in the presence of ritanserin (100 pM) (Fig. 1A).
Fig. 1.
Dose–response curves for increases in perfusion pressure by tryptamine of rat isolated perfused mesenteric arterial bed. Doses of tryptamine were administered as individual boluses (nmoles/100 μl). Each response is the mean ± S.E.M. increase in perfusion pressure. A. Dose–response curves for vasoconstrictor responses to tryptamine in the absence (□) and repeated in the presence (■, n = 3) of ritanserin (100 pM). * Significantly different from the absence of ritanserin, P < 0.05. B. Dose–response curves for tryptamine with intact endothelium (□, n = 4) and in de-endothelialised (■, n = 4) mesenteric arterial bed. * Significantly different from intact endothelium, P < 0.05. C. Dose–response curves in the absence (□) and presence (■, n = 7) of l-NAME (100 μM). All points significantly different between tryptamine alone and with l-NAME, P < 0.05. D. Dose–response curves in the presence of ritanserin (◆, 100 pM) and in the additional presence (▲, n = 3) of l-NAME (100 μM). * Significantly greater than ritanserin alone, P < 0.05, ** P < 0.01.
3.2. De-endothelialization and inhibition of nitric oxide synthase (NOS)
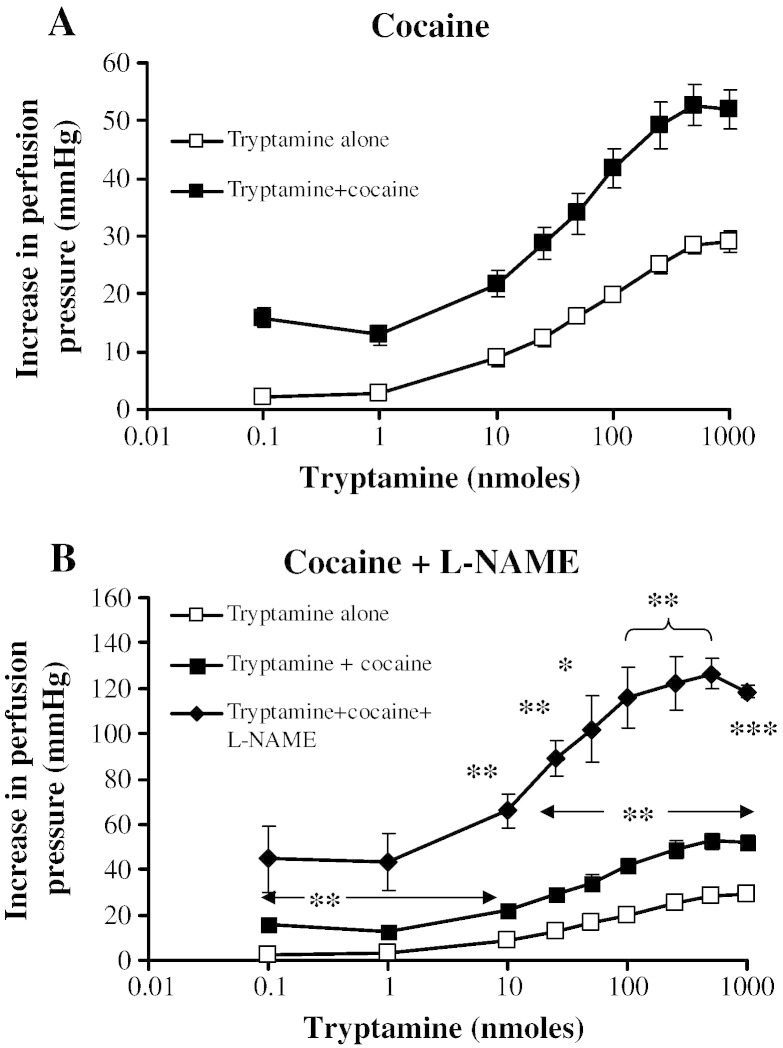
On denudation of the mesenteric arteries, the basal perfusion pressure was significantly increased (13 ± 1 vs 18 ± 1 mm Hg, P < 0.01). Moreover, the removal of endothelium augmented the maximum contractile response to tryptamine (Fig. 1B). In the presence of l-NAME (100 μM) the constrictor responses to tryptamine were potentiated (Fig. 1C) and the maximum response was significantly greater than in the control mesenteries (Table 1). When l-NAME was introduced in the presence of ritanserin (100 pM), small vasoconstrictor responses to tryptamine were reinstated (Fig. 1D).
Table 1.
Potency and maximum vasoconstrictor responses for tryptamine in the absence and presence of inhibitors in the rat isolated perfused mesenteric arterial beds.
| Signalling pathway and inhibitor | Potency (ED50, nmol/100 μl) |
Maximum effects (Emax, mm Hg) |
n |
|---|---|---|---|
| 5-HT2A receptors | |||
| Control | 32.0 (22.4–45.6) | 37.3 ± 3.3 | 9 |
| Ritanserin (100 pM) | 103.9 (14.2–760) | 2.0 ± 0.4$$ | 3 |
| Nitric oxide synthase | |||
| Control | 35.2 (25.9–47.8) | 32 ± 3 | 7 |
| l-NAME (100 μM) | 33.4 (17.4–64.2) | 90.1 ± 10.3⁎⁎⁎ | 7 |
| l-NAME + ritanserin | 9.0 (1.3–61.0) | 8.6 ± 1.2 | 3 |
| Denudation | |||
| + Endothelium | 81.0 (63.3–103.5) | 12.4 ± 1.5 | 4 |
| − Endothelium | 96.8 (24.7–379.2) | 20.0 ± 2.3⁎ | 4 |
| Monoamine transporter | |||
| Control | 46.9 (33.7–65.4) | 27 ± 1 | 4 |
| Cocaine (10− 5 M) | 46.4 (33.2–64. 7) | 51 ± 3⁎⁎ | 4 |
| Cocaine + l-NAME | 22.1 (6.5–75.5)## | 126.4 ± 6.9### | 4 |
| Phospholipase C | |||
| Control | 38.8 (24.1–62.5) | 43 ± 6 | 4 |
| U73,122 (10− 5 M) | ND | 8.6 ± 1.7⁎⁎ | 4 |
| Phospholipase A2 | |||
| Control | 29.6 (12.8–68.4) | 18 ± 2 | 3 |
| PACOCF3 (10− 5 M) | 92.1 (55.5–152.8)⁎ | 18 ± 2 | 3 |
| Rho-kinase | |||
| Control | 26.2 (12.5–54.7) | 20 ± 4 | 3 |
| Y-27,632 (10− 5 M) | 18.9 (5.9–61.0) | 11 ± 3⁎⁎ | 3 |
| Control | 27.50 (13.24–57.1) | 26 ± 3 | 3 |
| Fasudil (2 × 10− 5 M) | 18.9 (4.3–82.6) | 9 ± 1⁎⁎ | 3 |
| COX-1 and COX-2 | |||
| Control | 29.2 (17.0–50.1) | 25 ± 3 | 5 |
| Indomethacin (10− 5 M) | ND | 10.4 ± 1.7⁎⁎ | 5 |
| COX-2 | |||
| Control | 28.9 (12.7–65.7) | 45 ± 1 | 3 |
| Nimesulide (10− 5 M) | 57.6 (52.7–63.0)⁎ | 22 ± 2⁎⁎ | 3 |
| Prostanoid EP1 receptor | |||
| Control | 32.0 (21.1–48.7) | 20 ± 3 | 4 |
| AH-6809 (10− 5 M) | 49.0 (32.6–73.7)* | 14 ± 3 | 4 |
| Prostacyclin synthase/MAO | |||
| Control | 29.4 (21.2–40.8) | 28 ± 7 | 5 |
| Tranylcypromine (10− 5 M) | 20.1 (14.1–28.6)⁎ | 27 ± 3 | 5 |
| Thromboxane TP receptor | |||
| Control | 29.8 (20.5–43.4) | 24 ± 5 | 4 |
| ICI 192605 (10− 6 M) | 28.8 (15.6–53.5) | 27 ± 5 | 4 |
Potency is represented as the geometric mean (with 95% confidence intervals) ED50 (nmole/100 μl) and the maximum response is displayed as arithmetic mean ± S.E.M maximum increase in perfusion pressure (mm Hg). n is the number of animals. * Represents significant differences from paired control values by Student's paired t-test, P < 0.05, ** P < 0.01 and *** P < 0.001. $$ Significant difference from unpaired controls by Student's unpaired t-test, P < 0.01. ## Significant differences between the l-NAME plus cocaine group and control or cocaine alone by one-way ANOVA with Tukey's multiple comparison test, P < 0.003, ###P < 0.0001. ND, not determined.
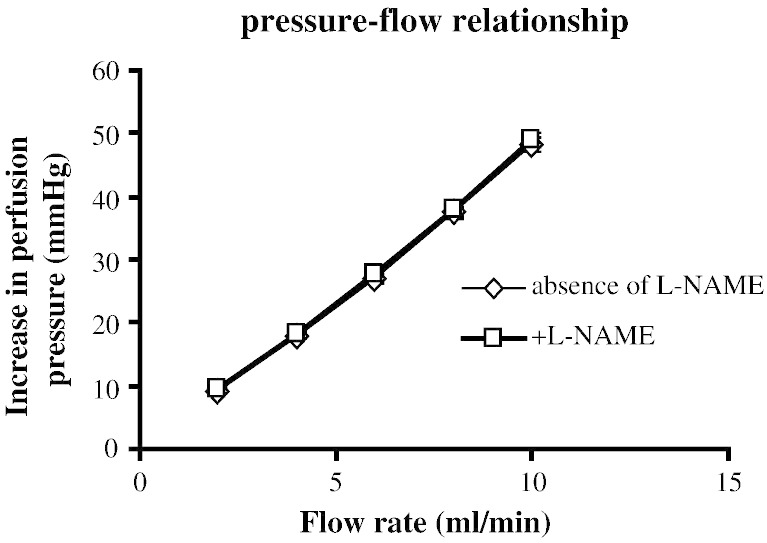
To examine the role of shear stress on the perfusion pressure and whether nitric oxide was released by the increasing shear stress during vasoconstrictor responses, the relationship between flow rate and perfusion pressure was examined. Increasing flow rate resulted in a linear increase in perfusion pressure (Fig. 2). This flow rate–perfusion pressure relationship was identical in the presence of l-NAME (100 μM) (Fig. 2).
Fig. 2.
Correlation between flow rate and increase in perfusion pressure in rat isolated perfused mesenteric arterial bed in the absence (⋄, n = 6, y = 4.9 × − 1.32, r2 = 0.99) and presence (□, n = 6, y = 4.90 × − 0.86, r2 = 0.99) of l-NAME (100 μM).
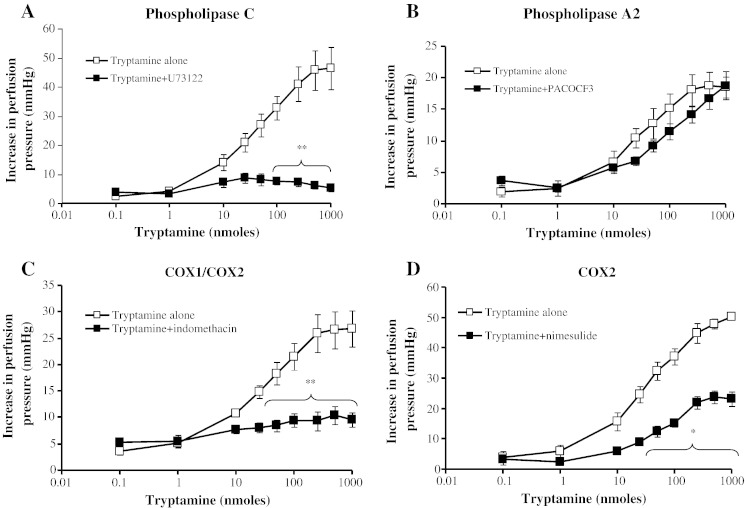
3.3. Phospholipase and cyclo-oxygenase pathways
The non-selective phospholipase C inhibitor, U73122 (1 μM), abolished the tryptamine-induced vasoconstrictor responses of the mesenteric arteries (Fig. 3A and Table 1), and it was not possible to ascertain an ED50 value. The competitive phospholipase A2 (iPLA2) inhibitor, PACOCF3, also antagonised the responses to tryptamine as a shift of the DRC to the right (Fig. 3B), reflected by the reduced potency (Table 1). Indomethacin (a non-specific COX inhibitor) almost completely abolished the vasoconstrictor responses to tryptamine (Fig. 3C), precluding determination of an ED50 value. Nimesulide, a specific COX-2 inhibitor, significantly attenuated the maximum response from 45 ± 1 to 22 ± 2 mm Hg (Fig. 3D).
Fig. 3.
Effects of phospholipase and COX inhibitors on dose–response curves for tryptamine-induced vasoconstriction of rat isolated perfused mesentery. Dose–response curves in the absence (□) and presence (■) of A. phospholipase C inhibitor, U73,122 (10 μM, n = 4), B. phospholipase A2 inhibitor, PACOCF3 (10 μM, n = 3), C. COX-1/COX-2 inhibitor, indomethacin (10 μM, n = 5), and D. COX-2 inhibitor, nimesulide (10 μM, n = 3). * Significantly different points from tryptamine alone, P < 0.05, ** P < 0.01. Each response is the mean ± S.E.M. increase in perfusion pressure.
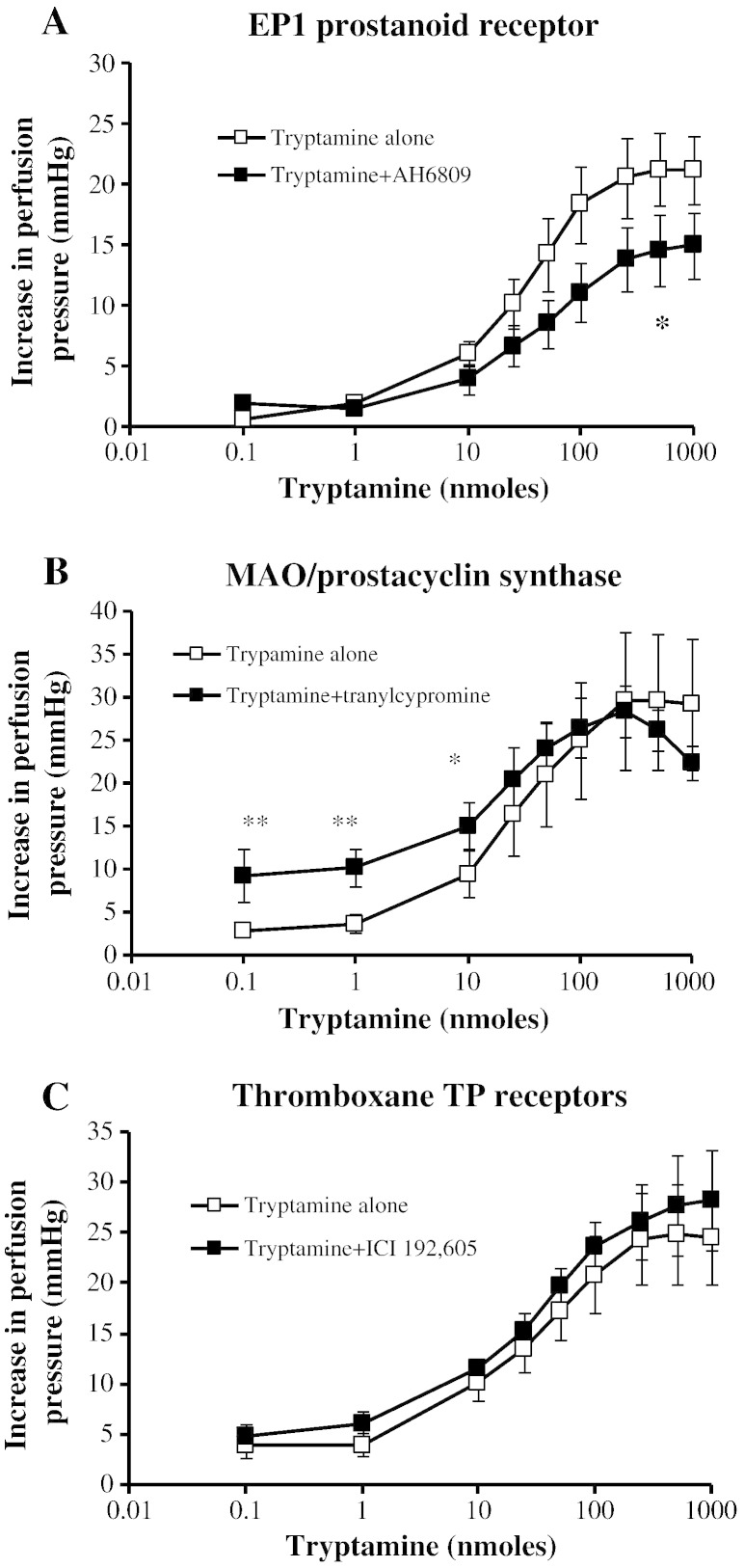
3.4. Prostanoid receptors
The EP1 prostanoid receptor inhibitor, AH6809 (10 μM), shifted the DRC for tryptamine to the right and significantly increased the ED50 value (Fig. 4A). Tranylcypromine (a prostacyclin synthase inhibitor and non-selective MAO-A and MAO-B inhibitor) increased the sensitivity to tryptamine only at the lower concentrations (Fig. 4B). The potency and maximum responses of the mesenteric arterial bed to tryptamine were unaltered in the presence of the thromboxane TP receptor antagonist ICI 192,605 (Fig. 4C).
Fig. 4.
Effects of prostanoid inhibitors on dose–response curves for tryptamine-induced vasoconstriction of rat isolated perfused mesentery. Dose–response curves in the absence (□) and presence (■) of A. prostaglandin EP1 receptor antagonist, AH-6809 (10 μM, n = 4), B. prostacyclin synthase/MAO inhibitor, tranylcypromine (10 μM, n = 5), and C. thromboxane TP receptor antagonist, ICI 192,605 (1 μM, n = 4). * Significantly different points from tryptamine alone, P < 0.05, ** P < 0.01. Each response is the mean ± S.E.M. increase in perfusion pressure.
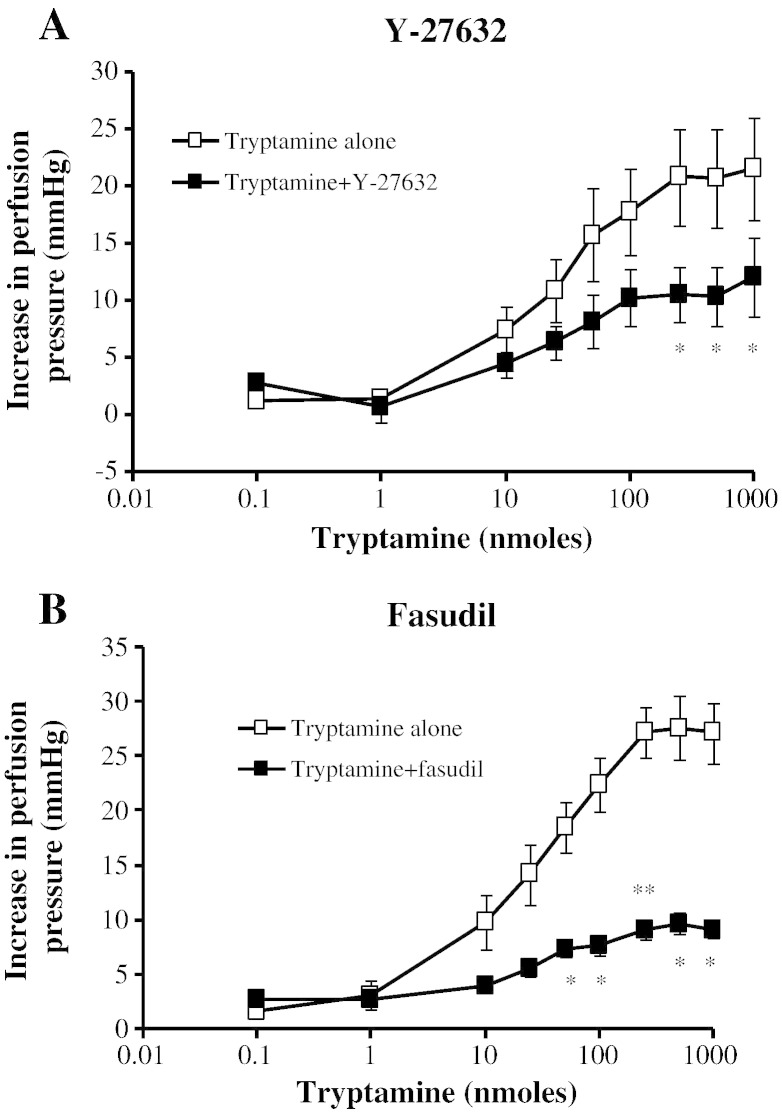
3.5. Rho-kinase inhibitors
The tryptamine-dependent vasoconstriction was inhibited by the Rho/Rho-kinase inhibitors. The maximum response to tryptamine was significantly inhibited to 45% by Y-27632 (Fig. 5A) and to 65% by fasudil (Fig. 5B).
Fig. 5.
Effects of Rho-kinase inhibitors on dose–response curves for tryptamine-induced vasoconstriction of rat isolated perfused mesentery. Dose–response curves in the absence (□) and presence (■) of A. Y-27632 (10 μM, n = 3) and B. fasudil (HA-1077, 20 μM, n = 3). * Significantly different from tryptamine alone, P < 0.05, ** P < 0.01. Each response is the mean ± S.E.M. increase in perfusion pressure.
3.6. Monoamine transporter
The presence of the neuronal amine transport inhibitor, cocaine (10 μM), significantly enhanced the tryptamine vasoconstrictor responses without altering the sensitivity (Fig. 6A and Table 1). When cocaine (10 μM) and l-NAME (100 μM) were combined, there was an additional potentiation, the maximum vasoconstriction reaching 126.4 ± 6.9 mm Hg, which was significantly greater than with l-NAME alone (90.1 ± 10.3 mm Hg).
Fig. 6.
Effects of the monoamine transport inhibitor, cocaine, on dose–response curves for tryptamine-induced vasoconstriction of rat isolated perfused mesentery. Dose–response curves in the absence (□) and presence (■) of cocaine (10 μM, n = 4) and, in separate experiments (B.), in the additional presence (♦) of l-NAME (100 μM, n = 4). All points in A. significantly different between tryptamine alone and with cocaine, P < 0.05. In B. * Significantly different from tryptamine alone, P < 0.05, ** P < 0.01, *** P < 0.001. Each response is the mean ± S.E.M. increase in perfusion pressure.
4. Discussion
This study has confirmed previous reports that tryptamine exerts vasoconstrictor properties in the rat isolated mesenteric arteries (Watts et al., 1994) and in the rat isolated perfused mesenteric arterial bed (Anwar et al., 2012). Tryptamine also causes vasoconstriction in rat (Fehler et al., 2010) and rabbit (Stollak and Furchgott, 1983) aorta and rat caudal arteries (Hicks and Langer, 1983, Bradley et al., 1985). This response would explain the increases in blood pressure observed when trypamine is administered to dogs (Eble, 1965). The tryptamine induced pressor response of the mesenteric bed was inhibited by the 5-HT2A receptor antagonist, ritanserin, indicating that the vasoconstriction is largely via activation of 5-HT2A receptors. This is at variance with the vasoconstriction in other vessels such as the rat aorta where the vasoconstriction by tryptamine is not inhibited by another 5-HT2A receptor antagonist, ketanserin (Fehler et al., 2010), although in the rabbit aorta (Stollak and Furchgott, 1983) and rat tail artery (Bradley et al., 1985), 5-HT antagonists were effective. The main aim of this study was to determine the signalling pathways for this 5-HT2A-mediated vasoconstriction by tryptamine by the use of appropriate inhibitors. Secondly, we examined whether the response was modulated through co-activation of relaxant signalling mechanisms.
4.1. Contractile transducers
4.1.1. Phospholipid signalling cascades
Activation of G protein-coupled receptors, such as 5-HT2A receptors, stimulates phospholipase C, the catalysed products of which funnel out to further amplify downstream signalling transducers of smooth muscle contractile responses. Stimulation of phospholipase C catalyses the hydrolysis of the phosphorylated lipid, phosphatidylinositol 4, 5-bisphosphate (PIP2) to produce second messengers, inositol 1, 4, 5-trisphosphate (IP3) and diacylglycerol (DAG), (Rhee, 2001, Fig. 7). IP3 through the activation of IP3 receptors (IP3Rs), located on store-operated calcium channels, mobilises calcium into the cytosol from intracellular stores (sarcoplasmic/endoplasmic reticulum, Golgi complex and the nuclear envelope), leading to contraction (Berridge, 1993). Tryptamine activates heterotrimeric G protein-coupled 5-HT2A receptors, which are linked to the PLC signalling system since the contractile response was completely eliminated by U73122, a putative blocker of PLC (Osol et al., 1993). DAG, the other transducer arising from PLC activation, stimulates protein kinase C (PKC), (Nishizuka, 1995). DAG activation of PKC is the initial step in the prostaglandin biosynthetic pathway initiated by activation of a family of phospholipase A2 (PLA2) isozymes [secretory PLA2s (sPLA2), the cytosolic PLA2s (cPLA2), calcium-independent PLA2s (iPLA2) and the platelet activating factor (PAF) acid hydrolases]. These are primarily responsible for agonist-induced hydrolysis of the sn-2 ester bonds in membrane phospholipids, such as phosphotidyl choline and phosphotidylethanolamines, releasing arachidonic acid and lysophospholipids (Schaloske and Dennis, 2006; Fig. 7).
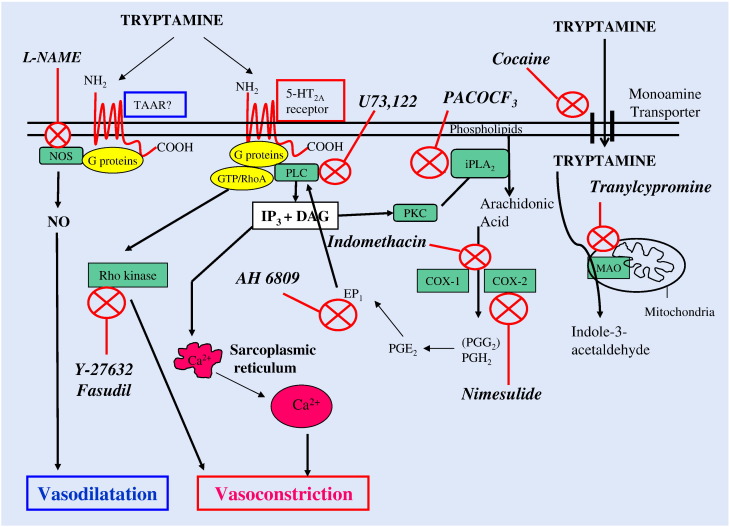
Fig. 7.
Schematic diagram showing the proposed signal transduction pathways for the vasoconstrictor and vasodilator responses to tryptamine of rat isolated mesenteric vascular bed mediated via 5-HT2A and trace amine-associated receptors (TAAR), respectively. Inhibitors of signalling intermediates are shown in italics and their site of interaction by a red cross ( ).
).
The present investigation has shown that a Ca2 +-independent PLA2 is involved in tryptamine-induced vasoconstriction, since the response was significantly attenuated by the iPLA2 inhibitor, PACOCF3. Previous studies have confirmed the iPLA2 blocking activity of PACOCF3 (Ackerman et al., 1995) and demonstrated the PKC-dependent promoting activity of calcium-independent phospholipase A2 β (iPLA2β, group VIB PLA2) and liberation of arachidonic acid (Jenkins et al., 2002, Akiba and Sato, 2004). Moreover, diacylglycerol has been shown to be a substrate for DAG lipase, which leads to the synthesis of 2-arachidonylglycerol, which is metabolised by monoacylglycerol lipase or fatty acid amidohydrolase to also yield arachidonic acid (Tang et al., 2006).
Interestingly, our results differ from the 5-HT2A receptor-mediated PLC and PLA2 signalling pathways in NIH3T3–5HT2A fibroblast cells, which are independently coupled to the receptor (Kurrasch-Orbaugh et al., 2003). This is perhaps due to a. overexpression of the receptor in NIH3T3 fibroblasts, b. the different cell types involved, or c. the static milieu of cell culture as opposed to the dynamic environment of the perfused isolated vascular bed.
4.1.2. Cyclooxygenases
Conversion of arachidonic acid to PGG2 is via a cyclooxygenase reaction, which is followed by a peroxidase reaction to PGH2, these are the committed steps in prostanoid biosynthesis, and both are mediated by two prostaglandin synthases or cyclooxygenases (COX-1 and COX-2) (Simmons et al., 2004).
Cyclooxygenases play a pivotal role in prostaglandin and/or thromboxane synthesis and their consequent vasoconstrictor effects (Simmons et al., 2004). The present study implicates both COX isoforms as constitutive enzymes in mediating vasoconstriction of the mesenteric arteries by tryptamine. The non-selective COX inhibitor, indomethacin, virtually abolished the vasoconstrictor responses to tryptamine whereas the COX-2-selective inhibitor, nimesulide, only halved the maximum response. This leads to the conclusion that both isoforms are involved in the vasoconstrictor response. Previous reports of expression of the two COX isozymes in different tissues, including the vasculature, from human and animal studies are in accord with our results (Ermert et al., 1998, Wang et al., 2005, Ho and Randall, 2007, Trappe et al., 2008). It is important to note that the abolition of the vasopressor responses by indomethacin indicates that the lipoxygenase and the cytochrome P-450 monoxygenase pathways are not involved in the tryptamine-mediated vasoconstriction.
4.1.3. Prostanoids
The PGH2 derived from cyclooxygenase activation is subsequently converted to a variety of bioactive prostanoids, such as thromboxane (TxA2) and prostaglandins F2α, D2, E2 and I2 (prostacyclin), depending on the downstream enzymatic machinery present in a particular cell type (Coleman et al., 1994, Breyer et al., 2001). Prostaglandin PGE2 has been implicated in a plethora of physiological processes in vascular smooth muscle and endothelial cells, including vascular tone, cellular signalling, proliferation, migration and tubulogenesis (Sugimoto and Narumiya, 2007). PGE2 exerts the majority of these pleiotropic actions in diverse tissues through a family of four G protein-coupled heptahelical cell surface receptors (EP1 to EP4), (Narumiya et al., 1999). The EP1 receptor antagonist, AH-6809, significantly inhibited the tryptamine-induced vasoconstriction, illustrating that PGE2 is probably the main prostanoid generated which induces tryptamine vasoconstriction in the mesenteric arterial bed via EP1 receptors. Stimulation of the EP1 receptor, through a Gq protein, activates PLC/inositol triphosphate and protein kinase C (PKC) signalling and is coupled to intracellular Ca2 + elevation (Boie et al., 1997), resulting in vasoconstriction (Fig. 7). In support of a role for EP1 receptors in mediating vasoconstrictor responses, PGE2- and 17-phenyl-trinor-PGE2 (selective EP1 receptor agonist)-induced vasoconstriction in isolated pressurised gracilis muscle arterioles of db/db mice was attenuated by pre-treatment with AH6809 (Rutkai et al., 2009). We next examined whether thromboxanes and thromboxane receptors (TP) were involved in the vasoconstrictor response. The thromboxane receptor inhibitor ICI 192,605 had no effect on the vasoconstrictor responses to tryptamine. Previous studies have shown that vasoconstriction of rat mesenteric arteries by PGE2 was unaffected by the thromboxane A2 (TxA2) receptor blocker SQ 29,548, whereas the response to PGF2α was abolished. The thromboxane A2 mimetic, U-46619, also contracted the rat mesenteric resistance arteries but was inhibited by an EP1 receptor antagonist (SC-19220) and attributed to release of PGE2 by U-46619 into the mesenteric perfusate (Bolla et al., 2004). A link can therefore be established in the vasoconstrictor response to tryptamine between activation of phospholipases C and A2, liberation of arachidonic acid and generation of the vasoconstrictor prostaglandin E2 via cyclooxygenase. A similar link has been shown in cultures of A-10 vascular smooth muscle cells isolated from wild-type mice, where iPLA2β catalysed the liberation of arachidonic acid which binds to COX-2 to produce PGE2. In contrast, the concentration of PGE2 was dramatically reduced in media obtained from iPLA2β-null mice VSMC cultures (Moon et al., 2008). Thus, tryptamine-induced contraction appears to be mediated via the prostanoid PGE2 through EP1 receptors, but there is no participation of thromboxanes or receptors for TxA2.
4.1.4. Rho-kinases
Heterotrimeric G-proteins of the Gα12 and Gα13 family transduce signals emanating from GPCRs to activate the low molecular weight guanosine triphosphate (GTP)-binding protein RhoA, a member of the Ras family of proteins, and its downstream target, Rho-kinase (a p160 Rho-associated coiled-coil-containing protein kinase, a serine/threonine specific kinase). Rho-kinase has 2 isoforms: ROKα/ROCKII and ROKβ/ROCKI, which are important regulators of vascular tone (Somlyo and Somlyo, 2003). During this calcium-independent process, Rho-kinase causes inhibition of myosin light chain phosphatase activity by phosphorylation of its myosin-binding subunit (a regulatory domain), resulting in elevated vascular tension. This phenomenon is referred to as calcium sensitisation (Uehata et al., 1997).
We have demonstrated that the 5-HT2A receptors stimulated by tryptamine are coupled to the Rho/ROCK signalling pathway by way of attenuation of the vasoconstrictor response to tryptamine by fasudil (active component: hydroxyfasudil) and Y-27632. Fasudil and Y-27632 block the activity of Rho-kinase by competing with the ATP-binding site on the enzyme (Jacobs et al., 2006).
4.2. Dilator mediators
4.2.1. Nitric oxide
Inhibition of NO release with the nitric oxide synthase inhibitor, l-NAME, caused almost three-fold increase in the maximum vasoconstriction of the rat mesentery by tryptamine. Similarly, endothelium denudation potentiated the maximum response. The heterotrimeric G protein Gα12 is coupled to eNOS leading to elevated intracellular concentrations of eNOS (Andreeva et al., 2006). The effects of NO are mediated primarily through the direct activation of soluble guanylate cyclase, generating cyclic guanosine monophosphate (cGMP) (Andreopoulos and Papapetropoulos, 2000, Lucas et al., 2000), which stimulates protein kinase G (PKG, also termed cGMP-targeted kinase, cGK), which can suppress Gαq stimulation by interaction with regulator of G-protein coupled signalling 2 (RGS2) (Hofmann et al., 2000). To note, vasodilation of mice aortic rings by PGE2 interaction with the EP4 receptor results in NO formation (Hristovska et al., 2007). The high bioavailability of NO counteracts the contractile actions of COX-metabolites (Miyamoto et al., 2007) and Rho-kinase (Sauzeau et al., 2000). Consequently, NO serves as a homeostatic buffer against incremental or excessive vasoconstriction and smoothes out excessive fluctuations in blood pressure.
When NO was inhibited by l-NAME after blockade of the tryptamine vasoconstriction by the 5-HT2A antagonist, ritanserin, small vasoconstrictor responses were restored. These cannot be due to 5-HT2A receptor stimulation overcoming the ritanserin blockade as the doses of tryptamine are unchanged. Our previous study showed that in the presence of 5-HT2A receptor blockade and with perfusion pressure raised by perfusion with phenylephrine, tryptamine causes dose-related vasodilatation which is NO-mediated (Anwar et al., 2012). When this vasodilator action is removed by l-NAME, a vasoconstriction appears. This was not examined further but may be due to activation of trace amine-associated receptors, which appear to mediate vasoconstrictor responses to tryptamine and other trace amines in other blood vessels such as coronary arteries (Herbert et al., 2008) and rat aorta (Fehler et al., 2010).
4.2.2. Shear stress
According to the Hagen–Poiseuille equation, vascular resistance is a function of vascular geometry (radius and length of vessel) and viscosity of fluid (η). The resistance of a blood vessel is related to the inverse of the fourth power of vessel diameter and therefore small reductions in diameter have significant consequences for vascular resistance. Graded increases in flow rate through the rat mesenteric bed induced by raising the pump flow rate were associated with corresponding rises in perfusion pressure. This is a reflection of an increase in vascular smooth muscle tone due to elevations in wall shear stresses, which leads to an increase in vascular resistance. The possibility was considered that these increases in shear stress might cause release of vasodilator NO which could dampen the pressure increases. However, inhibition of NO synthesis with l-NAME had no influence on flow rate–perfusion pressure relationship. Our results are consistent with the findings of unchanged perfusion pressure after incubation with l-NAME in the non-pregnant rat isolated uterine bed (Fulep et al., 2002) and non-pregnant rat isolated perfused mesenteric arteries (Cockell and Poston, 1996). However, they contrast with flow experiments on cultured endothelial cells (Kuchan and Frangos, 1994), where NO was found to be released. Therefore, in our study NO release can be attributed to tryptamine stimulation of post-receptor pathways and not as a result of any shear forces exerted on the luminal wall by the vasoconstriction. The possibility, however, arises that increasing perfusion pressure by pump-mediated increases in flow does not entirely mimic the increase due to vasoconstriction. The possibility must be considered that a part of the NO release by tryptamine results from conformational changes of endothelial cells arising from vasoconstriction. The effects of l-NAME on a wider range of vasoconstrictor agents acting via different receptors would be required to test this idea further.
It is worth mentioning that links have been established between nitric oxide and the Rho-kinase signal transduction pathways. NO can cause vasodilatation through inhibition of the RhoA/Rho-kinase (ROCK) signalling pathway in vascular smooth muscle (Sauzeau et al., 2000), rat coelic artery (Teixeira et al., 2005) and rat aorta (Chitaley and Webb, 2002). On the other hand, the RhoA/Rho-kinase pathway prevents protein kinase B/Akt-dependent eNOS activity in human endothelial cells (Ming et al., 2002). A more recent study has provided evidence that Rho-kinase signalling activity was amplified in endothelial nitric oxide synthase (eNOS) null mice (Williams et al., 2006). Further complexity arises from results suggesting that arachidonic acid generated by phospholipids can activate ROCK (Araki et al., 2001, Guo et al., 2003), and perhaps contribute to Ca2 +-sensitization by tryptamine.
4.2.3. Prostacyclin
Prostacyclin (PGI2) is a vasodilator prostanoid produced by endothelial cells as a key mediator in the regulation of vascular tone and blood pressure. Thus potentiation of the tryptamine-elicited vasoconstriction by endothelial cell denudation can be partly explained by removal of prostacyclin as well as removal of vasodilator nitric oxide. PGI2 exerts its cellular effects by binding to a G protein-coupled receptor, IP. Stimulation of the IP receptor, coupled to Gs-type G protein, activates adenylate cyclase leading to cAMP formation, and therefore to vasodilatation of the mesenteric vessels (Hata and Breyer, 2004). We examined whether tryptamine would release prostacyclin by use of a potent prostacyclin synthase antagonist, tranylcypromine (Xavier et al., 2008). The vasoconstrictor response was augmented at the lower doses of tryptamine, suggesting that at lower concentrations prostacyclin was indeed released by tryptamine. However, tranylcypromine is also a non-selective inhibitor of monoamine oxidases (MAO) A and B (Blackwell, 1963). MAO A and B activities appear to be associated with the mesenteric arteries of various species (Coquil et al., 1973, Caramona, 1982). Thus, an alternative explanation for the enhanced responses at lower doses is that tryptamine is metabolised by MAO in the mesenteric bed and its inhibition by tranylcypromine allows elevated levels to reach the receptors.
4.3. Monoamine transporters
Cocaine is a nonselective, competitive inhibitor of monoamine reuptake, inhibiting the dopamine (DAT), noradrenaline (NAT) and 5-HT (serotonin, SERT) transporters with Ki values of 267 nM, 872 nM and 392 nM, respectively (Torres et al., 2003). Therefore, cocaine is over 2-fold more potent at the serotonin than the noradrenaline transporter. Tryptamine is a substrate for the serotonin transporter (Segonzac et al., 1985, Adkins et al., 2001) and mesenteric arteries are known to express the SERT (Ni et al., 2004). Thus, inhibition of the transporter would be expected to increase the tryptamine concentration in mesenteric circulation. Indeed, cocaine moderately potentiated the contractile response of mesenteric arteries to tryptamine. When cocaine was combined with l-NAME, there was an additional potentiation which we assume is due to the combined additive effects of inhibition of tryptamine transport and removal of the opposing vasodilator effects of released NO. There is one recent report of regulation of nitric oxide enhancing the activity of the noradrenaline transporter to control blood pressure responses to tyramine in anaesthetized rats (Simaan and Sabra, 2011). However, this does not appear to apply to tryptamine responses here, as l-NAME would have opposed the action of cocaine rather than enhancing it.
4.4. Summary
In summary, tryptamine causes vasoconstriction of rat mesenteric arterial beds which is mediated via 5-HT2A receptors. This response is due to a coupling between the tryptaminergic receptors, phospholipases C and A2 and contractile prostaglandins (PGE2). Signalling through the RhoA/ROCK pathway is also implicated. There is a simultaneous release of vasodilator nitric oxide and possibly prostacyclin from the endothelium which oppose and homeostatically balance the increases in pressure. Thus, circulating levels of tryptamine derived from endogenous synthesis or from dietary intake can exert a regulatory control of mesenteric blood flow and thus the digestive and absorptive activities of the gastrointestinal tract.
Conflict of interest disclosure
The authors declare no conflicts of interest.
Acknowledgement
This work was supported in part by a Wellcome Trust Fellowship (#: 069301/Z/02/Z) awarded to M.A.A. and by the British Heart Foundation (Project Grant PG/09/021).
References
- Ackerman E.J., Conde-Frieboes K., Dennis E.A. Inhibition of macrophage Ca2 +-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J. Biol. Chem. 1995;270:445–450. doi: 10.1074/jbc.270.1.445. [DOI] [PubMed] [Google Scholar]
- Adkins E.M., Barker E.L., Blakely R.D. Interactions of tryptamine derivatives with serotonin transporter species variants implicate transmembrane domain 1 in substrate recognition. Mol. Pharmacol. 2001;59:514–523. doi: 10.1124/mol.59.3.514. [DOI] [PubMed] [Google Scholar]
- Akiba S., Sato T. Cellular function of calcium-independent phospholipase A2. Biol. Pharm. Bull. 2004;28:1174–1178. doi: 10.1248/bpb.27.1174. [DOI] [PubMed] [Google Scholar]
- Andreeva A.V., Vaiskunaite R., Kutuzov M.A., Profirovic J., Skidgel R.A., Voyno-Yasenetskaya T.A. Novel mechanisms of G protein-dependent regulation of endothelial nitric-oxide synthase. Mol. Pharmacol. 2006;69:975–982. doi: 10.1124/mol.105.018846. [DOI] [PubMed] [Google Scholar]
- Andreopoulos S., Papapetropoulos A. Molecular aspects of soluble guanylyl cyclase regulation. Gen. Pharmacol. 2000;34:147–157. doi: 10.1016/s0306-3623(00)00062-8. [DOI] [PubMed] [Google Scholar]
- Anwar M.A., Ford W.R., Broadley K.J. Mediators regulating tryptamine-induced tone of rat isolated mesenteric arterial bed. Br. Pharmacol. Soc. 2006 (pA2 online 4, 113P) [Google Scholar]
- Anwar M.A., Ford W.R., Broadley K.J. Role of cyclooxygenases (COX) metabolites in tryptamine-elicited vasopressor responses in rat isolated mesenteric arterial bed. J. Vasc. Res. 2008;45(Suppl. 2):109. (PTC2) [Google Scholar]
- Anwar M.A., Ford W.R., Broadley K.J., Herbert A.A. Vasoconstrictor and vasodilator responses to tryptamine of rat-isolated perfused mesentery: comparison with tyramine and β-phenylethylamine. Br. J. Pharmacol. 2012;165:2191–2202. doi: 10.1111/j.1476-5381.2011.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki S., Ito M., Kureishi Y., Feng J., Machida H., Isaka N., Amano M., Kaibuchi K., Hartshorne D.J., Nakano T. Arachidonic acid-induced Ca2 + sensitization of smooth muscle contraction through activation of Rho-kinase. Pflugers Arch. 2001;441:596–603. doi: 10.1007/s004240000462. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Blackwell B. Hypertensive crisis due to monoamine-oxidase inhibitors. Lancet. 1963;2:849–850. doi: 10.1016/s0140-6736(63)92743-0. [DOI] [PubMed] [Google Scholar]
- Boie Y., Stocco R., Sawyer N., Slipetz D.M., Ungrin M.D., Neuschäfer-Rube F., Püschel G.P., Metters K.M., Abramovitz M. Molecular cloning and characterization of four rat prostaglandin E2 prostanoid receptor subtypes. Eur. J. Pharmacol. 1997;340:227–241. doi: 10.1016/s0014-2999(97)01383-6. [DOI] [PubMed] [Google Scholar]
- Bolla M., You D., Loufrani L., Levy B.I., Levy-Toledano S., Habib A., Henrion D. Cyclooxygenase involvement in thromboxane-dependent contraction in rat mesenteric resistance arteries. Hypertension. 2004;43:1264–1269. doi: 10.1161/01.HYP.0000127438.39744.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky B., Adham N., Jones K.A., Raddatz R., Artymyshyn R., Ogozalek K.L., Durkin M.M., Lakhlani P.P., Bonini J.A., Pathirana S., Boyle N., Pu X., Kouranova E., Lichtblau H., Ochoa F.Y., Branchek T., Gerald C. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc. Natl. Acad. Sci. U. S. A. 2001;98:8966–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley P.B., Humphrey P.P.A., Williams R.H. Tryptamine-induced vasoconstrictor responses in rat caudal arteries are mediated predominantly via 5-hydroxytryptamine receptors. Br. J. Pharmacol. 1985;84:919–925. doi: 10.1111/j.1476-5381.1985.tb17386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breyer R.M., Bagdassarian C.K., Myers S.A., Breyer M.D. Prostanoid receptors: subtypes and signaling. Annu. Rev. Pharmacol. Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- Broadley K.J. The vascular effects of trace amines and amphetamines. Pharmacol. Ther. 2010;125:363–375. doi: 10.1016/j.pharmthera.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Broadley K.J., Anwar M.A., Herbert A.A., Fehler M., Jones E.M., Davies W.E., Kidd E.J., Ford W.R. Effects of dietary amines on the gut and its vasculature. Br. J. Nutr. 2009;101:1645–1652. doi: 10.1017/S0007114508123431. [DOI] [PubMed] [Google Scholar]
- Bunzow J.R., Sonders M.S., Arttamangkul S., Harrison L.M., Zhang G., Quigley D.I., Darland T., Suchland K.L., Pasumamula S., Kennedy J.L., Olson S.B., Magenis R.E., Amara S.G., Grandy D.K. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol. Pharmacol. 2001;60:1181–1188. doi: 10.1124/mol.60.6.1181. [DOI] [PubMed] [Google Scholar]
- Caramona M.M. Monoamine oxidase of types A and B in the saphenous vein and mesenteric artery of the dog. Naunyn Schmiedbergs Arch. Pharmacol. 1982;319:121–124. doi: 10.1007/BF00503923. [DOI] [PubMed] [Google Scholar]
- Chitaley K., Webb R.C. Nitric oxide induces dilation of rat aorta via inhibition of Rho-kinase signaling. Hypertension. 2002;39:438–442. doi: 10.1161/hy02t2.102960. [DOI] [PubMed] [Google Scholar]
- Cockell A.P., Poston L. Isolated mesenteric arteries from pregnant rats show enhanced flow-mediated relaxation but normal myogenic tone. J. Physiol. 1996;495:545–551. doi: 10.1113/jphysiol.1996.sp021614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R.A., Smith W.L., Narumiya S. International union of pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- Coquil J.F., Goridis C., Mack G., Neff N.H. Monoamine oxidase in rat arteries: evidence for different forms and selective localization. Br. J. Pharmacol. 1973;48:590–599. doi: 10.1111/j.1476-5381.1973.tb08245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P.F. Flow-mediated endothelial mechanotransduction. Physiol. Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble J.N. A study of the potentiation of tryptamine by monamine oxidase inhibitors in the dog. J. Pharmacol. Exp. Ther. 1965;148:48–53. [PubMed] [Google Scholar]
- Ermert L., Ermert M., Goppelt-Struebe M., Walmrath D., Grimminger F., Steudel W., Ghofrani H.A., Homberger C., Duncker H.-R., Seeger W. Cyclooxygenase isoenzyme localization and mRNA expression in rat lungs. Am. J. Respir. Cell Mol. Biol. 1998;18:479–488. doi: 10.1165/ajrcmb.18.4.2939. [DOI] [PubMed] [Google Scholar]
- Fehler M., Broadley K.J., Ford W.R., Kidd E.J. Identification of trace amine-associated receptors (TAAR) in the rat aorta and their role in vasoconstriction by β-phenylethylamine. Naunyn Schmiedebergs Arch. Pharmacol. 2010;382:385–398. doi: 10.1007/s00210-010-0554-1. [DOI] [PubMed] [Google Scholar]
- Fulep E.E., Vedernikov Y.P., Saade G.R., Garfield R.E. Flow rate-perfusion relationships in situ in the uterine circulation of pregnant rats. Am. J. Obstet. Gynecol. 2002;186:1022–1026. doi: 10.1067/mob.2002.122418. [DOI] [PubMed] [Google Scholar]
- Guo Z., Su W., Smith G.M., Gong M.C. Ca2 +-independent phospholipase A2 is required for agonist-induced Ca2 +-sensitization of contraction in vascular smooth muscle. J. Biol. Chem. 2003;278:1856–1863. doi: 10.1074/jbc.M211075200. [DOI] [PubMed] [Google Scholar]
- Hata A.N., Breyer R.M. Pharmacology and signalling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol. Ther. 2004;103:147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Herbert A.A., Kidd E.J., Broadley K.J. Dietary trace amine-dependent vasoconstriction in porcine coronary artery. Br. J. Pharmacol. 2008;155:525–534. doi: 10.1038/bjp.2008.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks P.E., Langer S.Z. Antagonism by tetra-hydro-β-carboline of the vasoconstrictor responses to tryptamine in rat tail arteries. Eur. J. Pharmacol. 1983;96:145–149. doi: 10.1016/0014-2999(83)90543-5. [DOI] [PubMed] [Google Scholar]
- Ho W.-S.V., Randall M.D. Endothelium-dependent metabolism by endocannabinoid hydrolases and cyclooxygenases limits vasorelaxation to anandamide and 2-arachidonoylglycerol. Br. J. Pharmacol. 2007;150:641–651. doi: 10.1038/sj.bjp.0707141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann F., Ammendala A., Schlossman J. Rising behind NO:cGMP-dependent protein kinases. J. Cell Sci. 2000;113:1671–1676. doi: 10.1242/jcs.113.10.1671. [DOI] [PubMed] [Google Scholar]
- Hristovska A.M., Rasmussen L.E., Hansen P.B., Nielsen S.S., Nüsing R.M., Narumiya S., Vanhoutte P., Skøtt O., Jensen B.L. Prostaglandin E2 induces vascular relaxation by E-prostanoid 4 receptor-mediated activation of endothelial nitric oxide synthase. Hypertension. 2007;50:525–530. doi: 10.1161/HYPERTENSIONAHA.107.088948. [DOI] [PubMed] [Google Scholar]
- Ishitani R., Kimura M., Takeichi M., Chuang D.-M. Tryptamine induces phosphoinositide turnover and modulates adrenergic and muscarinic cholinergic receptor function in cultured cerebellar granule cells. J. Neurochem. 1994;63:2080–2085. doi: 10.1046/j.1471-4159.1994.63062080.x. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Hayakawa K., Swenson L., Bellon S., Fleming M., Taslimi P., Doran J. The structure of dimeric ROCK1 reveals the mechanisms for ligand selectivity. J. Biol. Chem. 2006;281:260–268. doi: 10.1074/jbc.M508847200. [DOI] [PubMed] [Google Scholar]
- Jenkins C.M., Han X., Mancuso D.J., Gross R.W. Identification of calcium-independent phospholipase A2 (iPLA2) β, and not iPLA2γ, as the mediator of arginine vasopressin-induced arachidonic acid release in A-10 smooth muscle cells. Enantioselective mechanism-based discrimination of mammalian iPLA2s. J. Biol. Chem. 2002;277:32807–32814. doi: 10.1074/jbc.M202568200. [DOI] [PubMed] [Google Scholar]
- Kuchan M.J., Frangos J.A. Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. Am. J. Physiol. 1994;266:C628–C636. doi: 10.1152/ajpcell.1994.266.3.C628. [DOI] [PubMed] [Google Scholar]
- Kurrasch-Orbaugh D.M., Watts V.J., Barker E.L., Nichols D.E. Serotonin 5-hydroxytryptamine2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J. Pharmacol. Exp. Ther. 2003;304:229–237. doi: 10.1124/jpet.102.042184. [DOI] [PubMed] [Google Scholar]
- Lucas K.A., Pitari G.M., Kazerounians S., Ruiz-Stewart I., Park J., Schulz S., Chepenik K.P., Waldman S.A. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol. Rev. 2000;52:375–413. [PubMed] [Google Scholar]
- McGregor D.D. The effect of sympathetic nerve stimulation on vasoconstrictor response in perfused mesenteric blood vessels of the rat. J. Physiol. 1965;177:21–30. doi: 10.1113/jphysiol.1965.sp007572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X.F., Viswambharan H., Barandier C., Ruffieux J., Kaibuchi K., Rusconi S., Yang Z. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol. Cell. Biol. 2002;22:8467–8477. doi: 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto A., Hashiguchi Y., Obi T., Ishiguro S., Nishio A. Ibuprofen or ozagrel increases NO release and L-nitro arginine induces TxA2 release from cultured porcine basilar arterial endothelial cells. Vasc. Pharmacol. 2007;46:85–90. doi: 10.1016/j.vph.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Moon S.H., Jenkins C.M., Mancuso D.J., Turk J., Gross R.W. Smooth muscle cell arachidonic acid release, migration, and proliferation are markedly attenuated in mice null for calcium-independent phospholipase A2β. J. Biol. Chem. 2008;283:33975–33987. doi: 10.1074/jbc.M805817200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P., Takase H., Kung C.F., Shaw S., Luscher T.F. Blood pressure and vascular effects of endothelin blockade in chronic nitric oxide-deficient hypertension. Hypertension. 1997;29:763–769. doi: 10.1161/01.hyp.29.3.763. [DOI] [PubMed] [Google Scholar]
- Narumiya S., Sugimoto Y., Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Ni W., Thompson J.M., Northcott C.A., Lookingland K., Watts S.W. The serotonin transporter is present and functional in peripheral arterial smooth muscle. J. Cardiovasc. Pharmacol. 2004;43:770–781. doi: 10.1097/00005344-200406000-00006. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- Osborne N.N., Cutcliffe N., Peard A. Trace amines (ethylamine, octopamine, and tryptamine) stimulate inositol phospholipid hydrolysis in rat cerebral cortex slices. Neurochemistry. 1986;11:1525–1531. doi: 10.1007/BF00965771. [DOI] [PubMed] [Google Scholar]
- Osol G., Laher I., Kelley M. Myogenic tone is coupled to phospholipase C and G protein activation in small cerebral arteries. Am. J. Physiol. 1993;265:H415–H420. doi: 10.1152/ajpheart.1993.265.1.H415. [DOI] [PubMed] [Google Scholar]
- Rhee S.G. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkai I., Feher A., Erdai N., Henrion D., Papp Z., Edes I., Koller A., Kaley G., Bagi Z. Activation of prostaglandin E2 EP1 receptor increases arteriolar tone and blood pressure in mice with type 2 diabetes. Cardiovasc. Res. 2009;83:148–154. doi: 10.1093/cvr/cvp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauzeau V., Le Jeune H., Cario-Toumaniantz C., Smolenski A., Lohmann S.M., Bertoglio J., Chardin P., Pacaud P., Loirand G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2 + sensitization of contraction in vascular smooth muscle. J. Biol. Chem. 2000;275:21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- Schaloske R.H., Dennis E.A. The phospholipase A2 superfamily and its group numbering system. Biochim. Biophys. Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Segonzac A., Raisman R., Tateishi T., Schoemaker H., Hicks P.E., Langer S.Z. Tryptamine, a substrate for the serotonin transporter in human platelets, modifies the dissociation kinetics of [3H] Imipramine binding: possible allosteric interaction. J. Neurochem. 1985;44:349–356. doi: 10.1111/j.1471-4159.1985.tb05423.x. [DOI] [PubMed] [Google Scholar]
- Sewell R.A., Halpern J.H., Pope H.G., Jr. Response of cluster headache to psilocybin and LSD. Neurology. 2006;66:1920–1922. doi: 10.1212/01.wnl.0000219761.05466.43. [DOI] [PubMed] [Google Scholar]
- Simaan J., Sabra R. In-vivo evidence of a role for nitric oxide in regulating the activity of the norepinephrine transporter. Eur. J. Pharmacol. 2011;671:102–106. doi: 10.1016/j.ejphar.2011.09.165. [DOI] [PubMed] [Google Scholar]
- Simmons D.L., Botting R.M., Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- Somlyo A.P., Somlyo A.V. Ca2 + sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Stollak J.S., Furchgott R.F. Use of selective anatagonists for determining the types of receptors mediating the actions of 5-hydroxytryptamine and tryptamine in the isolated rabbit aorta. J. Pharmacol. Exp. Ther. 1983;224:215–221. [PubMed] [Google Scholar]
- Sugimoto Y., Narumiya S. Prostaglandin E receptors. J. Biol. Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- Tang X., Edwards E.M., Holmes B.B., Falck J.R., Campbell W.B. Role of phospholipase C and diacylglyceride lipase pathway in arachidonic acid release and acetylcholine-induced vascular relaxation in rabbit aorta. Am. J. Physiol. 2006;290:H37–H45. doi: 10.1152/ajpheart.00491.2005. [DOI] [PubMed] [Google Scholar]
- Teixeira C.E., Jin L., Ying Z., Palmer T., Priviero F.B.M., Webb R.C. Expression and functional role of the RhoA/Rho-kinase pathway in rat coeliac artery. Clin. Exp. Pharmacol. Physiol. 2005;32:817–824. doi: 10.1111/j.1440-1681.2005.04271.x. [DOI] [PubMed] [Google Scholar]
- Tipton K.F., Boyce S., O'sullivan J., Davey G.P., Healy J. Monoamine oxidases: certainties and uncertainties. Curr. Med. Chem. 2004;11:1965–1982. doi: 10.2174/0929867043364810. [DOI] [PubMed] [Google Scholar]
- Torres G.E., Gainetdinov R.R., Caron M.G. Plasma membrane monoamine transporters: structure, regulation and function. Nat. Rev. Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- Trappe T.A., Carroll C.C., Jemiolo B., Trappe S.W., Dossing S., Kjae M., Magnusson S.P. Cyclooxygenase mRNA expression in human patellar tendon at rest and after exercise. Am. J. Physiol. 2008;294:R192–R199. doi: 10.1152/ajpregu.00669.2007. [DOI] [PubMed] [Google Scholar]
- Trendelenburg U. Classification of sympathomimetic amines. In: Blaschko H., Muscholl E., editors. vol. 33. Springer-Verlag; Berlin: 1972. pp. 336–362. (Catecholamines, Handbook of Experimental Pharmacology). [Google Scholar]
- Uehata M., Ishizaki T., Satoh H., Ono T., Kawahara T., Morishita T., Tamakawa H., Yamagami K., Inui J., Maekawa M., Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Wang A., Nishihashi T., Trandafir C.C., Murakami S., Ji X., Shimizu Y., Kurahashi K. Involvement of endothelial cyclo-oxygenase metabolites in noradrenaline-induced contraction of rat coronary artery. Clin. Exp. Pharmacol. Physiol. 2005;32:628–632. doi: 10.1111/j.0305-1870.2005.04242.x. [DOI] [PubMed] [Google Scholar]
- Watts S.W., Gilbert L., Webb R.C. 5-hydroxytryptamine2B receptor mediates contraction in the mesenteric artery of mineralcorticoid hypertensive rats. Hypertension. 1994;26:1056–1059. doi: 10.1161/01.hyp.26.6.1056. [DOI] [PubMed] [Google Scholar]
- Weissbach H., King W., Sjoersdsma A., Udenfriend S. Formation of indole-3-acetic acid and tryptamine in animals. J. Biol. Chem. 1959;234:81–86. [PubMed] [Google Scholar]
- Williams J., Bogwu J., Oyekan A. The role of the RhoA/Rho-kinase signalling pathway in renal vascular reactivity in endothelial nitric oxide synthase null mice. J. Hypertens. 2006;24:1429–1436. doi: 10.1097/01.hjh.0000234125.01638.3b. [DOI] [PubMed] [Google Scholar]
- Wollman H., Nilsson E., Antonin K.H., Bieck P.R. Tryptamine kinetics in human volunteers. In: Boulton A.A., Maitre L., Beick P.R., Reiderer P., editors. Neuropsychopharmacology of Trace Amines. Humana Press; Clifton, N.J.: 1985. pp. 361–378. [Google Scholar]
- Xavier F.E., Aras-López R., Arroyo-Villa I., Campo L.D., Salaices M., Rossoni L.V., Ferrer M., Balfagó G. Aldosterone induces endothelial dysfunction in resistance arteries from normotensive and hypertensive rats by increasing thromboxane A2 and prostacyclin. Br. J. Pharmacol. 2008;154:1225–1235. doi: 10.1038/bjp.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi R., Chiellini G., Scanlan T.S., Grandy D.K. Trace amine-associated receptors and their ligands. Br. J. Pharmacol. 2006;149:967–978. doi: 10.1038/sj.bjp.0706948. [DOI] [PMC free article] [PubMed] [Google Scholar]