Abstract
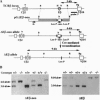
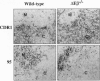
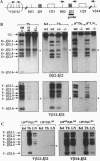
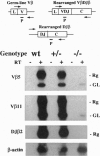
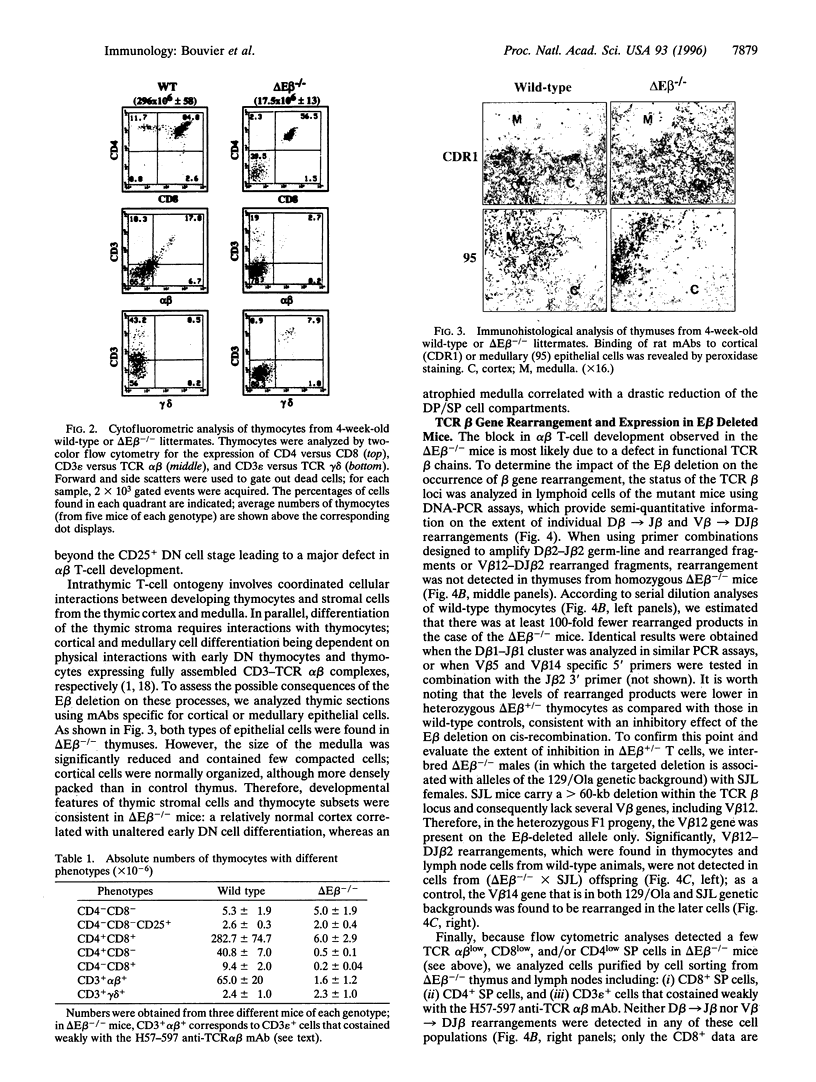
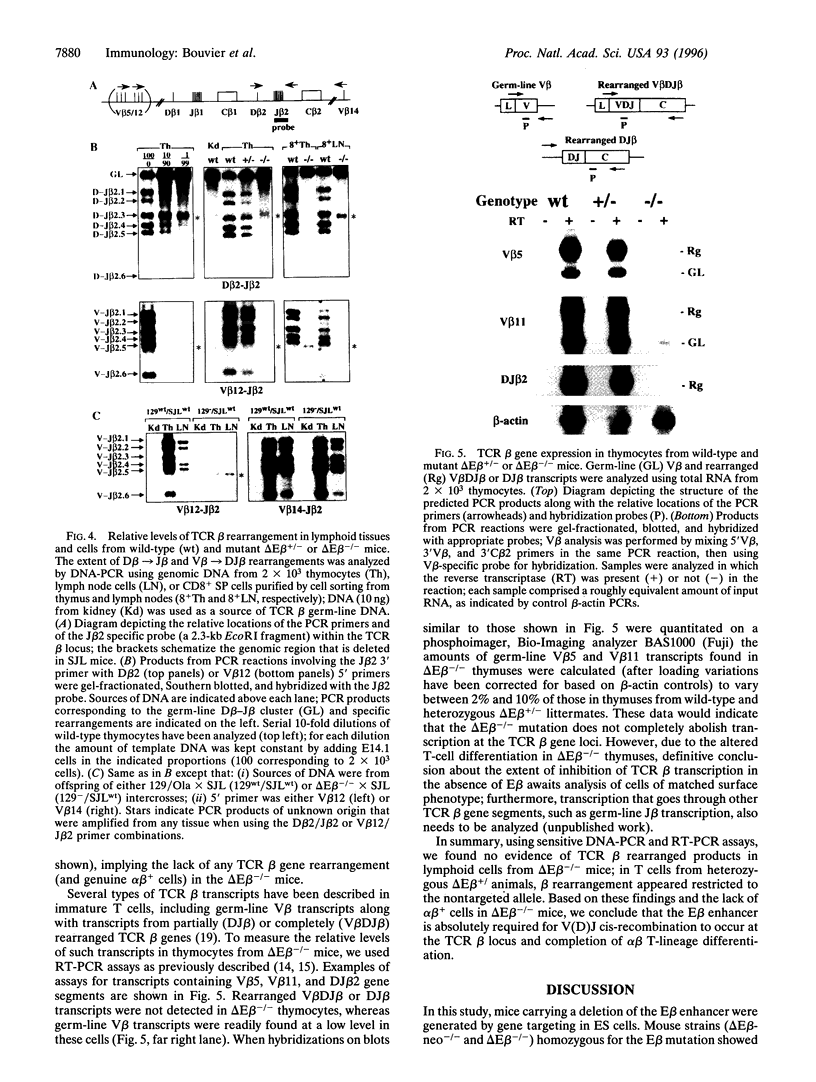
Intrathymic T-cell development requires temporally regulated rearrangement and expression of T-cell receptor (TCR) genes. To assess the role of the TCR beta gene transcriptional enhancer (Ebeta) in this process, mouse strains in which Ebeta is deleted were generated using homologous recombination techniques. We report that mice homozygous for the Ebeta deletion, whether a selectable marker gene is present or not, show a block in alphabeta T-cell development at the CD4-CD8- double-negative cell stage, whereas the number of gammadelta+ T cells is normal, few CD4+CD8+ double-positive thymocytes and no alphabeta+ T cells are produced. DNA-PCR and RNA-PCR analyses of thymic cells from homozygous mutants showed no evidence of TCR beta gene rearrangement although germ-line Vbeta transcripts were detected at a low level, in heterozygous T cells, the targeted allele is not rearranged. Thus, deletion of Ebeta totally prevents rearrangement, but not transcription, of the targeted beta locus. These data formally establish the critical role played by Ebeta in cis-activation of the TCR beta locus for V(D)J recombination during alphabeta T-cell development.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. J., Abraham K. M., Nakayama T., Singer A., Perlmutter R. M. Inhibition of T-cell receptor beta-chain gene rearrangement by overexpression of the non-receptor protein tyrosine kinase p56lck. EMBO J. 1992 Dec;11(13):4877–4886. doi: 10.1002/j.1460-2075.1992.tb05594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T., Nehls M., Kyewski B. Transcription factors that control development of the thymic microenvironment. Immunol Today. 1995 Dec;16(12):555–556. doi: 10.1016/0167-5699(95)80074-3. [DOI] [PubMed] [Google Scholar]
- Capone M., Curnow J., Bouvier G., Ferrier P., Horvat B. T cell development in TCR-alpha beta transgenic mice. Analysis using V(D)J recombination substrates. J Immunol. 1995 May 15;154(10):5165–5172. [PubMed] [Google Scholar]
- Capone M., Watrin F., Fernex C., Horvat B., Krippl B., Wu L., Scollay R., Ferrier P. TCR beta and TCR alpha gene enhancers confer tissue- and stage-specificity on V(D)J recombination events. EMBO J. 1993 Nov;12(11):4335–4346. doi: 10.1002/j.1460-2075.1993.tb06118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Young F., Bottaro A., Stewart V., Smith R. K., Alt F. W. Mutations of the intronic IgH enhancer and its flanking sequences differentially affect accessibility of the JH locus. EMBO J. 1993 Dec;12(12):4635–4645. doi: 10.1002/j.1460-2075.1993.tb06152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehling H. J., Krotkova A., Saint-Ruf C., von Boehmer H. Crucial role of the pre-T-cell receptor alpha gene in development of alpha beta but not gamma delta T cells. Nature. 1995 Jun 29;375(6534):795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- Ferrier P., Krippl B., Blackwell T. K., Furley A. J., Suh H., Winoto A., Cook W. D., Hood L., Costantini F., Alt F. W. Separate elements control DJ and VDJ rearrangement in a transgenic recombination substrate. EMBO J. 1990 Jan;9(1):117–125. doi: 10.1002/j.1460-2075.1990.tb08087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiering S., Epner E., Robinson K., Zhuang Y., Telling A., Hu M., Martin D. I., Enver T., Ley T. J., Groudine M. Targeted deletion of 5'HS2 of the murine beta-globin LCR reveals that it is not essential for proper regulation of the beta-globin locus. Genes Dev. 1995 Sep 15;9(18):2203–2213. doi: 10.1101/gad.9.18.2203. [DOI] [PubMed] [Google Scholar]
- Godfrey D. I., Zlotnik A. Control points in early T-cell development. Immunol Today. 1993 Nov;14(11):547–553. doi: 10.1016/0167-5699(93)90186-O. [DOI] [PubMed] [Google Scholar]
- Gu H., Zou Y. R., Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993 Jun 18;73(6):1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- Kisielow P., von Boehmer H. Development and selection of T cells: facts and puzzles. Adv Immunol. 1995;58:87–209. doi: 10.1016/s0065-2776(08)60620-3. [DOI] [PubMed] [Google Scholar]
- Krimpenfort P., de Jong R., Uematsu Y., Dembic Z., Ryser S., von Boehmer H., Steinmetz M., Berns A. Transcription of T cell receptor beta-chain genes is controlled by a downstream regulatory element. EMBO J. 1988 Mar;7(3):745–750. doi: 10.1002/j.1460-2075.1988.tb02871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R., Rajewsky K., Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991 Nov 1;254(5032):707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- Lauzurica P., Krangel M. S. Temporal and lineage-specific control of T cell receptor alpha/delta gene rearrangement by T cell receptor alpha and delta enhancers. J Exp Med. 1994 Jun 1;179(6):1913–1921. doi: 10.1084/jem.179.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt C. N., Eichmann K. Receptors and signals in early thymic selection. Immunity. 1995 Dec;3(6):667–672. doi: 10.1016/1074-7613(95)90056-x. [DOI] [PubMed] [Google Scholar]
- Levin S. D., Anderson S. J., Forbush K. A., Perlmutter R. M. A dominant-negative transgene defines a role for p56lck in thymopoiesis. EMBO J. 1993 Apr;12(4):1671–1680. doi: 10.1002/j.1460-2075.1993.tb05812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malissen M., Gillet A., Ardouin L., Bouvier G., Trucy J., Ferrier P., Vivier E., Malissen B. Altered T cell development in mice with a targeted mutation of the CD3-epsilon gene. EMBO J. 1995 Oct 2;14(19):4641–4653. doi: 10.1002/j.1460-2075.1995.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P., Clarke A. R., Hooper M. L., Tonegawa S. Creation of a large genomic deletion at the T-cell antigen receptor beta-subunit locus in mouse embryonic stem cells by gene targeting. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3084–3087. doi: 10.1073/pnas.88.8.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P., Clarke A. R., Rudnicki M. A., Iacomini J., Itohara S., Lafaille J. J., Wang L., Ichikawa Y., Jaenisch R., Hooper M. L. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992 Nov 19;360(6401):225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Iacomini J., Johnson R. S., Herrup K., Tonegawa S., Papaioannou V. E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992 Mar 6;68(5):869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Okada A., Alt F. W. Mechanisms that control antigen receptor variable region gene assembly. Semin Immunol. 1994 Jun;6(3):185–196. doi: 10.1006/smim.1994.1024. [DOI] [PubMed] [Google Scholar]
- Okada A., Mendelsohn M., Alt F. Differential activation of transcription versus recombination of transgenic T cell receptor beta variable region gene segments in B and T lineage cells. J Exp Med. 1994 Jul 1;180(1):261–272. doi: 10.1084/jem.180.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L., Theodor L., Carter M., Vu M. N., Sasaki A. W., Wilkinson M. F. T cell receptor-beta mRNA splicing: regulation of unusual splicing intermediates. Mol Cell Biol. 1993 Mar;13(3):1686–1696. doi: 10.1128/mcb.13.3.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse R. V., Bolin L. M., Bender J. R., Kyewski B. A. Monoclonal antibodies reactive with subsets of mouse and human thymic epithelial cells. J Histochem Cytochem. 1988 Dec;36(12):1511–1517. doi: 10.1177/36.12.2461413. [DOI] [PubMed] [Google Scholar]
- Saint-Ruf C., Ungewiss K., Groettrup M., Bruno L., Fehling H. J., von Boehmer H. Analysis and expression of a cloned pre-T cell receptor gene. Science. 1994 Nov 18;266(5188):1208–1212. doi: 10.1126/science.7973703. [DOI] [PubMed] [Google Scholar]
- Serwe M., Sablitzky F. V(D)J recombination in B cells is impaired but not blocked by targeted deletion of the immunoglobulin heavy chain intron enhancer. EMBO J. 1993 Jun;12(6):2321–2327. doi: 10.1002/j.1460-2075.1993.tb05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y., Rathbun G., Lam K. P., Oltz E. M., Stewart V., Mendelsohn M., Charron J., Datta M., Young F., Stall A. M. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992 Mar 6;68(5):855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Takeda S., Zou Y. R., Bluethmann H., Kitamura D., Muller U., Rajewsky K. Deletion of the immunoglobulin kappa chain intron enhancer abolishes kappa chain gene rearrangement in cis but not lambda chain gene rearrangement in trans. EMBO J. 1993 Jun;12(6):2329–2336. doi: 10.1002/j.1460-2075.1993.tb05887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Ardouin L., Gillet A., Lin S. Y., Magnan A., Malissen B., Malissen M. Early T-cell development in CD3-deficient mice. Immunol Rev. 1995 Dec;148:171–199. doi: 10.1111/j.1600-065x.1995.tb00098.x. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. Positive selection of lymphocytes. Cell. 1994 Jan 28;76(2):219–228. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]