Abstract
Management of meningioma includes observation, resection, and radiation therapy (RT). For patients with recurrent disease, similar options exist. However, the control rate following a second course of RT for recurrent disease is unknown. We reviewed an institutional database of patients with meningioma treated with stereotactic radiosurgery or fractionated stereotactic RT who underwent a second course for recurrent disease. Cox regression was used for analysis. Variables tested included tumor volume, RT type, tumor grade, age at diagnosis, time to progression, and interval between RT. Eleven of 19 patients (58%) experienced disease progression. Median time to second progression was 10 months. Freedom from progression at one year was lower in patients with grade II or III tumors compared to those with grade 1 or unknown histology (17% compared to 92%, p = 0.0054). Cox regression showed that a grade II–III tumor affects progression-free survival (PFS), with a hazard ratio of 5.37 (p = 0.011). Median time to progression (MTP) for patients with grade II–III tumors was eight months. MTP was not reached for patients with grade 1/unknown tumors. Reirradiation for recurrent meningioma yields modest tumor control rates but for patients with grade II or III tumors, outcomes are poor.
Keywords: Meningioma, Radiation Therapy, Recurrent, Radiosurgery, Tumor
1 Introduction
Meningioma is one of the most common tumors of the central nervous system, accounting for approximately one-third of all brain tumors 1. Management options include observation, surgical resection, and radiation therapy. Surgery is generally the mainstay of treatment of meningioma, and gross total resection can be achieved in approximately two-thirds of patients2. Radiation therapy (RT) is typically reserved for tumors that are unresectable, incompletely resected, high-grade, or recurrent after initial resection.
RT has been shown to be an effective salvage treatment for disease recurrence after surgical resection 3,4,5. For patients with disease recurrence after RT, the safety and efficacy of a second course of radiation is less well defined. Here we determine the outcome of patients with recurrent meningiomas who underwent a second course of RT.
2 Methods
We reviewed an institutional database of patients treated with stereotactic radiosurgery (SRS) or fractionated stereotactic radiotherapy (fSRT) between 1995 and 2009. Patients who underwent a second course of fSRT/SRS for disease recurrence or progression were identified for this analysis.
For patients treated with fSRT a dedicated 600SR linear accelerator (Varian, Palo Alto, CA, USA) was used to deliver 6 MV photons stereotactically. Before 2004, treatment planning was conducted with the X-Knife 3-D planning system (Radionics, Burlington, MA, USA). Patients were fitted with the Gill–Thomas–Cosman (GTC) relocatable frame and were taken to the MRI and/or CT scanning suite where the Brown–Roberts–Well fiducial cage was placed on the GTC frame and transaxial images were obtained. After June 2004, treatment planning was carried out with Brain Lab (Novalis TX™, Munich, Germany), with mMLC leaves and the ExacTrac feature. These patients were immobilized using a thermoplastic mask. For patients treated with SRS, either a Gamma Knife (Model U initially; model 4C after May 2006; Elekta Instruments, Atlanta, GA, USA) or Novalis TX were used. In patients treated with Gamma Knife, planning was performed with Leksell (Elekta Instruments). For patients treated with Novalis TX, Brain Lab was used for planning.
Gross tumor volume (GTV) was defined by the gadolinium-enhanced tumor edge using T1-weighted MRI series. The planning target volume was considered equivalent to the GTV, and edema, tumor “tail,” and the postoperative resection cavity were not included in the retreatment volume. Tumors were treated to the 85% to 90% isodose line in patients treated with fSRT/linear accelerator (LINAC)-based SRS, and to the 50% isodose line in patients receiving Gamma Knife radiosurgery. Critical structure constraints included the brain stem, optic nerve, and optic chiasm. For fractionated RT, limits of 54 Gy to 60 Gy to the brain stem, optic nerve, and chiasm were observed. For single fraction SRS the limits were 10 Gy to 12 Gy.
Clinical data, including patient presenting symptoms, tumor location, and tumor grade if available, according to the World Health Organization (WHO) Classification available at the time 6 were recorded. Treatment data included GTV, radiation modality (SRS or fSRT), and prescribed dose. Finally, outcome data including follow-up length and time to progression were obtained. Disease progression was defined as documented growth on follow-up imaging or need for additional intervention such as surgical resection. Time to progression was measured from completion of the second RT course.
The primary outcome measure was progression-free survival (PFS). Secondary outcomes included overall survival (OS) and treatment-related toxicity.
Kaplan–Meier curves were generated to examine the effect of several parameters on PFS and OS. These included tumor volume, type of radiation received (fSRT or SRS), tumor grade, age at diagnosis, gender, dose, time to progression, and timing of surgical intervention. Cox regression models were used to determine predictors of PFS and OS.
The Thomas Jefferson University institutional review board approved this single-institution, retrospective study.
3 Results
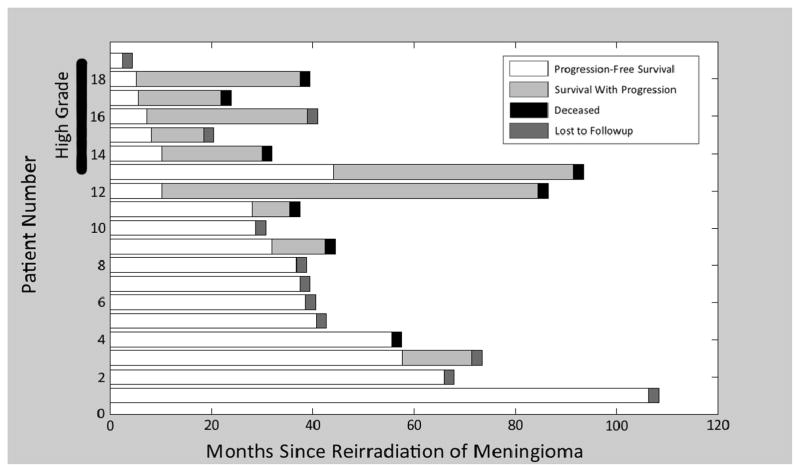
Our institutional database held 651 patients treated for meningioma from 1995 to 2009. All patients were treated with either fSRT (median dose = 54 Gy; range 41–64.8 Gy) or SRS (median dose = 15 Gy; range 14–18 Gy). Of this group, 19 patients underwent reirradiation with either fSRT (median dose = 50.4 Gy; range 35–54 Gy) or SRS (median Dose = 15 Gy; range 14–18 Gy) for a recurrent meningioma, giving a rate of reirradiation at our institution of 3%. A summary of each patient’s history is given in Table 1 with a corresponding representation of the disease course shown in Figure 1. The median time between the first and second courses of RT was 40 months (range 3–93 months). Of 19 patients, 14 underwent surgical resection prior to their initial course of RT, and six patients underwent surgical resection between courses of RT. Tumor grade information was available for the 15 patients who underwent surgical treatment at some point during therapy. Grading was according to the current WHO criteria available at the time 6. Eight patients were identified with WHO Grade I disease, one patient had grade II disease, and six had Grade III disease. The five patients who had no histologic sampling were grouped with the grade I patients for further analysis.
Table 1.
History of patients who underwent reirradiation of recurrent meningiomaa
| Patient No. | Age (Y) | Tumor location | Treatment prior to irradiation | Reirradiation | Treatment/s following reirradiation |
|---|---|---|---|---|---|
| 1 | 73 | Rt parafalcine | R (4 Y), SRS (16 Gy, 2 Y) | SRS to 15 Gy | None |
| 2 | 59 | L CPA angle | R (2 Y, grade 1), fSRT (1.5 Y, 54 Gy) | fSRT to 50.4 Gy | None |
| 3 | 47 | Rt mid cranial fossae | R (8 Y), SRS (15 Gy, 8 Y), R (3 M, grade 1) | SRS to 15 Gy | Reirradiation (5 Y) |
| 4 | 68 | L frontoparietal | fSRT (54 Gy, 7 Y) | SRS to 14 Gy | None |
| 5 | 33 | L optic nerve | R (3.5 Y, grade 1), fSRT (54 Gy, 2.5 Y) | fSRT to 50.4 Gy | None |
| 6 | 42 | L frontal/sphenoid | SRS (16 Gy, 4 Y) | fSRT to 54 Gy | R (3 Y) |
| 7 | 62 | Rt ant cranial fossa | R (5 Y, grade 1), SRS (14 Gy, 3 Y), R (2 Y), R (2 M) | fSRT to 46.8 Gy | None |
| 8 | 59 | L cavernous sinus | fSRT (54 Gy, 5 Y), R (4 M, grade 1) | fSRT to 50.4 Gy | None |
| 9 | 52 | Rt sphenoid wing | R (7.5 Y, grade 1), fSRT (54 Gy, 4 Y) | SRS to 14 Gy | None |
| 10 | 44 | L ONS | fSRT (50.4 Gy, 6 Y) | fSRT to 41.4 Gy | None |
| 11 | 57 | Rt sphenoid wing | Biopsy (4 Y, grade 1), fSRT (52.2 Gy, 4 Y) | fSRT to 45 Gy | None |
| 12 | 75 | L parasagittal | R (1 Y, grade 1), SRS to 18 Gy (4 M), R (3 M) | SRS to 18 Gy | Reirradiation (10 M), R (6 Y) |
| 13 | 67 | Rt occipital | R (3 Y, grade 3), fSRT (56 Gy, 3 Y) | SRS to 18 Gy | SRS (3.5 Y), RT (6.5 Y, grade II) |
| 14 | 61 | L temporal–parietal | R (5 Y, 1.5 Y, grade 3), fSRT (54 Gy, 15 M), R (2 M, grade 3) | fSRT to 35 Gy | R (10 M, grade 1), SRS (13 M, 16 Gy) |
| 15 | 53 | Bifrontal | SRS (14 Gy, 4 Y), R (1 Y, grade II) | fSRT to 54 Gy | R (1 Y, grade 3), reirradiation (1 Y), R (2 Y) |
| 16 | 78 | L frontal | R (1.5 Y, grade II), SRS (10 M, 15 Gy) | SRS to 18 Gy | SRS (2.5 Y, 15 Gy) |
| 17 | 69 | Rt infratemporal fossae | R (9 Y), fSRT (64.8 Gy, 3.5 Y), R (2.5 Y, 5 M, grade 3) | SRS to 18 Gy | SRS (7 M, 14 Gy) |
| 18 | 34 | Rt sphenoid wing | R (2.5 Y, 6 M), fSRT (41 Gy, 2 Y) | SRS to 14 Gy | R (9 M, 14 M, grade 3) |
| 19 | 73 | Bilateral parasagittal | R (3 Y, grade 3), SRS (15 Gy, 2 Y) | SRS to 14 Gy | None |
The disease course of each patient is shown in Figure 1.
Ant = anterior, CPA = cerebellopontine angle, fSRT = fractionated stereotactic radiotherapy, L = left, M = months, ONS = optic nerve sheath, R = resection, Rt = right, SRS = stereotactic radiosurgery, Y = years
Figure 1.
Timeline depicting the disease course of all 19 patients included in the study, divided into those with “high-grade” (World Health Organization grade II/III) and “low-grade” lesions. Progression, death, and loss to follow-up are indicated where appropriate.
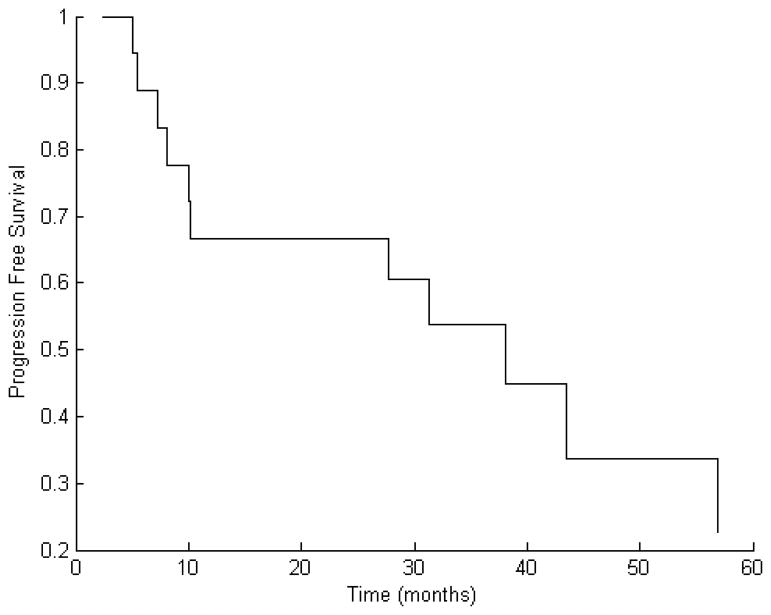
After a median follow-up time of 32 months (range 2–105 months) following reirradiation, 11 of 19 patients (58%) experienced further progression. Median time to this second progression was 10 months (range 5–57 months). The Kaplan–Meier estimate for PFS at one year was 66% (Fig. 2).
Figure 2.
Kaplan–Meier curve of progression free survival (PFS) for the entire patient cohort showing an estimate for the one-year PFS of 66%.
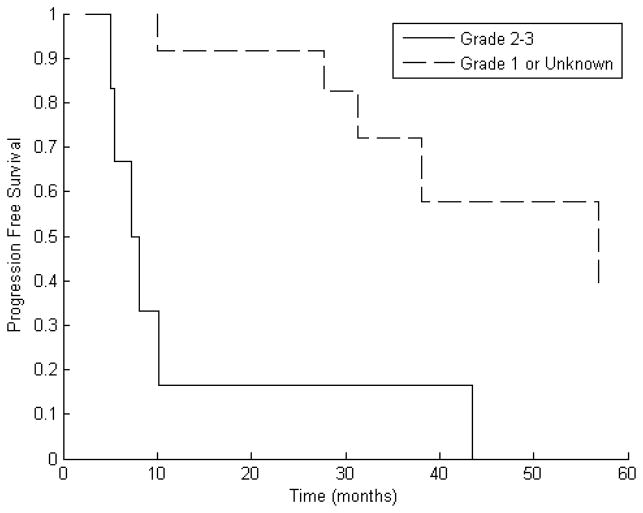
An analysis was performed to determine factors that may have a role in determining a patient’s likelihood of experiencing recurrence after reirradiation. GTV, type of radiation received (fSRT or SRS), interval between RT courses, tumor grade, timing of surgery, dose, gender, and age were all considered. Of these, only tumor grade was significantly correlated with PFS at one year. Compared to patients with grade I disease or unknown tumor grade, patients with grade II or III tumors demonstrated inferior PFS at one year (17% compared to 92%, p = 0.005 using Fisher Exact Test). Kaplan–Meier estimates for median PFS were eight months for the high-grade group and 57 months for other patients (Fig. 3). Cox Proportional Hazards modeling demonstrated that the presence of grade II or III disease significantly worsens PFS (hazard ratio [HR] = 5.37, 95% confidence interval [CI] 2.78–10.40, p = 0.011).
Figure 3.
Kaplan–Meier curve of progression free survival (PFS) separated by tumor grade showing that estimates for median PFS were eight months for patients with high-grade tumors and 57 months for other patients.
At the time of this analysis, eight of the 19 patients had died. The Kaplan–Meier estimate for median survival time was 90 months. Tumor grade did not significantly affect overall survival (HR = 2.11, 95% CI 2.11–8.25, p = 0.310). There were no reported serious (grade ≥ 3) toxic events attributed to reirradiation.
4 Discussion
Meningioma is traditionally thought of as a highly curable disease. When it is treated with radiotherapy, five-year PFS rates of 98% at five years7 and 92% at 10 and 15 years8 have been reported. Thus, the question of reirradiation for recurrent disease is relatively rarely encountered. To our knowledge, this is one of the first series describing the results of reirradiation for meningioma.
In this single-institution study, we looked at the PFS of patients with meningioma recurrent after RT. Recurrence was noted in 58% of patients after reirradiation, with a median time to recurrence of 10 months. The major predictor of PFS was tumor grade. For the 12 patients with low-grade or unbiopsied tumors, reirradiation was fairly effective, with Kaplan–Meier PFS estimates of 8% at one and two years. Six patients, however, experienced disease progression during follow-up.
Unfortunately, we detected disease recurrence in six out of seven patients with WHO grade II/III disease following a second course of RT. Furthermore, five of these recurrences occurred within one year of reirradiation. This is in agreement with a study by Mattozo et al. in 2007. In their series, six patients with grade III disease or evidence of malignant progression showed a similarly poor PFS of 11% at three years after reirradiation for recurrent meningioma 9.
As patients with recurrent, high-grade meningioma seem to have a poor prognosis, further investigation of treatment options is warranted. A report by Hug et al. in 2000 looked at a dose response for atypical and malignant meningiomas, finding that patients treated with combined photon/proton dose of at least 60 Gy achieved superior local tumor control compared to those who received a dose of less than 60 Gy 10. Kano et al. 11 used SRS as salvage therapy for recurrent tumors after surgical failure and showed a dose-dependent improvement in five-year PFS of patients with atypical or anaplastic meningioma with increasing dose. PFS increased from 29.4% to 63.1% when recurrent high-grade meningiomas were treated by SRS with a marginal dose exceeding 20 Gy compared to 15 Gy. Median GTV in that series was 4.4 cm3 compared to 12.9 cm3 in our series. Delivery of such a high single-fraction dose, especially in the setting of reirradiation, may not be safe with larger tumors or with tumors adjacent to critical normal structures, which might have received significant doses from the first course of RT. In retrospect, the subset of our patients who were known to have high-grade tumors at the time of their first RT course and received conventional doses (median SRS = 15 Gy, median fSRT = 55 Gy) may have benefitted from dose escalation. The currently active protocol RTOG 0539 incorporates dose escalation to 60 Gy for high risk patients with recurrent WHO grade II or grade III disease.
There are several weaknesses to this study that must be addressed. First, this is a retrospective review of a highly selected patient population. The WHO Grading Criteria for meningioma changed over time, being first characterized in 1993 and under revision in 2000 and 2007. Major revisions occurred in 2000, and upon reevaluation of old specimens, the prevalence of grade II meningiomas increased by 25% 12. Only minor changes to the grading criteria were made between 2000 and 2007. While these changes may have affected the grading of patients in our series, as the grading of meningiomas is still rather subjective with a significant amount of interobserver variability 13 and our findings rely on the simpler distinction of “benign versus non-benign”, we believe our conclusions are still valid, since a significant difference is seen between those two groups. Second, although we found no evidence of grade 3 or higher toxicity in our series, we are hesitant to comment on the rate of lower-grade complications, as they may not have been reliably recorded. Inherent to this retrospective review is an element of selection bias; reirradiation would not have been attempted in patients where it was deemed unsafe by the treating physicians.
As tumors are likely to recur in patients with high-grade tumors, other options beyond dose escalation should be considered in these patients. Although meningiomas are considered traditionally to be poorly chemo-responsive, aggressive treatment with radiosensitizing agents and chemotherapeutics can be considered in the management of patients in whom therapy has so-far failed. There has been some limited success with interferon alpha-2B treatment for recurrent disease13. Although temozolomide has been effective in the treatment of glioblastoma, it has not been in the treatment of meningioma 14. Hydroxyurea, however, has had some success in patients with meningiomas who are not amenable to other forms of therapy15. Future work exploring the effect of such radiosensitizing agents in patients with recurrent high-grade disease would be worthy of exploration.
5 Conclusions
Overall, we found that that WHO tumor grade is the most important factor for determining the prognosis of patients with recurrent meningioma treated with reirradiation. Patients with grade I disease (or unknown tumor grade) achieve an extended PFS following reirradiation and therefore should be offered such treatment. Conversely, patients with recurrent grade II/III disease are much more likely to have further progression even after a second course of RT, and might benefit from being offered investigational treatment options and enrollment in clinical trials.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Central Brain Tumor Registry of the United States; 2009. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2005 [database on the Internet] Available from: http://www.cbtrus.org. [Google Scholar]
- 2.Mirimanoff RDD, Linggood R, et al. Meningioma: analysis of recurrence and progression following neurosurgical resection. Journal of Neurosurgery. 1985;(62):18–24. doi: 10.3171/jns.1985.62.1.0018. [DOI] [PubMed] [Google Scholar]
- 3.Davidson L, Fishback D, Russin JJ, et al. Postoperative Gamma Knife surgery for benign meningiomas of the cranial base. Neurosurg Focus. 2007;23(4):E6. doi: 10.3171/FOC-07/10/E6. [DOI] [PubMed] [Google Scholar]
- 4.Miralbell R, Linggood RM, de la Monte S, et al. The role of radiotherapy in the treatment of subtotally resected benign meningiomas. J Neurooncol. 1992;13(2):157–64. doi: 10.1007/BF00172765. [DOI] [PubMed] [Google Scholar]
- 5.Taylor BW, Jr, Marcus RB, Jr, Friedman WA, et al. The meningioma controversy: postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1988;15(2):299–304. doi: 10.1016/s0360-3016(98)90008-6. [DOI] [PubMed] [Google Scholar]
- 6.Louis DN International Agency for Research on Cancer., World Health Organization. WHO classification of tumours of the central nervous system. 4. Lyon: International Agency for Research on Cancer; 2007. [Google Scholar]
- 7.DAGBWWWCL Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. Journal of Neurosurgery. 1994 Feb;80(2):195–201. doi: 10.3171/jns.1994.80.2.0195. [DOI] [PubMed] [Google Scholar]
- 8.WAMWMCARFKF Radiotherapy alone or after subtotal resection for benign skull base meningiomas. Cancer. 2003 Oct 1;98(7):1473–82. doi: 10.1002/cncr.11645. [DOI] [PubMed] [Google Scholar]
- 9.Mattozo CA, De Salles AA, Klement IA, et al. Stereotactic radiation treatment for recurrent nonbenign meningiomas. J Neurosurg. 2007;106(5):846–54. doi: 10.3171/jns.2007.106.5.846. [DOI] [PubMed] [Google Scholar]
- 10.Hug EB, Devries A, Thornton AF, et al. Management of atypical and malignant meningiomas: role of high-dose, 3D-conformal radiation therapy. J Neurooncol. 2000;48(2):151–60. doi: 10.1023/a:1006434124794. [DOI] [PubMed] [Google Scholar]
- 11.Kano H, Takahashi JA, Katsuki T, et al. Stereotactic radiosurgery for atypical and anaplastic meningiomas. J Neurooncol. 2007;84(1):41–7. doi: 10.1007/s11060-007-9338-y. [DOI] [PubMed] [Google Scholar]
- 12.Commins DL, Atkinson RD, Burnett ME. Review of meningioma histopathology. Neurosurg Focus. 2007;23(4):E3. doi: 10.3171/FOC-07/10/E3. [DOI] [PubMed] [Google Scholar]
- 13.Kaba SE, DeMonte F, Bruner JM, et al. The treatment of recurrent unresectable and malignant meningiomas with interferon alpha-2B. Neurosurgery. 1997;40(2):271–5. doi: 10.1097/00006123-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Chamberlain MC, Tsao-Wei DD, Groshen S. Temozolomide for treatment-resistant recurrent meningioma. Neurology. 2004;62(7):1210–2. doi: 10.1212/01.wnl.0000118300.82017.f4. [DOI] [PubMed] [Google Scholar]
- 15.Newton HB. Hydroxyurea chemotherapy in the treatment of meningiomas. Neurosurg Focus. 2007;23(4):E11. doi: 10.3171/FOC-07/10/E11. [DOI] [PubMed] [Google Scholar]