Highlights
► We analyzed the effect of Ca2+-calmodulin on membrane integration of tail-anchored proteins. ► We found that Ca2+-calmodulin inhibits membrane insertion of TA proteins. ► This inhibition was prevented by trifluoperazine, a CaM antagonist. ► The effect of Ca2+-CaM was also seen for a purified TA protein. Ca2+-CaM can be cross-linked to TA proteins.
Keywords: Calcium, Calmodulin, Membrane insertion, Tail-anchored membrane proteins, Trifluoperazine
Abbreviations: CaM, calmodulin; ER, endoplasmic reticulum; Get, guided entry of tail-anchored proteins; TA, tail-anchored; TRC, transmembrane recognition complex
Abstract
Cytosolic components and pathways have been identified that are involved in inserting tail-anchored (TA) membrane proteins into the yeast or mammalian endoplasmic reticulum (ER) membrane. Searching for regulatory mechanisms of TA protein biogenesis, we found that Ca2+-calmodulin (CaM) inhibits the insertion of TA proteins into mammalian ER membranes and that this inhibition is prevented by trifluoperazine, a CaM antagonist that interferes with substrate binding of Ca2+-CaM. The effects of Ca2+-CaM on cytochrome b5 and Synaptobrevin 2 suggest a direct interaction between Ca2+-CaM and TA proteins. Thus, CaM appears to regulate TA insertion into the ER membrane in a Ca2+ dependent manner.
1. Introduction
The endoplasmic reticulum (ER) membrane is one of the major cellular sites for the membrane integration of nascent membrane proteins and newly synthesized tail-anchored (TA) membrane proteins, i.e. proteins that are membrane-embedded via a carboxy-terminal transmembrane domain [1], [2], [3]. TA proteins that are first inserted into the ER membrane are involved in a variety of biological processes, including signal sequence-dependent protein transport into the ER, ER calcium leakage, ER-associated protein degradation, apoptosis, and vesicular trafficking. Thus TA proteins may remain in the ER membrane or relocate to the nuclear envelope or to any of the membranes involved in endo- or exocytosis. In the last five years, pathways and components have been identified that are involved in this posttranslational membrane insertion in yeast [4], [5] and mammals [6], [7], [8], [9], [10], [11], [12], [13], [14]. Some TA proteins can enter the ER membrane unassisted, including mammalian cytochrome b5 (Cytb5) and the human protein tyrosine phosphatase 1B (PTP1B) [15], [16], [17]. Other TA proteins interact with SRP [6], cytosolic molecular chaperones [7], [10], or TA-dedicated machinery, such as the cytosolic transmembrane recognition complex (TRC) in mammals [8], [14] or the guided entry of tail-anchored proteins (Get) system, in yeast [4], [5], and involve an ER membrane resident receptor and/or TA-insertase (WRB in mammals, Get1/2 in yeast) [4], [18]. Human Synaptobrevin 2 (Syb2) was the first TA protein shown to demonstrate an energy requirement for membrane insertion [19]. It was further demonstrated that this energy requirement was due to the involvement of either SRP or TRC40/Asna-1. Human Sec61ß appears to be a TA protein that requires Hsp70 and Hsp40 chaperones, SRP, or TRC40/Asna-1 [6], [10]. In addition, both model proteins depend on an ER-resident membrane receptor or TA-insertase [19].
To address regulatory mechanisms of TA protein biogenesis, we analyzed whether the membrane insertion of several types of mammalian TA proteins was affected by Ca2+-CaM. Specifically, we studied Cytb5, Sec61ß, and Syb2 as model TA proteins. All three proteins were extended at their carboxyl termini via an opsin-derived 13- or 28-amino acid N-glycosylation site to create a reliable assay for membrane insertion. We found that TA insertion into the mammalian ER membrane was generally inhibited by Ca2+-CaM and that this inhibition was irrespective of the cytosolic pathway. Furthermore, we showed that the inhibition of TA insertion by Ca2+-CaM could be prevented by the CaM antagonist trifluoperazine (TFP) which interferes with CaM substrate binding. Based on our observations that Cytb5 membrane insertion is inhibited by Ca2+-CaM even in the absence of cytosolic factors and that Syb2 can be cross-linked to CaM in the presence of cytosolic factors we propose that Ca2+-CaM binds directly to the TA proteins.
2. Materials and methods
2.1. Materials
Rabbit reticulocyte lysate was obtained from Promega and [35S]methionine was from Perkin Elmer. Apyrase (grade VII from potato), CaM (from bovine testes) and TFP were purchased from Sigma. Proteinase K was from Roche and the cross-linking reagent was from Pierce. Chemicals for electrophoresis were purchased from Serva. PMSF and all other chemicals were obtained from Merck. Canine pancreatic micosomes were purified as described previously [20]. Glutathione-S-transferase (GST) and the fusion protein consisting of amino terminal GST linked to rat CaM were produced in Escherichia coli and purified according to standard procedure.
2.2. Protein transport experiments
Precursor polypeptides were synthesized in reticulocyte lysate in the presence of [35S]methionine for 30 min at 30 °C. The translation reaction contained EGTA at a concentration of approximately 400 μM. Synthesis was inhibited by incubation for 5 min at 30 °C in the presence of cycloheximide (100 μg/ml) and RNaseA (80 μg/ml). Buffer, rough microsomes (RM), or semi-permeabilized HeLa cells [21] were added and incubated for 30 min at 30 °C for the posttranslational transport experiments. Where indicated, apyrase, Ca2+, CaM, or TFP were present at final concentrations of 2 units per ml, 0.7 mM, 0.26 mg/ml, or 200 μM, respectively. All samples were analyzed by SDS–PAGE and phosphorimaging (Typhoon-Trio imaging system with Image Quant TL software 7.7; GE Healthcare). Where indicated, microsomes were re-isolated by centrifugation or subjected to carbonate extraction. For the latter, microsomes were re-isolated, resuspended in 100 mM sodium carbonate pH 11.0 and incubated for 30 min at 0 °C. Subsequently, carbonate extract was separated from the carbonate-resistant membrane pellet by centrifugation at 190,000×g for 20 min at 2 °C.
Alternatively, a fusion protein consisting of amino terminal GST linked to Cytb5 with the opsin-derived 28-amino acid N-glycosylation site was produced in E. coli and the Cytb5-ops moiety was released from the GST by thrombin cleavage as previously described [17]. Cytb5-ops was radiolabeled with [14C]formaldehyde according to standard procedures [20] and re-isolated by gelfiltration in phosphate buffer (100 mM sodium phosphate, pH 7.4). Transport assays were carried out in buffer (0.2 M sucrose, 50 mM KCl, 2 mM Mg acetate, 1 mM DTT, 20 mM HEPES–KOH, pH 7.5).
2.3. Analytical procedures
Cytb5 was synthesized and incubated as described above. Subsequently, the samples were divided into five aliquots and incubated with increasing concentrations of proteinase K for 60 min at 0 °C as indicated. After inhibition of the protease with 10 mM phenylmethylsulphonyl fluoride (PMSF), all samples were subjected to SDS–PAGE and phosphorimaging.
Syb2 was synthesized and incubated as described above. Subsequently, the samples were incubated with 4 mM 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC) for 40 min at room temperature in 100 mM KCl, 0.1 mM CaCl2, 20 mM HEPES-KOH, pH 6 as described [22], [23]. All samples were analyzed by SDS–PAGE and phosphorimaging.
3. Results
3.1. Insertion of different model TA proteins into microsomes involves different cytosolic factors
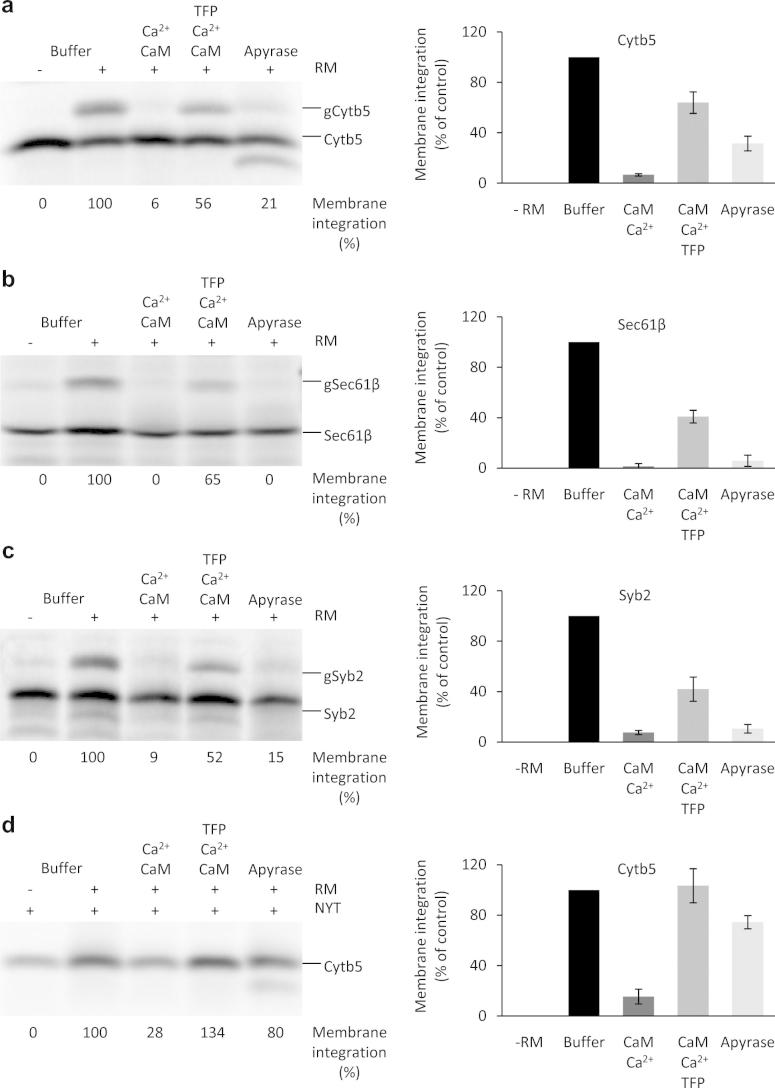
Before we investigated the effects of Ca2+-CaM on the insertion of mammalian TA proteins into the mammalian ER membrane, the energy dependence of insertion was confirmed for the model proteins. TA proteins were synthesized in the presence of [35S]methionine and the translation reactions were supplemented with pancreatic microsomes and divided into aliquots. Where indicated, the aliquots were supplemented with apyrase, which hydrolyzes ATP and ADP and leads to a depletion of GTP due to the action of nucleoside diphosphate kinases in the lysate. To allow membrane insertion, the aliquots were incubated further. One translation reaction was supplemented with buffer instead of microsomes and served as a negative control. Subsequently, all samples were subjected to SDS–PAGE and phosphorimaging analysis (Fig. 1a–f). As expected, there was no N-glycosylation in the absence of microsomes (Fig. 1a–f, lane 1 in the left panels), but there was membrane insertion as measured by glycosylation in the presence of microsomes for Cytb5, Sec61ß and Syb2 and as measured by carbonate resistance for Cytb5, PTP1B and Ubc6 (Fig. 1a–f, lane 2 in the left panels). However, when the translation reactions were depleted of nucleoside triphosphates, there was a significant reduction of glycosylation (Fig. 1a–f, compare lane 5 to lane 2, left panels). Thus, the in vitro insertion system proved suitable for the analysis of TA protein insertion into ER membranes and the known requirements for energy-dependent cytosolic factors were confirmed for the model proteins [10], [19]. As expected, the requirements for energy-dependent cytosolic factors varied for the different TA proteins (Sec61ß > Syb2 > PTP1B > Cytb5 > Ubc6).
Fig. 1.
Insertion of model tail-anchored (TA) membrane proteins into rough microsomes is inhibited by Ca2+-CaM. TA proteins were synthesized in the presence of [35S]methionine. After inhibition of protein synthesis, the translation reactions were supplemented with buffer (lane 1) and canine pancreatic microsomes (RM) or semi-permeabilized HeLa cells (SPC) (lanes 2–5) and divided into aliquots. Where indicated, the aliquots were supplemented simultaneously with apyrase, Ca2+, CaM, and TFP. The experiment that is depicted in d was carried out in the presence of the acceptor tripeptide NYT to prevent N-glycosylation. After further incubation for 30 min, samples were left untreated (a,g) or subjected to either centrifugation (b,c) or carbonate extraction (d–f). Samples or sample pellets after centrifugation or carbonate extraction were subjected to SDS–PAGE and phosphorimaging. Only the areas of interest in single gels are shown in the left panels. The right panels show the mean values and the standard errors of the mean from at least four individual experiments. The amount of carbonate-resistant protein was corrected using the buffer background value. The efficiency of membrane integration for the buffer control (as assayed as either N-glycosylation or carbonate resistance) was set as 100%.
3.2. Insertion of TA membrane proteins into the ER is inhibited by Ca2+-CaM
We showed previously that Ca2+-CaM can bind to the α-subunit of the Sec61 complex, thereby limiting Ca2+ leakage from the ER [24]. Binding of Ca2+-CaM to the Sec61 complex, however, does not interfere with signal peptide-dependent protein transport into the ER. Here, the question was whether Ca2+-CaM affects the Sec61-independent insertion of TA polypeptides into microsomal membranes. Posttranslational membrane insertion of TA proteins was carried out as described above. However, where indicated, the aliquots were supplemented with Ca2+-CaM (Fig. 1a–f). In all cases, the presence of Ca2+-CaM led to a significant reduction in glycosylation of the tail anchors (Fig. 1a–f, compare lane 3 to lane 2 in left panels). The inhibitory effect of Ca2+-CaM was more pronounced than the effect of energy depletion (Fig. 1a–f, compare lane 3 to lane 5). We note that this effect was not due to N-glycosylation inhibition per se, but was rather due to inhibition of membrane insertion. That is, the observed effect was similar to that seen when carbonate resistance was employed as an assay for membrane insertion (Cytb5, PTP1B, Ubc6) (Fig. 1d–f). Thus, the insertion of mammalian TA proteins into mammalian microsomes is sensitive to Ca2+-CaM and this sensitivity is irrespective of the cytosolic factors involved. Furthermore, Ca2+-CaM inhibited membrane insertion of Cytb5 when ER membranes derived from human cells in the form of semi-permeabilized cells were used instead of microsomes (Fig. 1g).
Next, we investigated whether the presence of a CaM-antagonist that interferes with substrate binding by CaM, such as TFP [25], interferes with the inhibitory effect of Ca2+-CaM on TA insertion into the ER. Posttranslational membrane insertion of TA proteins was carried out in the presence of Ca2+-CaM as before with the addition of TFP (Fig. 1a–g). For all of the model proteins, TFP partially relieved the inhibitory effect of Ca2+-CaM (Fig. 1a–g, compare lane 4 to lane 3 in all left panels). Thus, the observed inhibitory effect of Ca2+-CaM on TA protein biogenesis specifically involves the substrate binding-site of CaM.
We note that it is rather laborious to detect the effect of Ca2+-CaM. In particular, the rabbit reticulocyte lysate that is used for model protein synthesis contains CaM, Ca2+, and an excess of EGTA to quench the Ca2+ (about 400 μM EGTA). The concentrations of these compounds can vary from one batch of lysate to the next. Therefore, one can observe partial inhibition and relief of inhibition by TFP even without the addition of exogenous CaM (data not shown). In addition, high concentrations of TFP (>150 μM) are harmful to membranes in the absence of CaM at least in combination with reticulocyte lysate; however, the action of TFP requires a four-fold molar excess over CaM [25]. Furthermore, it has to be taken into account that CaM has four binding sites for Ca2+ and that all binding sites for TFP are active only in the presence of Ca2+ [25].
On first sight, the used CaM concentration of 0.26 mg/ml or approximately 15 μM appears to be rather high in comparison to the nM concentrations of in vitro translation products. However, the half-maximal effect of CaM on TA membrane insertion was observed at 7.5 μM (data not shown). For comparison, physiological concentrations of CaM vary between 3 and 30 μM [26] and a concentration of 30 μM CaM was used to mimic the stimulatory effect of recticulocyte lysate on nuclear protein import [27].
3.3. Ca2+-CaM directly affects TA proteins
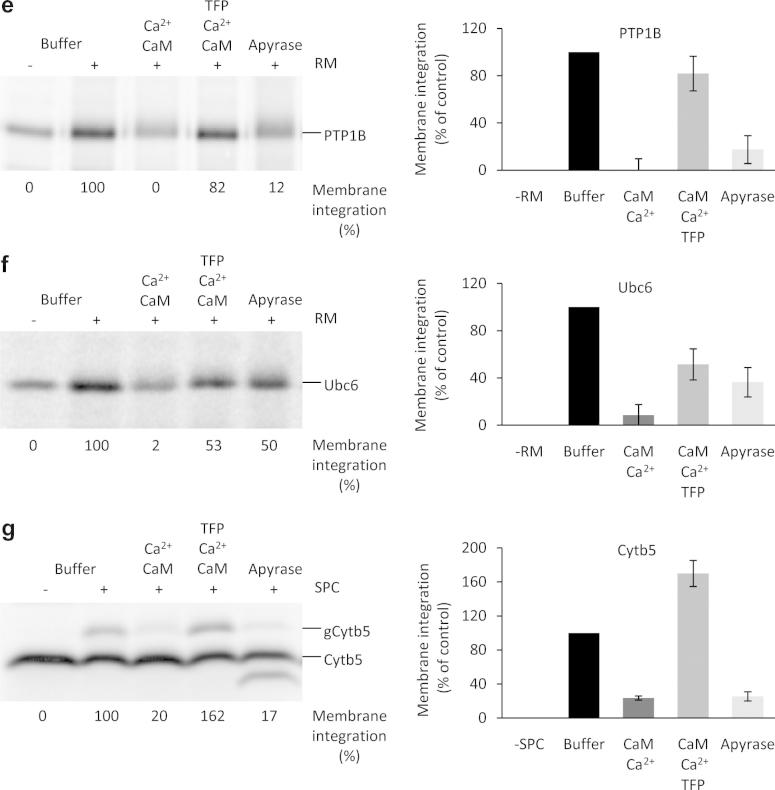
We next addressed the question of how Ca2+-CaM affects insertion of TA proteins into mammalian microsomes. Since both types of model TA proteins were affected, i.e. insertase-dependent and insertase-independent, irrespective of the cytosolic factors that aid insertion, it appeared that Ca2+-CaM could bind directly to TA proteins in solution. Three approaches were employed to address this question. First, differential protease sensitivity was used as a tool to detect differences in soluble Cytb5 in the presence and absence of Ca2+-CaM. Cytb5 was synthesized in the presence of [35S]methionine and the translation reactions were divided into several aliquots. The aliquots were supplemented and incubated with buffer, Ca2+-CaM, or Ca2+-CaM plus TFP. Subsequently, the aliquots were divided further and incubated with decreasing concentrations of protease. All samples were subjected to SDS–PAGE and phosphorimaging. Cytb5 was more sensitive to protease in the presence of Ca2+-CaM as shown by comparing the buffer samples with the Ca2+-CaM samples (Fig. 2). TFP prevented the increase in protease sensitivity of Cytb5 due to Ca2+-CaM. Thus, Ca2+-CaM either directly or indirectly affects the structure of Cytb5.
Fig. 2.
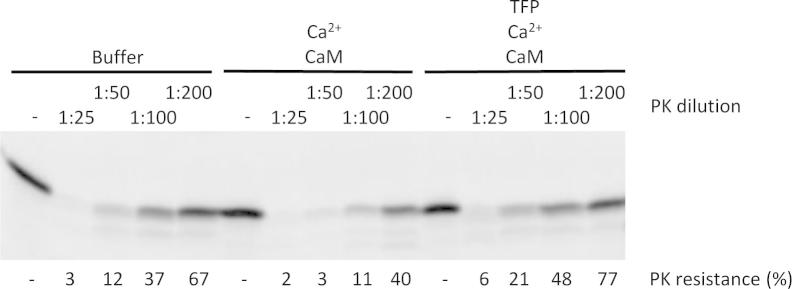
Protease accessibility of newly synthesized Cytb5 is altered by Ca2+-CaM. Cytb5 was synthesized in the presence of [35S]methionine and divided into three aliquots. The three translation reactions were supplemented with buffer, Ca2+-CaM, and TFP as indicated and incubated for 30 min at 30 °C. Subsequently, each of the three translation reactions was divided into five aliquots and incubated with increasing dilutions of proteinase K for 60 min at 0 °C as indicated (starting concentration 175 μg/ml). After protease inhibition, all samples were subjected to SDS–PAGE and phosphorimaging. Only the areas of interest of a single gel are shown. The experiment was carried out three times with similar results.
Second, we asked if membrane insertion of purified Cytb5 was affected in the absence of cytosolic factors. Cytb5-ops was purified from E. coli and 14C-labeled by reductive methylation. The labeled protein was then incubated with microsomes in the absence or presence of Ca2+-CaM. Ca2+-CaM inhibited membrane insertion of Cytb5-ops even in the absence of reticulocyte lysate (Fig. 3, compare lane 3 to 4). This inhibition was partially prevented by TFP (Fig. 3, compare lane 4 to 5). This indicates that Ca2+-CaM binds directly to Cytb5.
Fig. 3.
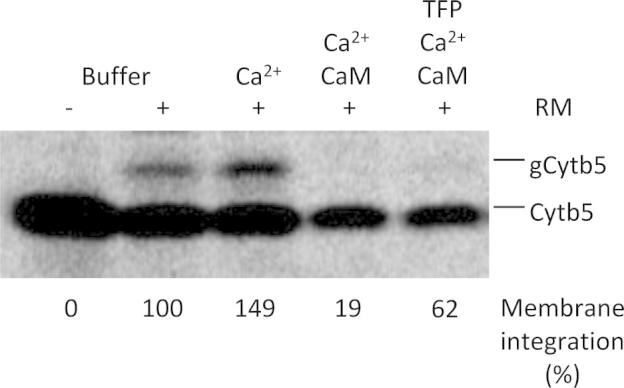
Insertion of purified radiolabeled Cytb5-ops into rough microsomes is inhibited by Ca2+-CaM. The experiment was performed in analogy to Fig. 1, except that purified Cytb5-ops rather than in vitro synthesized Cytb5 was employed and transport was carried out in buffer, i.e. in the absence of cytosolic factors. Only the areas of interest of a single gel are shown. The experiment was carried out three times with similar results.
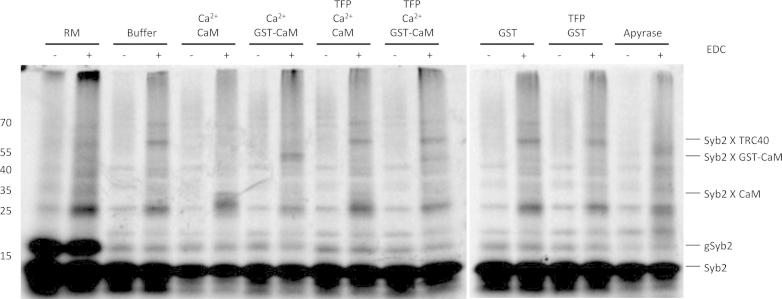
Third, we asked if Ca2+-CaM interfered with Syb2 binding to its dedicated cytosolic factor, TRC40/Asna-1. Syb2 was synthesized in reticulocyte lysate, i.e. in the presence of the cytosolic factors that facilitate its insertion into the membrane, and was then incubated with various agents before incubating with a zero-length cross-linking reagent. In the absence of any additional agents, one prominent cross-linking product was detected with an apparent mass of 65 kDa that most likely represents a cross-linking product between Syb2 and TRC40/Asna-1 (Fig. 4, lane 4). When Syb2 was incubated with microsomes to allow its membrane insertion, this cross-linking product was absent (Fig. 4, lane 2). Furthermore, the cross-linking product was absent after incubation with apyrase (Fig. 4, lane 18), The cross-linking product between Syb2 and TRC40/Asna-1was also absent after incubation with Ca2+-CaM or Ca2+-GST-CaM (Fig. 4, lanes 6 and 8), but was present when substrate binding of Ca2+-CaM was inhibited by TFP (Fig. 4, lanes 10 and 12). After incubation with Ca2+-CaM, however, a novel 35 kDa cross-linking product of Syb2 was detected (Fig. 4, lane 6). This product represents a cross-link between Syb2 (14 kDa) and CaM (17 kDa) since it was shifted to an apparent mass of about 55 kDa by employing GST-CaM (31 kDa) instead of CaM (Fig. 4, compare lane 6 to 8) and was absent after incubation with TFP or GST (Fig. 4, lanes 10, 12, 14 and 16). Thus, binding of Ca2+-CaM to Syb2 must displace cytosolic factors, such as TRC40/Asna-1, most likely by binding to the tail anchor.
Fig. 4.
Binding of Syb2 to TRC40/Asna-1 is displaced by Ca2+-CaM. Syb2 was synthesized in the presence of [35S]methionine. The translation reaction was divided into aliquots, then supplemented and incubated with buffer, Ca2+-CaM, Ca2+-GST-CaM, GST, TFP, and apyrase as indicated. The final concentrations of GST and GST-CaM were 0.26 mg/ml. The aliquots were then incubated with the heterobifunctional cross-linking reagent EDC and all samples were subjected to SDS–PAGE and phosphorimaging. The positions of molecular mass standards are indicated (kDa). The experiment was carried out three times with similar results. We note that GST-CaM was equally efficient in inhibiting membrane insertion of TA proteins in the presence of Ca2+ as compared to CaM and that this inhibitory effect was suppressed by TFP (data not shown).
4. Discussion
In this study we investigated whether membrane insertion of different types of mammalian TA proteins was affected by Ca2+-CaM. Specifically, we studied five model TA proteins that varied in terms of the auxiliary factors needed for membrane insertion: Cytb5 which involves but does not depend on cytosolic chaperones and which does not involve membrane insertase; Sec61ß which involves either chaperones, TRC40/Asna-1, or SRP plus a membrane-resident insertase; and Syb2 which involves TRC40/Asna-1 or SRP plus a membrane-resident insertase. We showed that TA protein insertion into the ER membrane was generally inhibited by Ca2+-CaM irrespective of the insertion pathway. Furthermore, we showed that the inhibition of TA insertion by CaM could be prevented by the CaM antagonist TFP which interferes with substrate binding by CaM. Based on TA insertion in the presence and absence of Ca2+-CaM in the absence of cytosolic factors and on chemical cross-linking of TA proteins in the presence of cytosolic factors, we propose that Ca2+-CaM binds directly to the tail anchors. Such binding is not entirely unexpected since Ca2+-CaM has been shown previously to bind to amphiphilic peptides in general [28] and to degradation products derived from signal peptides in particular [29]. It is also notable that TA protein biogenesis is regulated by the redox state of the cytosol in both yeast and mammals [9].
We propose that Ca2+-CaM can halt TA protein biogenesis prior to TA insertion into the ER membrane. For at least one TA protein, it must be important to prevent membrane insertion while CaM is saturated with Ca2+. We assume that such a TA protein is regulated by either Ca2+ or Ca2+-CaM in its membrane-resident form. Candidate proteins include TA proteins that are involved in vesicular transport (Syb2, VAMP2, and syntaxin), or apoptosis and autophagy (Bcl-2) [30], [31], [32]. Alternatively, binding of Ca2+-CaM may function to protect TA proteins from degradation prior to membrane integration. Future studies should address whether the inhibition of TA membrane insertion is limited to insertion into the ER membrane or whether insertion of TA proteins into the mitochondrial outer membrane can be inhibited in a similar way [33], [34].
Acknowledgments
We thank Dr. Nica Borgese (Milan, Italy) for stimulating discussions and Drs. Marcel Meissner and Veit Flockerzi (both Homburg) for supplying the GST-CaM coding plasmid. The cDNAs for human PTP1B and murine Ubc6 were kindly provided by A. Schmid (Homburg) and T. Sommer (Berlin), respectively. This work was supported by Grants from the Deutsche Forschungsgemeinschaft (FOR 967 and GRK 1326).
References
- 1.Zimmermann R., Eyrisch S., Ahmad M., Helms V. Protein translocation across the ER membrane. Biochem. Biophys. Acta. 2011;1808:912–924. doi: 10.1016/j.bbamem.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Mandon E., Gilmore R. The tail end of membrane insertion. Cell. 2007;128:1031–1332. doi: 10.1016/j.cell.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Borgese N., Fasana E. Targeting pathways of C-tail-anchored proteins. Biochem. Biophys. Acta. 2011;1808:937–946. doi: 10.1016/j.bbamem.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Schuldiner M., Metz J., Schmid V., Denic V., Rakwalska M., Schmitt H.D., Schwappach B., Weissman J.S. The GET complex mediates insertion of tail-anchored proteins into the ER membrane. Cell. 2008;134:634–645. doi: 10.1016/j.cell.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jonikas M.C., Collins S.R., Denic V., Oh E., Quan E.M., Schmid V., Weibezahn J., Schwappach B., Walter P., Weissman J.S., Schuldiner M. Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science. 2009;323:1693–1697. doi: 10.1126/science.1167983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abell B.M., Pool M.R., Schlenker O., Sinning I., High S. Signal recognition particle mediates post-translational targeting in eukaryotes. EMBO J. 2004;23:2755–2764. doi: 10.1038/sj.emboj.7600281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abell B.M., Rabu C., Leznicki P., Young J.C., High S. Post-translational integration of tail-anchored proteins is facilitated by defined molecular chaperones. J. Cell Sci. 2007;120:1743–1751. doi: 10.1242/jcs.002410. [DOI] [PubMed] [Google Scholar]
- 8.Stefanovic S., Hegde R.S. Identification of a targeting factor for posttranslational membrane protein insertion into the ER. Cell. 2007;128:1147–1159. doi: 10.1016/j.cell.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 9.Favaloro V., Spasic M., Schwappach B., Dobberstein B. Distinct targeting pathways fort the membrane insertion of tail-anchored (TA) proteins. J. Cell Sci. 2008;121:1832–1840. doi: 10.1242/jcs.020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabu C., Wipf P., Brodsky J.L., High S. A precursor-specific role for Hsp40/Hsc70 during tail-anchored protein integration at the endoplasmic reticulum. J. Biol. Chem. 2008;283:27504–27513. doi: 10.1074/jbc.M804591200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabu C., Schmid V., Schwappach B., High S. Biogenesis of tail-anchored proteins: the beginning for the end? J. Cell Sci. 2009;122:3605–3612. doi: 10.1242/jcs.041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leznicki P., Clancy A., Schwappach B., High S. Bat3 promotes the membrane integration of tail-anchored proteins. J. Cell Sci. 2010;123:2170–2178. doi: 10.1242/jcs.066738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Favaloro V., Vilardi F., Schlecht R., Mayer M.P., Dobberstein B. Asna1/TRC40-mediated membrane insertion of tail-anchored proteins. J. Cell Sci. 2010;123:1522–1530. doi: 10.1242/jcs.055970. [DOI] [PubMed] [Google Scholar]
- 14.Mariappan M., Li X., Stefanovic S., Sharma A., Mateja A., Keenan R.J., Hegde R.S. A ribosome-associating factor chaperones tail-anchored membrane proteins. Nature. 2010;466:1120–1124. doi: 10.1038/nature09296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brambillasca S., Yabal M., Soffientini P., Stefanovic S., Makarow M., Hegde R.S., Borgese N. Transmembrane topogenesis of a tail-anchored protein is modulated by membrane lipid composition. EMBO J. 2005;24:2533–2542. doi: 10.1038/sj.emboj.7600730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brambillasca S., Yabal M., Makarow M., Borgese N. Unassisted translocation of large polypeptide domains across phospholipid bilayers. J. Cell Biol. 2006;175:767–777. doi: 10.1083/jcb.200608101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colombo S.F., Longhi R., Borgese N. The role of cytosolic proteins in the insertion of tail-anchored proteins into phospholipid bilayers. J. Cell Sci. 2009;122:2383–2392. doi: 10.1242/jcs.049460. [DOI] [PubMed] [Google Scholar]
- 18.Vilardi F., Lorenz H., Dobberstein B. WRB is the recpetor for TRC40/Asna-1-mediated insertion of tail-anchored proteins into the ER membrane. J. Cell Sci. 2011;124:1301–1307. doi: 10.1242/jcs.084277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kutay U., Ahnert-Hilger G., Hartmann E., Wiedenmann B., Rapoport T.A. Transport route for synaptobrevin via a novel pathway of insertion into the endoplasmic reticulum membrane. EMBO J. 1995;14:217–223. doi: 10.1002/j.1460-2075.1995.tb06994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klappa P., Mayinger P., Pipkorn R., Zimmermann M., Zimmermann R. A microsomal protein is involved in ATP-dependent transport of presecretory proteins into mammalian microsomes. EMBO J. 1991;10:2795–2803. doi: 10.1002/j.1460-2075.1991.tb07828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson R., Allen A.J., Oliver J., Brookman J.L., High S., Bulleid N.J. The translocation, folding, assembly, and redox-dependent degradation of secretory an membrane proteins in semi-permeabilized mammalian cells. Biochem. J. 1995;387:679–687. doi: 10.1042/bj3070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto K., Sekine T., Sutoh K. The sites on calmodulin cross-linked to myosin light chain kinase and troponin I with water-soluble carbodiimide. J. Biochem. 1988;104:251–254. doi: 10.1093/oxfordjournals.jbchem.a122452. [DOI] [PubMed] [Google Scholar]
- 23.Minami Y., Kawasaki H., Suzuki K., Yahara I. The calmodulin-binding domain of the mouse 90-kDa heat shock protein. J. Biol. Chem. 1993;268:9604–9610. [PubMed] [Google Scholar]
- 24.Erdmann F., Schäuble N., Lang S., Jung M., Honigmann A., Ahmad M., Dudek J., Benedix J., Harsmann A., Kopp A., Helms V., Cavalié A., Wagner R., Zimmermann R. Interaction of calmodulin with Sec61a limits Ca2+ leakage from the endoplasmic reticulum. EMBO J. 2011;30:17–31. doi: 10.1038/emboj.2010.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandonselaar M., Hickie R.A., Quail J.W., Delbaere L.T.J. Trifuoperazine-induced conformational change in Ca2+-calmodulin. Nat. Struct. Biol. 1994;1:795–801. doi: 10.1038/nsb1194-795. [DOI] [PubMed] [Google Scholar]
- 26.Rogers M.S., Strehler E.E. In: Guidebook to the Calcium-binding Proteins. Celio M.R., Pauls T.L., Schwaller B., editors. Oxford University Press; Oxford, UK: 1996. Calmodulin; pp. 34–40. [Google Scholar]
- 27.Sweitzer T.D., Hanover J.A. Calmodulin activates nuclear protein import: a link between signal transduction and nuclear import. Proc. Natl. Acad. Sci. USA. 1996;93:14574–14579. doi: 10.1073/pnas.93.25.14574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher P.J., Prendergast F.G., Ehrhardt M.R., Urbauer J.L., Wand A.J., Sedarous S.S., McCormick D.J., Buckley P.J. Calmodulin interacts with amphiphilic peptides composed of all d-amino acids. Nature. 1994;368:651–653. doi: 10.1038/368651a0. [DOI] [PubMed] [Google Scholar]
- 29.Lyko F., Martoglio B., Jungnickel B., Rapoport T.A., Dobberstein B. Signal sequence processing in rough microsomes. J. Biol. Chem. 1995;270:19873–19878. doi: 10.1074/jbc.270.34.19873. [DOI] [PubMed] [Google Scholar]
- 30.Quetglas S., Iborra C., Sasakawa N., De Haro L., Kumakura C., Sato K., Leveque C., Seagar M. Calmodulin and lipid binding to synaptobrevin regulates calcium-dependent exocytosis. EMBO J. 2002;21:3970–3979. doi: 10.1093/emboj/cdf404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiGiovanni J., Iborra C., Maulet Y., Leveque C., El Far O., Seagar M. Calcium-dependent regulation of SNARE-mediated membrane fusion by calmodulin. J. Biol. Chem. 2010;285:23665–23675. doi: 10.1074/jbc.M109.096073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoyer-Hansen M., Bastholm L., Szyniarowski P., Campanella M., Szabadkai G., Farkas T., Biianchi K., Fehrenbacher N., Elling F., Rizzuoto R., Stenfeldt Mathiasen I., Jäättelä M. Control of macroautophagy by calcium-dependent kinase kinase-ß and Bcl-2. Mol. Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Kemper C., Habib S.J., Engl G., Heckmeyer P., Dimmer K.S., Rapaport D. Integration of tail-anchored proteins into the mitochondrial outer membrane does not require any known import components. J. Cell Sci. 2008;121:1990–1998. doi: 10.1242/jcs.024034. [DOI] [PubMed] [Google Scholar]
- 34.Wang F., Brown E.C., Mak G., Zhuang J., Denic V. A chaperone cascade sorts proteins for posttranslational membrane insertion into the endoplasmic reticulum. Mol. Cell. 2010;40:1–13. doi: 10.1016/j.molcel.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]