Abstract
Background
The effects of L-carnitine on the hemodynamic state of chronic hemodialysis patients have been debated. In order to clarify the effect of administered L-carnitine on cardiac function and hypotensive episodes during the hemodialysis procedure, a randomized double-blind placebo-controlled study was performed for 3 months.
Methods and Results
Twenty stable outpatients undergoing hemodialysis treatment were divided into two groups: controls (placebo) and treated patients (L-carnitine 900 mg p.o. daily). After 3 months, cardiac function was reevaluated by echocardiography, and hypotensive episodes during hemodialysis were assessed. Free and acyl carnitine levels increased significantly from 22.3 ± 7.1 to 140.3 ± 57.5 μmol/l and from 15.8 ± 2.8 to 94.8 ± 50.4 μmol/l, respectively, in the treated group. The ejection fraction significantly increased from 61.8 ± 16.0 to 64.4 ± 13.8% (p < 0.05) in the treated group. However, there was no difference in other echocardiographic parameters between the two groups. Hypotensive episodes significantly decreased from 4.0 ± 1.7 to 1.3 ± 0.9 times per month (p < 0.05), although patients' body weight did not change significantly.
Conclusions
Beneficial effects of L-carnitine on the hemodynamic state of chronic hemodialysis patients were observed. L-Carnitine supplementation might be considered especially for chronic hemodialysis patients with unstable hemodynamic conditions.
Key Words : L-Carnitine, Cardiac function, Hypotension, Hemodialysis
Introduction
It is well known that L-carnitine is a transporter of long-chain fatty acids into mitochondria, especially in skeletal and cardiac muscle tissues, which rely on fatty acids as an energy source under aerobic metabolic conditions [1]. Carnitine administration has been reported to be beneficial in patients with angina, ischemia-induced cardiac insufficiency, cardiogenic shock, cardiomyopathy and myocardial infarction [2].
The concentration of plasma and tissue carnitine in chronic hemodialysis patients decreases severely because of its impaired synthesis in the kidney and liver, as well as due to great losses across the dialysis membrane during hemodialysis [2,3,4,5]. It has also been reported that hypocarnitinemia is closely related to cardiomegaly and cardiac ischemia in these patients [6,7]. Therefore, an improvement in cardiac function of patients with chronic hemodialysis associated with carnitine therapy might be expected. However, the results of previous studies are conflicting [8,9,10,11].
Cardiovascular disease is a common finding in dialysis patients, accounting for 40% of deaths in this population [12]. It is important to clarify the effects of L-carnitine supplementation on the hemodynamic state of dialysis patients in terms of morbidity and mortality. In order to investigate whether the supplementation of L-carnitine might be associated with beneficial effects on cardiac function and clinical status of dialysis patients, a randomized double-blind placebo-controlled study was performed.
Methods
Subjects
Twenty stable and ambulatory hemodialysis patients were studied. The inclusion criteria were as follows: chronic hemodialysis for more than 2 years, dialysis frequency or duration unchanged for the previous 3 months, and stable laboratory data without severe anemia or hyperparathyroidism. Patients with prior myocardial infarction or valvular heart disease were excluded. The patients were randomly assigned either to an active treatment group (900 mg L-carnitine p.o. daily for 3 months) or a placebo group under double-blind conditions. In order to ascertain stable hemodynamic conditions, the real drug was administered after a 1-month observation period. In all patients, hematological parameters were measured every month during the study period. Plasma levels of total and free carnitine were determined at baseline and after 3 months of treatment according to the enzyme cycling method. The study protocol was approved by the local ethics committee and conformed to the recommendations of the Declaration of Helsinki. Written informed consent was obtained from each patient.
Echocardiographic Examination
The Toshiba Xario SSA-660A (Toshiba, Odawara, Japan) was used to determine cardiac function. Left ventricular ejection fraction and Tei index calculated from ejection time and the interval between cessation and onset of mitral inflow were measured using a 2.5-MHz transducer. Diastolic function of the left ventricle was measured in the supine position with pulsed Doppler echocardiography. The ratio of early ventricular filling (E) and late ventricular filling (A) velocities of mitral inflow wave (E/A), the ratio of tissue Doppler annular early (e) and late (e′) diastolic velocities (e/e′), and the ratio of peak systolic velocity (S) and peak anterograde diastolic velocity (D) of pulmonary venous waveforms (S/D) was evaluated. Left ventricular myocardial weight (LV mass) was calculated using the formula of Devereux. Echocardiography assessments were performed just after hemodialysis treatment at baseline and after 3 months of treatment. The same technician performed echocardiographic tests before and after the study period, without any previous information about the patient.
Episodes of Hypotension
Hypotension is defined as a drop in blood pressure necessitating a corrective intervention such as reduction in blood flow or negative pressure, or administration of saline or another medication. Hypotensive episodes were assessed every month during the study period.
Statistical Analysis
Descriptive results are shown as the mean ± standard deviation or standard error. Data were statistically analyzed by Student's t test for paired or unpaired samples and χ2 test. A value of p < 0.05 was regarded as statistically significant.
Results
Baseline Patient Characteristics
After randomization, it became clear that 1 patient suffered from gastric cancer, and another patient experienced bone fracture accidentally. Because there were 2 dropouts, the remaining 18 patients were divided into a placebo group (n = 8) and an L-carnitine treatment group (n = 10). There were no differences in examined parameters between the placebo and the treated group (table 1). The dialysis procedure, including dialysis time, blood flow, dialysate flow, and dialyzer, were not changed during the study in both groups.
Table 1.
Patient characteristics
| Placebo (n = 8) | Carnitine treated (n = 10) | p value | |
|---|---|---|---|
| Duration of dialysis, months | 109 ± 62.5 | 157.4 ± 115.3 | n.s. |
| Age, years | 67.8 ± 9.4 | 65.9 ± 6.4 | n.s. |
| Male/female | 4/4 | 4/6 | n.s. |
| Hypertension | 7 out of 8 | 8 out of 10 | n.s. |
| Diabetes mellitus | 0 out of 8 | 1 out of 10 | n.s. |
| Dry weight, kg | 52.4 ± 15.4 | 53.7 ± 12.2 | n.s. |
| Systolic blood pressure, mm Hg | 130.9 ± 17.4 | 136.0 ± 22.4 | n.s. |
| Diastolic blood pressure, mm Hg | 76.3 ± 5.3 | 75.1 ± 7.1 | n.s. |
| Hematocrit, % | 34.4 ± 3.1 | 34.4 ± 2.6 | n.s. |
| Hemoglobin, g/dl | 10.9 ± 1.0 | 10.9 ± 1.0 | n.s. |
| P, mg/dl | 5.8 ± 1.0 | 6.1 ± 1.3 | n.s. |
| i-PTH, pg/ml | 164.3 ± 108.5 | 140.0 ± 74.4 | n.s. |
| CRP, mg/dl | 0.19 ± 0.18 | 0.42 ± 0.8 | n.s. |
| BNP, pg/ml | 338.5 ± 343.9 | 309.7 ± 589.3 | n.s. |
| Drug therapy | |||
| Diuretics | 2 out of 8 | 3 out of 10 | n.s. |
| α-Blocker | 2 out of 8 | 0 out of 10 | n.s. |
| ß-Blocker | 2 out of 8 | 4 out of 10 | n.s. |
| Ca antagonist | 6 out of 8 | 6 out of 10 | n.s. |
| ACEi | 1 out of 8 | 0 out of 10 | n.s. |
| ARB | 6 out of 8 | 3 out of 10 | n.s. |
| α-Methyldopa | 1 out of 8 | 0 out of 10 | n.s. |
| Amezinium metilsulfate | 1 out of 8 | 1 out of 10 | n.s. |
P = Phosphate; i-PTH = intact parathyroid hormone; CRP = C-reactive protein; BNP = brain natriuretic peptide; ACEi = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; n.s. = not significant.
Plasma Carnitine Level
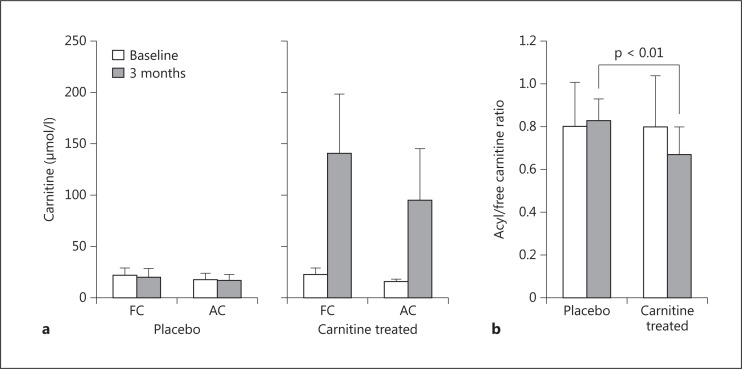
In the placebo group, free and acyl carnitine levels were 21.6 ± 7.9 and 17.4 ± 6.8 μmol/l at baseline, respectively, and 20.9 ± 7.8 and 17.2 ± 5.5 μmol/l after 3 months, respectively. In the treatment group, free and acyl carnitine levels were 22.3 ± 7.1 and 15.8 ± 2.8 μmol/l at baseline, respectively, and 140.3 ± 57.5 and 94.8 ± 50.4 μmol/l after 3 months, respectively. Differences in free and acyl carnitine levels at baseline versus after 3 months were highly significant (p < 0.001), representing a 6-fold increment in both cases (fig. 1a).
Fig. 1.
a Free carnitine and acyl carnitine concentrations before and after L-carnitine supplementation. b Acyl/free carnitine ratio before and after L-carnitine supplementation. FC = Free carnitine; AC = acyl carnitine.
The acyl/free carnitine ratio in the placebo group was 0.82 ± 0.19% at baseline and 0.83 ± 0.10% after 3 months. The acyl/free carnitine ratio in the treatment group decreased significantly from 0.80 ± 0.24% at baseline to 0.67 ± 0.13% after 3 months (p < 0.05). There was a significant difference in the acyl/free carnitine ratio between the placebo group and the treated group after 3 months of L-carnitine supplementation (p < 0.01; fig. 1b).
Effect of Oral L-Carnitine Treatment on Cardiac Function
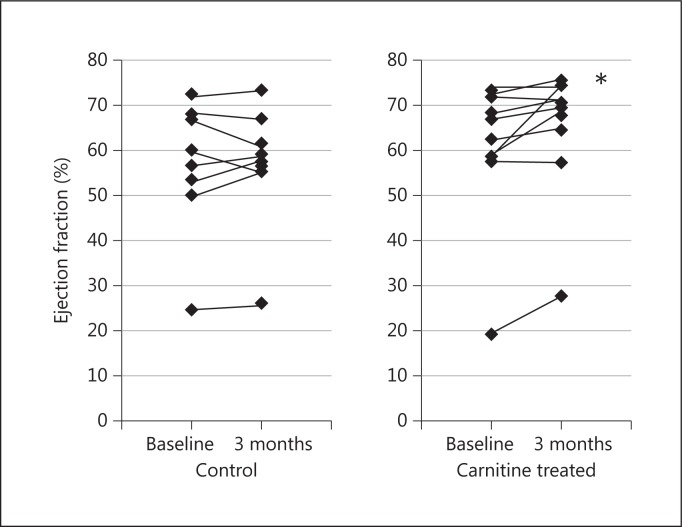
The ejection fraction significantly increased from 61.8 ± 16.0 to 64.4 ± 13.8% (p < 0.05) in the treated group (fig. 2). However, there was no difference in other echocardiographic parameters between the two groups (table 2).
Fig. 2.
Changes in ejection fraction. * p < 0.05.
Table 2.
Echocardiographic parameters of the patients before and after L-carnitine supplementation
| Baseline | After 3 months | |
|---|---|---|
| Placebo | ||
| Ejection fraction | 56.4 ± 15.0 | 56.9 ± 13.8 |
| Tei index | 0.65 ± 0.18 | 0.54 ± 0.21 |
| E/A | 0.69 ± 0.35 | 0.60 ± 0.25 |
| e/e′ | 15.9 ± 9.17 | 16.1 ± 9.51 |
| S/D | 1.73 ± 0.57 | 1.70 ± 0.50 |
| LV mass index | 109.4 ± 45.1 | 102.1 ± 22.3 |
| Body weight | 52.4 ± 15.4 | 52.9 ± 15.4 |
| Systolic blood pressure | 130.9 ± 17.4 | 136 ± 25.7 |
| Diastolic blood pressure | 76.3 ± 5.3 | 78.3 ± 13.2 |
| Carnitine treated | ||
| Ejection fraction | 61.8 ± 16.0 | 64.4 ± 13.8* |
| Tei index | 0.46 ± 0.1 | 0.44 ± 0.06 |
| E/A | 0.63 ± 0.12 | 0.66 ± 0.11 |
| e/e′ | 13.3 ± 4.25 | 13.1 ± 4.71 |
| S/D | 1.63 ± 0.51 | 1.93 ± 1.09 |
| LV mass index | 98.9 ± 15.0 | 97.1 ± 30.2 |
| Body weight | 53.7 ± 12.2 | 53.7 ± 12.1 |
| Systolic blood pressure | 136 ± 22.4 | 132.9 ± 17.2 |
| Diastolic blood pressure | 75 ± 7.1 | 73.5 ± 9.3 |
p values were calculated by paired t test.
p < 0.05.
Effect of Oral L-Carnitine Treatment on Intradialytic Hypotension
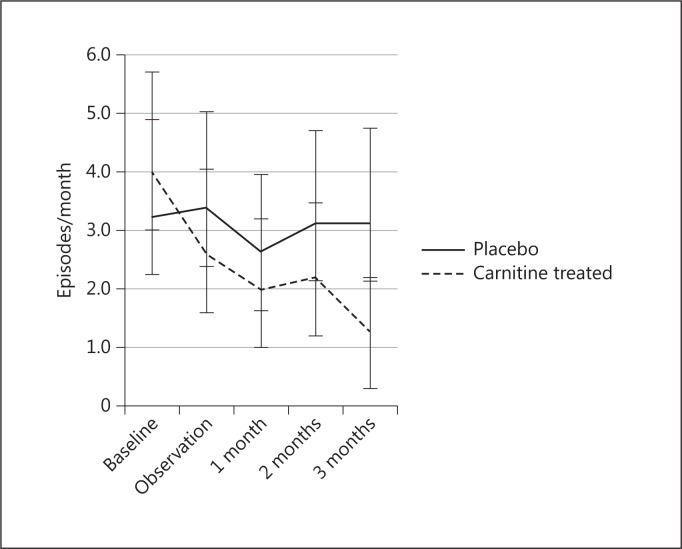
Hypotensive episodes significantly decreased from 4.0 ± 1.7 to 1.3 ± 0.9 times per month (p < 0.05) after 3 months of L-carnitine supplementation, although the patients' body weight did not change significantly (fig. 3).
Fig. 3.
Changes in hypotensive episodes after L-carnitine supplementation.
Discussion
Study Protocol
Despite many trials, the results regarding the effects of L-carnitine supplementation on cardiac function of chronic hemodialysis patients are controversial [8,9,10,11]. Some authors noted an improvement [13,14,15,16,17], while others found no change [18,19,20]. Firstly, study limitations such as heterogeneity of the study group, non-randomized, non-double-blind studies, and investigators' potential bias must be taken into account. Therefore, a randomized double-blind placebo-controlled study was performed here, rather than an open observational study.
Secondly, it has been reported that the cardiac function of hemodialysis patients is influenced not only by preload and afterload, but also by the hemodialysis procedure itself [21]. In order to evaluate cardiac function accurately, echocardiography was performed just after the hemodialysis procedure in our study (not on a predialysis or non-dialysis day) to keep preload constant and minimize uremic toxins which are potential cardiac depressants. Thirdly, it is well known that there is a negative correlation between plasma carnitine levels and the duration of dialysis [7]. In addition, it has been reported that there is a bell-shaped correlation between left ventricular hypertrophy and the duration of hemodialysis. Accordingly, patients with more than 2 years of hemodialysis treatment were selected in our study.
L-Carnitine and Cardiac Function
Only 1 randomized double-blind controlled study about the relationship of L-carnitine and cardiac function has been reported so far [18]. Fagher et al. [18] reported in 1985 that carnitine depletion is not responsible for cardiac dysfunction in patients on hemodialysis, but their patients had normal levels of skeletal and blood carnitine. Hemodialysis duration in their patients was not considered. Echocardiographic measurements were performed 16-18 h prior to dialysis. The duration of L-carnitine administration in their study was relatively short (6 weeks). In our study, it is not emphasized that L-carnitine has a positive inotropic effect, because the increase in the ejection fraction in the treated group was only 3% and statistical significance was marginal. However, taking the significant reduction of hypotensive episodes during the hemodialysis procedure into account, oral L-carnitine treatment might positively affect cardiac function even in patients with normal cardiac function.
In the systemic carnitine-deficient mouse [22], electron microscopy demonstrated an increase in mitochondria and lipid droplets in the cardiac muscle. Compression or distortion of the myofibril bundles suggested the possibility of functional abnormalities of the cardiac muscle. The cardiac myocytes contain autolysosomes or autophagic vacuoles with electron-dense membranous lamellar structures that are closely associated with mitochondria. Therefore, a potential reserve capacity of cardiac function might be damaged even in the phase of normal cardiac function assessed as total function. That might be the reason why a beneficial effect was observed in our study.
The echocardiographic parameters assessing diastolic cardiac function such as E/A, e/e′, and S/D were not affected after L-carnitine supplementation in our study. It has been reported that left ventricular mass measured by magnetic resonance imaging was reduced after 6 months of L-carnitine treatment [16]. However, such a reduction was not observed in our study. It is possible that the morphological changes might take a relatively longer time.
L-Carnitine and Hypotension
Hypotension is a frequent complication in hemodialysis patients. Cardiac dysfunction is a factor contributing to the occurrence of dialysis hypotension. It has been reported [23] that patients in whom hypotension occurred frequently had a significantly higher mortality rate than those in whom intradialytic hypotension was absent. Several studies have shown a significant reduction in the incidence of intradialytic hypotension with L-carnitine treatment [24,25]. Hypotensive episodes decreased significantly in our study, although patients' body weight did not change significantly. The findings of these studies indicate that L-carnitine has a great potential for use in the treatment of cardiac dysfunction, in particular in the treatment of dialysis-related hypotension.
Conclusions
Beneficial effects of administered L-carnitine on hemodynamic state of chronic hemodialysis patients were observed. L-carnitine supplementation might be considered especially in chronic hemodialysis patients with unstable hemodynamic conditions.
Disclosure Statement
There are no conflicts of interest.
References
- 1.Bahl JJ, Bressler R. The pharmacology of carnitine. Ann Rev Pharmacol Toxicol. 1987;27:257–277. doi: 10.1146/annurev.pa.27.040187.001353. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber B. Levocarnitine and dialysis: a review. Nutr Clin Pract. 2005;20:218–243. doi: 10.1177/0115426505020002218. [DOI] [PubMed] [Google Scholar]
- 3.Bonomini M, Zammit V, Pusey CD, Vecchi AD, Arduini A. Pharmacological use of L-carnitine in uremic anemia: has its full potential been exploited? Pharmacol Res. 2011;63:157–164. doi: 10.1016/j.phrs.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Reuter SE, Evans AM. Carnitine and acylcarnitines. Pharmacokinetic, pharmacological and clinical aspects. Clin Pharmacokinet. 2012;51:553–572. doi: 10.1007/BF03261931. [DOI] [PubMed] [Google Scholar]
- 5.Kudoh Y, Shoji T, Oimatsu H, Kikuchi K, Iimura O, Watarai I. Plasma L-carnitine in patients with chronic hemodialysis – pharmacokinetics of L-carnitine and its replacement therapy in these patients. Jpn J Nephrol. 1984;26:195–202. [PubMed] [Google Scholar]
- 6.Kudoh Y, Shoji T, Oimatsu H, Yoshida S, Kikuchi K, Iimura O. The role of L-carnitine in the pathogenesis of cardiomegaly in patients with chronic hemodialysis. Jpn Cir J. 1983;47:1391–1397. doi: 10.1253/jcj.47.1391. [DOI] [PubMed] [Google Scholar]
- 7.Kudoh Y, Shoji T, Oimatsu H, Yoshida S, Kikuchi K, Iimura O. Study on the risk factors of ischemic heart disease in patients with chronic hemodialysis with special reference of the role of plasma L-carnitine. Jpn J Nephrol. 1983;25:429–438. [PubMed] [Google Scholar]
- 8.Reuter SE, Faull RJ, Evans AM. L-carnitine supplementation in the dialysis population: are Australian patients missing out? Nephrology. 2008;13:3–16. doi: 10.1111/j.1440-1797.2007.00817.x. [DOI] [PubMed] [Google Scholar]
- 9.Schreiber B. Levocarnitine and dialysis: a review. Nutr Clin Pract. 2005;20:218–243. doi: 10.1177/0115426505020002218. [DOI] [PubMed] [Google Scholar]
- 10.Pauly DF, Pepine CJ. The role of carnitine in myocardial dysfunction. Am J Kidney Dis. 2003;41:S35–S43. doi: 10.1016/s0272-6386(03)00115-x. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad S. L-carnitine in dialysis patients. Semin Dial. 2001;14:209–217. doi: 10.1046/j.1525-139x.2001.00055.x. [DOI] [PubMed] [Google Scholar]
- 12.Harnett JD, Foley RN, Kent GM, Barre PE, Murray D, Parfrey PS. Congestive heart failure in dialysis patients; prevalence, incidence, prognosis and risk factors. Kidney Int. 1995;47:884–890. doi: 10.1038/ki.1995.132. [DOI] [PubMed] [Google Scholar]
- 13.Romagnoli GF, Naso A, Carraro G, Lidestri V. Beneficial effects of L-carnitine in dialysis patients with impaired left ventricular function: an observational study. Curr Med Res Opin. 2002;18:172–175. doi: 10.1185/030079902125000606. [DOI] [PubMed] [Google Scholar]
- 14.Trovato GM, Iannetti E, Murgo AM, Carpinteri G, Catalano D. Body composition and long term levo-carnitine supplementation. Clin Ther. 1998;149:209–214. [PubMed] [Google Scholar]
- 15.Van Es A, Henny FC, Kooistra MP, Lobbato S, Scholte HR. Amelioration of cardiac function by L-carnitine administration in patients on haemodialysis. Contrib Nephrol. 1992;98:28–35. doi: 10.1159/000421598. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto Y, Sato M, Ohashi H, Araki H, Tadokoro M, Osumi Y, Ito H, Morita H, Amano I. Effects of L-carnitine supplementation on cardiac morbidity in hemodialyzed patients. Am J Nephrol. 2000;20:201–207. doi: 10.1159/000013584. [DOI] [PubMed] [Google Scholar]
- 17.Khoss AE, Steger H, Legenstein E, Proll E, Salzer-Muhar U, Schlemmer M, Balzar E, Wimmer M. Effects of replacement therapy with L-carnitine on echocardiographic parameters in chronically hemodialyzed children. Wien Klin Wochenschr. 1989;101:17–20. [PubMed] [Google Scholar]
- 18.Fagher B, Cederblad G, Monti M, Olsson L, Rasmussen B, Thysell H. Carnitine and left ventricular function in haemodialysis patients. Scand J Clin Lab Invest. 1985;45:193–198. doi: 10.3109/00365518509160995. [DOI] [PubMed] [Google Scholar]
- 19.Sakurabayashi T, Takaesu Y, Haginoshita S, Takeda T, Aoike I, Miyazaki S, Koda Y, Yuasa Y, Sakai S, Suzuki M, Takahashi S, Hirasawa Y, Nakamura T. Improvement of myocardial fatty acid metabolism through L-carnitine administration to chronic hemodialysis patients. Am J Nephrol. 1999;19:480–484. doi: 10.1159/000013502. [DOI] [PubMed] [Google Scholar]
- 20.Topaloglu R, Celiker A, Saatci U, Kininc K, Bakkaloglu A, Besbas N, Sezaozen Tokel K. Effect of carnitine supplementation on cardiac function in hemodialyzed children. Acta Paediatr Japon. 1998;40:26–29. [PubMed] [Google Scholar]
- 21.Kudoh Y, Satoh S, Tsuchida A, Hikita S, Sasa Y, Iimura O. The dual effects of hemodialysis on cardiac function assessed by pulsed Doppler echocardiography. Jpn Circ J. 1988;52:13–20. doi: 10.1253/jcj.52.13. [DOI] [PubMed] [Google Scholar]
- 22.Horiuchi M, Yoshida H, Kobayashi K, Kuriwaki K, Yoshimine K, Tomomura M, Koizumi T, Nikaido H, Hayakawa J, et al. Cardiac hypertrophy in juvenile visceral steatosis (jvs) mice with systemic carnitine deficiency. FEBS Lett. 1993;326:267–271. doi: 10.1016/0014-5793(93)81805-a. [DOI] [PubMed] [Google Scholar]
- 23.Tislér A, Akócsi K, Borbás B, Fazakas L, Ferenczi S, Görögh S, Kulcsar I, Nagy L, Samik J, Szegedi J, Toth E, Wagner G, Kiss I. The effect of frequent or occasional dialysis-associated hypotension on survival of patients on maintenance haemodialysis. Nephrol Dial Transplant. 2003;18:2601–2605. doi: 10.1093/ndt/gfg450. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad S, Robertson HT, Golper TA, Wolfson M, Kurtin P, Katz LA, Hirshberg R, Nocora R, Ashbrook DW, Kopple JD. Multicenter trial of L-carnitine in maintenance of hemodialysis patients II. Clinical and biochemical effects. Kidney Int. 1990;38:912–918. doi: 10.1038/ki.1990.290. [DOI] [PubMed] [Google Scholar]
- 25.Casciani CU, Caruso U, Cravotto E, Corsi M, Maccari F. Beneficial effects of L-carnitine in post-dialysis syndrome. Curr Ther Res. 1982;32:116–127. [Google Scholar]