Abstract
Binding of Ricinus communis I agglutinin to the outer surface of resealed human erythrocyte ghosts results in an organizational perturbation that is translated to the inner membrane surface. The organizational change was detected by an enhancement in the chemical cross-linking of several erythrocyte membrane components by the bifunctional reagent, dimethyl malonimidate, resulting in their loss or reduction after sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the solubilized erythrocyte components. Of the components that failed to appear, or are reduced in amount, on the gels (protein bands Ia, Ib, IVa, and VII), two are known to be the subunits of spectrin (bands Ia and Ib), an inner-surface peripheral protein. A new band, which was identified as R. communis lectin, appeared on the polyacrylamide gels of lectin-treated ghosts with or without crosslinking. The loss of spectrin and other bands after lectin treatment and chemical crosslinking was due to a specific transmembrane event because: (a) β-lactose, an inhibitor of R. communis agglutinin, prevented labeling of ghosts by the lectin and loss of spectrin and other erythrocyte components on gels after crosslinking; (b) use of inactive bifunctional or active monofunctional crosslinking reagents did not result in loss of spectrin or other components from lectin-treated ghosts; (c) the loss of spectrin and other components after lectin treatment and crosslinking was sensitive to temperature and lectin concentration; (d) no new bands appeared on the gels except for the band identified as R. communis agglutinin; (e) R. communis agglutinin does not interact with purified spectrin; and (f) previously published data indicate the R. communis lectin binds exclusively to the outer membrane surface while spectrin is located on the inner membrane surface. Perturbation of components of the outer membrane surface that can be translated to the cell interior by transmembrane linkages may provide a structural means of membrane communication that could be important in a variety of cellular control processes.
Keywords: Ricinus communis agglutinin, receptor, spectrin, crosslinking, human erythrocyte membrane
Full text
PDF

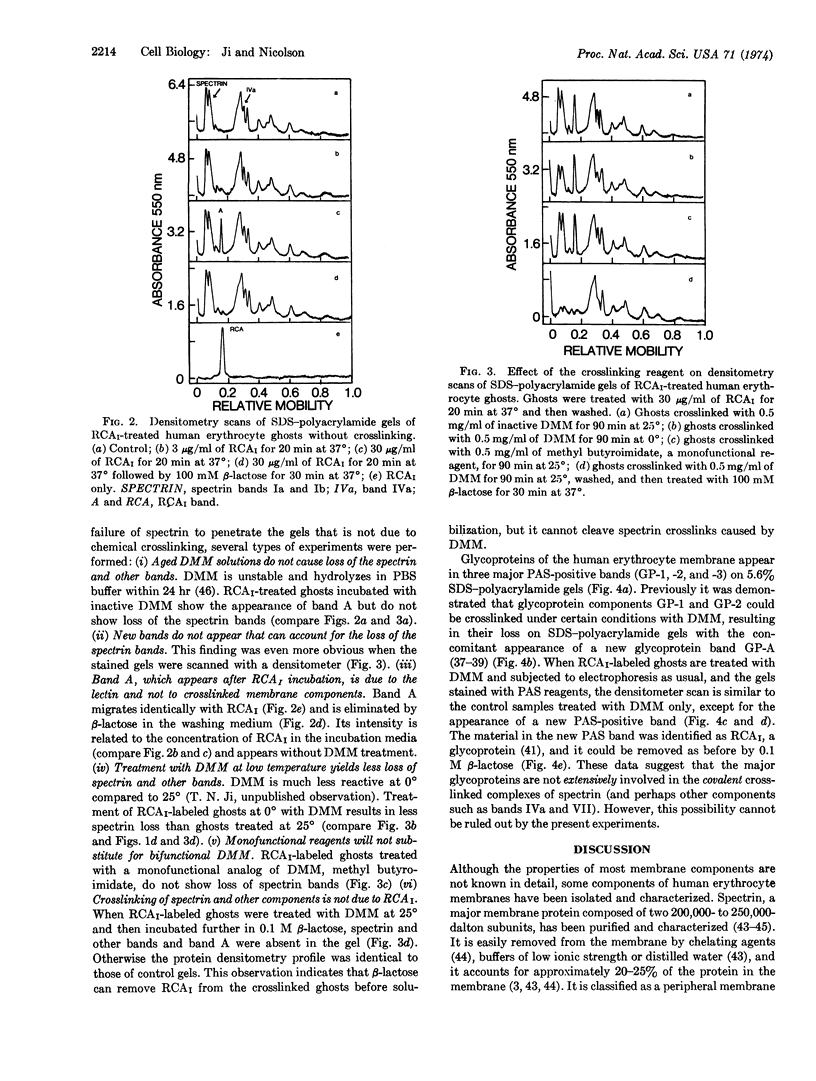
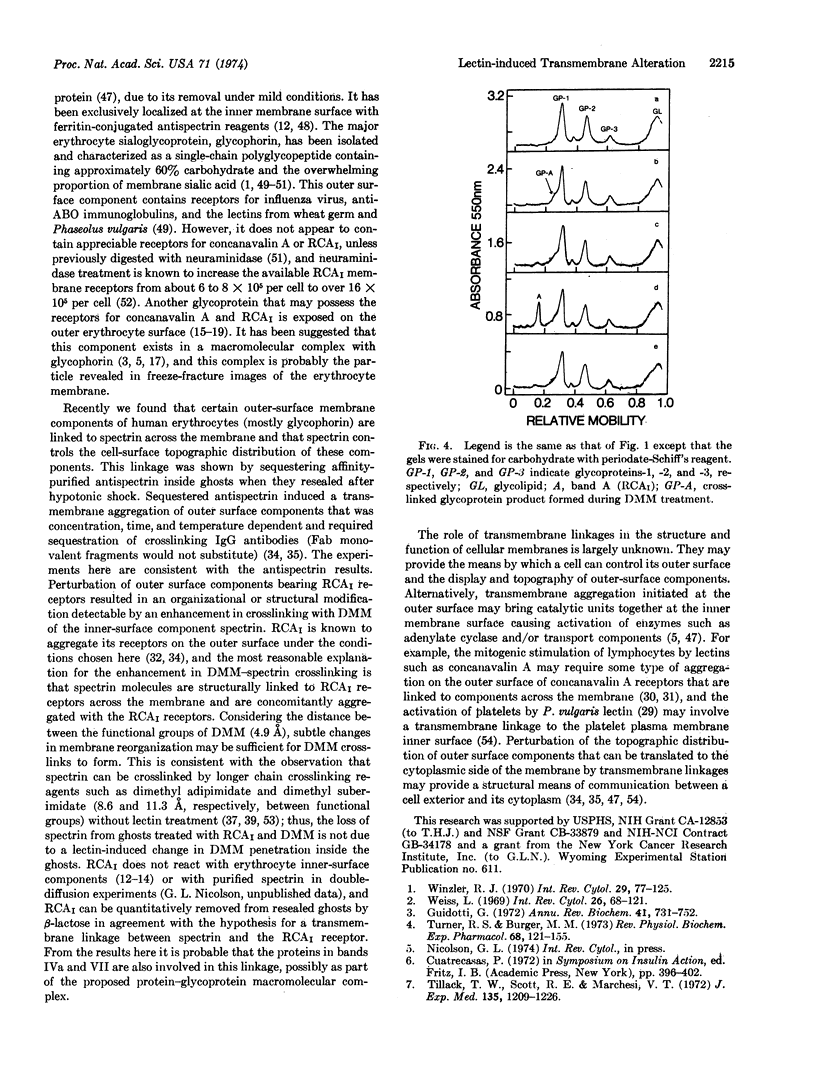

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Sjöberg O., Möller G. Mitogens as probes for immunocyte activation and cellular cooperation. Transplant Rev. 1972;11:131–177. doi: 10.1111/j.1600-065x.1972.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. A major protein which spans the human erythrocyte membrane. J Mol Biol. 1971 Jul 28;59(2):351–357. doi: 10.1016/0022-2836(71)90055-6. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Major human erythrocyte glycoprotein spans the cell membrane. Nat New Biol. 1971 Jun 23;231(25):229–232. doi: 10.1038/newbio231229a0. [DOI] [PubMed] [Google Scholar]
- Clarke M. Isolation and characterization of a water-soluble protein from bovine erythrocyte membranes. Biochem Biophys Res Commun. 1971 Nov;45(4):1063–1070. doi: 10.1016/0006-291x(71)90445-1. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. The insulin receptor. Diabetes. 1972;21(2 Suppl):396–402. doi: 10.2337/diab.21.2.s396. [DOI] [PubMed] [Google Scholar]
- De Petris S., Raff M. C., Mallucci L. Ligand-induced redistribution of concanavalin A receptors on normal, trypsinized and transformed fibroblasts. Nat New Biol. 1973 Aug 29;244(139):275–278. doi: 10.1038/newbio244275a0. [DOI] [PubMed] [Google Scholar]
- Dutton A., Adams M., Singer S. J. Bifunctional imidoesters as cross-linking reagents. Biochem Biophys Res Commun. 1966 Jun 13;23(5):730–739. doi: 10.1016/0006-291x(66)90462-1. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I., Wang J. L. Receptor mobility and receptor-cytoplasmic interactions in lymphocytes. Proc Natl Acad Sci U S A. 1973 May;70(5):1442–1446. doi: 10.1073/pnas.70.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Osawa T. Isolation and characterization of a glycoprotein from human group O erythrocyte membrane. J Biol Chem. 1973 Jul 25;248(14):5100–5105. [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973 Jun 25;248(12):4311–4317. [PubMed] [Google Scholar]
- Guidotti G. Membrane proteins. Annu Rev Biochem. 1972;41:731–752. doi: 10.1146/annurev.bi.41.070172.003503. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulla F. W., Gratzer W. B. Association of high-molecular weight proteins in the red cell membrane. FEBS Lett. 1972 Sep 15;25(2):275–278. doi: 10.1016/0014-5793(72)80502-7. [DOI] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Mobility of carbohydrate containing sites on the surface membrane in relation to the control of cell growth. FEBS Lett. 1973 May 15;32(1):124–128. doi: 10.1016/0014-5793(73)80753-7. [DOI] [PubMed] [Google Scholar]
- Ji T. H. Cross-linking sialoglycoproteins of human erythrocyte membranes. Biochem Biophys Res Commun. 1973 Jul 17;53(2):508–514. doi: 10.1016/0006-291x(73)90691-8. [DOI] [PubMed] [Google Scholar]
- Ji T. H. Crosslinking of the glycoproteins in human erythrocyte membranes. Proc Natl Acad Sci U S A. 1974 Jan;71(1):93–95. doi: 10.1073/pnas.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenard J. Protein and glycolipid components of human erythrocyte membranes. Biochemistry. 1970 Mar 3;9(5):1129–1132. doi: 10.1021/bi00807a012. [DOI] [PubMed] [Google Scholar]
- Loor F., Forni L., Pernis B. The dynamic state of the lymphocyte membrane. Factors affecting the distribution and turnover of surface immunoglobulins. Eur J Immunol. 1972 Jun;2(3):203–212. doi: 10.1002/eji.1830020304. [DOI] [PubMed] [Google Scholar]
- Majerus P. W., Brodie G. N. The binding of phytohemagglutinins to human platelet plasma membranes. J Biol Chem. 1972 Jul 10;247(13):4253–4257. [PubMed] [Google Scholar]
- Marchesi V. T., Tillack T. W., Jackson R. L., Segrest J. P., Scott R. E. Chemical characterization and surface orientation of the major glycoprotein of the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1445–1449. doi: 10.1073/pnas.69.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazia D., Ruby A. Dissolution of erythrocyte membranes in water and comparison of the membrane protein with other structural proteins. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1005–1012. doi: 10.1073/pnas.61.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Anionic sites of human erythrocyte membranes. I. Effects of trypsin, phospholipase C, and pH on the topography of bound positively charged colloidal particles. J Cell Biol. 1973 May;57(2):373–387. doi: 10.1083/jcb.57.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J., Etzler M. E. Characterization of two plant lectins from Ricinus communis and their quantitative interaction with a murine lymphoma. Biochemistry. 1974 Jan 1;13(1):196–204. doi: 10.1021/bi00698a029. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J. The interaction of Ricinus communis agglutinin with normal and tumor cell surfaces. Biochim Biophys Acta. 1972 May 9;266(2):543–547. doi: 10.1016/0005-2736(72)90109-5. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Cis- and trans-membrane control of cell surface topography. J Supramol Struct. 1973;1(4):410–416. doi: 10.1002/jss.400010419. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Marchesi V. T., Singer S. J. The localization of spectrin on the inner surface of human red blood cell membranes by ferritin-conjugated antibodies. J Cell Biol. 1971 Oct;51(1):265–272. doi: 10.1083/jcb.51.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Neuraminidase "unmasking" and failure of trypsin to "unmask" -D-galactose-like sites on erythrocyte, lymphoma, and normal and virus-transformed fibroblast cell membranes. J Natl Cancer Inst. 1973 Jun;50(6):1443–1451. doi: 10.1093/jnci/50.6.1443. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Painter R. G. Anionic sites of human erythrocyte membranes. II. Antispectrin-induced transmembrane aggregation of the binding sites for positively charged colloidal particles. J Cell Biol. 1973 Nov;59(2 Pt 1):395–406. doi: 10.1083/jcb.59.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Singer S. J. Electron microscopic localization of macromolecules on membrane surfaces. Ann N Y Acad Sci. 1972 Jun 20;195:368–375. [PubMed] [Google Scholar]
- Nicolson G. L., Singer S. J. Ferritin-conjugated plant agglutinins as specific saccharide stains for electron microscopy: application to saccharides bound to cell membranes. Proc Natl Acad Sci U S A. 1971 May;68(5):942–945. doi: 10.1073/pnas.68.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Singer S. J. The distribution and asymmetry of mammalian cell surface saccharides utilizing ferritin-conjugated plant agglutinins as specific saccharide stains. J Cell Biol. 1974 Jan;60(1):236–248. doi: 10.1083/jcb.60.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Temperature-dependent mobility of concanavalin A sites on tumour cell surfaces. Nat New Biol. 1973 Jun 13;243(128):218–220. doi: 10.1038/newbio243218a0. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. The relationship of a fluid membrane structure to cell agglutination and surface topography. Ser Haematol. 1973;6(3):275–291. [PubMed] [Google Scholar]
- Nicolson G. L. Topography of membrane concanavalin A sites modified by proteolysis. Nat New Biol. 1972 Oct 18;239(94):193–197. doi: 10.1038/newbio239193a0. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. Exposed protein on the intact human erythrocyte. Biochemistry. 1971 May 11;10(10):1766–1771. doi: 10.1021/bi00786a006. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Guidotti G. Fractionation of the protein components of human erythrocyte membranes. J Biol Chem. 1969 Oct 10;244(19):5118–5124. [PubMed] [Google Scholar]
- Rosenblith J. Z., Ukena T. E., Yin H. H., Berlin R. D., Karnovsky M. J. A comparative evaluation of the distribution of concanavalin A-binding sites on the surfaces of normal, virally-transformed, and protease-treated fibroblasts. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1625–1629. doi: 10.1073/pnas.70.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrest J. P., Kahane I., Jackson R. L., Marchesi V. T. Major glycoprotein of the human erythrocyte membrane: evidence for an amphipathic molecular structure. Arch Biochem Biophys. 1973 Mar;155(1):167–183. doi: 10.1016/s0003-9861(73)80019-0. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Steck T. L. Cross-linking the major proteins of the isolated erythrocyte membrane. J Mol Biol. 1972 May 14;66(2):295–305. doi: 10.1016/0022-2836(72)90481-0. [DOI] [PubMed] [Google Scholar]
- Tillack T. W., Scott R. E., Marchesi V. T. The structure of erythrocyte membranes studied by freeze-etching. II. Localization of receptors for phytohemagglutinin and influenza virus to the intramembranous particles. J Exp Med. 1972 Jun 1;135(6):1209–1227. doi: 10.1084/jem.135.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayer H. R., Nozaki Y., Reynolds J. A., Tanford C. Polypeptide chains from human red blood cell membranes. J Biol Chem. 1971 Jul 25;246(14):4485–4488. [PubMed] [Google Scholar]
- Triplett R. B., Carraway K. L. Proteolytic digestion of erythrocytes, resealed ghosts, and isolated membranes. Biochemistry. 1972 Jul 18;11(15):2897–2903. doi: 10.1021/bi00765a024. [DOI] [PubMed] [Google Scholar]
- Turner R. S., Burger M. M. The cell surface in cell interactions. Ergeb Physiol. 1973;68:121–155. doi: 10.1007/3-540-06238-6_6. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Perkins W. D., Karnovsky M. J. Ligand-induced movement of lymphocyte membrane macromolecules. I. Analysis by immunofluorescence and ultrastructural radioautography. J Exp Med. 1972 Oct 1;136(4):885–906. doi: 10.1084/jem.136.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzler R. J. Carbohydrates in cell surfaces. Int Rev Cytol. 1970;29:77–125. doi: 10.1016/s0074-7696(08)60033-9. [DOI] [PubMed] [Google Scholar]