Abstract
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal malignancy of the gastrointestinal tract. PET/CT is a common diagnostic tool and is also used for therapy monitoring. GISTs typically show strong 18F-fluorodeoxyglucose (FDG) uptake. Here we present two cases of GIST with unusually low/negative FDG uptake. FDG negativity does not preclude the diagnosis of a GIST.
Key Words: Gastrointestinal stromal tumors, PET/CT, Fluorodeoxyglucose
Introduction
Gastrointestinal stromal tumors (GISTs) represent the most common mesenchymal malignancy of the gastrointestinal tract [1]. They typically show 18F-fluorodeoxyglucose (FDG) uptake. FDG-PET/CT is therefore a common diagnostic tool: it can be valuable in interpreting ambiguous CT or MRI results and allows early assessment of treatment response [2]. Especially in cases in which biopsy remains inconclusive and radical surgery seems difficult, PET/CT can be an important measure, helping to direct the management of the patient.
Here we present two cases of GIST with unusually low/negative FDG uptake at the time of diagnosis.
Case Reports
Case 1
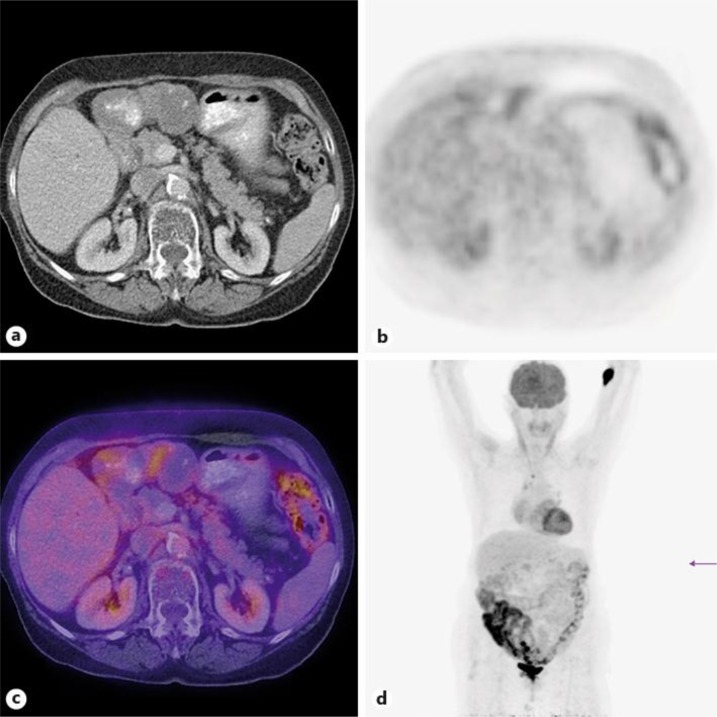
An 82-year-old patient had an abdominal ultrasound in order to rule out a postrenal cause of a urinary tract infection. A suspicious epigastric mass was found. A CT scan showed a 14 × 7 × 8 cm tumor of the anterior wall of the stomach. Endosonographic biopsy revealed a GIST. PET/CT (fig. 1) confirmed a partly cystic, partly solid tumor with a maximum FDG uptake standardized uptake value (SUV) of 2.2. A second mass in the right rectus abdominis muscle with a maximum SUV of 2.9 had disappeared in a CT scan 3 months later and was probably the equivalent of a postinterventional hematoma.
Fig. 1.
Partly cystic, partly solid tumor of the anterior stomach wall with a maximum FDG uptake SUV of 2.2.
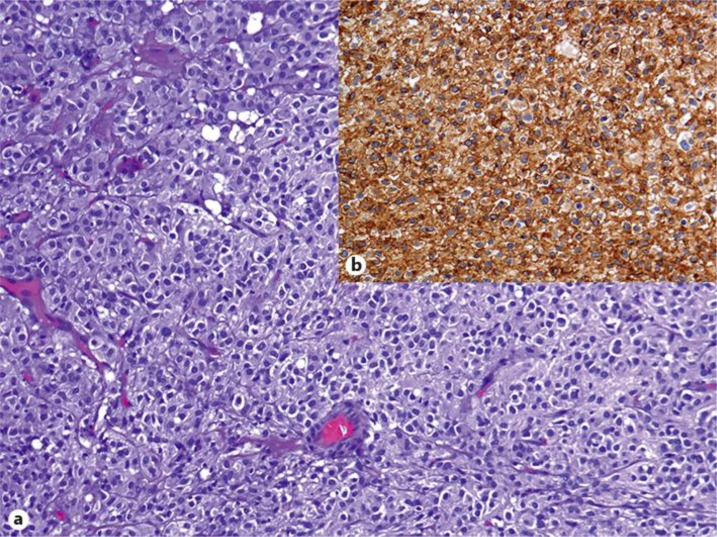
The patient received a sleeve tumorectomy. Histology (fig. 2a, b) showed a typical GIST with a mitotic count of 2/50 high power fields (HPF).
Fig. 2.
a Epithelioid variant of the GIST of case 1. b HE staining: strong CD117 expression.
Because of the intermediate risk profile [size >10 cm, mitotic count <5/50 HPF, location of the tumor (stomach favorable compared to small intestine 3)], no adjuvant therapy with imatinib was given. One year after surgery, there was no evidence of relapse.
Case 2
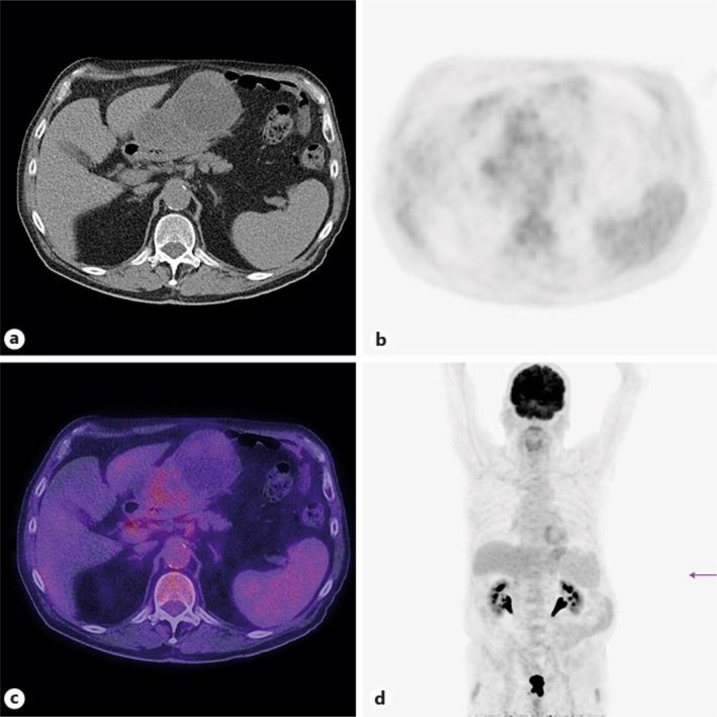
A 66-year-old woman presented with epigastric and right-sided chest pain. Endoscopy discovered a submucosal mass in the antrum. Percutaneous biopsy revealed a GIST. A subsequent PET/CT (fig. 3) showed a 4-cm paragastric tumor, with a locoregional suspicious lymph node of 6 mm in diameter. There was no evidence of other metastasis. The tumor was FDG negative, except for a central area of the tumor with a maximum SUV of 4.2.
Fig. 3.
FDG-negative paragastric tumor with a central area of the tumor with a maximum SUV of 4.2.
The patient initially refused the intended surgery because of fear of perioperative risks due to other medical conditions. She received palliative therapy with imatinib. After 3 months, PET/CT showed a slightly larger tumor with a lower FDG uptake with a SUV of maximum 3.0. Consequently, the patient agreed to be operated on. A distal gastrectomy was performed. Histology revealed a typical GIST of 7 cm in diameter with a mitotic count of <5/50 HPF. Because of the low-risk profile (low mitotic count, location of the tumor), the patient received no adjuvant therapy with imatinib. So far, there has been no evidence of tumor recurrence.
Discussion
GISTs have been documented in all parts of the gastrointestinal tract. A great majority of GISTs occur in the stomach (60–70%) and the small intestine (25–35%), with a rare occurrence in the colon and rectum (5%), the esophagus (<2%) and the appendix [4]. GISTs are usually asymptomatic in early stages. They are often unrecognized until serious symptoms such as bleeding or obstruction occur. Approximately 50% of patients have developed distant metastasis at the time of diagnosis, mostly of the liver or the peritoneum.
PET/CT is frequently used for staging purposes and is particularly indicated in ambiguous CT or MRI results [2]. GISTs typically show FDG uptake [5]. The sensitivity and positive predictive value for the detection of GISTs by PET/CT have been described as 86 and 98%, respectively, and false-negative PET/CTs were mostly related to small lesions [6]. This and the fact that these are relatively rare tumors might lead to the impression that GISTs are always FDG positive. However, PET/CTs of our 2 patients showed very low FDG uptake, although their tumors were large, leaving us uncertain about the diagnosis until we obtained histological proof of GIST.
The malignant potential of GISTs is difficult to predict preoperatively as risk stratification is assessed by pathological factors such as tumor size, mitotic count and lesion site. PET/CT has been described as a potential predictor for malignant potential of GISTs with low FDG uptake, indicating low-risk GISTs [5, 7]. Both of our patients had GISTs with low histological risk profiles, which could possibly explain the low FDG uptake.
PET/CT is a sensitive and specific method to assess early response to imatinib treatment [8, 9] as tumor size alone is unreliable for assessing the response in early imatinib treatment [8]. Consequently, when neoadjuvant imatinib therapy is considered, we believe that a baseline PET/CT is compulsory. This has not been suggested in the NCCN guidelines [10]. PET/CT cannot be used for therapy monitoring in patients whose baseline FDG-PET results are negative [8].
Conclusion
PET/CT is a very useful diagnostic tool for the management of GISTs. However, in tumors with typical morphological criteria, a GIST should always be considered even when FDG uptake is negative or low.
References
- 1.Kochhar R, Manoharan P, Leahy M, Taylor MB. Imaging in gastrointestinal stromal tumours: current status and future directions. Clin Radiol. 2010;65:584–592. doi: 10.1016/j.crad.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Kalkmann J, Zeile M, Antoch G, Berger F, Diederich S, Dinter D, Fink C, Janka R, Stattaus J. Consensus report on the radiological management of patients with gastrointestinal stromal tumours (GIST): recommendations of the German GIST Imaging Working Group. Cancer Imaging. 2012;12:126–135. doi: 10.1102/1470-7330.2012.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38(suppl 5):S39–S51. doi: 10.1016/s0959-8049(02)80602-5. [DOI] [PubMed] [Google Scholar]
- 5.Kamiyama Y, Aihara R, Nakabayashi T, Mochiki E, Asao T, Kuwano H, Oriuchi N, Endo K. 18F-fluorodeoxyglucose positron emission tomography: useful technique for predicting malignant potential of gastrointestinal stromal tumors. World J Surg. 2005;29:1429–1435. doi: 10.1007/s00268-005-0045-6. [DOI] [PubMed] [Google Scholar]
- 6.Gayed I, Vu T, Iyer R, Johnson M, Macapinlac H, Swanston N, Podoloff D. The role of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. J Nucl Med. 2004;45:17–21. [PubMed] [Google Scholar]
- 7.Park JW, Cho CH, Jeong DS, Chae HD. Role of F-fluoro-2-deoxyglucose positron emission tomography in gastric GIST: predicting malignant potential pre-operatively. J Gastric Cancer. 2011;11:173–179. doi: 10.5230/jgc.2011.11.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi H, Charnsangavej C, de Castro Faria S, Tamm EP, Benjamin RS, Johnson MM, Macapinlac HA, Podoloff DA. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. Am J Roentgenol. 2004;183:1619–1628. doi: 10.2214/ajr.183.6.01831619. [DOI] [PubMed] [Google Scholar]
- 9.Stroobants S, Goeminne J, Seegers M, Dimitrijevic S, Dupont P, Nuyts J, Martens M, van den Borne B, Cole P, Sciot R, Dumez H, Silberman S, Mortelmans L, van Oosterom A. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec) Eur J Cancer. 2003;39:2012–2020. doi: 10.1016/s0959-8049(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 10.Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, Sundar HM, Trent JC, Wayne JD. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8(suppl 2):S1–S41. doi: 10.6004/jnccn.2010.0116. quiz S42–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]